Abstract
Intracellular killing of Staphylococcus aureus by alveolar macrophages is known to be enhanced by exposure to alveolar lining material. Because this material may have a role in pulmonary host defenses, we have studied its effect on pneumococci and other nonstaphylococcal organisms. Alveolar lining material from rats caused rapid killing and lysis of pneumococci. The antipneumococcal activity was localized to the surfactant-containing fraction of the fluid and was not affected by trypsin. Phospholipid extracts of the surfactant fraction or purified lamellar bodies killed pneumococci. Lysis of pneumococci by the surfactant fraction appeared to be mediated by a detergent-like activation of pneumococcal autolysin, in that bacteriolysis was prevented by substitution of ethanolamine for choline in pneumococcal cell walls, and a pneumococcal transformant that lacked autolysin was not lysed. The surfactant fraction readily killed pneumococci containing ethanolamine or the autolysin-defective transformant, and studies with tritiated methyl-D-glucose loading and release showed that killing was associated with increased bacterial cell membrane permeability. Bactericidal activity (without lysis) was observed with several nonpneumococcal gram-positive bacteria, including Streptococcus viridans, unspeciated respiratory streptococci, Streptococcus pyogenes, Streptococcus bovis, and Bacillus species. Purified diacylphospholipids had no antibacterial activity, however, a lysophospholipid, palmitoyl lysophosphatidylcholine, had many properties resembling the surfactant-containing fraction of lavage, including autolysin-mediated pneumococcal lysis, altered cell membrane permeability, and antibacterial activity against several gram-positive bacteria.
Full text
PDF
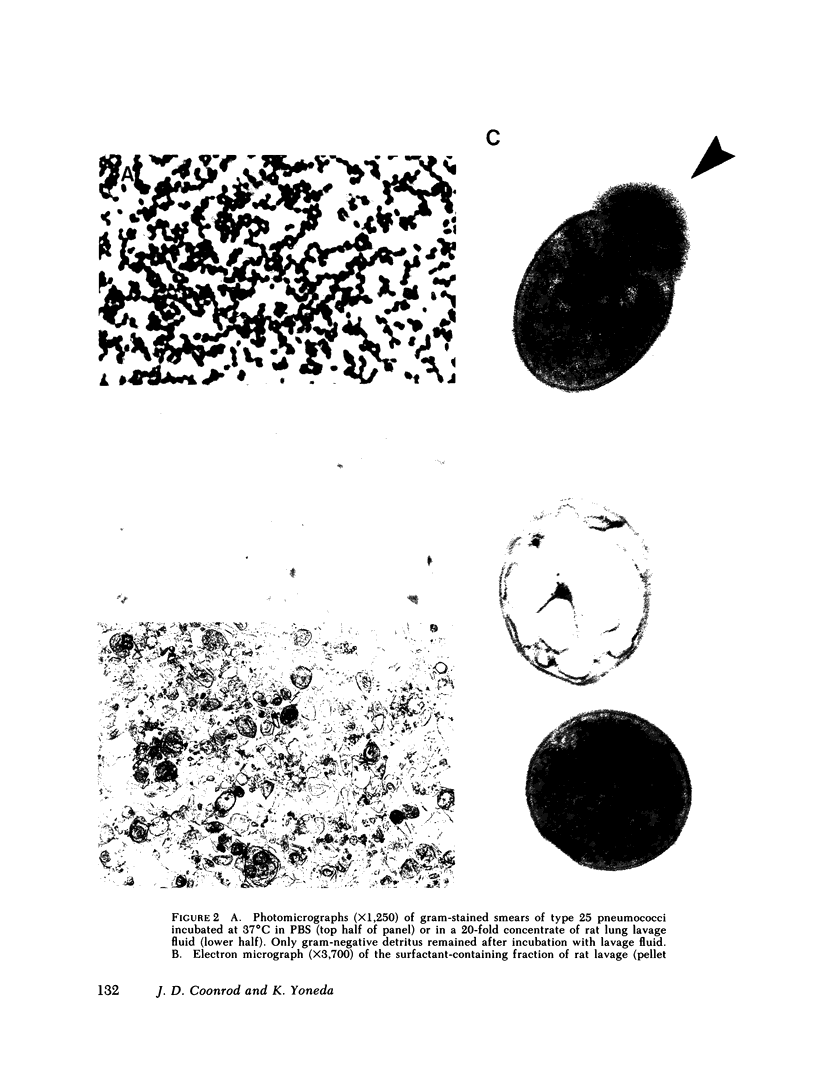


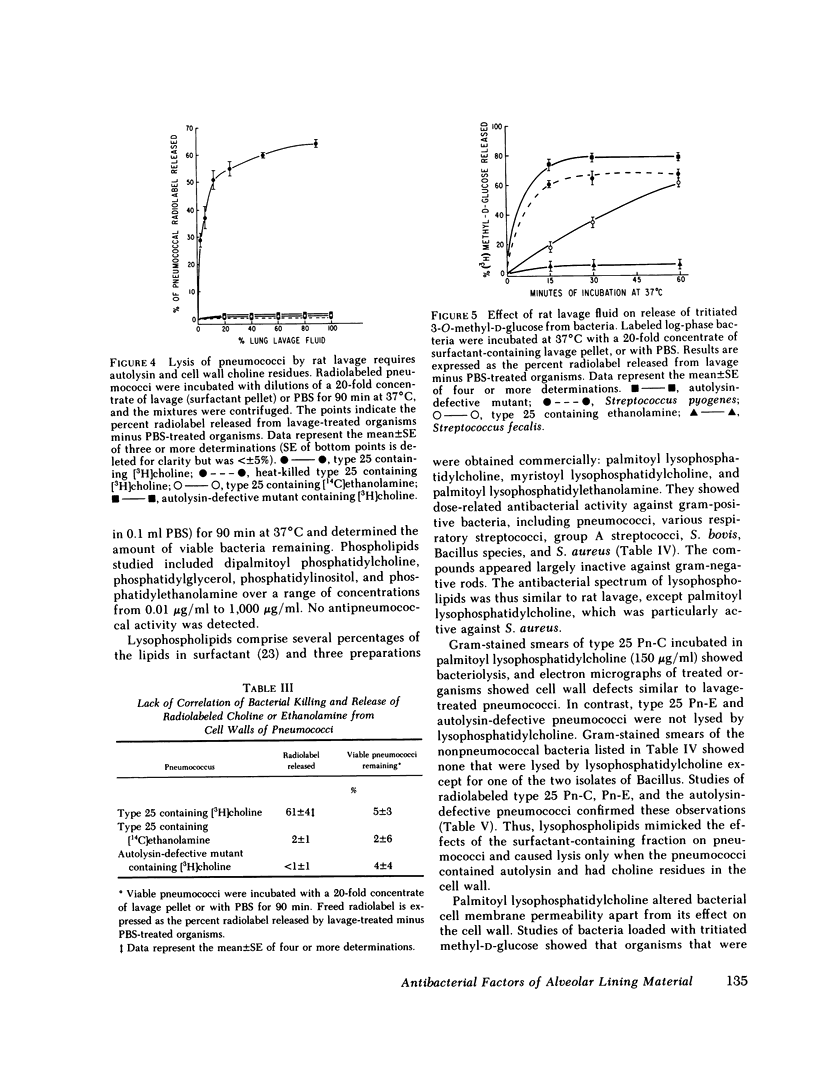
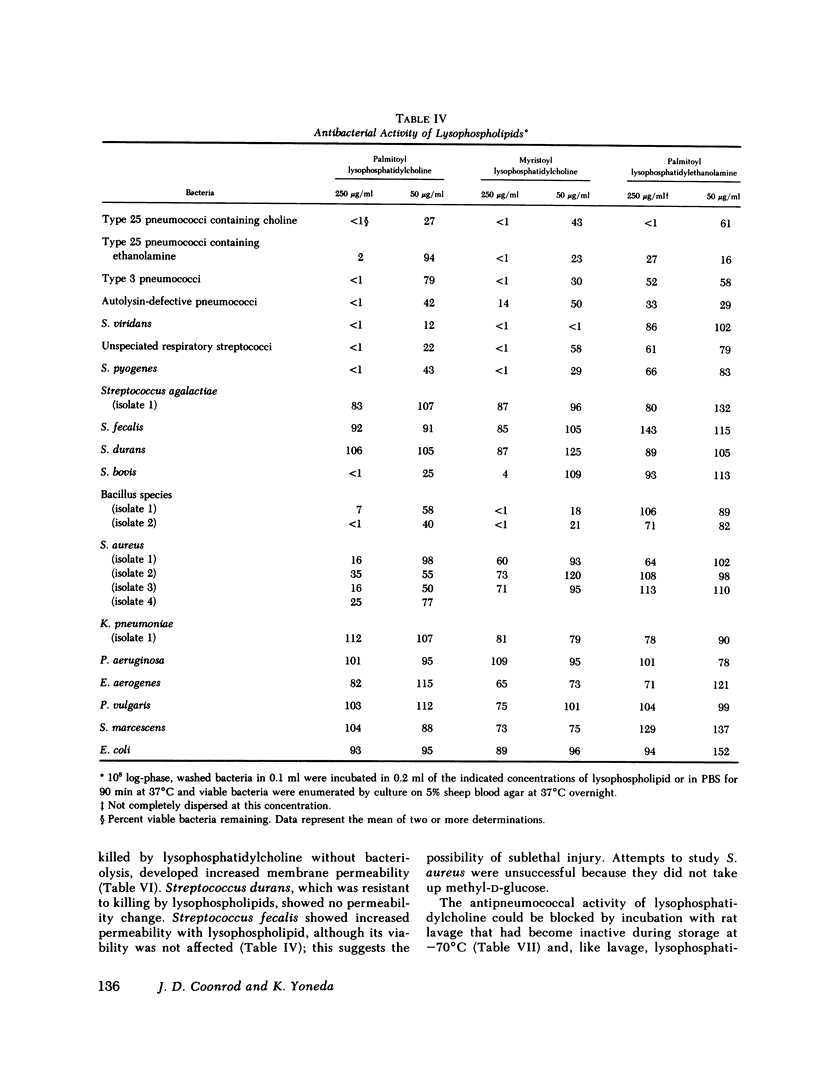
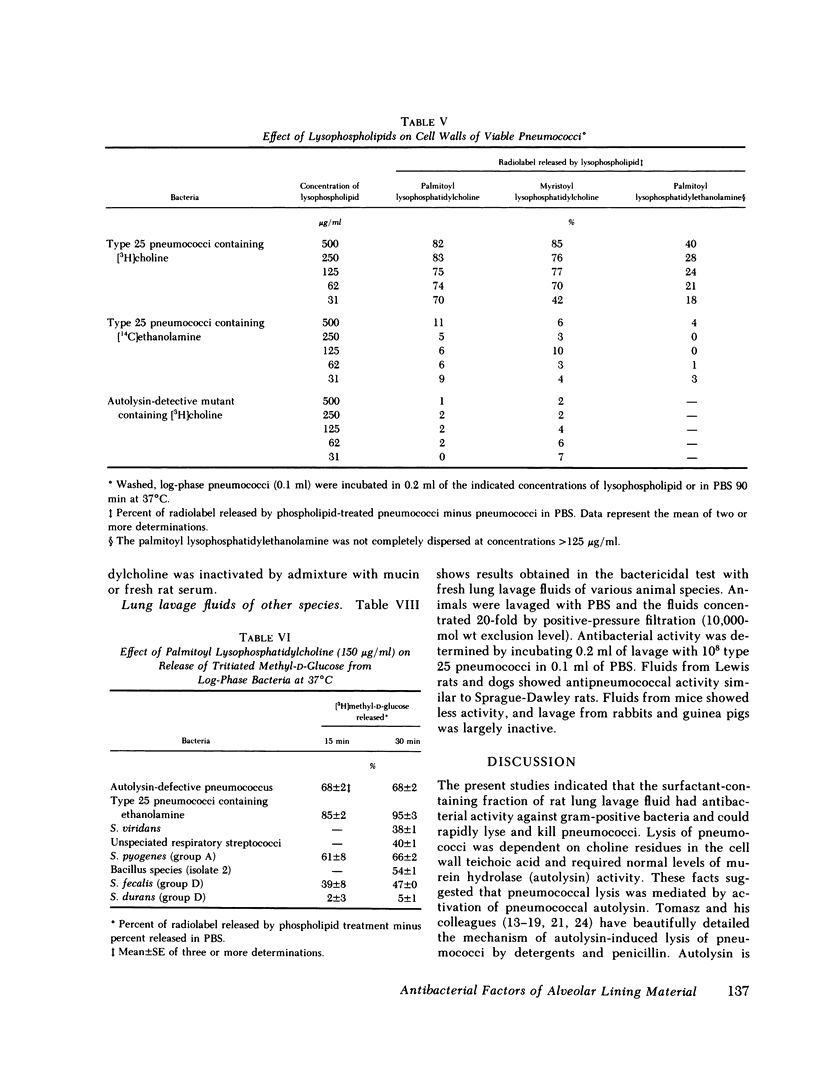



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baxter C. F., Rouser G., Simon G. Variations among vertebrates of lung phospholipid case composition. Lipids. 1969 May;4(3):243–244. doi: 10.1007/BF02532640. [DOI] [PubMed] [Google Scholar]
- Baxter C. F., Rouser G., Simon G. Variations among vertebrates of lung phospholipid case composition. Lipids. 1969 May;4(3):243–244. doi: 10.1007/BF02532640. [DOI] [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. A., Nellenbogen J., Trahan H. J. Pulmonary surfactant and evolution of the lungs. Science. 1970 Aug 7;169(3945):603–604. doi: 10.1126/science.169.3945.603. [DOI] [PubMed] [Google Scholar]
- Cohen A. B., Gold W. M. Defense mechanisms of the lungs. Annu Rev Physiol. 1975;37:325–350. doi: 10.1146/annurev.ph.37.030175.001545. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D., Jenkins S. D. Functional assay of the alternative complement pathway of rat serum. J Immunol Methods. 1979;31(3-4):291–301. doi: 10.1016/0022-1759(79)90142-x. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D., Yoneda K. Complement and opsonins in alveolar secretions and serum of rats with pneumonia due to Streptococcus pneumoniae. Rev Infect Dis. 1981 Mar-Apr;3(2):310–322. doi: 10.1093/clinids/3.2.310. [DOI] [PubMed] [Google Scholar]
- Fujiwara M. The Forssman antigen of pneumococcus. Jpn J Exp Med. 1967 Dec;37(6):581–592. [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. FACTORS INFLUENCING THE CLEARANCE OF BACTERIA BY THE LUNG. J Clin Invest. 1964 Apr;43:769–776. doi: 10.1172/JCI104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell C. G., Peterson P. K., Schmeling D., Kim Y., Mathews J., Wannamaker L., Quie P. G. Potentiation of opsonization and phagocytosis of Streptococcus pyogenes following growth in the presence of clindamycin. J Clin Invest. 1981 May;67(5):1249–1256. doi: 10.1172/JCI110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J., Reiss O. K. Isolation and characterization of lamellar bodies and tubular myelin from rat lung homogenates. J Cell Biol. 1973 Jul;58(1):152–171. doi: 10.1083/jcb.58.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heczko P. B., Lütticken R., Hryniewicz W., Neugebauer M., Pulverer G. Susceptibility of Staphylococcus aureus and group A, B, C, and G streptococci to free fatty acids. J Clin Microbiol. 1979 Mar;9(3):333–335. doi: 10.1128/jcm.9.3.333-335.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. The pulmonary-alveolar macrophage (first of two parts). N Engl J Med. 1979 Sep 13;301(11):580–587. doi: 10.1056/NEJM197909133011104. [DOI] [PubMed] [Google Scholar]
- Holtje J. V., Tomasz A. Biological effects of lipoteichoic acids. J Bacteriol. 1975 Nov;124(2):1023–1027. doi: 10.1128/jb.124.2.1023-1027.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juers J. A., Rogers R. M., McCurdy J. B., Cook W. W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976 Aug;58(2):271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Goldstein E., Lewis J. P., Lippert W., Warshauer D. Murine pulmonary alveolar macrophages: rates of bacterial ingestion, inactivation, and destruction. J Infect Dis. 1976 Mar;133(3):310–320. doi: 10.1093/infdis/133.3.310. [DOI] [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- Laforce F. M., Boose D. S. Sublethal damage of Escherichia coli by lung lavage. Am Rev Respir Dis. 1981 Dec;124(6):733–737. doi: 10.1164/arrd.1981.124.6.733. [DOI] [PubMed] [Google Scholar]
- Larsson K., Norén B., Odham G. Antimicrobial effect of simple lipids and the effect of pH and positive ions. Antimicrob Agents Chemother. 1975 Dec;8(6):733–736. doi: 10.1128/aac.8.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K., Norén B., Odham G. Antimicrobial effect of simple lipids with different branches at the methyl end group. Antimicrob Agents Chemother. 1975 Dec;8(6):742–750. doi: 10.1128/aac.8.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P. J., Wetherall B. L., Pruul H. Postantibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev Infect Dis. 1981 Jan-Feb;3(1):38–44. doi: 10.1093/clinids/3.1.38. [DOI] [PubMed] [Google Scholar]
- Morgan T. E., Finley T. N., Fialkow H. Comparison of the composition and surface activity of "alveolar" and whole lung lipids in the dog. Biochim Biophys Acta. 1965 Oct 4;106(2):403–413. doi: 10.1016/0005-2760(65)90049-4. [DOI] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Mårdh P. A., Taylor-Robinson D. The susceptibility of aerobic and anaerobic bacteria, L-phase variants, candida, protozoa and viruses to lysolecithin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):748–752. doi: 10.1111/j.1699-0463.1974.tb02372.x. [DOI] [PubMed] [Google Scholar]
- Pfleger R. C., Thomas H. G. Beagle dog pulmonary surfactant lipids. Lipid composition of pulmonary tissue, exfoliated lining cells and surfactant. Arch Intern Med. 1971 May;127(5):863–872. doi: 10.1001/archinte.127.5.863. [DOI] [PubMed] [Google Scholar]
- Rehm S. R., Coonrod J. D. Early clearance of pneumococci from the lungs of decomplemented rats. Infect Immun. 1982 Apr;36(1):24–29. doi: 10.1128/iai.36.1.24-29.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Isturiz R., Molavi A., Metcalf J. A., Malech H. L. Interactions between antibiotics and human neutrophils in the killing of staphylococci. J Clin Invest. 1981 Jan;67(1):247–259. doi: 10.1172/JCI110020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz L. V., Mårdh P. A. The lytic effect of lysolecithin on acholeplasmas and mycoplasmas. Acta Pathol Microbiol Scand B. 1977 Aug;85(4):255–261. doi: 10.1111/j.1699-0463.1977.tb01971.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science. 1967 Aug 11;157(3789):694–697. doi: 10.1126/science.157.3789.694. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Mosser J. L. On the nature of the pneumococcal activator substance. Proc Natl Acad Sci U S A. 1966 Jan;55(1):58–66. doi: 10.1073/pnas.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Surface components of Streptococcus pneumoniae. Rev Infect Dis. 1981 Mar-Apr;3(2):190–211. doi: 10.1093/clinids/3.2.190. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The role of autolysins in cell death. Ann N Y Acad Sci. 1974 May 10;235(0):439–447. doi: 10.1111/j.1749-6632.1974.tb43282.x. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Westphal M. Abnormal autolytic enzyme in a pneumococus with altered teichoic acid composition. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2627–2630. doi: 10.1073/pnas.68.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
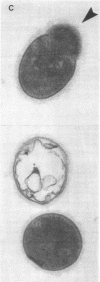
- Yoneda K., Coonrod J. D. Experimental type 25 pneumococcal pneumonia in rats: an electron-microscopic study. Am J Pathol. 1980 Apr;99(1):231–242. [PMC free article] [PubMed] [Google Scholar]