Abstract
Objectives
To determine the prevalence of conflicts of interest (COIs) among Danish physicians who are authors of clinical drug trial reports and determine the extent of undisclosed COIs in trial publications.
Design
Cross-sectional study.
Setting
The 100 most recent drug trial reports with at least one Danish non-industry employed physician author published in a journal adhering to the International Committee of Medical Journal Editors' (ICMJE) manuscript guidelines. For each article, two observers independently extracted trial characteristics and the authors' COIs. Disclosed COIs were compared to what was registered on the Danish Health and Medicines Authority's public disclosure list.
Participants
Trial authors who are Danish physicians.
Main outcome measures
Number of disclosed and undisclosed COIs.
Results
One observer screened 928 articles and two observers assessed 120 articles for eligibility. The 100 included trials were published from February 2011 to May 2013 and included 318 Danish non-industry employed authors. Eighty-six of the 318 authors (27%) reported one or more COIs in the journal article. We found undisclosed COIs for 40 of 318 authors (13%) related to the trial sponsor or manufacturer of trial drugs. Seventy-nine of 318 authors (25%) had undisclosed COIs related to competing companies manufacturing drugs for the same indication and 136 (43%) had undisclosed COIs with any drug manufacturer.
Conclusions
Almost half of all authors had undisclosed COIs in clinical trials reported in journals adhering to the International Committee of Medical Journal Editors’ manuscript guidelines. Self-declared COIs cannot be trusted, but public registries may assist editors in ensuring that more COIs are being reported.
Keywords: clinical trials, conflicts of interest, drug industry, disclosure
Introduction
Clinical trials are essential for evaluating effects of medical interventions.1 Most trials test drugs and around half of all trials are industry sponsored.2 Sponsorship by the drug industry is not without problems, as it may lead to bias in the design, conduct and reporting of the trials.3 Non-industry sponsored trials may also be biased, particularly if the trialists have affiliations with the companies whose products are being tested. Such relationships create a conflict of interest (COI) and may lead authors to perceive drugs to be more beneficial and less harmful than they really are.4–6
COIs are acknowledged as an important source of bias,7 and medical journals usually require authors to disclose their COIs, for example by using the Disclosure Form of the International Committee of Medical Journal Editors (ICMJE).8 However, journals rely on voluntary disclosure by authors and the ICMJE’s criteria are not entirely clear, e.g. ‘relevant financial activities’ may be interpreted differently by authors resulting in some COIs being undisclosed.9 Thus, it is imperative that we quantify the amount of under-reporting of COIs in medical journals.
In Denmark, physicians who wish to engage in collaboration with a pharmaceutical company are required by law to apply for permission from the Danish Health and Medicines Authority. More specifically, all Danish physicians who have permission to prescribe medication to patients and who wish to engage in paid collaboration or have long-term, unpaid collaboration (e.g. the equivalent of full-time work for 4 weeks) have to seek permission with the Danish Health and Medicines Authority before initiation of the collaboration. Furthermore, all pharmaceutical companies are required to report names and social security numbers of Danish physicians who are affiliated with the company. Failure to seek permission for a collaboration will result in a fine. All physicians with permissions are named on a publicly available list,10 similar to the US Physician Payments Sunshine Act.11 Travel expenses and honoraria covering the provision of meals that do not exceed what is reasonable for the service the doctor provided do not require permission from the Danish Health and Medicines Authority and are therefore not published on the list. The Danish Health and Medicines Authority list provides the names and specialty of the Danish physicians, the name of the pharmaceutical company, the type of collaboration, e.g. advisory board member and the expiration date for the collaboration. The amount paid by the pharmaceutical company to the physician is not published on the list. The Danish Health and Medicines Authority’s list made it possible to study the level of under-reporting of COIs in trials published in biomedical journals.12
The objectives are:
To determine the prevalence of COIs among Danish physicians who are authors of clinical drug trial reports irrespective of who sponsored the trial.
To determine the extent of undisclosed COIs in trial publications.
Methods
On 12 May 2013, we searched EMBASE using the limits function for ‘randomised controlled trials’ (RCTs) and ‘article’, and the index term ‘Denmark’ under Institutional Address for the 100 most recent and eligible drug trials.
Inclusion criteria
Eligible articles had to be reports of randomised drug trials with at least one Danish non-industry employed physician author (determined using the institutional address) and published in a journal that adheres to the ICMJE’s Uniform Requirements for Manuscripts Submitted to Biomedical Journals (identified via http://www.icmje.org/journals.html). The latter criterion was used to ensure that included articles contained COI statements. Both primary publications and secondary analyses (e.g. follow-up studies or subgroups) of trials were included.
Exclusion criteria
Trials were excluded if all Danish physician authors were employed by a drug company or a commercial contract research organisation, determined using the institution address reported in the journal. Trials of fluid therapy, vaccines and dietary supplements were excluded, as companies producing these products are not listed on the Danish Health and Medicines Authority’s list. Trials not related to specific drugs, but to general treatment strategies (e.g. initiation of antiretroviral therapy using a different cut-off for CD4 cell counts) were also excluded.
One observer (KR) screened title and abstract of articles, and final inclusion was based on full text screening conducted independently by two observers (AL, KR). Disagreements were settled by discussion.
Data extraction
Two observers (AL, KR) independently extracted trial characteristics and disclosed COIs for each included article into a pilot tested spreadsheet. Characteristics included name of first author, title of article, journal name, type of journal (general or specialist), journal Impact Factor (according to Journal Citation Reports 2011), publication date, generic names of drugs used in the trial, and type of comparator drug (placebo, active, multiple arms, non-drug comparator or no treatment).
We also extracted information on sponsorship and used four categories: industry sponsorship, mixed sponsorship, non-industry sponsorship and not stated. We extracted the name of the manufacturers of the tested trial drugs (both test drug and comparators) and if it was not stated in the article, we identified the manufacturer using the Danish Pharmaceutical Information’s website (www.pro.medicin.dk). If the study drug had multiple manufacturers, all names were extracted. We also extracted statements about industry sponsor’s involvement in the trial (e.g. industry employed co-authors, assistance with data analysis or writing of manuscript, including statements in acknowledgements like ‘XX provided editorial assistance’).
We extracted number of authors, industry employed authors (with company affiliation in their address) and Danish non-industry employed physician authors (registered as a physician by the Danish Registry of Authorization to Practice Medicine13 and with an affiliation to a non-industry institution, e.g. hospital, stated in their address). The names and COI statements of the Danish non-industry employed physician authors were also extracted.
Identification of conflicts of interest
We focused only on financial COIs and defined a COI as a paid or unpaid, but long-term affiliation with a drug company excluding affiliations that only consisted of honoraria for travel expenses and provision of meals. For each of the Danish non-industry employed physicians, two observers (JS, KR) independently categorised each disclosed COI as related to the trial industry sponsor or manufacturer of trial drug being studied, related to a competing drug manufacturer or as related to any drug manufacturer.
COIs related to the trial sponsor or manufacturer were categorised as:
Consultant/advisory board member/employee
Speaker/educational activities
Investigator/research collaboration/grants
Equity/stockholder
Other (e.g. provided legal testimony for the sponsor)
Two observers (JS, KR) independently compared the disclosed COIs with information about industry collaboration (type and drug company) on the Danish Health and Medicines Authority’s public disclosure list.10 Being an investigator for the trial sponsor or manufacturer of the drug being studied was not considered an undisclosed COI, as we could not determine whether the investigator role related to the included trial or a different trial reported elsewhere. Receiving reimbursement for conference expenses and travel expenses for single activities are not listed on the Danish Health and Medicines list and thus could not be identified as undisclosed COIs. Only the COIs that were present at the time of publication or three years prior, similar to ICMJE’s criteria, were included (we used multiple editions of the disclosure lists from June 2010 and forward). If there was any doubt about the start date of the involvement with the pharmaceutical company, we contacted the Danish Health and Medicines Authority and the dates were specified further to allow for a precise classification.
Undisclosed COIs were categorised as related to the trial sponsor or manufacturer of the trial drug, to a competing manufacturer or to any drug manufacturer. COIs related to a competing manufacturer of a drug with the same indication as the drug being studied was classified using the Danish Pharmaceutical Information’s website (www.pro.medicin.dk). This is a similar methodology as that employed by other studies.4,5 For example, in a trial of the beta-blocker metoprolol for heart failure, heart failure drugs from other companies were considered, whereas in a trial of beta-blockers for hypertension, antihypertensives from other companies were considered.
Data analysis
We calculated the number of Danish non-industry physician authors with one or more disclosed and undisclosed COIs related to trial sponsor or manufacturer, to competing manufacturers and to any manufacturer. The number of disclosed COIs that were not listed on the Danish Health and Medicines Authority’s website10 was also calculated.
Sensitivity analysis
We re-analysed the results using a more conservative approach where undisclosed COIs were excluded if the author’s only industry affiliation was participating as an investigator in an industry trial for a competing manufacturer or any manufacturer, as it is unclear whether such affiliation requires disclosure according to the ICMJE criteria.8 To avoid clustering due to some authors (prolific authors) having co-authored multiple articles, we conducted a second sensitivity analysis restricted to authors of single articles.
Results
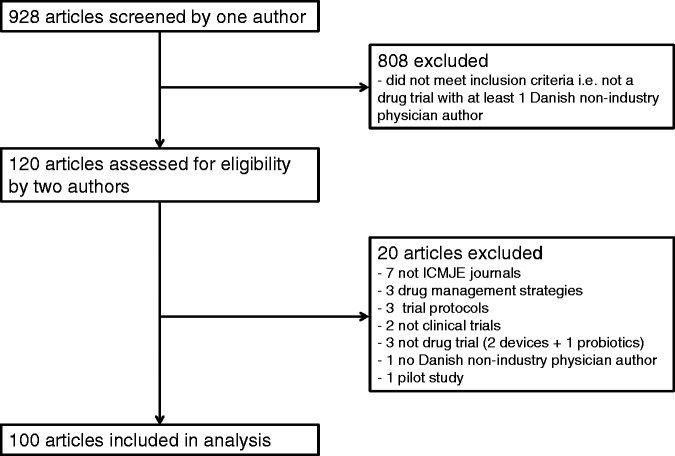
One observer screened 928 articles and two observers assessed 120 for eligibility of which 100 were included (Figure 1).
Figure 1.
Inclusion of drug trials for analysis.
The 100 articles included 318 Danish non-industry employed physician authors (median 1.5 per trial) (Table 1). There were 241 unique authors: 194 being of a single article and 47 of multiple articles (the maximum was 8 articles). Seventy articles were published in specialist journals, the median Impact Factor for the 100 included articles was 5.97 (IQR: 4.09 to 14.09), and 68 articles reported the analysis of primary results. Sixty-three trials were placebo controlled, 22 used an active comparator drug, four had several arms, one used a non-drug comparator, three were unclear and seven had a no-treatment control group. For the three trials classified as unclear, we could not determine whether the comparator was a placebo or no-treatment. Forty-nine trials were solely industry sponsored, 30 received both industry and public funding, 19 were non-industry sponsored and two did not report on sponsorship. We contacted the Danish Health and Medicines Authority to obtain information about start dates of collaborations for 11 of the 318 physicians, as it was unclear whether the publication of the paper had occurred before the collaboration started.
Table 1.
Trial characteristics (n = 100).
| Characteristics | |
|---|---|
| Journal type, n | |
| General medical journal | 30 |
| Specialist journal | 70 |
| Journal impact factor, median (IQR) | 5.97 (4.09–14.09) |
| Publication type, n | |
| Primary results | 68 |
| Secondary analyses | 32 |
| Comparator drug, n | |
| Active | 22 |
| Placebo | 63 |
| Multiple | 4 |
| Other* | 11 |
| Trial sponsorship, n | |
| Industry | 49 |
| Mixed | 30 |
| Non-industry | 19 |
| Not stated | 2 |
| Number of authors per trial, median (IQR) | |
| All authors | 10 (7–13.25) |
| Danish non-industry physician authors | 1.5 (1–5) |
| Industry employed authors | 0 (0–2) |
*Other includes 1 non-drug comparator, 3 unclear and 7 no comparator drug.
Eighty-six of the 318 authors (27%) disclosed one or more COIs in the article (Table 2). Seventy-two authors (23%) disclosed one or more COIs related to the trial sponsor or manufacturer of the trial drugs. Of these 72 authors, 58 had COIs related to consultancy or advisory board membership, 39 received grants, worked as investigator or received honoraria, 36 were paid for educational activities and 1 was a stockholder. Forty authors out of 318 (13%) had disclosed COIs related to a competing company manufacturing drugs for the same indication.
Table 2.
Overview of disclosed, undisclosed and no conflicts of interest for all Danish non-industry physician authors.
| All conflicts of interest disclosed n (%) | Some conflicts of interest undisclosed n (%) | All conflicts of interest undisclosed n (%) | No conflicts of interest identified* n (%) | Total (%) | |
|---|---|---|---|---|---|
| COI related to trial sponsor or manufacturer | 44 (14%) | 28 (9%) | 12 (4%) | 234 (74%) | 318 (100%) |
| COI related to competing manufacturer | 10 (3%) | 30 (9%) | 49 (15%) | 229 (72%) | 318 (100%) |
| COI related to any drug manufacturer | 15 (5%) | 71 (22%) | 66 (21%) | 166 (52%) | 318 (100%) |
*No conflicts of interest identified either in publication or in Danish Health Authority’s list.
We found undisclosed COIs for 40 of 318 authors (13%) related to the trial sponsor or manufacturer of trial drugs. Seventy-nine of 318 authors (25%) had undisclosed COIs related to competing companies manufacturing drugs for the same indication and 137 (43%) had undisclosed COIs with any drug manufacturer. For example, one author disclosed that he received advisory board fees from AstraZeneca (trial sponsor and manufacturer), Bristol-Myers Squibb and Bayer, but did not disclose that he was a speaker for AstraZeneca and that he was on the advisory board for Eli Lilly, a company manufacturing drugs for the same indication, and had additional undisclosed COIs related to six other drug companies. Thirty-five of 115 authors (30%) from non-industry sponsored trials had any undisclosed COI whereas 102 of 203 authors (50%) from industry sponsored trials had any undisclosed COI.
A sensitivity analysis excluding all undisclosed COIs in the investigator role showed similar results. There were no changes to the COIs related to trial sponsor or manufacturer, as these did not include investigator COIs. The undisclosed COIs related to a competing manufacturer were reduced from 25% to 20% and any COI was reduced from 43% to 38%, see Supporting Information Table S1. A second sensitivity analysis restricted to 194 authors of single articles had some effect on our results. The undisclosed COIs related to sponsor or manufacturer was reduced from 13% to 5%, COIs related to a competing manufacturer from 25% to 13%, and COIs related to any manufacturer from 43% to 31%. Additionally, the proportion of authors with no COIs related to the sponsor, competing manufacturers and any manufacturer was higher for the group of single article authors, see Supporting Information Table S2.
Forty-five (14%) authors disclosed COIs in the journal that were not found on the Danish Health and Medicines Authority’s list. Twenty-four authors had COIs related to a single company missing from the list and 21 related to several companies.
Discussion
Statement of principal findings
Almost half of all authors had undisclosed financial COIs in clinical trials reported in journals that adhere to the ICMJE’s manuscript guidelines, and one of eight authors had not even disclosed COIs related to the trial sponsor or manufacturer of the drug being studied.
Strengths and weaknesses of the study
A major strength of our study is the use of a list where authors and companies are required by law to report their type of collaboration. Failure to report collaboration will result in a fine.14 Our sample of 100 recent drug trials represents international publications of clinical trials in a wide range of journals and specialties. We discovered that 14% of authors declared COIs that were not listed on the Danish Health and Medicines Authority’s list. This could be due to the fact that we only looked at lists that were published up to two years prior to the publication date of the study in question, as earlier lists were not available to us. The lists provide an ‘up-to-the-minute account’ and do not provide information on collaborations that have expired years before the current version. Thus, the lack of knowledge about COIs that were present more than two years prior to publication could potentially give an underestimation on the amount of under-reporting. As Denmark has been rated the least corrupt country in the world,15 it is likely that the results we have reported here provide a ‘best case scenario’.
Two observers independently undertook data extraction, and disagreements were discussed so consensus could be reached. The comparison of COIs between trial publications and the Danish Health and Medicines Authority’s list was done using objective criteria pre-specified in the study protocol. However, this procedure could not be blinded, which could potentially have lead to bias.
Strengths and weaknesses in relation to other studies
Norris et al.16 also found a high rate of under-reporting of COIs, with two-thirds of US physicians not disclosing COIs listed in ProPublica’s Dollars for Docs database. However, this database only contains COIs related to 15 companies and it relies on voluntary information published on these companies’ websites. Thus, it is highly likely that the results underestimate the amount of under-reporting.16 A similar strategy was employed by Chimonas et al.17 in a study of COIs related to five orthopaedic device companies, whereas Wang et al.4 and Neuman et al.9 identified additional COIs with Google searches and by retrieving COI statements in previous articles by the authors. The Danish Health and Medicines Authority’s list provides us with more accurate and comprehensive data, as all Danish physicians are legally responsible for the reporting of the collaboration with the industry.
Interestingly, we found that a considerable proportion of COIs related to a competing manufacturer were undisclosed despite the fact that the ICMJE guidelines state that these COIs should be disclosed. To our knowledge, this finding has not previously been described.
Our definition of COIs could have influenced our results. However, our sensitivity analysis, where all COIs that were categorised as investigator role or research grants were excluded, gave similar results. We considered working as an investigator and receiving grants from the pharmaceutical industry as a COI, but some might consider it a less important relationship. However, we could not identify undisclosed COIs related to receiving reimbursement for conference and travel expenses because the Danish list currently does not contain this information.
Our second sensitivity analysis showed that the proportion of undisclosed COIs decreased when we restricted our sample to authors of single articles. This suggests that COIs and lack of disclosure may be particularly prevalent among the group of authors of multiple articles, likely representing key opinion leaders in their field. This finding of a higher non-disclosure rate among prolific authors is a concerning result, as prolific authors dominate the literature and the lack of disclosure misleads the readers.
Meaning of the study
Our results show that COIs reported in ICMJE journals by trialists are not reliable. The Institute of Medicine emphasises that transparency of COIs is an important, but limited first step in dealing with COIs.7 Public registries where both physicians and the drug companies are legally responsible for disclosure of their collaboration such as the Danish Health and Medicines Authority’s list are a model for other countries. With the Physicians Payments Sunshine act,12 it will become possible to get reasonably accurate information in the United States, similar to what is possible in Denmark. In a recent open letter to the General Medical Council (GMC) in the UK, the authors requested a similar registry where physicians can disclose all their COIs to ensure transparency in any collaboration with the industry.18 The GMC reports that the matter of a UK register for mandatory declaration of COIs is still ‘a work in progress’. Relying solely on the disclosures by the pharmaceutical industry such as the Association of the British Pharmaceutical Industry’s disclosures is not reliable and will miss COIs such as shares in pharmaceutical companies.19 However, a public registry cannot combat the bias that COIs can result in, but it can make physicians and the public aware of them.20
To ensure accurate and complete disclosure of financial COIs in the future, journals could require a report from a public registry providing information on paid collaboration for the past three years instead of relying on the authors’ voluntary disclosures.
Conclusions
Under-reporting of COIs is common in clinical trials reported in journals adhering to the ICMJE’s manuscript guidelines. Self-declared COIs cannot be trusted, but public registries may assist editors in ensuring that more COIs are being reported.
Declarations
Competing interests
None declared
Funding
The Nordic Cochrane Centre provided in-house resources. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
Ethical approval was not required for this study according to Danish law.
Guarantor
KR
Contributorship
AL conceived the idea for the study and developed the protocol with contributions from KR, JS and PCG. KR identified trials and AL verified the selection. AL and KR extracted trial data, and KR and JS identified conflicts of interest. All authors participated in data analysis and writing of the paper. All authors had full access to all the data in the study.
Acknowledgements
None
Provenance
Not commissioned; peer-reviewed by Jack Noble and James Brophy
References
- 1.Evans I, Thornton H, Chalmers I, Glasziou P. Testing Treatments. Better Research for Better Healthcare, 2nd edn London: Pinter & Martin Ltd, 2011. [PubMed] [Google Scholar]
- 2.Chan A, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 2005; 365: 1159–1162. [DOI] [PubMed] [Google Scholar]
- 3.Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2012; 12: MR000033–MR000033. [DOI] [PubMed] [Google Scholar]
- 4.Wang AT, McCoy CP, Murad MH, Montori VM. Association between industry affiliation and position on cardiovascular risk with rosiglitazone: cross sectional systematic review. BMJ 2010; 340: c1344–c1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stelfox HT, Chua G, O’Rourke K, Detsky AS. Conflict of interest in the debate over calcium-channel antagonists. N Engl J Med 1998; 338: 101–106. [DOI] [PubMed] [Google Scholar]
- 6.Lerner TG, Miranda MDC, Lera AT, Ueda A, Briones B, Del Giglio A, et al. The prevalence and influence of self-reported conflicts of interest by editorial authors of phase III cancer trials. Contemp Clin Trials 2012; 33: 1019–1022. [DOI] [PubMed] [Google Scholar]
- 7.Lo B, Field MJ. Conflict of Interest in Medical Research, Education, and Practice, Washington, DC: Institute of Medicine of the National Academies, 2009. [PubMed] [Google Scholar]
- 8.ICMJE Form for Disclosure of Potential Conflicts of Interests. See: http://www.icmje.org/conflicts-of- interest (last checked 21 September 2014).
- 9.Neuman J, Korenstein D, Ross JS, Keyhani S. Prevalence of financial conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study. BMJ 2011; 343: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Danish Medicines Agency. List of Permissions Granted to Physicians and Dentists Affiliated with Pharmaceutical Companies. See: http://ext. laegemiddelstyrelsen.dk/tilladelselaegertandlaeger/ tilladelse_laeger_tandlaeger_full_soeg.asp?vis=hele (last checked 21 September 2014).
- 11.Godlee F. A sunshine act for Europe. BMJ 2011; 343: d6593–d6593. [Google Scholar]
- 12.Carpenter D, Joffe S. A unique researcher identifier for the Physician Payments Sunshine Act. JAMA 2011; 305: 2007–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Board of Health. Danish Authorisation Register. See: http://www.sst.dk/DS/OpslagAutReg.aspx (last checked 21 September 2014).
- 14.Jensen HG, Ministeriet for Sundhed og Forebyggelse. Vejl læger og tandlæger – apotekerlovens § 3, stk. 2. 2009:1–8. See: https://sundhedsstyrelsen.dk/da/ medicin/tilknytning-til-virksomhed/spoergsmaal-og-svar (last checked 21 September 2014).
- 15.Transparency International. Transparency International Corruption Perceptions Index 2012. See: http://transparency.org/cpi2012/results (last checked 21 September 2014).
- 16.Norris SL, Holmer HK, Ogden LA, Burda BU, Fu R. Characteristics of physicians receiving large payments from pharmaceutical companies and the accuracy of their disclosures in publications: an observational study. BMC Med Ethics 2012; 13: 24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chimonas S, Frosch Z, Rothman DJ. From disclosure to transparency: the use of company payment data. Arch Intern Med 2011; 171: 81–86. [DOI] [PubMed] [Google Scholar]
- 18.McCartney M, Goldacre B, Chalmers I, Reynolds C, Mendel J, Smith S, et al. Why the GMC should set up a central registry of doctors’ competing interests. BMJ 2014; 348: 1–2. [DOI] [PubMed] [Google Scholar]
- 19.Dyer C. Who is paying your doctor? BMJ 2014; 4601: 2013–2015. [DOI] [PubMed] [Google Scholar]
- 20.Wilson M. Is transparency really a panacea? J R Soc Med 2014; 107: 216–217. [DOI] [PMC free article] [PubMed] [Google Scholar]