Abstract
Investigations into the role of microRNA (miRNA) in hepatitis C virus (HCV) infection, disease pathogenesis and host immune and treatment response have potential to produce innovations in diagnosis, prognosis and therapy. However, investigational challenges remain in generating clinically useful and reproducible results. We review the literature with a primary emphasis on methods and technologies used to construct our current understanding of miRNA and HCV disease. A second emphasis is to understand potential clinical research applications and provide clarification of previous study results. Many miRNA have key roles in viral and immunopathogenesis of HCV infection across multiple tissue compartments. Controversy exists among published studies regarding relative measurements, temporal changes and biological significance of specific miRNA and HCV infection. To reconcile diverging data, additional research into optimal sample processing, in vitro models, techniques for microarray differential expression of miRNAs, practices for sample result normalization, and effect of HCV genotype variation on expression are all necessary. Microarray and miRNA isolation techniques should be selected based on ability to generate reproducible results in the sample type of interest. More direct comparisons of efficacy and reliability of various multiplex microarrays and an improved consensus around miRNA normalization and quantitation are necessary so that data can be compared across studies.
Keywords: Hepatitis C, microRNA, inflammation, antiviral agents, review
Introduction
MicroRNAs (miRNA) are small ~22 nucleotide RNAs that are known to regulate gene transcription in all animals via interaction with messenger RNA (mRNA)(Bartel, 2004). At the time of this writing, over 2500 mature human miRNA have been isolated and described in a public database, miRBase (Kozomara and Griffiths-Jones, 2011; “miRBase 20,” 2013). Hepatitis C virus (HCV) is an RNA virus known to be a leading cause of liver disease, affecting approximately 185 million people worldwide (Mohd Hanafiah et al., 2013). Emerging literature describes the role of miRNA in HCV pathogenesis, host immunologic response and progression to cirrhosis and hepatocellular carcinoma (HCC).
The role of miRNA in HCV infection has been reviewed previously (Conrad and Neipmann, 2013; Gottwein, 2013; Gupta et al., 2012; Hoffman et al., 2012; Kerr et al., 2011; Shrivastava et al., 2013a; Takahashi et al., 2013). However, the technology to detect and quantify more miRNA and investigate their targets is rapidly evolving, as is the number of studies interrogating the precise details of a complex network of miRNA regulation that occurs across cell compartments. Changes in gene transcription regulated by miRNA and provoked by HCV may also explain HCV’s predilection for causing fibrosis and carcinoma in liver tissues. However, as diverse methods and techniques for miRNA detection and quantitation are employed, discrepancies among published results are emerging. We review the literature with a primary emphasis on the methods and technologies used to construct the current understanding of miRNA and HCV disease. Our goal is to provide a better understanding of what is currently known regarding best practices for investigation. A secondary goal is to provide an understanding of current miRNA-HCV research, and where opportunities for novel investigation and clarification of previous studies lie.
Methods
A PubMed and Medline search for medical subject headings (MeSH) “Hepatitis C” and “microRNA” on 11/3/13 revealed 270 papers, of which 55 papers were selected for further review as they tested hypotheses relevant to novel miRNA mediation of HCV infection, its pathogenesis and host response with experimental design (Supplemental table 1). Excluded were reviews, other published papers that did not involve primary research, and studies that did not have novel aspects of both HCV disease and miRNA in humans as their primary focus of investigation. This review identified 87 miRNA from 73 miRNA families (groups of miRNA that vary in sequence by only 1–2 nucleotides) that may have a role in HCV lifecycle, pathogenesis, host response to infection, and detection of various disease states relevant to HCV infection (Supplemental table 2). The specific miRNA under investigation, study populations, cell types, experimental models and study designs were abstracted, as were the specific technologies used to assay and count miRNA. Data from papers under review were used to illustrate what is currently proposed regarding miRNA-mediated effects of HCV infection in host cell compartments (Tables 1–3; Figures 1 and 2).
Table 1.
A Summary of The Possible Role of miRNA in Infection, Pathogenesis, and Treatment with Relevant Hypothesized Gene Targets.
| Immune Response to infection |
Fibrosis of Liver |
Oncogenesis | Direct effect on HCV lifecycle |
|
|---|---|---|---|---|
| Expected Change in miRNA Level with HCV Promotes*#& |
Hepatocytes: miR-1⇑(unknown) Let7b⇑( IGF2BP1) miR-142⇑(unknown) miR-196⇑(Bach1) miR-224⇑(OCLN) PBMC: miR-30⇑(IL28RA) miR-125⇓(TLR4) miR-128⇑(Unknown) miR-146a⇓(STAT1,IL 6,8, CCL8, CD40L) miR-155⇑(SOCS1) miR-196⇑(unknown) miR-296⇑(unknown) Plasma: miR-128⇑ miR-155⇑ |
Hepatocytes: miR-21⇑(SMAD7) miR-23b⇓(LHFPL2, CCDC62, ERBB2IP) miR-24⇓(SMADs) miR-27a⇑(PPARα) miR-30⇓(unknown) miR-34a ⇑(FUT8, MGAT4A, RCAN1) miR-92⇑(unknown) miR-155⇑(SEMA3A) miR-199,200⇑ (TGIF,SMAD,SMURF2) miR-449a⇓(NOTCH1) miR-455⇓(unknown) Stellate Cells: miR-29⇓(COL1A1,3A1) miR-214-5p⇑ (unknown) miR-221,222⇑(CDKN1B) miR-571⇑(CREBBP) PBMC: miR-652⇓(unknown) Plasma: miR-34a⇑ |
HCC: miR-10a⇑(unknown) miR-21⇑ (PDCD4,PTEN,TIMP3,RECK) miR-100⇑(unknown) miR-122⇑(unknown) miR-124⇓(SMYD3) miR-145⇓(unknown) miR-198⇓(unknown) miR-222⇑(unknown) miR-223⇓(STMN-1) miR-515⇑(unknown) miR-518⇑(unknown) miR-520f⇑(unknown) miR-525⇑(unknown) Hepatocytes: miR-141⇑(DLC-1) miR-155⇑(APC) miR-345⇑(p21) miR-491⇓(PI3k/akt) Plasma: miR-21⇑ |
Hepatocytes: miR-24⇓(unknown) miR-27a⇑(PPARα) miR-30⇓(unknown) miR-122⇑(HCV IRES) miR-128⇓(unknown) miR-149⇑(AAK1, PHLPPL) miR-194⇓(CLDN-1, OCLN) miR-351⇓(unknown) miR-431⇓(unknown) miR-448⇓(unknown) miR-638⇑ (NEUROD2, H1FX) miR-1181⇑(STARD10, NPAS4) |
| Expected Change in miRNA Level with HCV Counteracts*#& |
Hepatocytes: miR-16⇑(MAPK14,BID) miR-18a⇑(IRF1,2,4,6,7) miR-21⇑ (MyD88,IRAK1) miR-34b⇑ (IRF1,2,4,6,7) miR-125b⇑(TLR4) miR-128⇓( CAMTA1, CAST, PARK7, REPS1, RND3, SAA4) miR-130a⇑(IFITM1) miR-143⇑( BCL2, RARA, SMAD2) miR-145⇑(IRF1,2,4,6,7) miR-146⇑(STAT1,IL 6,8) miR-155⇓(SEMA3A) miR-296⇓(unknown) Plasma: miR-16⇑ miR-125⇑ miR-146⇑ |
Hepatocytes: Let-7e⇑(TLR4) miR-19a⇑(EGFR) miR-20a⇑(TGFβ, VEGF) miR-146a⇑(SMAD4, EGFR) miR-361⇑(MGAT4A) |
HCC: miR-193b⇑(Mcl-1) Hepatocytes: miR-199⇑(PAK4) |
Hepatocytes: miR-1⇑(unknown) Let-7b⇑(HCV NS5A, HCV IRES) miR-196⇑(unknown) miR-199⇑( HCV IRES) miR-296⇑(unknown) |
Relative increase or decrease in miRNA in given tissue type denoted by arrows
Proposed relevant gene target in parenthesis, targets may be different for same miRNA depending on tissue type and disease process in question.
Definitions of increase and decrease vary based on the study in question. Many studies used significant differences defined p <.05 vs. healthy comparators as defining characteristic. However, some studies used fold change cut points to define up and down regulated. These ranged from fold changes of +/− 1.2 to +/− 2.0.
Table 3.
Possible Baseline miRNA Changes That May Be Predictive Of Treatment Failure or Fibrosis
| Treatment failure | Fibrosis | |
|---|---|---|
| Relative upregulation at baseline leads to outcome* |
Hepatocytes: miR-18a, miR-34b, miR- 143, miR-145, miR-652 PBMCs: miR-296 |
Plasma: miR-20a, miR-34a, miR-92a Hepatocytes: miR-34a, miR-92a, miR- 155, miR-222 |
| Relative downregulation at baseline leads to outcome* |
Hepatocytes: miR-27b, miR-122, miR- 148a, miR-378, miR-422b, miR-652 PBMCs: miR-128, miR-196 Serum: miR-122 |
Hepatocytes: let-7e, miR-19a, miR- 20a, miR-23b, miR-27b, miR-30b/c, miR-146a, miR-361, miR-455 |
Definitions of upregulation and downregulation vary based on the study in question. Many studies used significant differences defined p <.05 vs. baseline as definition of up and downregulation However, some studies used fold change cut points to define up and down regulated. These ranged from fold changes of +/− 1.2 to +/− 2.0.
Figure 1.
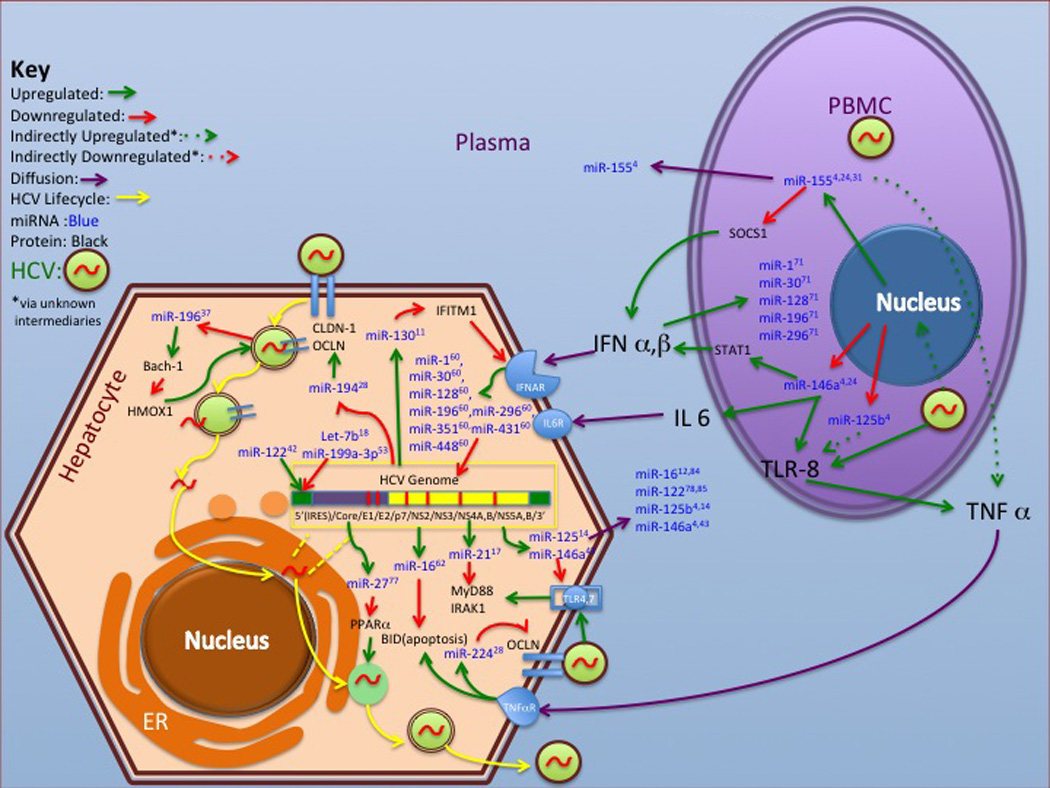
MicroRNA Involved in the HCV Life Cycle and Immune Response
Figure 2.
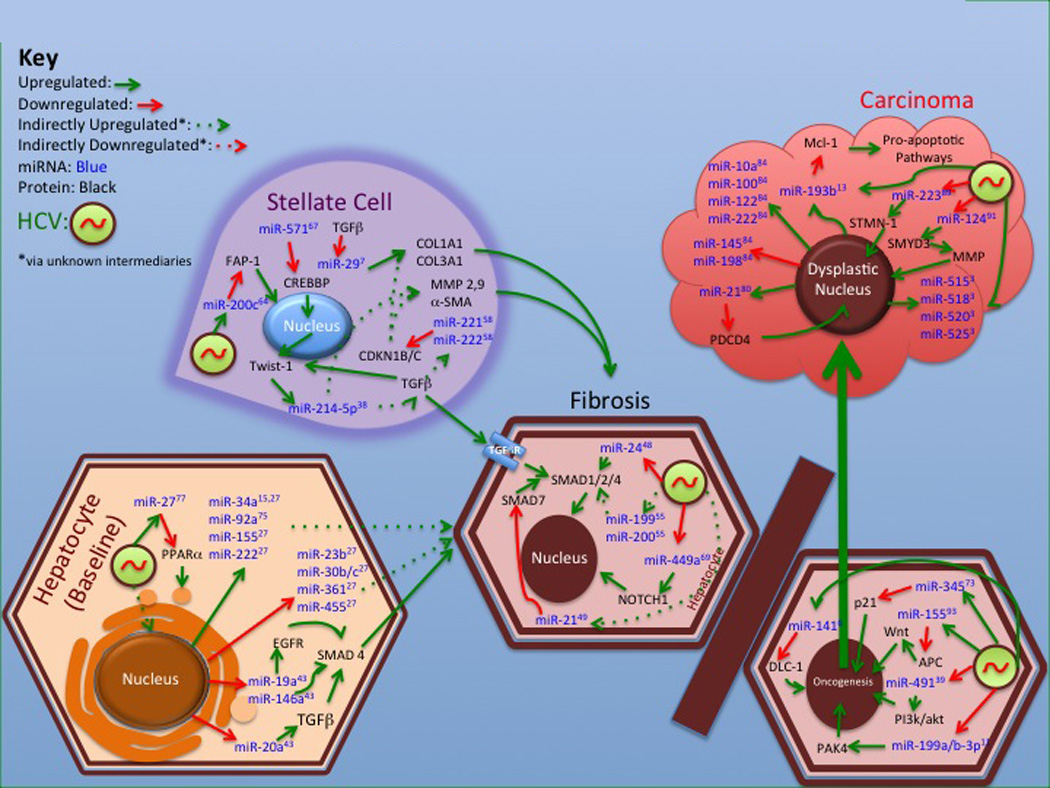
MicroRNA involved in progression to Fibrosis and Carcinoma in HCV disease
miRNA biogenesis, nomenclature and HCV lifecycle review
Biogenesis of miRNA and HCV lifecycle in humans have been reviewed extensively elsewhere, and will only briefly be discussed (Bartel, 2004; Ray and Thomas, 2010; Shwarz et al., 2003). MiRNA are transcribed, often in polycistronic form as single stranded hairpin RNA known as pri-mRNA, which are cleaved in the nucleus by Drosha (a Class 2 RNase III enzyme) into 60–90 base pair pre-miRNAs, and actively exported out of the nucleus by Exportin 5 (Bartel, 2004). Once in the cytoplasm, pre-miRNA are processed by Dicer (an endoribonuclease) into 20–22 base pair double-stranded RNA fragments. The active or “guide” strand is separated and loaded onto the RNA-induced silencing complex (RISC), which facilitates its interaction with target mRNA. The excess strand or “passenger” strand is most often degraded, but in some cases is loaded on to RISC itself in relatively low abundance having its own targets for regulation (Bartel, 2004; Shwarz et al., 2003). Based on complementarity with its target, the target is either cleaved or degraded by RISC (if perfect complementarity) or its translation is simply repressed (if imperfect complementarity). In this manner, each miRNA can target and down-regulate multiple mRNAs therefore modulating the expression of many genes (Shwarz et al., 2003).
MiRNA nomenclature is sequential numbering from the time of discovery. The first designation is the species from which it is isolated (i.e., has in humans). The next prefix is miR, with the exception of let and lin families whose discovery predated systematic miRNA naming (Kozomara and Griffiths-Jones, 2011). The number may then refer to either a single miRNA or a “family” of miRNA, which are groups based on shared sequences with 1–2 nucleotide variations, and designated by a letter appended to the number. If the passenger strand is preserved and is actively regulating genes, it can be designated one of two ways. The older designation was to append a “*” after the number of its higher abundance complementary strand, and was then known as the “star form” of the higher abundance miRNA molecule. To remove the issue of abundance, this designation is now made by noting whether the molecule is from the 5’ or 3’ end of the original hairpin pre-RNA by attaching the suffix 3p or 5p as appropriate (Kozomara and Griffiths-Jones, 2011; “miRBase 20,” 2013).
HCV is a single stranded positive sense RNA virus with a 9.6 kb genome and a 9.0 kb open reading frame (ORF). HCV enters host cells via binding to one of several cell surface receptors including claudin-1 (CLDN-1), occludin (OCLN), cluster of differentiation 81 (CD81), scavenger receptor class BI (SR-BI) and low-density lipoprotein receptor (LDL-R) and is taken up by endosomes inside the cell (Ray and Thomas, 2010). HCV uses various receptors for entry allowing it access to tissue compartments beyond the liver. The most notable extra-hepatic cellular reservoir is peripheral blood mononuclear cells or PBMCs, e.g., B and T lymphocytes and monocytes, although it is controversial as to whether active viral replication occurs in these cells or if the presence of virus is due to passive diffusion (Bernardin et al., 2008; Chary et al., 2012; Fujiwara et al., 2013; Radkowski et al., 2005; Ray and Thomas, 2010). After incorporation into an endosome, the decreased pH causes a conformational change in viral envelope proteins, resulting in fusion with the endosomal membrane and extrusion of viral RNA into the cytoplasm at the rough endoplasmic reticulum where it is translated by host ribosomes. The 5’ untranslated end of HCV includes a 300-nucleotide internal ribosome entry site (IRES) essential for loading into host ribosomes. The HCV ORF encodes a single polyprotein co-translationally processed into 10 proteins. These include 3 structural proteins, a Core Protein (C), 2 envelope proteins (E1, E2), 2 proteins (p7 and NS2) necessary for virion construction, and 5 proteins encoding the viral replicase complex (NS3, NS4A/B, NS5A/B) (Ray and Thomas, 2010). After translation and protein processing, the viral replicase complex produces new viral RNA, which is then loaded into nascent virions, forming lipid droplets on the endoplasmic reticulum; a process mediated by host enzymes including retinoid X receptor α (RXRα) and peroxisome proliferator-activated receptor α (PPARα) (Shirasaki et al., 2013; Singaravelu et al., 2013). HCV is then released outside the cell via the vesicular secretory network. There are 7 HCV genotypes with numerous subtypes, which vary regarding virulence and susceptibility to treatment. Exogenous pegylated interferon-α (IFN-α) combined with ribavirin has been standard HCV therapy for many years (Ray and Thomas, 2010). However, several novel directly acting antivirals (DAAs) have been approved (including HCV protease and polymerase inhibitors), and many more are in development and are expected to result in the disappearance of interferon-based regimens (Lawitz et al., 2013).
Investigational Challenges: Cell Line Systems and Disease Modeling
It is evident that human miRNA are essential for HCV pathogenesis and host immunologic response to infection (figure 1, table 1). Effects of miRNA on the HCV lifecycle, particularly miR-122, has been ably reviewed elsewhere and will be discussed briefly to provide context and review subsequent relevant studies (Conrad and Niepmann, 2013; Gottwein, 2013; Kerr et al., 2011; Shrivastava et al., 2013a). Jopling et al. (2005) used HCV genotype 1b transfected human hepatoma cell lines (Huh 7 replicons) to show that miR-122 was necessary for HCV replication by using a specific miR-122 inhibitor and demonstrating loss of infectivity. They also noted that miR-122 had complimentarity with the HCV RNA 5’ UTR and proposed that this interaction was stabilizing and facilitated interaction of IRES with host ribosomes, allowing viral genome translation. This realization that host miRNA were utilized by HCV to sustain itself, led to subsequent research based on the hypothesis that HCV modulated changes in hepatocyte transcription that were favorable to it and allowed for continual infection and evasion of host immunity.
One important aspect of HCV therapy is that it utilizes a pharmacologic form of an endogenous mammalian protein, IFN-α, to induce the host to clear HCV. Acknowledging prior work in plants and invertebrates demonstrating that interfering RNA was utilized in those hosts as a direct antiviral, Pederson et al. (2007) hypothesized that human interferon response utilized miRNA in a similar way. Using a microarray they developed, they noted that both primary murine hepatocyte cultures and Huh 7 cells exposed to interferon α and β upregulated 8 miRNA (miR-1, miR-30, miR-128, miR-196, miR-296, miR-351, miR-431 and miR-448) that had complimentarity in their binding sequences with key portions of the HCV genome. They then used Huh 7.5 replicons with variants of a JFH-1 genotype 2a version of HCV to show that replication decreased with transfection of mimics of these 8 miRNA, and that mutations in relevant regions of HCV caused a loss of this inhibitory effect in at least 2 of the 8 (miR-196 and miR-448).
These experiments provided a template for further research, using HCV replicon systems in permissive Huh 7.5 cells to extrapolate the role of miRNA in the HCV lifecycle and immune response. Huh 7.5 cell lines with JFH-1 and other full genome viral clones have been a solution to the intractability of successfully establishing in vitro HCV models using primary hepatocyte cultures (Lindenbach et al., 2005). Hypothesizing that miRNA can have several related targets of regulation that cause a multiplicative effect, Hou W et al. (2010) noted that miR-196 also had sequence complimentarity with a basic leucine zipper mammalian transcriptional repressor (Bach-1) gene, which itself downregulated expression of heme oxygenase decycling 1(HMOX-1). The resulting upregulation of HMOX-1 caused changes in intracellular oxygen tension, which were less favorable to HCV infectivity. They then used a HCV replicon luciferase reporter assay to confirm their hypothesis. Downregulation of miR-196 expression may be one way HCV evades the immune response. Using Huh 7 cells with both sub-genomic and full genome replicons, Murakami Y et al. (2009) used miR-199-3p mimics and inhibitors to show that miR-199a-3p overexpression decreased HCV infectivity. In silico, they showed that miR-199-3p had a possible target on domain II of the IRES.
However, presence of a binding sequence within HCV RNA may not necessarily mean that the primary effect of a specific miRNA is by direct binding with the viral genome. Using Huh 7.5 cells, there is growing evidence that let-7b plays a role in host antiviral response, but there is controversy in understanding the exact nature of how it mediates this response. Cheng JC et al. (2012) noted in silico that the HCV genome had a let-7b recognition sequence on its 5’ end. They then used luciferase reporter assays showing that let-7b mimics decreased NS5A expression, and therefore HCV replication, the opposing effect to miR-122. The let-7b effect potentiated the action of IFN-α in reducing HCV infectivity. Cheng M. et al. (2013) used a Huh 7.5 replicon system before and after IFN-α and/or IL-28 treatment to broadly assess differential expression of 750 miRNA. They found that let-7b expression correlated with IFN-α and IL-28 effects in reducing HCV replication, and used in silico target scanning to identify insulin-like growth factor 2 mRNA-binding protein 1(IGF2BP1), a growth factor thought to mediate HCV-IRES interaction with the host ribosome as its target. They theorized that let-7b’s HCV inhibitory effect on HCV replication may be secondary to downregulation of IGF2BP1 and showed this with a luciferase assay. From these two studies, it is unclear if let-7b’s effect is due to direct binding with viral RNA or from regulating transcription of IGF2BP1, although these hypotheses are not mutually exclusive. MiRNA gene targets involved in the pathogenesis of HCV are often difficult to assess because each miRNA can have complimentarity with multiple targets, allowing each miRNA to regulate expression of several genes. As these two and other studies show, it is often necessary for researchers to identify genes of interest and analyze for potential matching with miRNA in silico and then confirm hypotheses in vitro, although this methodology can introduce confirmatory bias.
Although studies often use similar cell models, use of different techniques and technologies for assessment of effect have led to diametrically opposing conclusions in some cases. It had been noted using RT-PCR methods in human liver tissue that miR-27a and b appeared upregulated in HCV infection more so than in hepatitis B virus (HBV) infection and that the miRNA 27 family appeared to actively regulate a suite of genes involved in both regulating and promoting lipogenesis (Shirasaki et al., 2013; Ura et al., 2009). Shirasaki et al. (2013) attempted to delineate specific actions of miR-27a by transfecting Huh 7.5 cells with both mimic and inhibitory molecules and JFH-1 and H77Sv2 Gluc2A viral clones and showed that miR-27a appeared to significantly decrease HCV infectivity and intracellular lipid concentrations, and theorized that overexpression of miR-27a was a mechanism of host antiviral response. However, in a separate study, Singarvelu et al. (2013) also used Huh 7.5 cells with JFH-1 replicons and although they confirmed that transfection of miR-27 family mimics decreased abundance of HCV, they also noted that overproduction of miR-27 appeared to be directly induced by viral proteins, not by any component of the host immune system. They also used a different technique, coherent anti-stokes Raman scattering (CARS), a laser-tweezer based microscopy system that uses Raman spectroscopy imaging to assess molecular content of cells and noted that intracellular lipid concentration appeared increased instead of decreased with miR-27 transfection and theorized that this was due to regulation of PPAR-α, a negative regulator of triglyceride synthesis which increased hepatic steatosis. The authors were aware of the Shirasaki study and theorized that the difference was cell line specific due to shifts in cellular metabolism in the prior study.
Although permissive Huh 7 and Huh 7.5 cell lines infected with HCV clones are important models for generating hypotheses and delineating miRNA function in relation to HCV infection in laboratory controlled settings, they are both physiologically different from primary hepatocytes and exist outside of the normal cellular milieu of ex-vivo samples. Bruni et al. (2011) directly compared 3 different cell lines transfected with full-length genotype 1b (Sfl) replicon HCV and measured miRNA expression versus uninfected Huh 7 cells before and after interferon-β stimulation using a TaqMan PCR based array. There were discordant variations in miRNA expression between cell lines, although three miRNA of most interest, 128a, 196a and 142-3p, had concordant expression across the three different replicon containing cell lines. These three miRNA were differentially regulated by HCV infection, but this differential regulation was reversed by interferon-β administration. These data suggests that these miRNA may be differentially regulated by HCV to evade immunity and may be mediators of response to exogenous interferon, but the study also suggests that there may be variation between Huh 7 and 7.5 replicon systems.
Another possibility in modeling in vitro HCV infection, which may reconcile the difference between cell line models and in vivo hepatocytes by using more physiologically similar cells, is to use primary hepatocyte cultures. Although permissive, Huh 7 and Huh 7.5 cells have been utilized in much of the in vitro HCV literature due to the difficulty of successfully inoculating and culturing primary hepatocytes. Banaudha et al. (2010) did report the ability to do this with full-length HCV clones of genotype 1a, 1b and 2a by using CD81+ primary hepatocyte cell lines. The same group of authors (Banaudha et al., 2011) then used a 355 miRNA array with a Klenow enzyme-based PCR assay to examine primary hepatocyte cultures transfected with those three genotypes, and found that miR-141 had a significant 4-fold increase with transfection of genotype 1a and b virus. Subsequent in silico analysis identified a tumor suppressor (Deleted in Liver Cancer 1, DLC-1) as a likely target, and this was confirmed in primary hepatocytes with a luciferase reporter assay. These findings add an additional layer of complexity to the HCV and miRNA relationship suggesting that viral genotype may be a key factor in provoking changes in miRNA expression. However, the finding of miR-141 upregulation in HCV infection has yet to be confirmed in human hepatic tissue samples or other cell line models. Genotype specific effects on replicon systems may also confound generalizability. Gu et al. (2012) transfected Huh 7 cells with plasmids containing cDNA for core protein from both genotype 1b and 3a HCV and noted that differential regulation as measured by chip-based microarrays were different for 16 miRNA between the genotypes.
Challenges in Investigation: Assaying Differential Expression in Host Tissues
Identifying miRNA of significance in human samples is clearly preferable and necessary for translational applications, but given the difficulty of accurately measuring and assessing function in tissue samples, this can be challenging. Using broad multiplex assays to identify miRNA of interest and generate hypotheses by evaluating the differential expression of specific miRNA in tissue beds of choice and assessing fold changes relative to the comparator of choice can be an important but possibly limited tool. Of the 53 papers reviewed 25 used multiplex arrays (supplemental table 1). Currently, a standard approach is to confirm any differential regulation signal of interest found on multiplex arrays using qRT-PCR with specific primers for the miRNA warranting further investigation (supplemental table 1). However, it is not always clear that results across modalities are comparable.
Comprehensive comparisons of various microRNA arrays and detection technologies have been published (Git et al., 2010; Sato et al., 2009; Wang et al., 2011). Three broad classes of oligonucleotide arrays are generally used for miRNA profiling that vary in their use of carriers and amplification: chip or slide-based microarrays in which dye-labeled cDNA or cRNA are hybridized to detection probes on flat glass, silicon or nylon array carriers; bead-based arrays in which capture probe-coated beads are used to hybridize biotinylated miRNA, and PCR-based arrays generally with microfluidic cards with up to 384 wells on each card (Wang et al., 2011). Of the 25 papers using multiplex arrays reviewed here, 12 used slide or chip-based hybridization microarrays from various vendors, predominantly Agilent, Affymetrix, CapitolBio, Exiqon and Toray, one used a bead-based hybridization array from Illumina (which uses PCR product as its hybridization substrate), and 12 used PCR-based arrays, predominantly TaqMan low density arrays from Applied Biosystems. Each of these technologies has their unique strengths and limitations for miRNA. For both slide and bead-based arrays, the short length of miRNA often requires the entire sequence to be used as a probe, which can cause melting temperatures to vary by over 20°C (Git et al., 2010). This can cause particular difficulty in distinguishing between different members of miRNA families using hybridization based assays which require high enough temperatures to limit confounding by cross-hybridization (Git el al., 2010; Sato et al., 2009). Accurate and reproducible quantification using PCR-based assays are limited by biases induced by RNA ligation and amplification steps (Git et al., 2010). A published comparison by Wang et al. (2011) of these three general types of arrays found the best signal to noise ratio and intraplatform reproducibility for the bead-based array assessed as compared to slide-based and PCR arrays, although trends in expression were more consistent in the slide-based array. However, the 3 assays selected were not necessarily representative of their respective technologies. Somewhat concerning was the low degree of interplatform consistency demonstrated by this study particularly between PCR arrays and the other technologies, which indicates that cross-platform comparisons likely cannot be made reliably.
It is also clear that selecting an appropriate normalization algorithm for miRNA microarray data is crucial for obtaining biologically valid results. A comparative study by Sato et al. (2009) of normalization techniques demonstrated that results varied significantly depending on the technique chosen and showed that LoessM normalization yielded the most comparable results between platforms. LoessM normalization is a modified non-parametric regression method that differs from the standard Loess algorithm in that it scales expression data on the global median expression rather than on zero. Within the glass slide-based hybridization array category, inter-platform data may be more comparable. A study by Meyer et al. (2012) demonstrated that results were similar for over 300 miRNAs between Agilent, Ambion, Invitrogen, Exiqon and Toray brand microarrays. The significance of differential regulation with multiplex arrays across miRNAs is not uniform, however. The significance of fold changes may vary with the abundance of the miRNA in question. For more abundant miRNA, small fold changes may be biologically significant, but for less abundant miRNA, larger fold changes may not matter (Chugh and Dittmer, 2012).
Another potential issue with producing reproducible results from both multiplex and single miRNA techniques is the variety of miRNA isolation technologies available, which can result in widely different amounts and purity of extracted miRNA as well as their compatibility with various microarrays (Eldh et al., 2012; Hammerle-Fickinger et al., 2010; Nanostring Technologies, 2012). Three broad types of isolation techniques have been used in the reviewed papers: phenol based (TRIzol- Invitrogen), column based (RNeasy- Qiagen, modified RNeasy- Qiagen, miRCURY- Exiqon), or combined phenol and column separations (TRIzol+clean up- Invitrogen, miRNeasy- Qiagen, mirVana isolation - Ambion, mirVana PARIS - Ambion) (Eldh et al., 2012). Isolation of miRNA is complicated by the small size of miRNA, and in extracellular fluid such as blood serum and plasma, or the presence of miRNA in exosomes, which can be difficult to lyse further complicating isolation (Eldh et al., 2012; Hammerle-Fickinger, 2010). Depending on the array used for assaying the miRNA, phenol or other contaminants from collection of tissue or blood including heparin or citrate may interfere with hybridization and confound results (Hammerle-Fickinger, 2010; Nanostring Technologies, 2012). Some isolation methods may be optimized for isolation from cells, others may be more efficient for exosome rich fluid such as serum or plasma (Eldh et al., 2012). There is some published evidence from mouse mast cells and bovine serum that column based methods are more effective for miRNA extraction from exosome rich fluid, and that combined phenol /column based methods are effective in extraction from cells (Eldh et al., 2012; Hammerle-Fickinger, 2010). To produce optimal results, researchers must choose a method and vendor demonstrated to be optimum for the particular sample type and array of choice. No direct comparison of extraction methods has been made in the setting of HCV infection.
An example of the strengths and limitations of multiplex microarrays was shown by Peng et al. (2009), who used paired fold changes of miRNA and mRNA extracted with TRIzol in post-transplant liver biopsies with an Agilent multiplex microarray of 470 miRNA. They identified miR-16 as differentially expressed in HCV infection as a possible gene of interest for potentially arresting the cell cycle and a negative regulator of the BH3 interacting-domain death agonist (BID) gene, a mediator of apoptosis. However, this finding and target analysis has not been confirmed with further published investigation. Confirming findings from tissue in vitro can be challenging due to differences between Huh cells with modified virus and hepatocytes from liver tissue with wild type virus. For example, at least one other study in Huh 7.5 cells saw no significant change in miR-16 with transfection of full genome HCV clones (Cheng JC et al., 2012). Liu et al. (2010) also used paired miRNA and mRNA expression data to identify miRNA and genes of interest with a 988 human miRNA CapitolBio microarray in Huh 7.5 cells, and then synthesized mimics of 8 miRNA and inhibitors of 10 miRNA to assess the effect of miRNAs of interest in HCV replication. However, the 4 miRNA they identified as most interesting using this technique, miR-24, miR-149, miR-638, and miR-1181, have not yet been further investigated in other studies and the author’s conclusion that these miRNA may be of special relevance to the HCV life cycle has yet to be confirmed.
Another possible approach is to begin with the genes of interest, assess for complimentary miRNA sequences in silico, then assay for them specifically in tissue samples of interest. Gelley et al. (2013) assayed liver biopsy tissue from transplant recipients with qRT-PCR for miRNA of interest that were known to regulate expression of cell surface proteins integral to HCV binding and incorporation. They were able to identify 4 miRNA (21, 99a-3p, 194 and 224) that were differentially regulated at the time of recurrence of HCV infection post-transplant and responded to 48 weeks of therapy with a likely effect on expression of relevant cell surface proteins such as CLDN1 and OCLN. However, their finding of miR-21 downregulation at the time of HCV recurrence was somewhat surprising given previous data from Chen Y et al. (2013) using Huh 7 cells that miR-21 is consistently upregulated with HCV infection. In that study, miR-21 was found to increase 12-fold after transfection with viral clones. The effect was mediated by viral proteins, the NS3/4A complex and NS5A, which induced host transcription enhancer activator protein 1(AP1) activity, and was in turn shown to promote miR-21 expression. The authors also found that a likely gene target was Myeloid differentiation primary response gene 88 (MyD88), a key enzyme in the toll-like receptor pathway. Therefore, overexpression of miR-21 may contribute to viral evasion of host immunity.
Assessing for differential expression of miRNA between HCV infected and uninfected samples are a common starting point for identifying miRNAs of interest (Table 2). However, inconsistencies with the direction of differential regulation between studies are not uncommon, especially between tissue samples and in vitro cell models and may reflect differences in physiology, assay performance and disease progression. Differential change may be easier to detect with abundant miRNA in large multiplex assays, however, given that miRNA are regulatory molecules at various stages of signaling and metabolic cascades, small changes in low abundance miRNA may be important. However, since many studies use differential regulation from large multiplex arrays to identify miRNA of interest, this can be problematic. Bhanja Chowdhury et al. (2012) identified miR-130a as a molecule of interest when looking in silico for possible regulators of interferon-induced transmembrane proteins (IFITMs) integral to cell surface binding and recognition of interferon, and found complimentarity with the IFITM-1gene. They then assayed liver biopsy specimens of nine infected patients as compared to controls with qRT-PCR and found an approximately 8-fold relative increase in miR-130a. A luciferase activity assay was then used in Huh 7 cells to confirm that transfection of a mimic led to decreased IFITM-1 gene activity and transfection of anti-miR-130a led to an increase. However, in a separate study, Zhang et al. (2013) found miR-130a was decreased approximately 3-fold in Huh 7.5 cells using a TaqMan 365 miRNA PCR-based multiplex array confirmed with qRT PCR at baseline prior to exposure to IFN-α which corrected the downregulation. In silico analysis performed in the study to identify potential targets of relevance did not identify IFITM-1. The difference between these two studies again likely shows how the differential regulation observed in permissive Huh 7 cell lines may differ from what is found in tissue as they both used the same technology, TaqMan qRT-PCR assays, to assess fold changes.
Table 2.
Proposed Upregulation and Downregulation of miRNA Provoked by HCV Infection by Compartment
| Hepatocytes (non carcinoma) |
Hepatic Stellate Cells |
Hepatocellular Carcinoma |
Plasma | PBMC | |
|---|---|---|---|---|---|
| Upregulated* | miR-1, let 7b/e,miR-16, miR-21, miR27a/b, miR- 34a, miR 99a, miR-122, miR-125a/b, miR-130a, miR-142, miR-146a/b, miR-149, miR-196a/b, miR-200a/b/c, miR-224, miR-296, miR-351, miR- 638, miR-652, miR-1181 |
miR-200c, miR-214- 5p, miR- 221, miR- 222 |
miR-10a, miR-16, miR- 21, miR-100, miR- 193b, miR-222, miR- 224, miR-245, miR- 515-3p, miR-518-3p, miR-520f, miR-525-3p |
miR-20a, miR-34a, miR-92a, miR-122, miR-125b, miR-146a, miR-155, miR-320, miR-1207, miR-1225, miR-1275 |
miR-1, miR- 30, miR- 128, miR- 155, miR- 296 |
| Downregulated* | miR-23b, miR-24, miR- 30b/c, miR-128a/b, miR- 155, miR-192, miR-194, miR-301, miR-320c, miR- 324-5p, miR-361, miR-431, miR-448, miR-449a, miR- 455, miR-491, miR-560 |
miR-29 | miR-124, miR-145, miR-223 |
miR-451, miR-652, miR-762, miR-1914 |
miR-125b, miR-146a, miR-652 |
Definitions of upregulation and downregulation vary based on the study in question. Many studies used significant differences defined p <.05 vs. healthy comparators as definition of up and downregulation. However, some studies used fold change cut points to define up and down regulated. These ranged from fold changes of +/− 1.2 to +/− 2.0.
Indeed, differential regulation may not always be a key indicator of miRNA of interest, especially when assessing host response to viral infection where there may be pseudo-normalization of an expected inflammatory response. This factor may be of crucial relevance given that another replicative reservoir for HCV may be PBMCs, particularly lymphocytes, although this is controversial. There is some thought that presence of HCV RNA in PBMCs may be due to passive diffusion from plasma, not replication competent virus, and not of clinical importance (Fujiwara et al., 2013). However, other studies have shown presence of HCV in PBMCs when none is found in plasma and have confirmed that it is replication competent negative strand virus (Bernardin et al., 2008; Chary et al., 2012; Januszkiewicz-Lewandowska et al., 2007; Radkowski et al., 2005). Several studies have examined miRNA expression in PBMCs in the context of HCV infection (see figure 1, tables 1–2).
There appears to be several miRNA with immune modulatory targets that may be differentially expressed between hepatocytes and PBMCs, which may provide further insight into how HCV evades the immune system. These miRNA include miR-30a, miR-125b, miR-128a, miR-146a, miR-155, miR-296, and miR-652 (Bala et al., 2012; El-Ekiaby et al., 2012; Grek et al., 2011; Scagnolari et al., 2010; Sidorkiewicz et al., 2010). As discussed above, pseudonormalization may also be a factor, as was argued by El-Ekiaby et al. (2012) when they assessed for miR-146a and miR-155 with qRT-PCR, both thought to be important regulators of the interferon cascade, in the pooled PBMCs of HCV genotype 4 infected patients and found them to be unchanged in contrast to previous findings in other viral infections such as HSV and EBV, but differentially regulated when exposed to either interferon-α2a or Imiquimod (a TLR-7 agonist). However, the finding of pseudonormalized miR-155 levels with untreated HCV infection contradicts three other studies, which found elevated miR-155, or precursor B-cell Integration Cluster (BIC) molecules in HCV infected PBMCs (Bala et al., 2012; Sidorkiewicz et al., 2010; Grek et al., 2011). A study by Grek et al. (2011) used qRT-PCR to demonstrate that elevations in miR-155 and 196b in PBMCs correlated with the presence of replication competent virus.
This difference between the findings of El-Ekiaby et al. (2012) and the other three studies may be due to differences in methodology, as the former study used only PBMCs from patients with HCV genotype 4, as opposed to the Bala et al. (2012) study which examined sera and PBMCs from genotype 1, 2 and 3 patients, but had no genotype 4 patients. The other two studies do not mention the genotype of the HCV infected patients included. The other major methodology difference was that the El-Ekiaby et al. (2012) study used fold changes in the pooled PBMCs of 20 HCV infected patients as compared to 10 healthy controls to reach their conclusions rather than the direct comparison of individually measured samples as did the other studies. The pooling of samples can introduce an outlier bias as extreme production of microRNA in a single individual can influence the entire population. However, they did validate their findings in a subset of 6 patients, in an attempt to evaluate and correct for this bias.
In addition, the study from Grek et al. (2011) also demonstrates that normalization to endogenous controls is a major challenge in discerning reliable data about fold changes for both hepatic and extra-hepatic tissues in studies that use single miRNA qRT-PCR based-assays as well as the microarrays discussed above. Normalization is a necessary step to minimize variation secondary to varying amounts of total RNA from samples in order to assess for true biologically significant change. For qRT-PCR of miRNA extracted from cells and tissue, quantitation of certain small nuclear RNAs such as RNU6, RNU44 and RNU48 among others are thought to vary little with disease states and be reflective of the total amount of RNA and have therefore been commonly used for normalization. However, there is no consensus on this practice, and there is some evidence that expression of small nuclear RNA may vary significantly independent of total RNA amount (Meyer et al., 2012; Peltier and Latham, 2008). In the above study by Grek et al. (2011), the significant elevations of miR-155 and miR-196b that were reported may have been influenced by the use of RNU6 as a sole endogenous control for normalization. The authors report a highly consistent level (Cycle threshold, Ct range, 24.72–26.03) of miR-155 and miR-196 across the studied PBMCs with TaqMan qRT-PCR miRNA assays, and the differences reported only became apparent after normalization to RNU6, which may have biased the data if RNU6 was not a reliable endogenous control, which may be possible. Although RNU6 is the small nuclear RNA most often used in the literature in both PBMCs and hepatocytes, a review of miRNA normalization by Peltier and Latham (2008) came to the conclusion that these small nuclear RNA are often of high variance and do not necessarily correlate to relative total amounts of RNA. This was particularly true of extracellular samples from the plasma where small RNA is easily degraded, but can still be true in both PBMCs and hepatocytes.
Many of the papers reviewed here use single miRNA TaqMan qRT-PCR assays to assess for fold changes or to validate microarray results, and thus require normalization to a single molecule (see Supplemental table 1). The specific choice of method and identity of the chosen endogenous control can be a key determinant in biasing data (Meyer et al., 2012). Strategies to avoid this problem include using exogenous spiked-in controls of known quantity, data from microarrays to select a miRNA for normalization with minimal variance from a particular assay or to use a miRNA described in the literature to be minimally variable such as miR-16 (Meyer et al., 2012; Peltier and Latham, 2008). However, all of these methods can have their own bias: exogenous controls correct for variation in RNA extraction efficiency but not for variation in initial input (tissue mass, cell number or plasma volume); selecting an endogenous control based on minimal variance in microarray analysis may be itself biased by variation in input and RNA extraction efficiency; and abundant miRNA such as miR-16 as detailed above may also be actively modified by HCV infection and may not be reliable endogenous controls (Meyer et al., 2012; Peltier and Latham, 2008; Peng et al., 2009). Normalization is somewhat more reliable for microarrays as global normalization based on algorithms analyzing large populations of measured miRNA are possible (Meyer et al., 2012).
Translation and Application: Treatment Prognosis
Although there have been challenges with consistency and measurement reliability as described above, progress has been made along many fronts in translating basic miRNA research into clinically useful applications. Prediction of treatment response may be one of many translational applications of ongoing miRNA research (table 3). Scagnolari et al. (2010) evaluated miRNA expression in the context of interferon treatment for HCV infection on the levels of 5 miRNAs (miR-1, miR-30, miR-128, miR-196, miR-296) using qRT-PCR and RNU6B for normalization in HCV infected patient PBMCs as compared to normal controls. They then stratified their data based on subsequent treatment response to pegylated interferon-α and ribavirin. This study suggested that decreased baseline levels of miR-296 and increased baseline levels of miR-128 and 196 in PBMCs may be predictive of treatment response (see table 3), although the sample size used was not powered to detect significance. Murakami Y et al. (2010) examined a similar question of using miRNA for treatment prognosis in liver biopsy samples, assessing for fold changes with an Agilent microarray, and found that increased relative baseline levels of miR-27b, miR-378, miR-422b (mean fold changes of 1.3, 1.43, and 1.41 respectively) and decreased baseline levels of miR-18a, 143, 145 and 652 (fold changes of 0.82, 0.76, 0.74, and 0.78 respectively) were significantly correlated (p <.001) with attaining a sustained viral response (SVR). The relatively small fold changes associated with significance reported in this study demonstrate how small percentage changes in abundant miRNA using a sensitive assay can reach statistical significance, further study may be necessary to ensure that these results are reproducible in a clinical context and not obscured by assay measurement variability.
Even if validated, miRNA levels from hepatic biopsy tissue may be harder to obtain and process clinically. Importantly, miRNA can also complex with Argonaute (Ago-2) proteins which allows it to persist extracellularly in blood plasma for up to 2 months by preventing RNA-ase mediated degradation, and there may also be exosome carriage of miRNA, although the protein-complexed form appears to be the dominant mode of circulation (Arroyo et al., 2011; Turchinovich et al., 2011). Although persistence of miRNA in serum is likely at least partially as a result of cell lysis, it may also have cell to cell or paracrine signaling functions yet uncertain (Turchinovich et al., 2011). Given the relative ease of obtaining patient sera, there has been an effort to use circulating miRNA, particularly miR-122 as a prognostic marker for HCV treatment success. However, two separate studies have come to opposing conclusions. Waidmann et al. (2012) evaluated miR-122 levels from RNA isolated with the mirVana isolation kit from the sera of 52 HCV genotype 1, treatment naive patients using TaqMan qRT-PCR which was normalized to endogenous miR-16. Assays were performed on samples obtained immediately prior to treatment with pegylated interferon-α and ribavirin and no significant association between baseline serum levels of miR-122 and eventual treatment response was found. Su et al. (2013) used the same qRT-PCR assay to assess miR-122 levels, however differed in that they normalized to an exogenous control, cea-miR-39, isolated miRNA with TRIzol and included both HCV genotype 1 and 2 patients. In contrast to the prior study, there was a significantly higher baseline level of miR-122 in patients who went on to SVR (n=98) as compared to non-responders (n=28). However, in subset analysis, this difference appears to have been concentrated among HCV genotype 2 infected patients not included in the other study, as well as to a lesser degree in patients with the less treatment responsive IL-28B TT genotype. The presence of genotype 2 patients in the Su et al. (2013) study is likely the reason for the discrepancy between the two studies, although the physiologic rationale for this is not yet known. Interestingly, no significant dynamic change or decrease in circulating miR-122 was noted with successful treatment in this study which is perhaps contrary to expectations if circulating miR-122 is a marker of liver disease as was hypothesized by the authors (Su et al., 2013).
One hope of miRNA investigation is that it may lead to new drug development and better therapy for both HCV infection and HCV related malignancy. Miravirsen (Santaris Pharma A/S), an antisense inhibitor of miR-122 showed promising results in stage II trials for genotype 1 HCV infection, inducing significant reductions in serum HCV viral load as a monotherapy (Janssen et al., 2013). This approach holds promise, and Miravirsen may be one of a new generation of drugs to treat HCV infection. Using a novel gene therapy approach, Yang et al. (2013) reported that a polycistronic cluster of 7 miRNA, miR-17–92, could be used as a scaffold for insertion of a plasmid encoding a synthetic polycistronic cluster of 5 anti-HCV miRNAs complementary to target sites on the HCV genome. This would be delivered by an adenovirus vector, and demonstrated efficacy without toxicity in mice.
Translation and Application: Diagnosis, Prognosis and Potential Treatment of Hepatic Fibrosis
MiRNA as mediators and predictors of HCV induced liver pathogenesis may be another important facet of translational investigation (Figure 2, Table 3). Several studies have assessed changes in miRNA expression in stellate cells from liver biopsy tissue and explored their likely gene targets in vitro using LX2 fibroblast lineage cell lines (Banyopadhyay et al., 2011; Iizuka et al., 2012; Murakami Y et al., 2011; Ogawa et al., 2012; Ramachandran et al., 2013; Roderburg et al., 2012). Overexpression of the miR-199 and 200 families in 105 liver biopsy samples from HCV infected patients as measured by an Agilent v1.5 microarray was strongly correlated with the degree of fibrosis and results confirmed with qRT-PCR (Murakami Y et al., 2011). In a separate study, miR-200c was identified as differentially regulated in liver stellate cells using an Illumina Human HT-12 v3 Expression BeadChips array, a bead-based assay, and its mechanism of action was hypothesized to be through immortalizing fibroblasts by downregulating Fas-associated phosphatase-1 (FAP-1), a promoter of apoptosis (Ramachandran et al., 2013). This hypothesis was confirmed using fibroblast cultures in vitro. Furthermore, it appears that in LX2 fibroblast lineage cells, the Twist-1 enhancer promotes expression of both miR-199a and miR-214-5p, and that miR-214-5p increases expression of matrix metalloproteases (MMPs) by an unknown mechanism to promote fibrosis (Iizuka et al., 2012). Along with increases in miR-199, miR- 222 was noted to be upregulated (mean fold change 1.8, p <.01) in 7 liver biopsy tissues with F3/F4 fibrosis versus 15 liver biopsy tissues with F1/F2 fibrosis from genotype 1 HCV infected patients when measured by a Toray microarray. The closely related miR-221 was noted to be similarly upregulated (mean fold change 2.4, p <.01) in mouse livers 4 days after liver injury was induced, and the target for both in LX2 cells appeared to be cyclin-dependent kinase inhibitor 1B (CDKN1B), a negative regulator of both collagen, type I and III, alpha (COL1A and COL3A) gene activity and MMPs (Ogawa et al., 2012). A more direct regulator of collagen expression via COL1A and COL3A is thought to be miR-29 which was found to be decreased in vitro by transforming growth factor beta (TGFβ) and downregulated in HCV infected stellate cells versus healthy controls by qRT-PCR (Bandyopadhyay et al., 2011). There is also some evidence that HCV infection may induce these expression changes by upregulating miR-571, which in turn, downregulates the transcription suppressor cAMP Response Element-Binding Protein (CREB)-binding protein (CREBBP)(Roderburg et al., 2012). Downregulation of miR-449a may also be a key mediator of fibrosis in the liver of HCV infected patients as it appears to regulate NOTCH-1 expression and by extension its downstream effectors YL40 and tumor necrosis factor alpha (TNFα) (Sarma et al., 2012).
Profiling circulating miRNA in the serum may also have an important role in monitoring for the progression of HCV related liver disease. Cermelli et al (2011) used in vitro analysis of Huh 7.5 replicon supernatants to hypothesize that miR-16, 34a and 122 in serum may be useful in predicting liver fibrosis, and then demonstrated using TaqMan qRT-PCR that serum elevations in miR-34a (undetectable in controls versus mean of 44,000 copies/mL in HCV infected patients, p <.0001) and miR-122 (mean fold change 10.8, p <.0001) were significantly correlated with progression to cirrhosis in 53 HCV infected patients as compared to 19 healthy controls. Elevations in miR-34a in liver biopsy tissue (mean fold change not explicitly reported in paper, p = .0105) as measured by an Affymetrix microarray were also associated with progression to fibrosis in 43 HCV infected post-transplant patients in at least one subsequent study (Gehrau et al., 2013).
The association of elevated miR-122 with fibrosis in HCV patients suggested by Cermelli et al. (2011) may be more complicated, however, particularly in late stage cirrhosis. A prospective study of all cirrhotic inpatients regardless of etiology (66 of 250 patients enrolled had HCV) on a hepatology ward demonstrated that relatively lower levels of serum miR-122 as isolated by mirVana miRNA isolation kit and measured by TaqMan qRT-PCR (not normalized) was a predictor of mortality (p= 0.016) as well as decompensated cirrhosis (p= 0.012), particularly if the cause of decompensation was hepatorenal syndrome (p= 0.037), spontaneous bacterial peritonitis (p= 0.003 or ascites (p= 0.007) (Waidmann et al., 2012b). Lack of normalization and use of raw CT numbers is a limitation of the study that may limit its comparability to other studies. However, these findings of decreased circulating miR-122 correlating with increasing level of fibrosis were confirmed in HCV patients in a study by Tribecka et al. (2013) which evaluated miR-122 levels with SYBR-green qRT PCR from both hepatic tissues (isolated with Trizol reagents and normalized to RNU6) of 84 patients and serum (isolated with Qiazol reagents and normalized to exogenous SV40) from 164 patients. This study found that mean hepatic levels appeared to peak with stage F1 fibrosis, and mean serum levels with stage F2 fibrosis with incrementally lower mean levels in patients with F3 and F4 disease (p <.001 for mean hepatic levels, p =0.043 for mean serum levels), suggesting that loss of hepatocytes by the liver drove this reduction in miR-122 as fibrosis progressed. This contradicts the earlier Cermelli et al. (2011) study, which Tribecka et al. (2013) were aware of and attributed the conflict to differences in populations, although the demographics and HCV genotypes of the patients in the Cermelli et al. (2011) study were not explicitly stated, so it is unclear if this is a sufficient explanation. It should be noted that the real-time PCR techniques used between the two studies, TaqMan and SYBR-green differ, although on its own this may not be sufficient to explain the difference in findings.
Shrivastava et al. (2013b) used a Qiagen MIHS-106Z PCR array with an exogenous control in serum samples from 44 HCV infected patients with various stages of liver fibrosis to identify miR-20a and miR-92a (both part of proto-oncogenic cluster miR-17–92) as possible markers of disease severity and miR-574-3p as a miRNA with minimal across sample variance possibly useful for normalization in qRT-PCR in serum. MiR-20a correlated most closely with disease severity across categories of fibrosis and was proposed as a diagnostic marker for fibrosis. However, Joshi et al (2013), noted that in liver biopsy tissue from 20 HCV positive transplant patients with recurrence divided into slow (less than or equal to F2 fibrosis at 12 months after transplant, n=11) and rapid progressors (greater than F2 fibrosis at 12 months after transplant, n=9), significant decreases in hepatic miR-20a (mean fold change not explicitly stated, p <.01), as measured by an Affymetrix GeneChip version 2.0 array and confirmed by miRCURY (Exiqon) PCR were associated with rapid progression to fibrosis. This was associated with a corresponding upregulation of the TGF-β pathway. The increase in serum miR-20a noted by Shrivastava et al. (2013b) and decrease in hepatic miR-20a noted with rapid progression to fibrosis by Joshi et al. (2013) post transplant are difficult to explain biologically if the hypothesis that a fibrotic liver is the source of the increase in circulating miR-20a, although this could be due to an increase in hepatocyte lysis.
Murakami Y et al. (2012) used an Agilent microarray in 95 HCV infected patient’s sera as compared to 24 health volunteers, 20 HBV infected patients and 20 patients with non-alcoholic steatohepatitis to identify 16 miRNAs whose expression pattern significantly correlated with fibrosis. The correlation was determined using “Leave One Out Cross-Validation”, a model validation method that assesses generalizability of results by assessing each observation sequentially versus a training set composed of all the other observations. Using this methodology and the relative expression data from all 16 miRNA, the authors were able to predict the degree of fibrosis regardless of cause with between 65 and 88% accuracy depending on the stage of disease. This study was also notable for how they isolated the miRNA from serum, using Exoquick to enrich for exosomes prior to extraction with a miRNeasy kit, which appeared to improve the amount of miRNA isolated. Although these studies appear to validate the principle that serum miRNA profiles may be useful surrogates for fibrosis, they have not yet been validated prospectively in trials in which the investigators are blinded to liver pathology findings. In addition, the best technology and procedures to assess for miRNA in serum for this purpose is still not clear.
Translation and Application: Diagnosis, Prognosis and Potential Treatment of HCV Related Malignancy
Malignancy, particularly HCC, intraductal colangiocarcinoma (ICC), and non-Hodgkin’s lymphoma (NHL) are a consequence of HCV pathogenesis that is also mediated in part by miRNA (Figure 2). Differential expression of miRNA in dysplastic tissues in HCV related liver carcinomas compared to cirrhotic tissue has been described in the literature for some time (Ura et al., 2009; Varnholt., 2008). Further study has delineated specific pathway regulation by miRNA that may be useful in drug discovery for HCV associated cancer treatment. Zeng et al. (2012) noted that miR-124 was specifically downregulated in HCV-associated cholangiocarcinoma tissue. They showed that HCV core protein provoked expression of DNA (cytosine-5)-methyltransferase 1 (DMNT-1) in vitro, which methylated the miR-124 gene as a form of epigenetic regulation. Further in vitro analysis showed that miR-124 was a regulator of SET and MYND domain-containing protein 3 (SMYD3), an activator of MMPs. Zhang et al. (2012) theorized that increased miR-155 noted in hepatic tissues with HCV infection was likely oncogenic and demonstrated in vitro in both BALB/c mice and Huh 7 replicon systems that it upregulated the Wnt oncogenesis pathway by downregulating adenomatous polyposis coli (APC). Although the effect did not appear specific to HCV infected carcinomas, Wong et al. (2008) noted that downregulation of miR-223 in HCC tissue appeared to promote oncogenesis by disinhibiting the Stathmin 1/oncoprotein 18 (STMN-1) oncogene. An in vitro study in Huh 7 and HEK 293 human hepatoma cell lines demonstrated a likely HCV core protein induced upregulation of miR-345, which appeared to downregulate the p21waf1/cip to promote carcinogenesis (Shiu et al., 2013). In another in vitro study of Huh 7.5 replicons, miR-491 was noted to both increase full genome HCV clones to replicate and to downregulate cell growth inhibition on the PI3K/Akt pathway (Ishida et al., 2011). Other strategies involving miRNA also hold hope for novel HCV related HCC therapy. Mir-193b mimics have been shown to potentiate the effect of sorafenib to induce apoptosis of HCC cells in vitro (Braconi et al., 2010). A novel cluster of miRNA on chromosome 19, C19MC, appears to be strongly associated with dysplastic tissues and may be a future drug target (Augello et al., 2012).
Leveraging serum miRNA profiles to better detect early carcinoma or to better assess risk is a potentially useful application of miRNA research in the setting of HCV. Fognani et al. (2013) assessed the serum of patients with HCV associated hematologic disease, namely 11 NHL patients and 75 mixed cryoglobulinemia (MC) patients with qRT-PCR normalized to let-7d and compared to 81 HCV infected controls without malignancy and 35 healthy controls. They found that in the HCV infected NHL patients versus HCV infected patients without malignancy and healthy controls, miR-16 (mean fold changes 2.15 and 3.16 respectively, p<.01), miR-21 (mean fold changes 4.34 and 24.51 respectively, p<.001) and miR-155 (mean fold changes 3.68 and 13.12 respectively, p<.001) were all significantly elevated. However, these levels were not significantly elevated in the 75 MC patients as compared to HCV infected patients without malignancy and healthy controls. MiR-26b was noted to be significantly downregulated in both NHL and MC patients versus healthy controls (fold changes 1.94 and 2.19 respectively, p <.001).
MiR-21 has specifically been noted to be a tumor promoter potentially active by downregulating tumor suppressors like programmed cell death protein 4 (PDCD4). However, in the context of HCV related fibrosis it had also been noted in at least one study in hepatic biopsy tissues of cirrhotic livers to be upregulated, and in vitro was found to be a potential downregulator of mothers against decapentaplegic homolog 7 (SMAD7) which promotes TGF-β mediated fibrosis and carcinogenesis (Marquez et al., 2010). As a result of these findings, there has been interest in miR-21 as a possible serum marker for HCC, however, study results are unclear as to its efficacy as a biomarker.
Bihrer et al. (2011) isolated miRNA with a mirVana isolation kit and then assayed the sera of 29 HCV infected HCC patients compared with 67 chronic HCV patients without malignancy and 19 healthy controls for miR-21 with qRT-PCR normalized to miR-16 and found that although levels were elevated as compared to healthy controls (mean fold change 1.54 p<.001), they were not significantly different from HCV infected patients without carcinoma when matched by level of necroinflammation. Indeed, they noted that miR-21 sera levels appeared to correlate independently with the level of necroinflammation in biopsy tissues, not with the presence of HCC. However, Tomimaru et al. (2012) used very similar techniques albeit in a greater number of patients, isolating miRNA with the mirVana PARIS kit and assaying 126 HCV infected HCC patient’s sera for miR-21 with qRT-PCR normalized to miR-16 compared to 30 patients with chronic HCV and no malignancy and 50 healthy controls and did find a significant elevation (precise fold changes were not explicitly stated in publication) in HCC patients as compared to patients with chronic HCV infection, and noted that when combined with serum alpha fetoprotein (AFP) levels using cut-offs with optimal area-under-curves in receiver-operator curve comparisons had an approximately 81% sensitivity and 76% specificity at predicting the presence of HCC of any size. The sensitivity and specificity both dropped to 55% if only tumors < 2cm were considered, which is the most relevant screening population. Furthermore, in this study, there was no difference in miR-21 levels between cirrhotic patients with HCC and non-cirrhotic patients with HCC, although a specific comparison by necroinflammation was not made.
Although the assay techniques used were similar, Bihrer et al. and Tomimaru et al. came to differing conclusions regarding the efficacy of miR-21 as a biomarker for HCC. Bihrer et al. (2011) were aware of the Tomimaru et al. (2012) study and stated that they felt that the latter study was not sufficiently controlled for fibrosis/necroinflammation to draw conclusions about its efficacy as a biomarker for HCC in cirrhotic patients. Tomimaru et al. (2012) did state that they compared miR-21 expression in the 59 cirrhotic patients with HCC and 67 non-cirrhotic patients with HCC included in their sample and found no difference, therefore concluding that the presence of cirrhosis did not bias their sample. However, this was likely not as robust an assessment as stratifying their comparisons by level of fibrosis/necroinflammation as in the Bihrer et al. (2011) study, which would have demonstrated more clearly if the specificity of serum miR-21 for HCC was lost as fibrotic liver disease worsened. However, it may be also be true that the larger patient sample sizes used in the Tomimaru et al. (2012) study significantly improved the power to detect a difference. Regardless, further study and confirmation is needed before serum miR-21 levels can be used for HCC screening and diagnosis and they may be a better marker for necroinflammation than for HCC.
Beyond miRNA as serum markers for HCV related HCC, polymorphisms in miRNA genes may also predict HCC risk. Akkiz et al. (2011) reported a polymorphism (rs11614913) in the pre-miR-196a-2 gene that was associated with an odds ratio of 2.41 for developing HCC in a case control study of 185 patients matched with 185 controls in a Turkish population. Although this study was focused on delineating the risk of HCC, it demonstrates that there may be a role for investigating polymorphisms of genes encoding miRNA in predicting treatment response to various drugs or development of fibrosis. As IL-28B allele variations have been shown to predict HCV treatment response, allele variations for genes encoding miRNA may have similarly useful predictive applications (Pasha et al., 2013).
Conclusion
Investigations into the role of miRNA in HCV infection, immune response, pathogenesis and carcinogenesis hold great translational promise as a source of novel diagnostic, prognostic and therapeutic tools. However, additional research into optimal techniques for assessing differential expression of miRNA with microarrays, optimal sample processing including extracting miRNA, best practices for normalization of samples, optimal in vitro models and the effect of variations in HCV genotype on expression are all necessary.
Microarrays and miRNA isolation techniques should be selected based on their ability to produce reproducible results in the sample type of interest. Sample collection, preservation and isolation techniques should also be chosen with the downstream assay or array in mind to minimize confounding. Extraction of miRNA should take into account exosomes when dealing with extracellular fluid such as serum or plasma from blood. The low level of correlation observed between hybridization based microarrays and PCR arrays is concerning, and effort may be needed to reconcile this and better understand which method is more reflective of biologically important abundance and changes. More direct comparisons of the efficacy and reliability of various multiplex microarrays and an improved consensus around normalization for microRNA are necessary so that data can be compared across studies. It is not possible to draw conclusions currently regarding best practices for normalization of qRT-PCRs for specific miRNA with available data, however, specific abundant miRNA with minimal variance in expression in HCV disease states have been found and may be more useful than small nuclear RNA or miR-16 which have been traditionally used. The appropriate miRNA for normalization will likely vary based on tissue type. Exogenous controls may be useful to control for variation in sample processing, but cannot fully replace endogenous controls to control for confounding based on variance in total RNA isolated from sample to sample.
As with most areas of translational investigation, caution must be used when generalizing in-vitro data to the bedside, particularly in HCV infection where the most established model system, the Huh 7 cell line is physiologically different from hepatocytes in-vivo. Whenever possible, cell line data should be confirmed with assessment of patient samples. However, patient samples can be variable and comparisons and generalizations must be adjusted to account for stage of liver disease, fibronecrosis, medical co-morbidities, and medications which can all effect expression of miRNA involved in multiple pathways and confound conclusions regarding the interaction between HCV and miRNA. As the field advances, it will be important to assess differences in miRNA expression based on gender, race and age, which are likely confounders when generalizing results. Published results in leiomyoma and myocardial infarction patients suggest that race may effect miRNA expression in those disease states, and may help explain observed differences in HCV treatment outcomes between racial groups (Beinhardt et al., 2013; Edelstein et al., 2013; Wang et al., 2007). Limited literature also suggests that gender difference may also affect miRNA expression (Rao et al.; 2009).
It should also be understood that differences in HCV genotype may have large implications in the relevance of specific miRNA, and this should be noted when attempting to generalize results, particularly as most in-vitro models use genotype 1b or 2a based clones. Studies using patient samples should make note of viral genotypes and whenever possible compare samples within genotype instead of across genotypes. Further investigation into these areas may improve the reproducibility of data and further define the translational applications of research into the intersection of HCV and miRNA.
Supplementary Material
Acknowledgements
The views expressed are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Funding: This work was supported in part by Department of Veterans Affairs intramural funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Akkız H, Bayram S, Bekar A, Akgöllü E, Ulger Y. A functional polymorphism in pre-microRNA-196a-2 contributes to the susceptibility of hepatocellular carcinoma in a Turkish population: a case-control study. J Viral Hepat. 2011 Jul;18(7):e399–e407. doi: 10.1111/j.1365-2893.2010.01414.x. [DOI] [PubMed] [Google Scholar]
- 2.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011 Mar 22;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augello C, Vaira V, Caruso L, Destro A, Maggioni M, Park YN, et al. MicroRNA profiling of hepatocarcinogenesis identifies C19MC cluster as a novel prognostic biomarker in hepatocellular carcinoma. Liver Int. 2012 May;32(5):772–782. doi: 10.1111/j.1478-3231.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 4.Bala S, Tilahun Y, Taha O, Alao H, Kodys K, Catalano D, et al. Increased microRNA-155 expression in the serum and peripheral monocytes in chronic HCV infection. J Transl Med. 2012 Jul 30;10:151. doi: 10.1186/1479-5876-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banaudha K, Orenstein JM, Korolnek T, St Laurent GC, 3rd, Wakita T, Kumar A. Primary hepatocyte culture supports hepatitis C virus replication: a model for infection-associated hepatocarcinogenesis. Hepatology. 2010 Jun;51(6):1922–1932. doi: 10.1002/hep.23616. [DOI] [PubMed] [Google Scholar]
- 6.Banaudha K, Kaliszewski M, Korolnek T, Florea L, Yeung ML, Jeang KT, et al. MicroRNA silencing of tumor suppressor DLC-1 promotes efficient hepatitis C virus replication in primary human hepatocytes. Hepatology. 2011 Jan;53(1):53–61. doi: 10.1002/hep.24016. [DOI] [PubMed] [Google Scholar]
- 7.Bandyopadhyay S, Friedman RC, Marquez RT, Keck K, Kong B, Icardi MS, et al. Hepatitis C virus infection and hepatic stellate cell activation downregulate miR-29: miR-29 overexpression reduces hepatitis C viral abundance in culture. J Infect Dis. 2011 Jun 15;203(12):1753–1762. doi: 10.1093/infdis/jir186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Beinhardt S, Rutter K, Stättermayer AF, Ferenci P. Revisiting the predictors of a sustained virologic response in the era of direct-acting antiviral therapy for hepatitis C virus. Clin Infect Dis. 2013 Jan;56(1):118–122. doi: 10.1093/cid/cis843. [DOI] [PubMed] [Google Scholar]
- 10.Bernardin F, Tobler L, Walsh I, Williams JD, Busch M, Delwart E. Clearance of hepatitis C virus RNA from the peripheral blood mononuclear cells of blood donors who spontaneously or therapeutically control their plasma viremia. Hepatology. 2008 May;47(5):1446–1452. doi: 10.1002/hep.22184. [DOI] [PubMed] [Google Scholar]
- 11.Bhanja Chowdhury J, Shrivastava S, Steele R, Di Bisceglie AM, Ray R, Ray RB. Hepatitis C virus infection modulates expression of interferon stimulatory gene IFITM1 by upregulating miR-130A. J Virol. 2012 Sep;86(18):10221–10225. doi: 10.1128/JVI.00882-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bihrer V, Waidmann O, Friedrich-Rust M, Forestier N, Susser S, Haupenthal J, Welker M, Shi Y, Peveling-Oberhag J, Polta A, von Wagner M, Radeke HH, Sarrazin C, Trojan J, Zeuzem S, Kronenberger B, Piiper A. Serum microRNA-21 as marker for necroinflammation in hepatitis C patients with and without hepatocellular carcinoma. PLoS One. 2011;6(10):e26971. doi: 10.1371/journal.pone.0026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braconi C, Valeri N, Gasparini P, Huang N, Taccioli C, Nuovo G, et al. Hepatitis C virus proteins modulate microRNA expression and chemosensitivity in malignant hepatocytes. Clin Cancer Res. 2010 Feb 1;16(3):957–966. doi: 10.1158/1078-0432.CCR-09-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruni R, Marcantonio C, Tritarelli E, Tataseo P, Stellacci E, Costantino A, et al. An integrated approach identifies IFN-regulated microRNAs and targeted mRNAs modulated by different HCV replicon clones. BMC Genomics. 2011 Oct 4;12:485. doi: 10.1186/1471-2164-12-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6(8):e23937. doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chary A, Winters MA, Eisen R, Knight TH, Asmuth DM, Holodniy M. Quantitation of hepatitis C virus RNA in peripheral blood mononuclear cells in HCV-monoinfection and HIV/HCV-coinfection. J Med Virol. 2012 Mar;84(3):431–437. doi: 10.1002/jmv.23210. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Chen J, Wang H, Shi J, Wu K, Liu S, Liu Y, Wu J. HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1. PLoS Pathog. 2013 Apr;9(4):e1003248. doi: 10.1371/journal.ppat.1003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng JC, Yeh YJ, Tseng CP, Hsu SD, Chang YL, Sakamoto N, et al. Let-7b is a novel regulator of hepatitis C virus replication. Cell Mol Life Sci. 2012 Aug;69(15):2621–2633. doi: 10.1007/s00018-012-0940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng M, Si Y, Niu Y, Liu X, Li X, Zhao J, et al. High-throughput profiling of alpha interferon- and interleukin-28B–regulated microRNAs and identification of let-7s with anti-hepatitis C virus activity by targeting IGF2BP1. J Virol. 2013 Sep;87(17):9707–9718. doi: 10.1128/JVI.00802-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chugh P, Dittmer DP. Potential pitfalls in microRNA profiling. Wiley Interdiscip Rev RNA. 2012 Sep-Oct;3(5):601–616. doi: 10.1002/wrna.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conrad KD, Niepmann M. The role of microRNAs in hepatitis C virus RNA replication. Arch Virol. 2013 Oct 25; doi: 10.1007/s00705-013-1883-4. [DOI] [PubMed] [Google Scholar]
- 22.Edelstein LC, Simon LM, Montoya RT, Holinstat M, Chen ES, Bergeron A, et al. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat Med. 2013 Dec;19(12):1609–1616. doi: 10.1038/nm.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eldh M, Lötvall J, Malmhäll C, Ekström K. Importance of RNA isolation methods for analysis of exosomal RNA: evaluation of different methods. Mol Immunol. 2012 Apr;50(4):278–286. doi: 10.1016/j.molimm.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 24.El-Ekiaby N, Hamdi N, Negm M, Ahmed R, Zekri AR, Esmat G, et al. Repressed induction of interferon-related microRNAs miR-146a and miR-155 in peripheral blood mononuclear cells infected with HCV genotype 4. FEBS Open Bio. 2012 Jul 20;2:179–186. doi: 10.1016/j.fob.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fognani E, Giannini C, Piluso A, Gragnani L, Monti M, Caini P, et al. Role of microRNA profile modifications in hepatitis C virus-related mixed cryoglobulinemia. PLoS One. 2013 May 1;8(5):e62965. doi: 10.1371/journal.pone.0062965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiwara K, Allison RD, Wang RY, Bare P, Matsuura K, Schechterly C, et al. Investigation of residual hepatitis C virus in presumed recovered subjects. Hepatology. 2013 Feb;57(2):483–491. doi: 10.1002/hep.25921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gehrau RC, Mas VR, Villamil FG, Dumur CI, Mehta NK, Suh JL, et al. MicroRNA signature at the time of clinical HCV recurrence associates with aggressive fibrosis progression post-liver transplantation. Am J Transplant. 2013 Mar;13(3):729–737. doi: 10.1111/ajt.12047. [DOI] [PubMed] [Google Scholar]
- 28.Gelley F, Zadori G, Nemes B, Fassan M, Lendvai G, Sarvary E, Doros A, Gerlei Z, Nagy P, Schaff Z, Kiss A. MicroRNA profile before and after antiviral therapy in liver transplant recipients for Hepatitis C virus cirrhosis. J Gastroenterol Hepatol. 2013 Aug 22; doi: 10.1111/jgh.12362. [DOI] [PubMed] [Google Scholar]
- 29.Git A, Dvinge H, Salmon-Divon M, Osborne M, Kutter C, Hadfield J, et al. Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA. 2010 May;16(5):991–1006. doi: 10.1261/rna.1947110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottwein E. Roles of microRNAs in the life cycles of mammalian viruses. Curr Top Microbiol Immunol. 2013;371:201–227. doi: 10.1007/978-3-642-37765-5_8. [DOI] [PubMed] [Google Scholar]
- 31.Grek M, Piekarska A, Bartkowiak J, Fendler W, Kuydowicz J, Wroblewski P, et al. Coordinated increase of miRNA-155 and miRNA-196b expression correlates with the detection of the antigenomic strand of hepatitis C virus in peripheral blood mononuclear cells. Int J Mol Med. 2011 Nov;28(5):875–880. doi: 10.3892/ijmm.2011.748. [DOI] [PubMed] [Google Scholar]
- 32.Gu Y, Xu Y, Jiang L, Cao X, Liu F, Li H, et al. Differentially expressed microRNAs in Huh-7 cells expressing HCV core genotypes 3a or 1b: potential functions and downstream pathways. Int J Mol Med. 2012 Aug;30(2):374–382. doi: 10.3892/ijmm.2012.991. [DOI] [PubMed] [Google Scholar]
- 33.Gupta A, Swaminathan G, Martin-Garcia J, Navas-Martin S. MicroRNAs, hepatitis C virus, and HCV/HIV-1 co-infection: new insights in pathogenesis and therapy. Viruses. 2012 Oct 26;4(11):2485–2513. doi: 10.3390/v4112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammerle-Fickinger A, Riedmaier I, Becker C, Meyer HH, Pfaffl MW, Ulbrich SE. Validation of extraction methods for total RNA and miRNA from bovine blood prior to quantitative gene expression analyses. Biotechnol Lett. 2010 Jan;32(1):35–44. doi: 10.1007/s10529-009-0130-2. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann TW, Duverlie G, Bengrine A. MicroRNAs and hepatitis C virus: toward the end of miR-122 supremacy. Virol J. 2012 Jun 12;9:109. doi: 10.1186/1743-422X-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011 Feb 15;19(2):232–243. doi: 10.1016/j.ccr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Hou W, Tian Q, Zheng J, Bonkovsky HL. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatology. 2010 May;51(5):1494–1504. doi: 10.1002/hep.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iizuka M, Ogawa T, Enomoto M, Motoyama H, Yoshizato K, Ikeda K, et al. Induction of microRNA-214-5p in human and rodent liver fibrosis. Fibrogenesis Tissue Repair. 2012 Aug 1;5(1):12. doi: 10.1186/1755-1536-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida H, Tatsumi T, Hosui A, Nawa T, Kodama T, Shimizu S, et al. Alterations in microRNA expression profile in HCV-infected hepatoma cells: involvement of miR-491 in regulation of HCV replication via the PI3 kinase/Akt pathway. Biochem Biophys Res Commun. 2011 Aug 19;412(1):92–97. doi: 10.1016/j.bbrc.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 40.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013 May 2;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 41.Januszkiewicz-Lewandowska D, Wysocki J, Pernak M, Nowicka K, Zawada M, Rembowska J, et al. Presence of hepatitis C virus (HCV)-RNA in peripheral blood mononuclear cells in HCV serum negative patients during interferon and ribavirin therapy. Jpn J Infect Dis. 2007 Feb;60(1):29–32. [PubMed] [Google Scholar]
- 42.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005 Sep 2;309(5740):1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 43.Joshi D, Salehi S, Brereton H, Arno M, Quaglia A, Heaton N, et al. Distinct microRNA profiles are associated with the severity of hepatitis C virus recurrence and acute cellular rejection after liver transplantation. Liver Transpl. 2013 Apr;19(4):383–394. doi: 10.1002/lt.23613. [DOI] [PubMed] [Google Scholar]
- 44.Kerr TA, Korenblat KM, Davidson NO. MicroRNAs and liver disease. Transl Res. 2011 Apr;157(4):241–252. doi: 10.1016/j.trsl.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011 Jan;39:D152–D157. doi: 10.1093/nar/gkq1027. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013 May 16;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 47.Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, et al. Complete replication of hepatitis C virus in cell culture. Science. 2005 Jul 22;309(5734):623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Wang T, Wakita T, Yang W. Systematic identification of microRNA and messenger RNA profiles in hepatitis C virus-infected human hepatoma cells. Virology. 2010 Mar 1;398(1):57–67. doi: 10.1016/j.virol.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 49.Marquez RT, Bandyopadhyay S, Wendlandt EB, Keck K, Hoffer BA, Icardi MS, et al. Correlation between microRNA expression levels and clinical parameters associated with chronic hepatitis C viral infection in humans. Lab Invest. 2010 Dec;90(12):1727–1736. doi: 10.1038/labinvest.2010.126. [DOI] [PubMed] [Google Scholar]
- 50.Meyer SU, Kaiser S, Wagner C, Thirion C, Pfaffl MW. Profound effect of profiling platform and normalization strategy on detection of differentially expressed microRNAs--a comparative study. PLoS One. 2012;7(6):e38946. doi: 10.1371/journal.pone.0038946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.miRBase 20. [on 11/03/2013]; Retrieved from http://www.mirbase.org.
- 52.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013 Apr;57(4):1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 53.Murakami Y, Aly HH, Tajima A, Inoue I, Shimotohno K. Regulation of the hepatitis C virus genome replication by miR-199a*. J Hepatol. 2009 Mar;50(3):453–460. doi: 10.1016/j.jhep.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 54.Murakami Y, Tanaka M, Toyoda H, Hayashi K, Kuroda M, Tajima A, et al. Hepatic microRNA expression is associated with the response to interferon treatment of chronic hepatitis C. BMC Med Genomics. 2010 Oct 22;3:48. doi: 10.1186/1755-8794-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murakami Y, Toyoda H, Tanaka M, Kuroda M, Harada Y, Matsuda F, et al. The progression of liver fibrosis is related with overexpression of the miR-199 and 200 families. PLoS One. 2011 Jan 24;6(1):e16081. doi: 10.1371/journal.pone.0016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murakami Y, Toyoda H, Tanahashi T, Tanaka J, Kumada T, Yoshioka Y, et al. Comprehensive miRNA expression analysis in peripheral blood can diagnose liver disease. PLoS One. 2012;7(10):e48366. doi: 10.1371/journal.pone.0048366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nanostring Technologies. nCounter Plasma-Serum miRNA Tech Note. [on 11/03/2013]; retrieved from www.nanostring.com.
- 58.Ogawa T, Enomoto M, Fujii H, Sekiya Y, Yoshizato K, Ikeda K, et al. MicroRNA-221/222 upregulation indicates the activation of stellate cells and the progression of liver fibrosis. Gut. 2012 Nov;61(11):1600–1609. doi: 10.1136/gutjnl-2011-300717. [DOI] [PubMed] [Google Scholar]
- 59.Pasha HF, Radwan MI, Hagrass HA, Tantawy EA, Emara MH. Cytokines genes polymorphisms in chronic hepatitis C: impact on susceptibility to infection and response to therapy. Cytokine. 2013 Feb;61(2):478–484. doi: 10.1016/j.cyto.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, et al. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007 Oct 18;449(7164):919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008 May;14(5):844–852. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng X, Li Y, Walters KA, Rosenzweig ER, Lederer SL, Aicher LD, et al. Computational identification of hepatitis C virus associated microRNA-mRNA regulatory modules in human livers. BMC Genomics. 2009 Aug 11;10:373. doi: 10.1186/1471-2164-10-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Radkowski M, Gallegos-Orozco JF, Jablonska J, Colby TV, Walewska-Zielecka B, Kubicka J, et al. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology. 2005 Jan;41(1):106–114. doi: 10.1002/hep.20518. [DOI] [PubMed] [Google Scholar]
- 64.Ramachandran S, Ilias Basha H, Sarma NJ, Lin Y, Crippin JS, Chapman WC, et al. Hepatitis C virus induced miR200c down modulates FAP-1, a negative regulator of Src signaling and promotes hepatic fibrosis. PLoS One. 2013 Aug 12;8(8):e70744. doi: 10.1371/journal.pone.0070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao PK, Toyama Y, Chiang HR, Gupta S, Bauer M, Medvid R, et al. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ Res. 2009 Sep 11;105(6):585–594. doi: 10.1161/CIRCRESAHA.109.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ray S, Thomas D, Hepatitis C. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th ed. Mandell GM, Bennett JE, Dolin R, editors. Philadelphia: Churchill Livingston Elsevier; 2010. pp. 2157–2177. [Google Scholar]
- 67.Roderburg C, Mollnow T, Bongaerts B, Elfimova N, Vargas Cardenas D, Berger K, et al. Micro-RNA profiling in human serum reveals compartment-specific roles of miR-571 and miR-652 in liver cirrhosis. PLoS One. 2012;7(3):e32999. doi: 10.1371/journal.pone.0032999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sato F, Tsuchiya S, Terasawa K, Tsujimoto G. Intra-platform repeatability and inter-platform comparability of microRNA microarray technology. PLoS One. 2009;4(5):e5540. doi: 10.1371/journal.pone.0005540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarma NJ, Tiriveedhi V, Subramanian V, Shenoy S, Crippin JS, Chapman WC, et al. Hepatitis C virus mediated changes in miRNA-449a modulates inflammatory biomarker YKL40 through components of the NOTCH signaling pathway. PLoS One. 2012;7(11):e50826. doi: 10.1371/journal.pone.0050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwarz DS, Hutvágner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003 Oct 17;115(2):199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 71.Scagnolari C, Zingariello P, Vecchiet J, Selvaggi C, Racciatti D, Taliani G, et al. Differential expression of interferon-induced microRNAs in patients with chronic hepatitis C virus infection treated with pegylated interferon alpha. Virol J. 2010 Nov 12;7:311. doi: 10.1186/1743-422X-7-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirasaki T, Honda M, Shimakami T, Horii R, Yamashita T, Sakai Y, et al. MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J Virol. 2013 May;87(9):5270–5286. doi: 10.1128/JVI.03022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shiu TY, Huang SM, Shih YL, Chu HC, Chang WK, Hsieh TY. Hepatitis C virus core protein down-regulates p21(Waf1/Cip1) and inhibits curcumin-induced apoptosis through microRNA-345 targeting in human hepatoma cells. PLoS One. 2013;8(4):e61089. doi: 10.1371/journal.pone.0061089. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Shrivastava S, Mukherjee A, Ray RB. Hepatitis C virus infection, microRNA and liver disease progression. World J Hepatol. 2013a Sep 27;5(9):479–486. doi: 10.4254/wjh.v5.i9.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shrivastava S, Petrone J, Steele R, Lauer GM, Di Bisceglie AM, Ray RB. Up-regulation of circulating miR-20a is correlated with hepatitis C virus-mediated liver disease progression. Hepatology. 2013b Sep;58(3):863–871. doi: 10.1002/hep.26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sidorkiewicz M, Grek M, Jozwiak B, Majda-Stanislawska E, Piekarska A, Bartkowiak J. Expression of microRNA-155 precursor in peripheral blood mononuclear cells from Hepatitis C patients after antiviral treatment. Acta Virol. 2010;54(1):75–78. doi: 10.4149/av_2010_01_75. [DOI] [PubMed] [Google Scholar]
- 77.Singaravelu R, Chen R, Lyn RK, Jones DM, O'Hara S, Rouleau Y, et al. Hepatitis C virus induced up-regulation of microRNA-27: A novel mechanism for hepatic steatosis. Hepatology. 2013 Jul 29; doi: 10.1002/hep.26634. [DOI] [PubMed] [Google Scholar]
- 78.Su TH, Liu CH, Liu CJ, Chen CL, Ting TT, Tseng TC, Chen PJ, Kao JH, Chen DS. Serum microRNA-122 level correlates with virologic responses to pegylated interferon therapy in chronic hepatitis C. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7844–7849. doi: 10.1073/pnas.1306138110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi K, Yan I, Wen HJ, Patel T. microRNAs in liver disease: from diagnostics to therapeutics. Clin Biochem. 2013 Jul;46(10–11):946–952. doi: 10.1016/j.clinbiochem.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomimaru Y, Eguchi H, Nagano H, Wada H, Kobayashi S, Marubashi S, et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012 Jan;56(1):167–175. doi: 10.1016/j.jhep.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 81.Trebicka J, Anadol E, Elfimova N, Strack I, Roggendorf M, Viazov S, et al. Hepatic and serum levels of miR-122 after chronic HCV-induced fibrosis. J Hepatol. 2013 Feb;58(2):234–239. doi: 10.1016/j.jhep.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 82.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011 Sep 1;39(16):7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ura S, Honda M, Yamashita T, Ueda T, Takatori H, Nishino R, et al. Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology. 2009 Apr;49(4):1098–1112. doi: 10.1002/hep.22749. [DOI] [PubMed] [Google Scholar]
- 84.Varnholt H, Drebber U, Schulze F, Wedemeyer I, Schirmacher P, Dienes HP, et al. MicroRNA gene expression profile of hepatitis C virus-associated hepatocellular carcinoma. Hepatology. 2008 Apr;47(4):1223–1232. doi: 10.1002/hep.22158. [DOI] [PubMed] [Google Scholar]
- 85.Waidmann O, Bihrer V, Kronenberger B, Zeuzem S, Piiper A, Forestier N. Pretreatment serum microRNA-122 is not predictive for treatment response in chronic hepatitis C virus infection. Dig Liver Dis. 2012 May;44(5):438–441. doi: 10.1016/j.dld.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 86.Waidmann O, Köberle V, Brunner F, Zeuzem S, Piiper A, Kronenberger B. Serum microRNA-122 predicts survival in patients with liver cirrhosis. PLoS One. 2012;7(9):e45652. doi: 10.1371/journal.pone.0045652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang B, Howel P, Bruheim S, Ju J, Owen LB, Fodstad O, et al. Systematic evaluation of three microRNA profiling platforms: microarray, beads array, and quantitative real-time PCR array. PLoS One. 2011 Feb 11;6(2):e17167. doi: 10.1371/journal.pone.0017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007 Apr;46(4):336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 89.Wong QW, Lung RW, Law PT, Lai PB, Chan KY, To KF, et al. MicroRNA-223 is commonly repressed in hepatocellular carcinoma and potentiates expression of Stathmin1. Gastroenterology. 2008 Jul;135(1):257–269. doi: 10.1053/j.gastro.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 90.Yang X, Marcucci K, Anguela X, Couto LB. Preclinical evaluation of an anti-HCV miRNA cluster for treatment of HCV infection. Mol Ther. 2013 Mar;21(3):588–601. doi: 10.1038/mt.2012.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeng B, Li Z, Chen R, Guo N, Zhou J, Zhou Q, et al. Epigenetic regulation of miR-124 by hepatitis C virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3. FEBS Lett. 2012 Sep 21;586(19):3271–3278. doi: 10.1016/j.febslet.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 92.Zhang X, Daucher M, Armistead D, Russell R, Kottilil S. MicroRNA expression profiling in HCV-infected human hepatoma cells identifies potential anti-viral targets induced by interferon-α. PLoS One. 2013;8(2):e55733. doi: 10.1371/journal.pone.0055733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Wei W, Cheng N, Wang K, Li B, Jiang X, Sun S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology. 2012 Nov;56(5):1631–1640. doi: 10.1002/hep.25849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


