Abstract
Purpose
Tumor biopsies are central to the diagnosis and management of cancer and are critical to efforts in personalized medicine and targeted therapeutics. We sought to evaluate the safety and accuracy of biopsies in children with cancer.
Patients and Methods
All biopsies performed in children at our institution with a suspected or established diagnosis of cancer from 2003 to 2012 were retrospectively reviewed. Patient characteristics, and disease- and procedure-related factors were correlated with procedure-related complications and diagnostic accuracy, using logistic regression analysis.
Results
One thousand seventy-three biopsies were performed in 808 patients. Of 1025 biopsies with adequate follow-up, 79 (7.7%) were associated with an adverse event, 35 (3.4%) of which were minor (Grade 1–2) and 32 (3.1%) were major (Grade 3–4). The most common major adverse events were blood transfusion (>10cc/kg, 24 cases) and infection requiring intravenous antibiotics (6 cases). Eleven deaths (1.4%) occurred within 30 post-procedure days but the procedure may have contributed to the outcome in only two. Nine hundred twenty-six (90.3%) biopsies provided definitive histologic diagnoses. Using multivariable analysis, biopsy site, pre-procedure hematocrit, and body mass index (BMI) were associated with risk of post-procedural complications (P<0.0001, P<0.0001, and P=0.0029, respectively). Excisional biopsy and biopsy site were independently associated with obtaining a diagnostic result (P=0.0002 and P=0.0008, respectively).
Conclusion
Tumor biopsies in children with cancer are associated with a low incidence of complications and a high rate of diagnostic accuracy. The predictive factors identified for adverse outcomes may aid risk assessment and pre-procedural counseling.
Keywords: Safety, Accuracy, Biopsy, Tumors, Pediatrics
INTRODUCTION
Tumor biopsies are central to the current and evolving management strategies for patients with cancer1. Risk-based therapies rely on histologic features and molecular markers for stratification2–11. In addition, targeted agents are being increasingly used in pediatric cancers, and the need for individualized comprehensive genomic evaluation to identify potential molecular targets has become increasingly important12–15. An estimated 5% of pediatric solid tumors may harbor actionable genetic mutations, with 10% of mutations found in the germ-line16–18. Additionally, pharmacogenomic and pharmacodynamic studies utilize biomarkers from tumor tissue to evaluate modulation of the intended molecular targets19.
While most biopsies in cancer patients are performed for diagnostic purposes, the role of research-related biopsies is also being increasingly explored. Genomic analysis of serial biopsies has been used to study tumor heterogeneity and clonal evolution of cancers20–27. Sequential tumor biopsies have also been used in Phase I trials to evaluate putative predictive biomarkers and proof of target alteration in adult cancers19, 28–30. The potential incorporation of serial biopsies in pediatric cancer management may similarly help guide treatment and provide insights into the mechanisms of chemoresistance and acquired mutations20. Yet the safety and accuracy of tumor biopsies have not been assessed in the pediatric population31–33. This information is of critical importance in planning patient management and in the informed consent process, particularly when the biopsies are performed as part of research protocols.
We sought to assess the safety and diagnostic accuracy of tissue biopsies in pediatric cancer patients, and identify factors that predict for post-procedural adverse events and sub-optimal diagnostic accuracy.
PATIENTS AND METHODS
Patients and procedures
Following Institutional Review Board approval, we retrospectively reviewed the medical records of all patients who underwent tissue biopsies at St. Jude Children’s Research Hospital between January 1, 2003 and December 31, 2012. We collected data regarding patient characteristics including age at the time of procedure, weight, height, race, gender, primary diagnosis, histologic result of biopsy and pre-procedure laboratory values; and procedure characteristics including the type of anesthesia used, biopsy site, mode and extent of biopsy, imaging modality used (if any), and the department performing the biopsy.
Patients with and without a final diagnosis of cancer were included, so long as cancer was in the pre-procedure differential diagnosis. Excisional, incisional, and core needle biopsies performed by either a surgeon or an interventional radiologist were included (Fig 1A).
Fig 1.
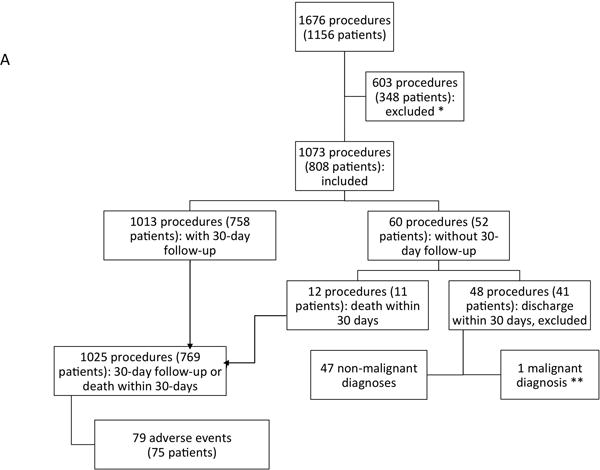
Breakdown of 1025 biopsies analyzed for adverse events (A) and diagnostic accuracy (B).
* Excluded biopsies performed at the time of or as part of a larger operation, fine needle aspirations, centeses, bone marrow aspiration and biopsies, brain biopsies, endoscopic biopsies including gastrointestinal endoscopies beyond the oropharynx, bronchoscopies, cystoscopies, any biopsy where the differential diagnosis did not include malignancy, biopsies performed to assess for graft-versus-host disease, and all skin biopsies ** sought treatment elsewhere.
All adverse events occurring within the 30-day post-procedure period were reviewed and graded 1–4 according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.034. Sixty procedures in 52 patients did not have the 30-day follow-up: 11 patients (one of whom had two procedures) died within 30 post-procedure days and were included in the analysis; 40 patients had non-malignant diagnoses, and were, therefore, not followed at our institution, and so were excluded from the analysis.
The diagnostic accuracy of a biopsy was determined by (1) evaluating the conclusiveness of the pathologist’s report, (2) comparing the congruency of the histologic result obtained with all subsequent biopsies, if done, taken at that site, and (3) the patient’s clinical course. In accordance with Society of Interventional Radiology guidelines35, a biopsy was considered “diagnostic” if it acquired an adequate volume of lesional material that yielded a definitive histologic diagnosis, “inadequate” if lesional material was present but a definitive, pathologic diagnosis could not be obtained, or “non-diagnostic” if there was no representation of lesional material from the target biopsy site or if the biopsy failed to provide any diagnostic information whatsoever.
Statistical analysis
Correlation of study variables with diagnostic accuracy and incidence of complications were analyzed using univariate logistic regression. Using stepwise selection, all factors entered into multivariable logistic regression models at level of P≤0.236. The relationship of selected laboratory test values and the occurrences of post-procedural blood transfusions and infections were analyzed using Pearson Correlation and univariate logistic regression.
RESULTS
Patient and procedure characteristics
One thousand seventy-three biopsies were performed in 808 patients. Of these, 769 patients (1025 procedures) with adequate follow-up, and 11 patients who died within 30 days of the procedure were included in the analysis. Patient, procedure, and disease characteristics are summarized in Table 1. Fifty-nine (7.7%) patients undergoing 81 (7.9%) procedures were over the age of 21 and 7 (0.9%) patients undergoing 9 (0.9%) procedures were over the age of 25. Eighty-one (7.9%) patients were obese with a BMI above 30 at the time of procedure. Four hundred eighty-nine (47.7%) biopsies were performed for initial diagnosis (within 30 days of presentation), and 536 (52.3%) for follow-up of disease.
Table 1.
Univariate analysis of variables associated with occurrence of post-operative complications and inadequate or non-diagnostic biopsy result
| Characteristic | No. of procedures | Post-procedural adverse events | Inadequate / non-diagnostic result | ||
|---|---|---|---|---|---|
| Patient characteristics | |||||
| All patients | 1025 | 79(7.7) | 99 (9.7) | ||
|
| |||||
| Categorical variables | n (%) | n(%) | P | n (%) | P |
|
| |||||
| Gender | 0.7134 | 0.5326 | |||
| Male | 591 (57.7) | 44 (55.7) | 60 (60.6) | ||
| Female | 434 (42.3) | 35 (44.3) | 39 (39.4) | ||
|
| |||||
| Ethnicity | 0.8548 | 0.1907 | |||
| White | 656 (64.0) | 50 (63.3) | 69 (69.7) | ||
| African-American | 320 (31.2) | 25 (31.6) | 23 (23.2) | ||
| Others | 49 (4.8) | 5 (7.0) | 7 (7.1) | ||
|
| |||||
| Continuous variables | Median (range) | Median (range) | P | Median (range) | P |
|
| |||||
| BMI | 19.0 (10.1–61.1) | 19.1 (10.1–61.1) | 0.4806 | 19.0 (13.2–42.5) | 0.9628 |
| Weight (kg) | 46.7 (2.8–165.0) | 47.0 (3.1–117.5) | 46.6 (2.9–120.1) | ||
| Height (cm) | 153.2 (30.0–198.4) | 153.8 (30.0–180.0) | 153.1 (47.0–198.4) | ||
|
| |||||
| Age at biopsy (years) | 12.7 (0.0–33.7) | 12.8 (0.1–33.7) | 0.1283 | 12.7 (0.1–25.5) | 0.9102 |
|
| |||||
| Procedure characteristics | |||||
|
| |||||
| Categorical variables | n (%) | n(%) | P | n (%) | P |
|
| |||||
| Anesthesia | 0.0908 | 0.6067 | |||
| General anesthesia | 961 (93.8) | 78 (98.7) | 94 (94.9) | ||
| Local or regional | 64 (6.2) | 1 (1.3) | 5 (5.1) | ||
|
| |||||
| Biopsy Site | <0.0001 | 0.0001 | |||
| Head and neck | 206 (20.1) | 3 (3.8) | 11 (11.1) | ||
| Thorax | 172 (16.8) | 31 (39.2) | 23 (23.2) | ||
| Abdomen and pelvis | 323 (31.5) | 30 (38.0) | 18 (18.2) | ||
| Musculoskeletal | 324 (31.6) | 15 (19.0) | 47 (47.5) | ||
|
| |||||
| Mode of biopsy | 0.0344 | <0.0001 | |||
| Open | 443 (43.2) | 18 (22.8) | 20 (20.2) | ||
| Minimally Invasive | 64 (6.2) | 11 (13.9) | 1 (1.0) | ||
| Percutaneous | 518 (50.5) | 50 (63.3) | 78 (78.8) | ||
|
| |||||
| Use of radiographic image guidance | 0.5793 | <0.0001 | |||
| Yes | 645 (62.9) | 52 (65.8) | 83 (83.8) | ||
| No | 380 (37.1) | 27 (34.2) | 16 (16.2) | ||
|
| |||||
| Extent of Biopsy | 0.1786 | <0.0001 | |||
| Incisional | 808 (78.8) | 67 (84.8) | 96 (97.0) | ||
| Excisional | 217 (21.2) | 12 (15.2) | 3 (3.0) | ||
|
| |||||
| Repeat biopsy at a prior biopsy site | 0.7003 | 0.6197 | |||
| Yes | 141 (13.8) | 12 (15.2) | 12 (12.1) | ||
| No | 884 (86.2) | 67 (84.8) | 87 (87.9) | ||
|
| |||||
| Timing of biopsy | 0.1837 | 0.7080 | |||
| At initial diagnosis | 489 (47.7) | 32 (40.5) | 49 (49.5) | ||
| On follow-up | 536 (52.3) | 47 (59.5) | 50 (50.5) | ||
|
| |||||
| Continuous variables | Median (range) | Median (Range) | P | Median (Range) | P |
| WBC (×109/L)** | 6.6(0.1, 91.9) | 6.4 (0.1, 26.4) | 0.7593 | 6.5 (0.1, 91.9) | 0.2723 |
| Hematocrit (%)** | 35.4(5.7, 49.7) | 31.1 (20.4, 43.8) | <0.0001 | 36.0 (20.4, 49.1) | 0.1737 |
| Platelets (×109/L)** | 294 (17, 1526) | 294 (46, 1045) | 0.6940 | 288.5 (45, 784) | 0.6940 |
| INR (s)*** | 1.1 (0.70, 2.30) | 1.1 (0.8, 2.3) | * | 1.0 (0.7, 1.40) | * |
|
| |||||
| Disease characteristics | |||||
|
| |||||
| Categorical variables | n (%) | n(%) | P | n (%) | P |
|
| |||||
| Primary diagnosis | 0.0231 | 0.1777 | |||
| Leukemias and lymphomas | 285 (27.8) | 21 (26.6) | 25 (25.3) | ||
| Central nervous system tumors and retinoblastoma | 30 (2.9) | 4 (5.1) | 2 (2.0) | ||
| Solid organ tumors (neuroblastic, renal, liver tumors; extracranial germ cell tumors, and other solid tumors) | 174 (17.0) | 23 (29.1) | 15 (15.2) | ||
| Soft tissue sarcomas and bone tumors | 357 (34.8) | 19 (24.1) | 30 (30.3) | ||
| Rule out malignancy/non-malignant diagnosis | 178 (17.4) | 12 (15.2) | 26 (26.3) | ||
| No pathological material* | 1 (0.1) | 0 (0.0) | 1 (1.0) | ||
Omitted from analysis.
n=1024.
n=478. Bold values are significant at P<0.05.
General anesthesia was used in 961 (93.8%) procedures, conscious sedation in 59 (5.8%) procedures, and local anesthesia in 5 procedures. The most common biopsy sites were musculoskeletal (324, 31.6%) and abdomen/pelvis (323, 31.5%), followed by head and neck (206, 20.1%) and thoracic sites (172, 16.8%) – the latter defined as the lungs or mediastinum.
Biopsies were performed via an open, “minimally-invasive”, or percutaneous approach in 443 (43.2%), 64 (6.2%) and 518 (50.5%) of cases, respectively. Radiographic image guidance was used in almost all percutaneous biopsies; in only 17 was image guidance not used. Among the 645 image-guided biopsies, the most common imaging modalities used were ultrasound (299, 46.4%), fluoroscopy (178, 27.6%) and computed tomography (CT) (168, 26.0%). The most common primary diagnoses for patients undergoing biopsies are listed in Table 1.
Adverse events – severity and grading
Among 1,025 procedures in 769 patients, 79 adverse events occurred in 76 patients – an incidence of 7.7% (Fig 1A). Using CTCAE criteria v4.0, there were 35 (51.5%) minor Grade 1 or 2 and 32 (48.5%) major Grade 3 or 4 adverse events. During the 30-day follow-up period, 11 deaths occurred after 12 procedures (Supplemental Table 1). The total of 79 adverse events is represented by the total number of procedures performed including the 67 CTCAE graded adverse events and the 12 procedures with deaths within 30 days. The most common minor complications were pulmonary contusions/hematoma or pneumothorax after lung biopsies (17 procedures), and wound infections requiring oral antibiotics (3 procedures). Six patients with pneumothorax required admission for observation, with two additionally requiring supplemental oxygen, and two requiring tube thoracostomy. The CTCAE considers pneumothorax, even if an intervention is required, to be a minor complication.
The most common major complication was post-procedural transfusion of >10cc/kg packed red blood cells (PRBCs) (24 procedures). The CTCAE considers any pediatric patient receiving more than 10cc/kg packed red blood cell (PRBC) transfusion a major complication. The median amount of PRBCs transfused was 12.7 (range: 8.1–60.8) cc/kg. The median pre-procedure hematocrit for these patients was abnormally low at 27.2% (range: 20.4–36.3), but was not significantly lower than in patients not receiving blood transfusions (P=0.5923). Approximately one third of all patients also had a pre-procedural hematocrit of less than 33% (data not shown). Fourteen patients had a greater than 3% drop in post-procedural hematocrit. The estimated blood loss (EBL) was greater than 10% during or after 5 procedures (0.5%) that required blood transfusions – 2 were open procedures and 3 were percutaneous core biopsies. Eleven procedures had negligible blood loss or had no documented record of blood loss. The low pre-procedural hematocrit in these patients and negligible blood loss after these procedures may indicate these patients were likely to receive transfusions regardless of the procedure.
There were 9 (0.9%) post-procedural infections requiring intervention. Three patients required oral antibiotics (Grade 2), and 6 patients required intravenous antibiotics (Grade 3), 3 of whom required opening of the surgical wound.
Two other major (Grade 3) complications included one patient with reactive airway disease post-extubation requiring re-intubation, and another patient with a post-procedural lymph leak after an excisional biopsy of a groin mass requiring re-operation.
There were three Grade 4 adverse events (0.3%). One patient with acute lymphocytic leukemia and multi-system organ failure had bronchopulmonary hemorrhage during an image-guided lung biopsy requiring resuscitation, transfusion of blood products, and vasopressor support. The second patient had post-procedural respiratory failure secondary to fluid overload after an exploratory laparotomy and a liver wedge biopsy for neuroblastoma. The third patient had a wound infection and sepsis after an open thigh mass biopsy.
Three procedure-related adverse events were thought to be secondary to anesthesia. One patient mentioned above had reactive airway disease requiring re-intubation. The second patient also mentioned above had post-procedural respiratory failure secondary to fluid overload. Lastly, a third patient was noted to have aspirated during endotracheal intubation leading to right lower lobe consolidation not requiring any antibiotics.
Adverse Events – Deaths
Within the 30-day post-procedural period, 11 deaths occurred after 12 procedures (Supplemental Table 1). The median duration from procedure to death was 17 days (range: 1–30). Of the 11 deaths, only two biopsies may have contributed to the demise of these patients (#1 and #9). Patient #1, with progressive non-Hodgkin lymphoma, died on post-procedure day 1, with the biopsy having been performed in the setting of sepsis and multi-organ system failure. Although the biopsy seemed to have been uncomplicated, other procedural factors (e.g. anesthesia, positioning) may have contributed to the patient’s demise. Patient #9 required a blood transfusion of 16.7 cc/kg immediately following an open biopsy of a hepatoblastoma. On post-procedure day 5, the patient, had a hematocrit of 33%. However, the patient expired the next day, 6 days after the procedure likely due to delayed intra-tumoral hemorrhage. Seven patients died from rapidly progressive cancer; the biopsy procedure probably did not contribute to their demise. Two patients (#2 and #4) expired without malignant diagnoses. The first patient had aplastic anemia and a fungal infection diagnosed by biopsy, and died of overwhelming sepsis following lobectomy. The second patient had sclerosing mediastinitis diagnosed by biopsy and died 7 days after surgical debulking of disease.
Adverse events – predictive factors
On univariate analysis, low pre-procedure hematocrit, biopsy site, percutaneous mode of biopsy, and primary diagnosis were associated with post-procedure adverse events (P<0.0001, P<0.0001, P=0.0344, and P=0.0231, respectively) (Table 1). On multivariable analysis, all possible explanatory variables were entered or removed based on the stepwise selection rule of P=0.20. The model convergence criterion was satisfied and Hosmer and Lemeshow Goodness-of-Fit had a P-value 0.2781, which showed that the model fit the data well. Based on multivariable analysis, low pre-procedure hematocrit, biopsy site, and higher BMI were associated with adverse events (P= 0.0001, P<0.0001, and P=0.0389 respectively) (Table 3). Post-procedure adverse events were more likely in patients with lower pre-procedure hematocrit (Odds ratio (O.R.) 0.913 (95% confidence interval (CI): 0.875–0.953). Twenty-three of 24 patients requiring transfusions had a pre-procedural hematocrit <33%, and 18 had a hematocrit <30%. Adverse events were also more likely to occur with thoracic sites compared to musculoskeletal sites and abdomen/pelvic sites (O.R. 4.650 (95% CI: 2.340–9.239), and 2.650 (95% CI: 1.488–4.719), respectively). Adverse events were less likely to occur with head and neck sites compared to thoracic and abdomen/pelvic sites (O.R. 0.071 (95% CI: 0.020–0.250), and 0.189 (95% CI: 0.055–0.653), respectively). Lastly, post-procedural adverse events were more likely per unit (kg/m2) increase of BMI. For example, an increase of BMI from 19 to 20 caused a patient’s odds of having an adverse event to increase to 1.067 times (CI: 1.023–1.114).
TABLE 3.
Multivariable Analysis of Variables Associated With the Occurrence of Postoperative Complications and an Inadequate or Nondiagnostic Biopsy Result
|
Complication
|
Inadequate or Nondiagnostic Histologic Result
|
|||||
|---|---|---|---|---|---|---|
| Factor | OR (95% CI)a | Wald Chi-Square Test | P | OR (95% CI) | Wald Chi-Square Test | P |
| Biopsy site | <.0001 | .0008 | ||||
| Head and neck vs musculoskeletal | 0.332 (0.090–1.234) | 2.7092 | 0.410 (0.196–0.854) | 5.6602 | ||
| Head and neck vs thorax | 0.072 (0.020–0.250) | 0.490 (0.217–1.105) | ||||
| Head and neck vs abdomen and pelvis | 0.189 (0.55–0.653) | 1.250 (0.552–2.830) | ||||
| Thorax vs musculoskeletal | 4.650 (2.340–9.239) | 19.2481 | 0.836 (0.464–1.506) | |||
| Abdomen and pelvis vs musculoskeletal | 1.755 (0.885–3.477) | 0.328 (0.181–0.594) | 13.4774 | |||
| Thorax vs abdomen and pelvis | 2.650 (1.488–4.719) | 2.5963 | 2.551 (1.309–4.969) | |||
| Hematocrit | <.0001 | |||||
| Per unit increase | 0.913 (0.875–0.953) | 17.3030 | ||||
| Biopsy type | <.0001 | |||||
| Open vs percutaneous | 0.311 (1.73–0.558) | 15.3521 | ||||
| BMI | .0029 | |||||
| Per unit increase | 1.067 (1.023–1.114) | 8.8928 | ||||
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index; OR, odds ratio.
Bold type indicates confidence intervals that do not cross 1.
Diagnostic Accuracy
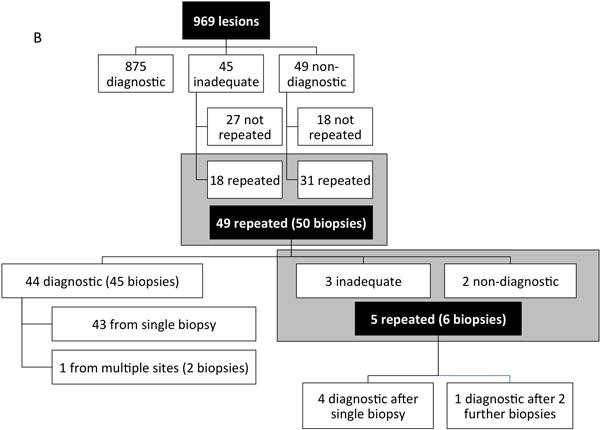
A definitive diagnosis was obtained after 1 biopsy attempt in 875 of 969 (90.3%) cases; a second biopsy in 49 patients (50 biopsies) yielded an additional 45 diagnostic samples; 5 patients required third and fourth biopsies to obtain a diagnosis for an overall diagnostic yield of 94.9% (Fig 1B). Of the 49 patients that had repeat biopsies, 39 (79.6%) had an initial percutaneous needle biopsy. Of these 39 initial percutaneous needle biopsies requiring repeat biopsies, 20 (51.2%) went on to open procedures of which only 2 were unsuccessful, 4 (10.2%) went on to minimally invasive biopsies all of which were successful, and the remaining 15 (38.5%) had repeat percutaneous biopsies of which 4 were unsuccessful. Eventually all 49 patients had a diagnostic result–14 had a final non-neoplastic diagnosis, 5 had benign neoplasms and 30 were malignant. The most common malignant diagnoses that required repeat biopsies were Hodgkin lymphoma (3 new diagnoses, 3 relapses), osteosarcoma (3 high grade and 3 low grade), and Ewing sarcoma (4 patients). Of 136 patients with Hodgkin’s lymphoma, 47 had initial biopsies for diagnosis. Of these 47, 20 (42.6%) were performed via an open or minimally-invasive approach, all of which yielded a diagnostic result. In contrast, of the remaining 27 procedures that were performed percutaneously, the diagnostic yield from these procedures was only 88.9% (24 of 27 procedures).
In 27 patients new information was obtained on the repeat biopsy that changed the final diagnosis. Perioperative adverse events occurred in 3 (6%) of the 50 patients that had repeat biopsies – 1 patient had post-procedural hematomas during initial and both repeat lung biopsies, requiring an unplanned ICU admission on one episode; 1 patient had an initial biopsy aborted due to technical difficulties; and 1 patient had pulmonary hemorrhage on an initial biopsy not requiring chest tube insertion.
On univariate analysis, failing to obtain a diagnostic result from a biopsy was significantly associated with mode of biopsy, use of radiographic image guidance, and extent of biopsy (P<0.0001), as well as biopsy site (P=0.0001) (Table 1). Whereas the diagnostic yield of open and minimally-invasive procedures was 95.9%, the diagnostic yield of percutaneous biopsies was 78.0%. Similarly, the diagnostic yield of excisional biopsies was 98.6% as compared to incisional biopsies being 88.1%. On multivariable analysis, the final model was constructed based on the stepwise selection of P=0.20. The model convergence criterion was satisfied and Hosmer and Lemeshow Goodness-of-Fit had a P-value 0.6195, which showed that the model fit the data well. Based on multivariable analysis, obtaining a diagnostic result from a biopsy was independently associated with mode of biopsy (P<0.0001) and biopsy site (P<0.0001) (Table 3). A diagnostic result was more likely with open biopsies compared to percutaneous biopsies (O.R. 5.006 (95% CI: 1.388–18.053), and when the biopsy site was thoracic compared to abdomen/pelvis (O.R. 2.551 (95% CI: 1.309–4.969). A non-diagnostic or inadequate biopsy result was more likely when the biopsy site was the head and neck or abdomen/pelvis, compared to musculoskeletal sites (O.R. 0.410 (95% CI: 0.196–0.854) and 0.328 (95% CI: 0.181–0.594), respectively).
DISCUSSION
This large, single-institution, retrospective review demonstrates that tumor biopsy in the pediatric population is associated with a low rate of adverse events, and a high rate of diagnostic accuracy. Adverse events occurred in association with 7.7% of the procedures that included 32 major adverse events and 11 deaths (4.2%). However, careful review suggested the biopsy procedure may have contributed to the demise of only 2 patients and 24 of the major adverse events were due to the need for blood transfusion of >10cc/kg, with most of these patients having anemia prior to the procedure and only 8 of whom had clearly documented post-procedural blood loss. Although pre-procedural hematocrit was not associated with transfusion, it was also not associated with any other individual adverse events. These results suggest that the incidence of clinically significant, procedure-related adverse events is probably <2%. In addition, in this review, we attempted to capture only the most invasive procedures by excluding all endoscopic and skin or subcutaneous biopsies as these biopsies were likely to be associated with an even lower rate of adverse events.
Multivariable analysis revealed that low pre-procedure hematocrit, higher BMI, and site of biopsy (thoracic>musculoskeletal>head/neck) were significantly associated with adverse events, with the risk of procedure-related complications being nearly 4 times higher when biopsying a lesion in a thoracic site compared to a musculoskeletal site. However, a variable confounding this result likely was the frequent use of post-procedure radiographic imaging following pulmonary biopsies, which may have increased the detection of minor pulmonary contusions and parenchymal bleeds that were generally of no clinical consequence.
The risks of adverse events of these procedures must be weighed in individual patients against potential benefits gained both by the patient’s therapy and through research-related analyses37. Some of the adverse events we observed in ill, neutropenic patients illustrate the importance of rational patient selection, particularly for research biopsies. Although we did not find any correlation between adverse events and neutropenia, approximately 5% of patients had an absolute neutrophil count of <1,000 ×106/L (data not shown). When biopsies are used to determine the use of experimental agents, a patient’s willingness to participate in multiple biopsies depends on their understanding of the scientific rationale and the potential risks and benefits of the procedure and the test agent19, 29, 31, 38. While the emerging consensus is that it is ethical to implement mandatory biopsies in clinical trials, concerns remain over issues of patient autonomy, perceived benefit, and impact on protocol accrual, despite informed consent29, 38, 39.
Consistent with our results, other published studies suggest that a definitive result can be obtained at the initial biopsy attempt in up to 90% of cases of childhood solid tumors40–44. In addition, when a definitive result was not obtained initially, we found that a definitive result could be obtained with additional biopsies, without a greater likelihood of complications following repeat biopsies – potentially improving the diagnostic yield by changing from core needle biopsy to an open or minimally-invasive excisional biopsy. This is an important observation for institutions such as ours, that are often referred patients following an initial biopsy performed at another institution where the tissue obtained was not processed in a way that would permit full molecular genetic analysis, and so a repeat biopsy might be considered.
Notably, the majority of cases that required repeat biopsies in our series had a percutaneous biopsy initially, suggesting that percutaneous biopsies, despite usually being performed with imaging guidance, had a poorer diagnostic accuracy as compared to open biopsies, likely due to the smaller volume of tissue obtained. As nearly all percutaneous biopsies were performed with image guidance, this was the likely reason use of image guidance was associated with failure to obtain diagnostic tissue in univariate analysis39–41,43–47. Thus the benefits of using the generally least invasive, percutaneous approach to performing a biopsy must be weighed against the possibility of obtaining a non-diagnostic sample and the risk and delay with performing a second biopsy40, 41, 45.
Our study was limited by its retrospective nature. This study also does not address potential long-term adverse events following open, surgical biopsies. It also does not consider possible long-term oncologic adverse events such as local recurrence or tumor dissemination, as a consequence of the biopsy. Biopsy procedures could have been associated with potential tumor cell dissemination46–49. Sampling error is a key concern of biopsies in childhood cancers, particularly solid tumors, due to their heterogeneity. We did not evaluate diagnostic inaccuracy in relation to tumor heterogeneity, but this could be the subject of future studies analyzing diagnostic discrepancies in serial tumor biopsies.
We believe that the safety and accuracy data from this study will assist in the pre-procedure counseling of patients and families regarding the potential risks of biopsies. These data will also help in developing research-directed tumor biopsy protocols, which may contribute to the growing body of genomic and molecular marker data in targeted pediatric cancer therapy.
Supplementary Material
Table 2.
Multivariable analysis of variables associated with occurrence of post-operative complications and inadequate or non-diagnostic biopsy result
| Factor | Complication | Inadequate or non-diagnostic histologic result | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Odds ratio (95% CI) | Wald Chi-square | P | Odds ratio (95% CI) | Wald Chi-square | P | |
| Biopsy site | <0.0001 | 0.0008 | ||||
| Head and neck vs. musculoskeletal | 0.332 (0.090–1.234) | 2.7092 | 0.410 (0.196–0.854) | 5.6602 | ||
| Head and neck vs. thorax | 0.072 (0.020–0.250) | 0.490 (0.217–1.105) | ||||
| Head and neck vs. abdomen and pelvis | 0.189 (0.55–0.653) | 1.250 (0.552–2.830) | ||||
| Thorax vs. musculoskeletal | 4.650 (2.340–9.239) | 19.2481 | 0.836 (0.464–1.506) | |||
| Abdomen and pelvis vs. musculoskeletal | 1.755 (0.885–3.477) | 2.5963 | 0.328 (0.181–0.594) | 13.4774 | ||
| Thorax vs. abdomen and pelvis | 2.650 (1.488–4.719) | 2.551 (1.309–4.969) | ||||
|
| ||||||
| Hematocrit | <0.0001 | |||||
| Per unit increase | 0.913(0.875–0.953) | 17.3030 | ||||
|
| ||||||
| Biopsy Type | <0.0001 | |||||
| Open vs. percutaneous | 0.311 (1.73–0.558) | 15.3521 | ||||
|
| ||||||
| BMI | 0.0029 | |||||
| Per unit increase | 1.067 (1.023–1.114) | 8.8928 | ||||
Abbreviation: CI, confidence interval.
Acknowledgments
The authors thank Liza Emanus, Leslie White and Tracey Daley for administrative assistance.
Funding support: National Cancer Institute Cancer Center Support (CORE) grant CA-21765
Footnotes
Disclaimers: None
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Conception and design: Rodrigo B. Interiano, Amos H.P. Loh, Alberto Pappo, Andrew M. Davidoff
Financial support: Andrew M. Davidoff
Provision of study materials or patients: Andrew M. Davidoff
Collection and assembly of data: Rodrigo B. Interiano, Amos H.P. Loh, Nathan Hinkle, Fazal N. Wahid, Alpin Malkan
Data analysis and interpretation: Rodrigo B. Interiano, Amos H.P. Loh, Shenghua Mao, Jianrong Wu
Manuscript writing: Rodrigo B. Interiano, Amos H.P. Loh, Andrew M. Davidoff
Final approval of manuscript: All authors
References
- 1.Basik M, Aguilar-Mahecha A, Rousseau C, Diaz Z, Tejpar S, Spatz A, et al. Biopsies: next-generation biospecimens for tailoring therapy. Nature reviews Clinical oncology. 2013 Aug;10(8):437–50. doi: 10.1038/nrclinonc.2013.101. [DOI] [PubMed] [Google Scholar]
- 2.Maris JM. Recent advances in neuroblastoma. The New England journal of medicine. 2010 Jun 10;362(23):2202–11. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Pediatric clinics of North America. 2008 Feb;55(1):97–120. doi: 10.1016/j.pcl.2007.10.014. , x. [DOI] [PubMed] [Google Scholar]
- 4.Metzger ML, Dome JS. Current therapy for Wilms’ tumor. The oncologist. 2005 Nov-Dec;10(10):815–26. doi: 10.1634/theoncologist.10-10-815. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson GE, Breslow NE, Dome J, Guthrie KA, Norkool P, Li S, et al. Rhabdoid tumor of the kidney in the National Wilms’ Tumor Study: age at diagnosis as a prognostic factor. Journal of clinical oncology. 2005 Oct 20;23(30):7641–5. doi: 10.1200/JCO.2004.00.8110. [DOI] [PubMed] [Google Scholar]
- 6.Green DM. The evolution of treatment for Wilms tumor. Journal of pediatric surgery. 2013 Jan;48(1):14–9. doi: 10.1016/j.jpedsurg.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Meza JL, Anderson J, Pappo AS, Meyer WH, Children’s Oncology G. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children’s Oncology Group. Journal of clinical oncology. 2006 Aug 20;24(24):3844–51. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 8.Breneman JC, Lyden E, Pappo AS, Link MP, Anderson JR, Parham DM, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma–a report from the Intergroup Rhabdomyosarcoma Study IV. Journal of clinical oncology. 2003 Jan 1;21(1):78–84. doi: 10.1200/JCO.2003.06.129. [DOI] [PubMed] [Google Scholar]
- 9.Huang HY, Illei PB, Zhao Z, Mazumdar M, Huvos AG, Healey JH, et al. Ewing sarcomas with p53 mutation or p16/p14ARF homozygous deletion: a highly lethal subset associated with poor chemoresponse. Journal of clinical oncology. 2005 Jan 20;23(3):548–58. doi: 10.1200/JCO.2005.02.081. [DOI] [PubMed] [Google Scholar]
- 10.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. Journal of clinical oncology. 2005 Mar 20;23(9):2004–11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. Journal of clinical oncology. 2003 May 15;21(10):2011–8. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 12.Andre F, McShane LM, Michiels S, Ransohoff DF, Altman DG, Reis-Filho JS, et al. Biomarker studies: a call for a comprehensive biomarker study registry. Nature reviews Clinical oncology. 2011 Mar;8(3):171–6. doi: 10.1038/nrclinonc.2011.4. [DOI] [PubMed] [Google Scholar]
- 13.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009 Apr 9;458(7239):719–24. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahal R, Frick M, Romero R, Korn JM, Kridel R, Chan FC, et al. Pharmacological and genomic profiling identifies NF-kappaB-targeted treatment strategies for mantle cell lymphoma. Nature medicine. 2014 Jan;20(1):87–92. doi: 10.1038/nm.3435. [DOI] [PubMed] [Google Scholar]
- 15.Desai AN, Jere A. Next-generation sequencing: ready for the clinics? Clinical genetics. 2012 Jun;81(6):503–10. doi: 10.1111/j.1399-0004.2012.01865.x. [DOI] [PubMed] [Google Scholar]
- 16.Janeway KA, Place AE, Kieran MW, Harris MH. Future of clinical genomics in pediatric oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 May 20;31(15):1893–903. doi: 10.1200/JCO.2012.46.8470. [DOI] [PubMed] [Google Scholar]
- 17.Downing JR, Wilson RK, Zhang J, Mardis ER, Pui CH, Ding L, et al. The Pediatric Cancer Genome Project. Nature genetics. 2012 Jun;44(6):619–22. doi: 10.1038/ng.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung NK, Zhang J, Lu C, Parker M, Bahrami A, Tickoo SK, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012 Mar 14;307(10):1062–71. doi: 10.1001/jama.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowlati A, Hoppel CL, Ingalls ST, Majka S, Li X, Sedransk N, et al. Phase I clinical and pharmacokinetic study of rebeccamycin analog NSC 655649 given daily for five consecutive days. Journal of clinical oncology. 2001 Apr 15;19(8):2309–18. doi: 10.1200/JCO.2001.19.8.2309. [DOI] [PubMed] [Google Scholar]
- 20.Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. British Society of Gastroenterology. Gut. 1999 Oct;45(Suppl 4):IV1–IV11. doi: 10.1136/gut.45.2008.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010 Apr 15;464(7291):999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009 Oct 8;461(7265):809–13. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 23.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012 Jun 21;486(7403):395–9. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008 Nov 28;322(5906):1377–80. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer JA, Wang J, Hogan LE, Yang JJ, Dandekar S, Patel JP, et al. Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nature genetics. 2013 Mar;45(3):290–4. doi: 10.1038/ng.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England journal of medicine. 2012 Mar 8;366(10):883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh SS, Wei JP, Li X, Huang RR, Doan NB, Scolyer RA, et al. Differential gene expression profiling of primary cutaneous melanoma and sentinel lymph node metastases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012 Jun;25(6):828–37. doi: 10.1038/modpathol.2012.32. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Han JJ, Altwerger G, Kohn EC. Proteomics and biomarkers in clinical trials for drug development. Journal of proteomics. 2011 Nov 18;74(12):2632–41. doi: 10.1016/j.jprot.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Roca CA, Lacroix L, Massard C, De Baere T, Deschamps F, Pramod R, et al. Sequential research-related biopsies in phase I trials: acceptance, feasibility and safety. Annals of oncology. 2012 May;23(5):1301–6. doi: 10.1093/annonc/mdr383. [DOI] [PubMed] [Google Scholar]
- 30.Cannistra SA. Performance of biopsies in clinical research. Journal of clinical oncology. 2007 Apr 10;25(11):1454–5. doi: 10.1200/JCO.2006.10.0115. [DOI] [PubMed] [Google Scholar]
- 31.Lee JM, Hays JL, Noonan AM, Squires J, Minasian L, Annunziata C, et al. Feasibility and safety of sequential research-related tumor core biopsies in clinical trials. Cancer. 2013 Apr 1;119(7):1357–64. doi: 10.1002/cncr.27916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agulnik M, Oza AM, Pond GR, Siu LL. Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. Journal of clinical oncology. 2006 Oct 20;24(30):4801–7. doi: 10.1200/JCO.2005.03.4496. [DOI] [PubMed] [Google Scholar]
- 33.Anderson BD, Adamson PC, Weiner SL, McCabe MS, Smith MA. Tissue collection for correlative studies in childhood cancer clinical trials: ethical considerations and special imperatives. Journal of clinical oncology. 2004 Dec 1;22(23):4846–50. doi: 10.1200/JCO.2004.02.138. [DOI] [PubMed] [Google Scholar]
- 34.NCI. Common Terminology Criteria for Adverse Events v4.0. (NIH publication # 09-74732009; (NIH publication # 09-7473)). [Google Scholar]
- 35.Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC, et al. Quality improvement guidelines for percutaneous needle biopsy. Journal of vascular and interventional radiology : JVIR. 2010 Jul;21(7):969–75. doi: 10.1016/j.jvir.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3. Hoboken, New Jersey: John Wiley & Sons, Inc; 2013. Assessing the Fit of the Model; pp. 153–225. (Wiley Series in Probability and Statistics). Chapter 5. [Google Scholar]
- 37.Olson EM, Lin NU, Krop IE, Winer EP. The ethical use of mandatory research biopsies. Nature reviews Clinical oncology. 2011 Oct;8(10):620–5. doi: 10.1038/nrclinonc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Osta H, Hong D, Wheler J, Fu S, Naing A, Falchook G, et al. Outcomes of research biopsies in phase I clinical trials: the MD anderson cancer center experience. The oncologist. 2011;16(9):1292–8. doi: 10.1634/theoncologist.2011-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peppercorn J, Shapira I, Collyar D, Deshields T, Lin N, Krop I, et al. Ethics of mandatory research biopsy for correlative end points within clinical trials in oncology. Journal of clinical oncology. 2010 May 20;28(15):2635–40. doi: 10.1200/JCO.2009.27.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Squire R, Willetts I. The International Society of Paediatric Surgical Oncology. Montreal, Canada: Sep 13–14, 1999. Problems with biopsying solid tumours in children; p. 344. 1999. [Google Scholar]
- 41.Shin HJ, Amaral JG, Armstrong D, Chait PG, Temple MJ, John P, et al. Image-guided percutaneous biopsy of musculoskeletal lesions in children. Pediatric radiology. 2007 Apr;37(4):362–9. doi: 10.1007/s00247-007-0421-5. [DOI] [PubMed] [Google Scholar]
- 42.Waldhausen JH, Tapper D, Sawin RS. Minimally invasive surgery and clinical decision-making for pediatric malignancy. Surgical endoscopy. 2000 Mar;14(3):250–3. doi: 10.1007/s004640000033. [DOI] [PubMed] [Google Scholar]
- 43.Sebire NJ, Roebuck DJ. Pathological diagnosis of paediatric tumours from image-guided needle core biopsies: a systematic review. Pediatric radiology. 2006 May;36(5):426–31. doi: 10.1007/s00247-006-0123-4. [DOI] [PubMed] [Google Scholar]
- 44.Sklair-Levy M, Lebensart PD, Applbaum YH, Ramu N, Freeman A, Gozal D, et al. Percutaneous image-guided needle biopsy in children–summary of our experience with 57 children. Pediatric radiology. 2001 Oct;31(10):732–6. doi: 10.1007/s002470100533. [DOI] [PubMed] [Google Scholar]
- 45.Garrett KM, Fuller CE, Santana VM, Shochat SJ, Hoffer FA. Percutaneous biopsy of pediatric solid tumors. Cancer. 2005 Aug 1;104(3):644–52. doi: 10.1002/cncr.21193. [DOI] [PubMed] [Google Scholar]
- 46.Raa ST, Oosterling SJ, van der Kaaij NP, van den Tol MP, Beelen RH, Meijer S, et al. Surgery promotes implantation of disseminated tumor cells, but does not increase growth of tumor cell clusters. Journal of surgical oncology. 2005 Nov 1;92(2):124–9. doi: 10.1002/jso.20273. [DOI] [PubMed] [Google Scholar]
- 47.Fermor B, Umpleby HC, Lever JV, Symes MO, Williamson RC. Proliferative and metastatic potential of exfoliated colorectal cancer cells. Journal of the National Cancer Institute. 1986 Feb;76(2):347–9. [PubMed] [Google Scholar]
- 48.Guller U, Zajac P, Schnider A, Bosch B, Vorburger S, Zuber M, et al. Disseminated single tumor cells as detected by real-time quantitative polymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Annals of surgery. 2002 Dec;236(6):768–75. doi: 10.1097/00000658-200212000-00009. discussion 75–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oosterling SJ, van der Bij GJ, van Egmond M, van der Sijp JR. Surgical trauma and peritoneal recurrence of colorectal carcinoma. European journal of surgical oncology. 2005 Feb;31(1):29–37. doi: 10.1016/j.ejso.2004.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


