Abstract
Serial serum samples from 27 patients who underwent double umbilical cord blood transplantation (dUCBT) were analyzed for BK polyomavirus (BKPyV) DNA by real-time PCR and BKPyV-specific immune globulin by ELISA. Clinical data were collected on all patients. All pre-transplant sera had detectable anti-BKPyV IgG. Fifteen patients (56%) had detectable serum BKPyV DNA (median 8.9×104 copies/mL; range 4.1×103–7.9×106 copies/mL) a median of 40 days (range, 27–733 days) after dUCBT, with highest viral loads on Day 100 assessment. The cumulative probability of developing BKPyV viremia by Day 100 was 0.52 (95% CI, 0.33–0.71). Six of 15 patients with BKPyV viremia experienced hemorrhagic cystitis by Day 100. By Day 100, there was a trend towards higher BKPyV viral loads in sera of patients with hemorrhagic cystitis than in those BKPyV viremic patients without hemorrhagic cystitis (p=0.06). BKPyV viremia was associated with significantly higher anti-BKPyV IgM values at 6 months post-dUCBT (P=0.003). BKPyV viremia occurs early after dUBCT and is associated with a detectable humoral immune response by 6 months post-dUBCT.
Keywords: BK polyomavirus, hemorrhagic cystitis, viral reactivation
2. Introduction
The mammalian species of BK polyomavirus (BKPyV) is a small non-enveloped double-stranded DNA virus in the family Polyomaviridae, genus Orthopolyomavirus [1]. Primary infection usually occurs during childhood and is usually asymptomatic. After primary infection, BKPyV remains latent in the urothelium of the kidneys and urinary tract [2]. BKPyV has been identified as a cause of nephropathy, ureteral stenosis, and cystitis in renal transplant recipients [3–7] and has also been implicated as an etiologic agent of hemorrhagic cystitis in hematopoietic stem cell transplantation (HSCT) recipients [8, 9].
3. Objectives
While several studies have shown an association between BKPyV viruria and post-HSCT hemorrhagic cystitis [9–12], few studies have linked BKPyV viremia to post-HSCT hemorrhagic cystitis [13, 14]. Specific risk factors for the development of BKPyV-associated hemorrhagic cystitis have included myeloablative conditioning and use of a graft from an unrelated donor [15, 16]. Studies have reported that umbilical cord blood transplant recipients are at a higher risk of developing BKPyV-associated hemorrhagic cystitis [17, 18]. These patients are known to have an impaired and delayed immune recovery, increasing their susceptibility to infectious complications [19, 20]. As umbilical cord blood transplantation becomes more common, it is important to better characterize these infectious complications, including those linked to BKPyV reactivation. In the present study, we examined BKPyV reactivation and the humoral immune response to BKPyV in a cohort of double umbilical cord blood transplantation (dUCBT) recipients.
4. Materials and Methods
This research protocol was approved by the Office for Human Research Studies at Dana-Farber/Harvard Cancer Center. Written informed consent was obtained from all patients for laboratory studies at the time of transplantation.
4.1 Patients and Treatment Details
Eligibility criteria and study details have been previously published [21]. Briefly, patients included in this analysis underwent dUCBT between October 2005 and November 2007. UCB units were obtained from national and international cord blood banks. Both units were required to be a 4/6 or greater Human Leukocyte Antigen (HLA) A, HLA B, and HLA DRB1 allele-level match with each other and the patient.
Patients underwent conditioning with fludarabine 30 mg/m2 per day from Day −8 through Day −3 (total dose of 180 mg/m2), melphalan 100 mg/m2 on Day −2 only, and rabbit antithymocyte globulin 1.5mg/kg per day on Days −7, −5, −3, and −1. Prophylaxis for graft-versus-host disease (GVHD) included tacrolimus and sirolimus initiated on Day −3. In the absence of GVHD, tacrolimus and sirolimus were tapered from Day +100 through Day +180. Patients received filgrastim at 5 μg/kg per day from Day +5 until an absolute neutrophil count higher than 2.0 × 109 cells/L was reached for 2 consecutive days [21].
4.2 Sample Collection
Peripheral blood samples were collected prospectively at the following time points: immediately before transplantation (before administration of conditioning chemotherapy), 4 weeks, 8 weeks, 100 days, 6 months, 12 months, and 24 months after transplantation. Serum was separated with centrifugation and stored at −80°C. Urine testing was clinically triggered.
4.3 Detection of BKPyV DNA and Antibody
Using 150μl of serum, DNA extraction was performed with the QIAamp® MinElute Virus Spin Kit (Qiagen, CA) following the kit protocol. BKPyV DNA was quantified by Quantitative PCR (qPCR) using a 7300 Real Time PCR System (Applied Biosystems, CA). The primer pair 5′-AGTGGATGGGCAGCCTATGTA-3′ (nt 2511–2531) and 5′-TCATATCTGGGTCCCCTGGA-3′ (nt 2586–2605), and probe 6FAM-AGGTAGAAGAGGTTAGGGTGTTTGATGGCACA-TAMRA (nt 2546–2578) (Applied Biosystems, CA), located in the VP1 gene, were used for qPCR detection, as previously described, with a C to G modification of nucleotide 2569 [22]. For each sample, the extraction volume was 200 μl and the elution volume was 150 μl. Each qPCR reaction was run in triplicate and all results were expressed in copies per ml.
BKPyV ELISA was used to quantify anti-BKPyV IgM and IgG and results were reported as mean values of duplicates [23]. The serum dilution in assays for anti-BKPyV IgM and IgG was 1:100. The cut-off for seropositivity was an OD value > 0.075 for anti-BKPyV IgM and an OD value > 0.150 for anti-BKPyV IgG.
4.4 Covariates and Definitions
Medical records were reviewed for covariates of interest including: gender, age at transplant, underlying disease, disease status at the time of dUCBT, creatinine and estimated glomerular filtration rate [24] at each time point of sera collection, urine analysis collected within two weeks before and after each sera time point, clinician notes documenting symptoms of cystitis around and at each time point, all clinical urine and blood BKPyV PCR results obtained on each patient, onset of graft-versus-host disease (GVHD), bacterial urine culture results, and treatments intended for BKPyV. Neutrophil engraftment was defined as the first day of three consecutive days where the absolute neutrophil count was 500 cells/mm3 or greater. Platelet engraftment was defined as the first day of three consecutive days where the platelet count was 20,000/mm3 or greater and unsupported by a platelet transfusion. Hematuria was defined as having three or more RBC per high power field on urine analysis [25]. Hemorrhagic cystitis was defined as having hematuria and symptoms of cystitis documented by clinicians. Laboratory investigators were blinded to clinical data.
4.5 Statistical Analysis
Non-parametric continuous variables were compared using the Wilcoxon-Rank Sum and Mann-Whitney tests and all categorical variables were compared using Fisher’s Exact Test. Non-parametric ordinal variables were compared using Spearman’s correlation coefficient. The cumulative probability of having BKPyV viremia by Day 100 was determined by the Kaplan-Meier Method. Repeated measurements were adjusted for by Generalized Estimator Equation (GEE). Statistical analysis was performed using JMP Pro 10.0 2012 (SAS Institute Inc., Cary, NC), Stata® 12 (StataCorp, College Station, TX), and GraphPad Prism 5 (GraphPad Software, Inc. La Jolla, CA).
5. Results
5.1 Clinical Characteristics
A total of 32 patients with hematologic malignancies underwent dUCBT on the protocol. Five patients were excluded from the analyses – 4 patients died prior to Day 100 due to causes unrelated to BKPyV reactivation, and 1 patient had insufficient sera specimens after transplantation. Baseline characteristics and clinical outcomes of the 27 patients included in the analysis are shown in Table 1. Approximately half of this cohort of patients was male, half had lymphoma as the indication for transplantation, and half died prior to completion of the study [21].
Table 1.
Patient characteristics and transplant outcomes.
| Total Number of Patients | n = 27 (Range or %) |
|---|---|
| Median Age (range) | 48 (19–67) |
| Male sex (percentage) | 14 (52%)) |
| Baseline Disease | |
| Acute Leukemia | 9 (33%) |
| Chronic Leukemia | 2 (7%) |
| Lymphoma | 13 (48%) |
| Myeloproliferative Disease | 3 (11%) |
| Clinical Outcome | |
| Completed Study | 13 (48%) |
| Death Prior to Study Completion | 13 (48%) |
| Lost to Follow Up | 1 (4%) |
| Acute GVHD1 | 7 (26%) |
| Chronic GVHD2 | 2 (7%) |
| Median Days to Neutrophil Engraftment (range) | 21 (13–70) |
| Median Days to Platelet Engraftment (range) | 42 (25–162) |
Five patients with Grade 1 acute GVHD and two patients with Grade 2 acute GVHD
Four with limited chronic GVHD and two with extensive chronic GVHD
5.2 BKPyV DNA detection in serum
No BKPyV DNA was detected in pre-transplant sera (Table 2). Fifteen of 27 patients (56%) had detectable BKPyV DNA in at least one sample after transplant. Of these fifteen viremic patients, 1 patient had 1 positive sample, 3 patients had 2, 6 patients had 3, 3 patients had 4, and 2 patients had 5 positive samples. Serum specimens were available for all fifteen viremic patients at week 4, week 8, Day 100 days, and 6 months. Ten serum specimens were available at 1 year and 5 serum specimens at 2 years. BKPyV DNA was detected in as early as the week 4 sample and as late as in the 2 year post-dUCBT sample.
Table 2.
Incidence of BKPyV DNA and anti-BKPyV IgM at all study time points.
| Time Point | Number tested | BKPyV DNA | Anti-BKPyV IgM |
|---|---|---|---|
| (Number of positive (%)) | |||
| Pre-Transplant | 24 | 0 | 3 (13%) |
| 4 weeks | 23 | 8 (35%) | 1(4%) |
| 8 weeks | 26 | 14 (54%) | 1 (4%) |
| 100 days | 26 | 14 (54%) | 3 (12%) |
| 6 months | 21 | 7 (33%) | 5 (24%) |
| 1 year | 16 | 3 (19%) | 7 (44%) |
| 2 years | 9 | 1 (11%) | 7 (78%) |
Seven patients had additional BKPyV clinical blood specimens obtained by treating physicians. Two of these 7 patients had positive clinical specimens prior to Week 4 and the first positive BKPyV lab PCR. Of the remaining five, 2 patients had no detectable BKPyV DNA by clinical lab and 3 had detectable BKPyV DNA after Week 4 and the first positive BKPyV study PCR. The cumulative incidence of BKPyV viremia was highest between 8 weeks and 100 days, where more than 50% of the patients had BKPyV viremia (Table 2).
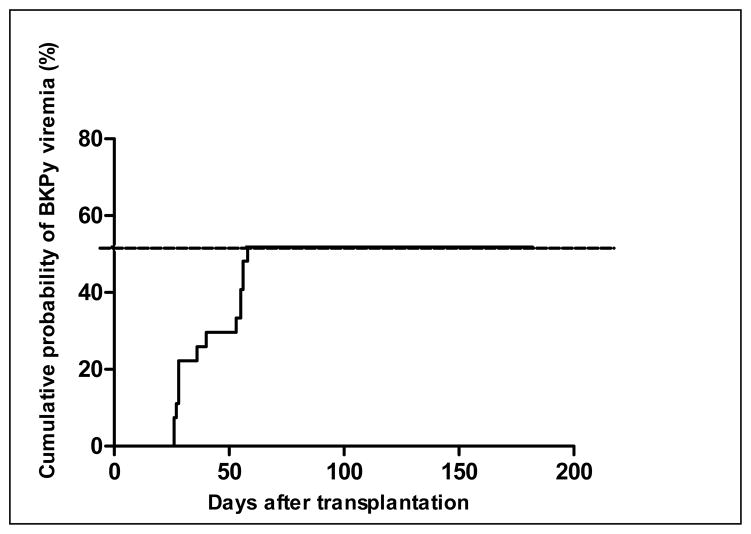
The probability of developing BKPyV viremia by Day 100 was 0.52 (95% CI, 0.33–0.71) (Figure 1) and stayed constant until 6 months post-dUCBT.
Figure 1.
Time to BKPyV viremia analysis showed the cumulative probability of developing BKPyV viremia by Day 100 was 52%.
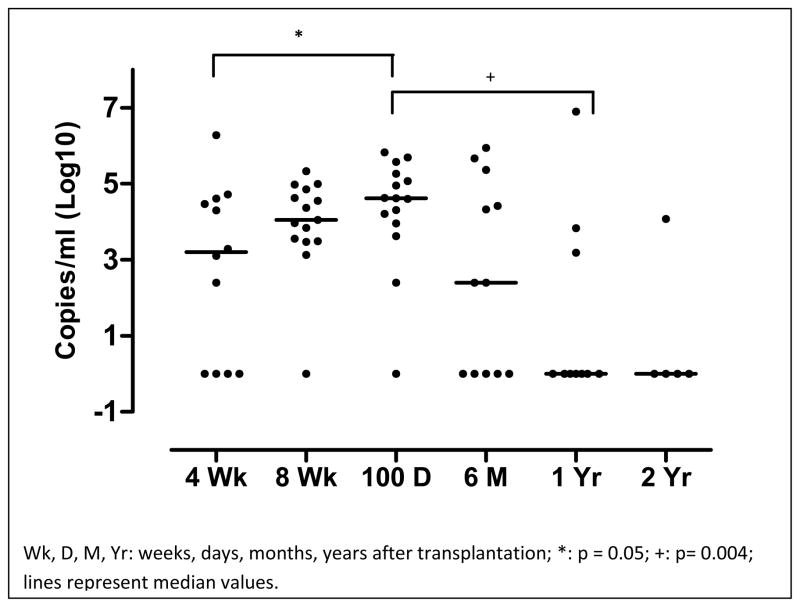
The median quantity of BKPyV viral loads of 15 viremic patients who had at least 1 positive serum specimen was examined and found to be highest on the Day 100 assessment (4.1 × 104 copies/ml; range, 0 – 6.6 × 105 copies/ml) after dUBCT as compared the week 4 assessment (1.2 × 103copies/ml, range 0 – 1.9 × 106 copies/ml; p = 0.05) and 1 year (0 copies/ml, range 0 – 7.9 × 106 copies/ml; p = 0.004), (Figure 2).
Figure 2.
BKPyV viral loads in sera of 15 BKPyV viremic patients peaked at 100 days after dUBCT
5.3 BKPy viremia and clinical factors
Platelet and neutrophil engraftment between BKPyV viremic and non viremic patients was similar in 26 patients. Data for one patient was not available.
We analyzed factors that may be associated with BKPyV viremia in 14 patients who developed viremia by Day 100. We found no significant difference in median age, gender, transplant indication, disease status, change in creatinine by Day 100, or development of GVHD.
5.4 BKPyV-specific immunoglobulins
Pre-transplant sera were available in 24 of 27 patients and all 24 had detectable anti-BKPyV IgG (IgG) through 1 year, and 3 of 24 had detectable anti-BKPyV IgM (Table 2). Of the 21 patients with negative anti-BKPyV IgM pre-transplant, 7 developed detectable anti-BKPyV IgM post-transplant and all had BKPy viremia.
5.5 BKPy viremia and humoral immune response
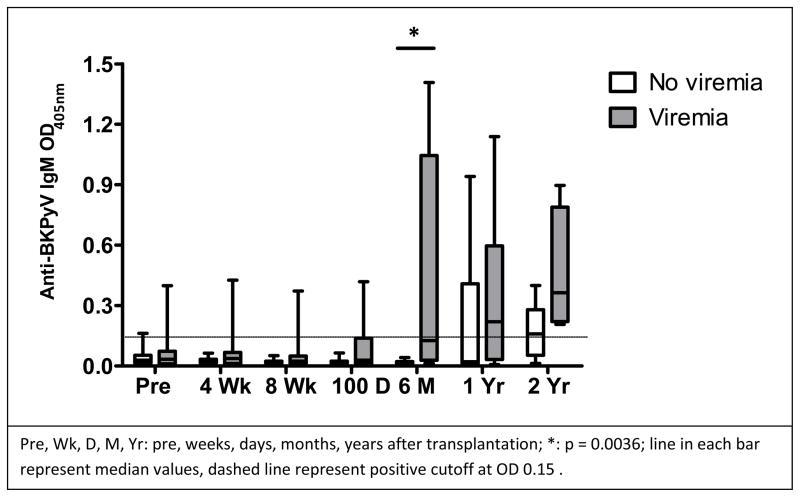
We compared IgM values in BKPyV viremic patients to those with no viremia at each study time point. The BKPyV viremic group contained higher individual IgM values than the non-viremic group at each of the study time points and the median value of viremic patients was significantly elevated at 6 months post dUCBT than non-viremic patients (p=0.003) (Figure 3).
Figure 3.
Anti-BKPyV IgM levels in the patients with BKPyV viremia were elevated as compared to those patients with no viremia at 6 months after transplantation
The median IgM values in the viremic group were higher than in the non-viremic group at 1 year and at 2 years, but the difference did not remain statistically significant, as the median IgM values of the viremic group decreased after 6 months. BKPyV viral loads at 1 year post-dUBCT correlated inversely to the quantity of IgM (Spearman coefficients: −0.79, p = 0.019).
5.6 BKPyV viremia and hemorrhagic cystitis
Of 27 patients who underwent dUCBT, 8 (30%) developed hemorrhagic cystitis by Day 100. Five patients had hemorrhagic cystitis before neutrophil engraftment, 1 patient had hemorrhagic cystitis on the date of neutrophil engraftment, and 2 patients had hemorrhagic cystitis after neutrophil engraftment. Six of the 8 patients (75%) who developed hemorrhagic cystitis by Day 100 had concurrent BKPyV viremia. The quantitative serum BKPyV viral loads of those patients who had BKPyV viremia and hemorrhagic cystitis by Day 100 (median, 4 × 104 copies/ml) was non-significantly higher than the quantitative viral loads of those patients who had BKPyV viremia and no hemorrhagic cystitis (median, 7 × 103 copies/ml) (p=0.06 GEE). There was no significant difference in the median age, gender, transplant indication, disease status, change in creatinine by Day 100, or development of GVHD in patients who had hemorrhagic cystitis compared to those who did not have hemorrhagic cystitis by Day 100.
5.7 BKPyV Viruria
BKPyV urine PCR was performed in 8 of 14 patients with detectable serum BKPyV by Day 100 and all 8 were positive. Clinically-driven urine specimens were collected at a median of 16 days (range 0–23 days) after transplant in 7 patients and 237 days post-transplant in 1 patient. Five of 6 (83%) patients with both hemorrhagic cystitis and BKPyV viremia had urine studies sent and all had detectable BKPyV DNA by PCR.
6. Discussion
BKPyV viremia and humoral response to BKPy virus infection after dUCBT were examined in this study. The cumulative incidence of BKPyV reactivation in this cohort of dUCBT recipients was 56%, higher than reported in previous studies of non-dUCBT allogeneic HSCT recipients [14, 26]. The donor cell source and conditioning regimen likely contributed to this observation, as recipients of dUCBT are known to have delayed reconstitution of granulocytes and an impaired immune response to viral pathogens compared to other sources of stem cells [20, 27, 28]. Additionally, umbilical cord blood grafts may have higher populations of regulatory T cells (Tregs), which have known suppressive effects on effector cells in the graft which would normally respond to viral antigens [28]. Although sera collection time points in our study were less frequent than in other studies [14, 26], we still found a higher incidence of BKPyV viremia. Thus, a more frequent collection of serum samples in dUCBT populations post transplantation may demonstrate an even higher incidence of BKPyV viremia.
Six of 8 (75%) patients with hemorrhagic cystitis by Day 100 developed this complication on or before neutrophil engraftment, a clinical surrogate marker of bone marrow engraftment. Although some patients in this dUCBT cohort experienced hemorrhagic cystitis prior to neutrophil engraftment, it is unclear if BKPyV viremia was also present prior to or at the time of neutrophil engraftment, as the first serum specimen in this study was collected only four weeks after the date of dUCBT. Erard and colleagues reported the onset of BKPyV viremia prior to the onset of hemorrhagic cystits in their cohort [26]. If BKPyV viremia occurs prior to neutrophil engraftment, then symptomatic BKPyV disease, such as hemorrhagic cystitis, could be a result of replicating BKPyV in the bladder epithelium followed by host-mediated uroepithelial cell damage with immune system recovery, as previously described [29, 30].
Patients with BKPyV viremia by Day 100 and hemorrhagic cystitis had non-significantly higher BKPyV viral loads than patient with BKPyV viremia and no HC. This result suggests that the degree of viral injury in the uroepithelium may correlate with the quantity of BKPyV viral load. Larger studies will need to be performed to better characterize the correlation between absolute copies/mL BKPyV viremia and the degree of hemorrhagic cystitis.
We demonstrated a statistically significant association between anti-BKPyV IgM levels and detection of BKPyV DNA in serum at 6 months post-transplant and a non-significant trend persisting up to 2 years post-transplant. This association shows the capability of the developing humoral immune response to produce IgM in response to active BKPyV replication. Due to the small number of patients in this cohort, it is difficult to conclude if this IgM response was able to control BKPyV viremia or if it was a measure of de novo priming of B-cells with no correlation to immune protection. The inverse correlation between IgM level and BKPyV viral load 1 year post transplant is suggestive of a possible protective effect of a humoral immune response. Such protective effect of the BKPyV humoral immune response has been proposed by Koskenvuo et al. [31], who identified a small subset of children post-HCT unable to clear BKPyV viremia secondary to a lack of anti-BKPyV IgM and IgG responses. Immunoglobulin (IVIG) may interfere with the accurate quantification of anti-BKPyV IgM and IgG. Randhawa and colleagues [32] identified the presence of BKPyV neutralizing antibodies in human IVIG preparations; however, the prevalence of BKPyV-specific IgM and IgG in human IVIG is unknown. Although IVIG administration was not captured in our patient cohort, as it was administered when clinically indicated, anti-BKPyV IgM correlated to the detection of BKPyV viremia. Therefore, we surmise that our detections of anti-BKPyV IgM were most likely a result of host immune response to viremia. Another factor that could have affected anti-BKPyV IgM detection was cross-reactivity in the assay with another virus, such a JC virus. Although re-adsorption studies were not done for anti-BKPyV IgM and cross-reactivity could theoretically occur, this is unlikely to explain the temporal association between anti-BKPyV IgM response and BKPyV viral load. Additional studies are warranted since passive or active immunotherapy would be feasible therapeutic interventions to prevent or control hemorrhagic cystitis. Further studies are needed to examine if this IgM response along with detection of BKPyV in sera is unique to dUCBT or present in all patients post-allogeneic HSCT.
Strengths of this study include analysis of patients treated in a standardized protocol, with uniform conditioning and GVHD prophylaxis. This enabled examination of specific factors that related to BKPyV reactivation and insights into the humoral immune response in a unique cohort of patients. There are several limitations of the study including the relatively small number of patients in the cohort, which precludes a more detailed analysis, a retrospective design using prospectively collected specimens, lack of systematic urine collection, limited number of serum collection time points, especially in the time period between transplant and week 4, and lack of data regarding immunoglobulin administration. Furthermore, future studies examining cellular immune responses targeting BKPyV in the HSCT population are needed to fully define host immune interactions with BKPyV.
In conclusion, this study shows a higher rate of BKPyV reactivation after reduced intensity conditioning dUCBT than traditional allogeneic HSCT. BKPyV reactivation is an event that occurs early post-HSCT, with the majority of events in this cohort occurring prior to Day 100, and possibly prior to neutrophil engraftment. A higher BKPyV viral load might be a result of increased virus reactivation and lead to more urothelial damage in patients with hemorrhagic cystitis. Anti-BKPyV IgG did not protect patients from BKPyV reactivation, but IgM response to BKPyV was demonstrated amongst viremic patients.
Highlights.
BK polyomavirus reactivation occurred in 56% of patients in this cohort
BK polyomavirus reactivation peaked by Day 100 after HCT
At 6 months after HCT, anti-BKPyV IgM was higher in those patients who had BKPyV viremia
Acknowledgments
This work was conducted in part with support from NIH grants R01 NS 047029 and NS 074995, NS R56 041198, and K24 NS 060950 to IJK, and NIH grant K08 NS 064215-01 to CST.
Footnotes
Financial Disclosure Statement
FMM has received grant support and consulting honoraria from Chimerix and Vertex
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johne R, Buck CB, Allander T, Atwood WJ, Garcea RL, Imperiale MJ, et al. Taxonomical developments in the family Polyomaviridae. Archives of Virology. 2011;156:1627–34. doi: 10.1007/s00705-011-1008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorries Human polyomaviruses. Principles and Practice of Clinical Virology. 2004:675–702. [Google Scholar]
- 3.Bressollette-Bodin C, Coste-Burel M, Hourmant M, Sebille V, Andre-Garnier E, Imbert-Marcille BM. A prospective longitudinal study of BK virus infection in 104 renal transplant recipients. Am J Transplant. 2005;5:1926–33. doi: 10.1111/j.1600-6143.2005.00934.x. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25–30. doi: 10.1034/j.1600-6143.2002.020106.x. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–96. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 6.Pires EP, Bernardino-Vallinoto CV, Alves DM, Migone SR, Machado LF, Ishak MO, et al. Prevalence of infection by JC and BK polyomaviruses in kidney transplant recipients and patients with chronic renal disease. Transpl Infect Dis. 2011;13:633–7. doi: 10.1111/j.1399-3062.2011.00614.x. [DOI] [PubMed] [Google Scholar]
- 7.Randhawa PS, Demetris AJ. Nephropathy due to polyomavirus type BK. N Engl J Med. 2000;342:1361–3. doi: 10.1056/NEJM200005043421809. [DOI] [PubMed] [Google Scholar]
- 8.Apperley JF, Rice SJ, Bishop JA, Chia YC, Krausz T, Gardner SD, et al. Late-onset hemorrhagic cystitis associated with urinary excretion of polyomaviruses after bone marrow transplantation. Transplantation. 1987;43:108–12. doi: 10.1097/00007890-198701000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–4. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 10.Azzi A, Cesaro S, Laszlo D, Zakrzewska K, Ciappi S, De Santis R, et al. Human polyomavirus BK (BKV) load and haemorrhagic cystitis in bone marrow transplantation patients. J Clin Virol. 1999;14:79–86. doi: 10.1016/s1386-6532(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 11.Bogdanovic G, Priftakis P, Giraud G, Kuzniar M, Ferraldeschi R, Kokhaei P, et al. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. Journal of Clinical Microbiology. 2004;42:5394–6. doi: 10.1128/JCM.42.11.5394-5396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung aYH, Chan M, Cheng VCC, Lie aKW, Yuen K-Y, Kwong Y-L. Polyoma BK viruria in patients undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2005;35:1029–30. doi: 10.1038/sj.bmt.1704944. [DOI] [PubMed] [Google Scholar]
- 13.Erard V, Kim HW, Corey L, Limaye A, Huang M-L, Myerson D, et al. BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood. 2005;106:1130–2. doi: 10.1182/blood-2004-12-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell PH, Swanson K, Josephson Ma, Artz AS, Parsad SD, Ramaprasad C, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biology of Blood and Marrow Transplantation. 2009;15:1038–48. e1. doi: 10.1016/j.bbmt.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giraud G, Priftakis P, Bogdanovic G, Remberger M, Dubrulle M, Hau A, et al. BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA-mismatched grafts. Bone Marrow Transplantation. 2008;41:737–42. doi: 10.1038/sj.bmt.1705962. [DOI] [PubMed] [Google Scholar]
- 16.Satyanarayana G, Marty FM, Tan CS. The polyomavirus puzzle: is host immune response beneficial in controlling BK virus after adult hematopoietic cell transplantion? Transpl Infect Dis. 2014;16:521–31. doi: 10.1111/tid.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rorije NM, Shea MM, Satyanarayana G, Hammond SP, Ho VT, Baden LR, et al. BK Virus Disease after Allogeneic Stem Cell Transplantation: A Cohort Analysis. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Silva LDP, Patah Pa, Saliba RM, Szewczyk Na, Gilman L, Neumann J, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–90. doi: 10.3324/haematol.2009.016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr MJ, McCormack GP, Mutton KJ, Crowley B. Unique BK virus non-coding control region (NCCR) variants in hematopoietic stem cell transplant recipients with and without hemorrhagic cystitis. J Med Virol. 2006;78:485–93. doi: 10.1002/jmv.20566. [DOI] [PubMed] [Google Scholar]
- 20.Ruggeri A, Peffault de Latour R, Carmagnat M, Clave E, Douay C, Larghero J, et al. Outcomes, infections, and immune reconstitution after double cord blood transplantation in patients with high-risk hematological diseases. Transpl Infect Dis. 2011;13:456–65. doi: 10.1111/j.1399-3062.2011.00632.x. [DOI] [PubMed] [Google Scholar]
- 21.Cutler C, Stevenson K, Kim HT, Brown J, McDonough S, Herrera M, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46:659–67. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Trofe J, Gordon J, Du Pasquier RA, Roy-Chaudhury P, Kuroda MJ, et al. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol. 2006;80:3495–505. doi: 10.1128/JVI.80.7.3495-3505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viscidi RP, Khanna N, Tan CS, Li X, Jacobson L, Clifford DB, et al. JC virus antibody and viremia as predictors of progressive multifocal leukoencephalopathy in human immunodeficiency virus-1-infected individuals. Clin Infect Dis. 2011;53:711–5. doi: 10.1093/cid/cir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. London: 2008. [PubMed] [Google Scholar]
- 25.Wein AJ, Kavoussi LR, Campbell MF. In: Campbell-Walsh urology/editor-in-chief. 10. Wein Alan J, Kavoussi Louis R, et al., editors. Philadelphia, PA: Elsevier Saunders; 2012. [Google Scholar]
- 26.Erard V, Storer B, Corey L, Nollkamper J, Huang M-L, Limaye A, et al. BK virus infection in hematopoietic stem cell transplant recipients: frequency, risk factors, and association with postengraftment hemorrhagic cystitis. Clin Infect Dis. 2004;39:1861–5. doi: 10.1086/426140. [DOI] [PubMed] [Google Scholar]
- 27.Auletta JJ, Lazarus HM. Immune restoration following hematopoietic stem cell transplantation: an evolving target. Bone Marrow Transplant. 2005;35:835–57. doi: 10.1038/sj.bmt.1704966. [DOI] [PubMed] [Google Scholar]
- 28.Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol. 2008;127:286–97. doi: 10.1016/j.clim.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binet IVN, Hans H. Polyomavirus infections in transplant recipients. Curr Opinion in Organ Transplant. 2000;5:210–6. [Google Scholar]
- 30.Leung aYH, Yuen K-Y, Kwong Y-L. Polyoma BK virus and haemorrhagic cystitis in haematopoietic stem cell transplantation: a changing paradigm. Bone Marrow Transplantation. 2005;36:929–37. doi: 10.1038/sj.bmt.1705139. [DOI] [PubMed] [Google Scholar]
- 31.Koskenvuo M, Dumoulin A, Lautenschlager I, Auvinen E, Mannonen L, Anttila VJ, et al. BK polyomavirus-associated hemorrhagic cystitis among pediatric allogeneic bone marrow transplant recipients: treatment response and evidence for nosocomial transmission. J Clin Virol. 2013;56:77–81. doi: 10.1016/j.jcv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Randhawa PS, Schonder K, Shapiro R, Farasati N, Huang Y. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation. 2010;89:1462–5. doi: 10.1097/tp.0b013e3181daaaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]