Abstract
Background
Some centers have advocated selecting patients with small papillary thyroid cancer (PTC) to undergo active surveillance without surgical treatment. The objectives of this study were to analyze thyroid cancer–related mortality (TC-mortality) in a population-based cohort and to determine the impact of small PTC (in which the greatest dimension of the tumor is ≤ 2 cm) on TC-mortality.
Methods
Data from patients with thyroid cancer of follicular cell origin from the National Cancer Institute’s Surveillance, Epidemiology and End Results 17 Registries Database (1988–2007) were used to analyze characteristics of PTC ≤ 2 cm in patients with TC-mortality. The effects of clinical features on disease-specific survival were analyzed.
Results
Over the 20-year period, TC-mortality rate was 2.8% (n=1,753/61,523). Of patients with TC-mortality, 38% had PTC, 10% had follicular thyroid cancer, and 31.3% had anaplastic thyroid cancer. PTC ≤ 2 cm accounted for 12.3% of patients with TC-mortality. Compared to patients without TC-mortality from PTC ≤ 2 cm, there were significantly higher rates of men (30%-vs.−17%, p<0.01), patients ≥45 years (92%-vs.−52%, p<0.01), tumor >1cm (59%-vs. −46%, p<0.01), extrathyroidal extension (41%-vs.−11%, p<0.01), lymph node (77%-vs. −28%, p<0.01) and distant metastases (31%-vs. −1%, p<0.01) in patients who died from PTC ≤ 2cm. Independent risk factors for death from PTC ≤ 2 cm included age ≥45 years, lymph node and distant metastases, extrathyroidal extension, and less-than-lobectomy surgery.
Conclusions
Because 12.3% of patients with TC-mortality had PTC ≤ 2 cm, despite undergoing a thyroidectomy, nonoperative management for patients with PTC ≤ 2 cm should be used with caution. Patients 45 years of age or older with PTC ≤ 2cm should undergo thyroidectomy.
Keywords: thyroid cancer, mortality, SEER program, prognostic factor, cancer-specific survival
Thyroid cancer is the most common endocrine malignancy, accounting for 3.8% of all new cancer diagnoses (1). The American Cancer Society estimates that there will be almost 63,000 new cases of thyroid cancer and over 2,800 cases of thyroid cancer–related mortality (TC-mortality) in 2014. Thyroid cancer is currently the fifth most common cancer diagnosed in women, following breast, lung, colorectal, and uterine cancers (1). The age- and gender-adjusted incidence of thyroid cancer has increased faster than any malignancy in the last decade (2). The American Cancer Society has reported a remarkable increase in the incidence of thyroid cancer since the mid-1990s. The incidence has increased 5.6% per year in men and 7.0% per year in women since 2004, which represents the largest annual percentage increase of any cancer in both men and women (3).
The incidence of thyroid cancer is more than 3 times higher in women (4–6). However, men tend to have more advanced disease diagnosed at an older age, lower disease-free survival, and higher mortality (4, 5, 7). Over 90% of thyroid cancers originate from follicular cells and are commonly well-differentiated thyroid cancer (WDTC), which includes classical papillary thyroid cancer (PTC) and its follicular variant (FVPTC), follicular thyroid cancer (FTC), and Hürthle cell carcinoma (HCC). PTC, which accounts for over 80% of all thyroid malignancies, is associated with a good prognosis compared to other histologic subtypes and is the most common type, followed by FTC, HCC, medullary, and anaplastic thyroid cancer (ATC) (8).
The vast majority (87%) of thyroid cancers detected in the last 15 years are attributed to cases of small PTC (in which the tumor is ≤ 2 cm in greatest diameter) (9). Although most small thyroid cancers have an indolent clinical course, some exhibit aggressive behavior, with lymph node metastasis (up to 45%), (10, 11) extrathyroidal extension (6%), and/or distant metastasis (0.5%) (12). The need to treat the growing number of patients with low-risk, small PTC has been questioned as some investigators have shown that active surveillance in such patients results in no disease progression in the majority of patients, and few to no adverse events, with the possibility of salvage surgical treatment in patients who have disease progression during follow-up (13). Furthermore, health care expenditures for the management of thyroid cancer account for a substantial portion of medical care costs in the United States, and are likely to increase given the increasing incidence of thyroid cancer (14). Because the mortality rate for thyroid cancer, especially in patients with small, low-risk tumors, is low, a study of TC-mortality in a population-based cohort could help determine the trends and characteristics of TC-mortality and the impact of small PTC on TC-mortality, helping to drive management recommendations for the growing number of small, low-risk PTCs being diagnosed. Thus, the objective of this study was to determine the impact of PTC ≤ 2 cm on TC-mortality and to determine if any risk factors are associated with TC-mortality in patients with PTC ≤ 2 cm.
Methods
Data Acquisition
The Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute is a US population-based cancer registry that has been collecting information on patient demographics, incidence, prevalence, tumor characteristics, initial treatment, tumor histopathology, stage at diagnosis, and follow-up vital status since 1973. The data used in this study derived from 17 registries from various geographic locations throughout the United States, representing 28% of the US population. The SEER data contain unidentifiable patient information; therefore, this study was exempted from review and approval by the Office of Human Subject Research at the National Institutes of Health. Cases were identified and detailed information was captured using SEER*Stat software, Release 6.6.2 (April 2010; Cancer Statistics Branch, NCI, Bethesda, MD).
Because radioisotope therapy has been specifically coded in a separate category since 1988, we identified all adult patients (≥20 years of age) who were diagnosed with thyroid cancer, excluding medullary thyroid cancer, from 1988 to 2007, which provided at least a 5-year follow-up. All patients had thyroid cancer as the first and only primary cancer. The histologic subtypes and their International Classification of Disease codes (ICD-O-3)(15) included in the analysis were PTC (8050), papillary adenocarcinoma (8260), FTC (8330–8332, 8335), insular carcinoma (8337), FVPTC (8340), papillary thyroid microcarcinoma (FTMC) (8341), papillary carcinoma with oxyphilic cells (8342), encapsulated papillary carcinoma (8343), and papillary carcinoma with columnar cells (8344). The histologic differentiation grades analyzed were well, moderately, poorly, and undifferentiated (anaplastic) thyroid cancer. The variables analyzed were age (< 45 and ≥45 years of age), gender, race/ethnicity (white, black, other) according to the standards set forth by the US Office of Management and Budget for the classification of federal data on race and ethnicity, (16) year of diagnosis, size of the primary tumor in its greatest dimension, presence of extrathyroidal extension, cervical lymph node metastasis, distant metastasis, surgery type (less than lobectomy, lobectomy, subtotal or near or total thyroidectomy), radiotherapy (none, radioisotopes, external radiotherapy [XRT], combined radioisotopes and XRT, other), and disease-specific survival (DSS).
Statistical Analysis
We calculated DSS in years from the date of diagnosis to death caused by the disease. Survival was censored at the date of the last follow-up if no disease-specific death occurred. The Pearson chi-square test was used to assess the difference between patients with and without TC-mortality by ordinal and nominal categorical variables. The Mann–Whitney test (17) was used to compare continuous variables that were not normally distributed. The Pearson correlation and Spearman’s rho test were used to assess the correlation between normally distributed and non-normally distributed variables, respectively. The Kaplan–Meier estimator (18) with a log-rank (Mantel–Cox) test (19) was used to estimate and compare DSS for patients by various clinical features and treatments. Pairwise comparisons were used to test the difference in survival between subgroups. Univariate and multivariate Cox proportional hazard regressions (20) were performed to identify variables associated with DSS. A two-tailed p-value of less than 0.05 was considered statistically significant. We used TC-mortality and DSS as the primary outcome measures in this study.
Statistical analysis was performed using SPSS® v16.0 for Windows (SPSS, Inc., Chicago, IL).
Results
Of 61,523 patients with histologically confirmed thyroid cancer of follicular cell origin reported in the SEER program from 1988 to 2007, there were 1,753 cases of TC-mortality. The demographics, clinical and tumor characteristics, and treatment of these patients were analyzed and are summarized in Table 1. The median follow-up time in this cohort was 54 months (range: 1–239 months). Of 1,753 patients (2.8%) with TC-mortality, 28.8% (n = 504) had classical PTC, 9.4% (n = 165) had FVPTC, 10.1% (n = 177) had FTC, 3.7% (n = 66) had HCC, 16.7% (n = 292) had other histologic subtypes, such as tall-cell, columnar, insular, or poorly differentiated thyroid cancer, and 31.3% (n = 549) had ATC (Figure 1). As expected, there was an increase in the rate of PTC ≤ 2cm from 51% in 1988 to 59% in 2007 (Figure 2A). The rate of patients diagnosed with PTC ≤ 2 cm in the second decade of the study cohort (1998–2007) was significantly higher than that of the first decade (1988–1997) (58.2% vs. 52.8%, p < 0.01). The rate of patients diagnosed with PTMC (in which the tumor is < 1 cm in greatest dimension) was also significantly higher in the second decade of the study cohort (from 25% to 31%, p<0.01). Of patients with TC-mortality with available tumor staging, there were 153 patients who died from PTC ≤ 2 cm (12.3% of all patients with TC-mortality and 0.5% of patients with PTC ≤ 2 cm) Sixty three patients (5.1%) had PTMC. The rates of extrathyroidal extension, lymph node metastasis, and distant metastasis in patients who died from PTMC were 26%, 68.4%, and 26.7%, respectively. Only 5 patients (1.4%) who died from PTMC had no extrathyroidal extension, lymph node or distant metastasis. Median survival was 55 months (range 1–199 months). Compared to the group of patients with PTC ≤ 2 cm who did not die from their disease, we found significantly higher rates of male patients (30.1% vs. 17.7%, p < 0.01), patients ≥ 45 years of age (92% vs. 52%, p < 0.01), black patient (5.2% vs. 4.4%) and patients of other race/ethnicity (17.6% vs. 11.1%, p = 0.03), tumor sizes of 1–2 cm (58.8% vs. 46.1%, p < 0.01), lymph node metastasis (77.3% vs. 27.7%, p < 0.01), and distant metastasis (31% vs. 1.1%, p < 0.01) in the group of patients who died from their disease. Although there was no significant difference between the types of surgical treatments, XRT (8.1% vs. 1%, p < 0.01) and combined radioisotopes and XRT (5.4% vs. 0.4%, p < 0.01) were used more frequently in patients with PTC ≤ 2 cm who died from their disease than those who did not (Table 2).
Table 1.
Characteristics of Patients with Thyroid Cancer of Follicular Cell Origin as the First and Only Primary Tumors by Thyroid Cancer-Related Mortality, SEER, 1988–2007
| Patient Characteristics | Thyroid Cancer-Related Mortality
|
Total (%) | |
|---|---|---|---|
| Alive (%)1 | Dead (%) | ||
|
| |||
| No. of patients (%) | 59,770 (97.2) | 1,753 (2.8) | 61,523 |
|
| |||
| Sex | |||
| Male | 12,954 (21.7) | 688 (39.2) | 13,642 (22.2) |
| Female | 46,816 (78.3) | 1,065 (60.8) | 47,881 (77.8) |
|
| |||
| Age, years | |||
| < 45 | 29,262 (49) | 96 (5.5) | 29,358 (47.7) |
| ≥45 | 30,508 (51) | 1,657 (94.5) | 32,165 (52.3) |
|
| |||
| Race/ethnicity (16) | |||
| White | 49,212 (83) | 1,425 (81.3) | 50,637 (83) |
| Black | 3,340 (5.6) | 114 (6.5) | 3,454 (5.7) |
| Other | 6,782 (11.4) | 213 (12.2) | 6,995 (11.5) |
|
| |||
| Histopathology2 | |||
| PTC and FVPTC | 48,516 (83.6) | 669 (38.1) | 49,185 (82.3) |
| FTC | 3,981 (7) | 177 (10.1) | 4,158 (7) |
| HCC | 1,752 (3) | 66 (3.8) | 1,818 (3) |
| Other subtypes | 3,564 (6.1) | 292 (16.7) | 3,856 (6.5) |
| ATC | 191 (0.3) | 549 (31.3) | 740 (1.2) |
|
| |||
| Tumor size | |||
| ≤1 cm | 18,739 (34) | 95 (7.7) | 18,834 (33.4) |
| >1–2 cm | 16,001 (29) | 158 (12.8) | 16,159 (28.7) |
| >2–4 cm | 15,069 (27.3) | 371 (29.9) | 15,440 (27.4) |
| >4 cm | 5,345 (9.7) | 615 (49.6) | 5,960 (10.6) |
|
| |||
| Extrathyroidal extension | |||
| Yes | 8,989 (15.4) | 843 (67.7) | 9,832 (16.4) |
| No | 49,547 (84.6) | 402 (32.3) | 49,949 (83.6) |
|
| |||
| Stage T1 tumor | |||
| Yes | 30,686 (55.9) | 103 (9.4) | 30,789 (54.9) |
| No | 24,251 (44.1) | 1,092 (90.6) | 25,343 (45.1) |
|
| |||
| Lymph node metastasis | |||
| Yes | 12,015 (31.5) | 752 (77.1) | 12,767 (32.6) |
| No | 26,119 (68.5) | 223 (22.9) | 26,342 (67.4) |
|
| |||
| Distant metastasis | |||
| Yes | 996 (2.1) | 381 (47) | 1,377 (2.9) |
| No | 45,947 (97.9) | 430 (53) | 46,377 (97.1) |
|
| |||
| Surgery | |||
| Less than lobectomy | 421 (0.9) | 53 (6.5) | 474 (1) |
| Lobectomy | 7,206 (15.3) | 96 (11.7) | 7,302 (15.2) |
| Subtotal or total thyroidectomy | 39,531 (83.8) | 669 (81.8) | 40,200 (83.8) |
|
| |||
| Radiotherapy | |||
| None | 28,667 (48.8) | 515 (30.1) | 29,182 (48.2) |
| Radioisotopes | 27,932 (47.5) | 589 (34.4) | 28,521 (47.1) |
| XRT3 | 853 (1.5) | 478 (27.9) | 1,331 (2.2) |
| Combined XRT3 and radioisotopes | 327 (0.6) | 95 (5.5) | 422 (0.7) |
| Other | 1,024 (1.7) | 35 (2) | 1,059 (1.7) |
|
| |||
| Median follow-up (range) in months | 55 (1–239) | 19 (1–226) | 54 (1–239) |
Percentage of parameter by mortality status
PTC: papillary thyroid cancer; FVPTC: follicular variant of papillary thyroid cancer; FTC: follicular thyroid cancer; HCC: Hürthle cell cancer; Other subtypes include columnar, insular, tall cell, and poorly differentiated thyroid cancer; ATC: anaplastic or undifferentiated thyroid cancer
XRT: external radiotherapy.
Figure 1.
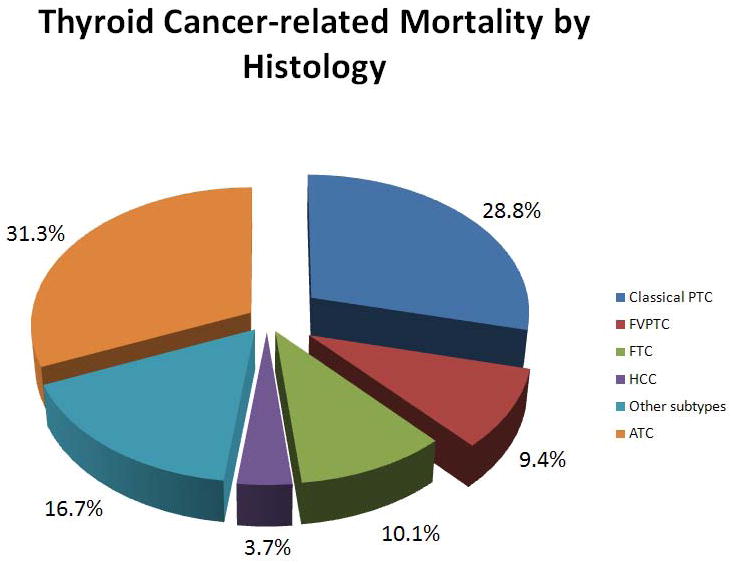
Thyroid cancer–related mortality (n = 1,753) in the SEER program from 1988 to 2007 by histology.
Figure 2.
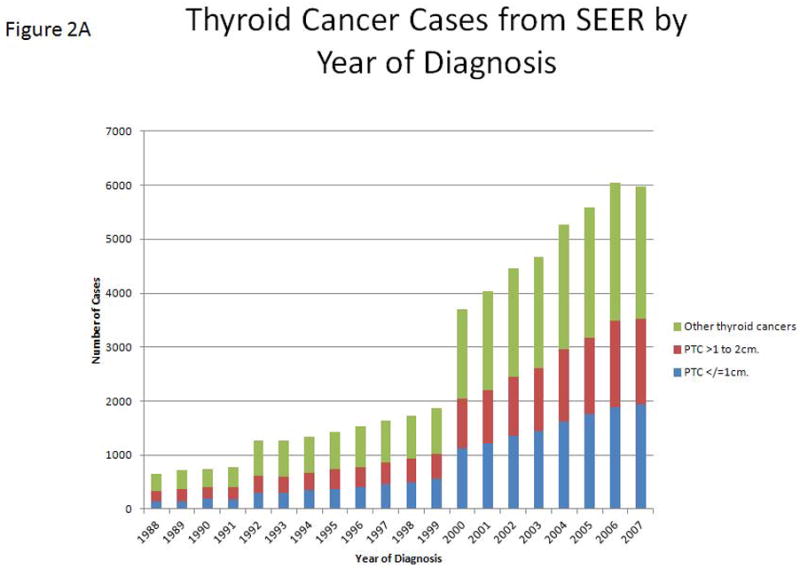
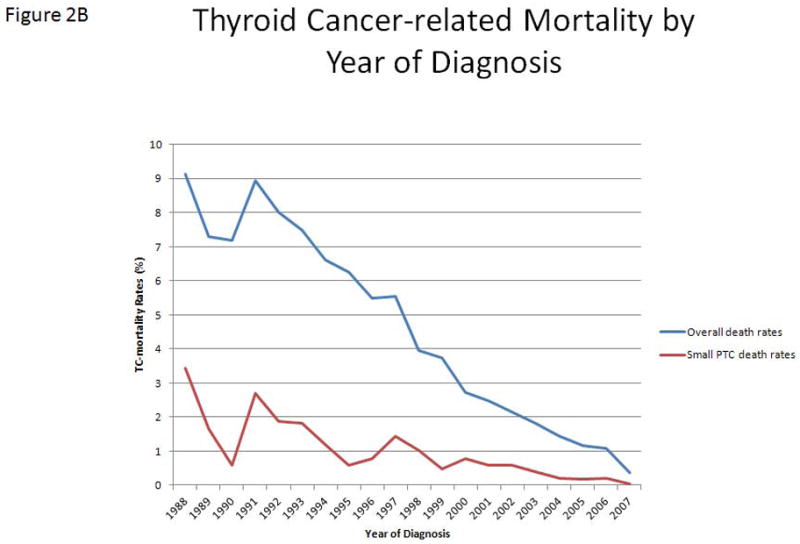
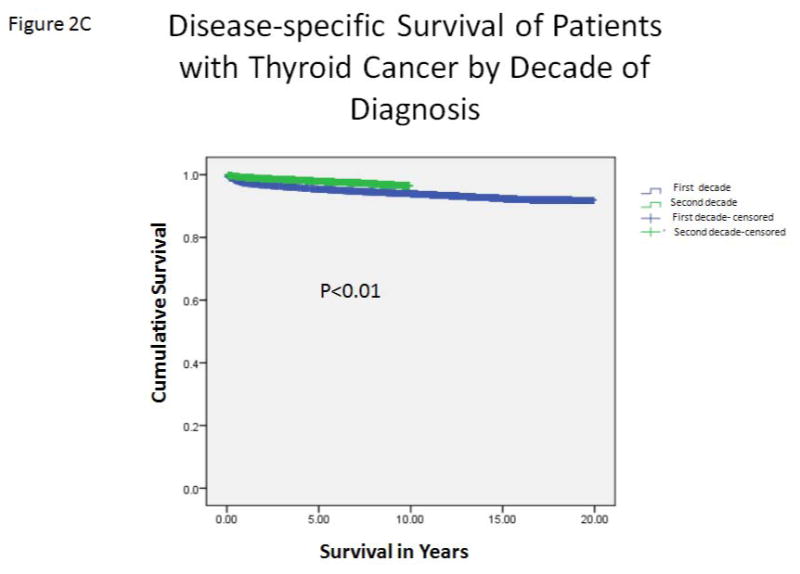
A, The incidence of thyroid cancer of follicular cell origin in the SEER program, from 1988 to 2007, by year of diagnosis. Blue bars represent PTMC. Red bars represent PTC with tumors ≥ 1–2 cm in size. B, The rates of thyroid cancer–related mortality (percent of number of annual deaths by total number of cases diagnosed each year) by the year of diagnosis. Blue line represents overall TC-mortality rate by year. Red line represents TC-mortality rate of patients with PTC ≤ 2 cm. C, Estimated disease-specific survival of patients with thyroid cancer of follicular cell origin by decade of diagnosis. Analysis was performed using a Kaplan–Meier estimator with a log-rank (Mantel–Cox) test.
Table 2.
Characteristics of Patients with PTC ≤ 2 cm as the First and Only Primary Tumors by Thyroid Cancer-Related Mortality, SEER, 1988–2007
| Patient Characteristics | Thyroid Cancer-Related Mortality
|
Total (%) | P | |
|---|---|---|---|---|
| Alive (%)1 | Dead (%) | |||
|
| ||||
| No. of patients (%) | 30,031 (99.5) | 153 (0.5) | 30,184 | |
|
| ||||
| Gender | < 0.01 | |||
| Male | 5,325 (17.7) | 46 (30.1) | 5,371 (17.8) | |
| Female | 24,706 (82.3) | 107 (69.9) | 24,813 (82.2) | |
|
| ||||
| Age, years | < 0.01 | |||
| < 45 | 14,520 (48.4) | 12 (7.8) | 14,532 (48.1) | |
| ≥45 | 15,511 (51.6) | 141 (92.2) | 15,652 (51.9) | |
|
| ||||
| Race/ethnicity (16) | 0.03 | |||
| White | 25,213 (84.5) | 118 (77.1) | 25,331 (84.4) | |
| Black | 1,327 (4.4) | 8 (5.2) | 1,335 (4.4) | |
| Other | 3,309 (11.1) | 27 (17.6) | 3,336 (11.1) | |
|
| ||||
| Tumor size | < 0.01 | |||
| ≤1 cm | 16,194 (53.9) | 63 (41.2) | 16,257 (53.9) | |
| >1–2 cm | 13,837 (46.1) | 90 (58.8) | 13,927 (46.1) | |
|
| ||||
| Extrathyroidal extension | < 0.01 | |||
| Yes | 3,337 (11.2) | 53 (40.8) | 3,390 (11.3) | |
| No | 26,509 (88.8) | 77 (40.8) | 26,586 (88.7) | |
|
| ||||
| Lymph node metastasis | < 0.01 | |||
| Yes | 5,459 (27.7) | 68 (77.3) | 5,527 (27.9) | |
| No | 14,266 (72.3) | 20 (22.7) | 14,286 (77.3) | |
|
| ||||
| Distant metastasis | < 0.01 | |||
| Yes | 258 (1.1) | 22 (31) | 280 (1.1) | |
| No | 24,023 (98.9) | 49 (69) | 24,072 (98.9) | |
|
| ||||
| Surgery | 0.31 | |||
| Less than lobectomy | 221 (0.9) | 1 (1.4) | 222 (0.9) | |
| Lobectomy | 3,987 (16.4) | 7 (9.9) | 3,994 (16.4) | |
| Subtotal or total thyroidectomy | 20,080 (82.7) | 63 (88.7) | 20,143 (82.7) | |
|
| ||||
| Radiotherapy | < 0.01 | |||
| None | 15,949 (53.9) | 56 (37.8) | 16,005 (53.9) | |
| Radioisotopes | 12,787 (43.2) | 68 (45.9) | 12,855 (43.3) | |
| XRT2 | 301 (1) | 12 (8.1) | 313 (1.1) | |
| Combined XRT2 and radioisotopes | 105 (0.4) | 8 (5.4) | 113 (0.4) | |
| Other | 428 (1.4) | 4 (2.7) | 432 (1.5) | |
|
| ||||
| Median follow-up (range) in months | 53 (1–239) | 55 (1–199) | 54 (1–239) | 0.14 |
Percentage of parameter by mortality status
XRT: external radiotherapy
Overall, the TC-mortality rate decreased from 9% in 1988 to 0.4% in 2007 (Figure 2B). There was a significantly lower TC-mortality rate in the second decade of this cohort (from 6.9% to 1.7%, p < 0.01). Patients who were diagnosed in the second decade (1998–2007) had significantly longer DSS than those diagnosed in the first decade (1988–1997) (p < 0.01) (Figure 2C). However, there was no significant difference in the total number of deaths per year from thyroid cancer over the entire study period. As expected, there was a strong, significant correlation between the increasing number of thyroid cancer cases in the SEER database and the number of PTC ≤ 2 cm cases (r = 0.99, p < 0.01). We found no difference in the rate of PTC ≤ 2 cm tumors in patients with TC-mortality by decade of diagnosis (15.6% in the first decade vs. 14.3% in the second decade, p = 0.52).
As expected, univariate analysis of patients with PTC ≤ 2 cm showed a significantly shorter DSS in men (p < 0.01), in patients ≥ 45 years of age (p < 0.01), and in patients with PTC 1–2 cm in size, compared to DSS in patients with PTMC (p < 0.01), extrathyroidal extension (p < 0.01), or lymph node (p < 0.01) and distant metastases (p < 0.01). Whites had significantly longer DSS than the “Other” race/ethnicity category (p = 0.02). The results of univariate analysis and estimated DSS are demonstrated in Table 3. However, gender, race/ethnicity, and tumor size were not independent prognostic factors by multivariate analysis. Independent prognostic factors for TC-mortality in patients with PTC ≤ 2cm included age ≥ 45 years (hazard ratio [HR] = 12.2, p < 0.01), extrathyroidal extension (HR = 2.8, p < 0.01), lymph node (HR = 5.4, p < 0.01) and distant metastases (HR = 6.4, p < 0.01), less than thyroid lobectomy (HR = 13.7, p = 0.01), and treatment with XRT (HR = 8.9, p < 0.01) or a combination of XRT and radioisotopes (HR = 8.1, p < 0.01)(Table 4). Compared to women, men with PTC ≤ 2 cm had significantly higher rates of PTC 1–2cm in size and several adverse prognostic factors, such as extrathyroidal extension, lymph node and distant metastases, and TC-mortality, regardless of age (Table 5).
Table 3.
Comparison of Disease-Specific Survival1 ± Standard Error of the Mean Estimated by Kaplan-Meier method in Patients with PTC ≤ 2 cm by Clinical Features.
| Patient Characteristics | Disease-Specific Survival (years) | P |
|---|---|---|
|
| ||
| Age at diagnosis, years | < 0.01 | |
| <45 | 19.9 ± 0.01 | |
| ≥45 | 19.5 ± 0.04 | |
|
| ||
| Gender | < 0.01 | |
| Female | 19.8 ± 0.02 | |
| Male | 19.6 ± 0.06 | |
|
| ||
| Race/ethnicity | 0.022 | |
| Black | 19.7 ± 0.08 | |
| White | 19.7 ± 0.02 | |
| Other | 19.6 ± 0.06 | |
|
| ||
| Tumor size | 0.01 | |
| ≤ 1 cm | 19.8 ± 0.02 | |
| 1–2 cm | 19.7 ± 0.03 | |
|
| ||
| Extrathyroidal extension | < 0.01 | |
| Yes | 19.3 ± 0.09 | |
| No | 19.8 ± 0.02 | |
|
| ||
| Regional lymph node metastasis | < 0.01 | |
| Yes | 19.5 ± 0.05 | |
| No | 19.8 ± 0.03 | |
|
| ||
| Distant metastasis | < 0.01 | |
| Yes | 8.9 ± 0.14 | |
| No | 9.9 ± 0.004 | |
Kaplan-Meier estimator with log-rank (Mantel–Cox) test was used.
Only when estimated DSS of the White category was compared to that of the Other category. No other pairwise comparison was statistically significant.
Table 4.
Multivariate Cox Proportional Hazard Regression Analysis of Clinical Characteristics and TC mortality in Patients with Primary PTC ≤ 2 cm.
| Patient Characteristics | Hazard Ratio | 95.0% CI for Exp (B)
|
P | |
|---|---|---|---|---|
| Lower | Upper | |||
|
| ||||
| Age ≥45 years* | 12.2 | 4.3 | 34.8 | < 0.01 |
|
| ||||
| Male | 0.8 | 0.4 | 1.6 | 0.48 |
|
| ||||
| Race/ethnicity | ||||
| Black (reference) | 0.59 | |||
| White | 1.5 | 0.2 | 11.5 | 0.68 |
| Other | 2.2 | 0.3 | 18.3 | 0.46 |
|
| ||||
| Tumor size | ||||
| ≤1 cm (reference) | ||||
| >1–2 cm | 0.8 | 0.4 | 1.7 | 0.61 |
|
| ||||
| Regional lymph node metastasis* | 5.4 | 2.5 | 11.8 | <0.01 |
|
| ||||
| Distant metastasis* | 6.4 | 2.8 | 14.4 | <0.01 |
|
| ||||
| Extrathyroidal extension* | 2.8 | 1.3 | 5.9 | <0.01 |
|
| ||||
| Subtotal or total thyroidectomy (reference) | 0.21 | |||
| Lobectomy | 2.4 | 0.9 | 6.7 | 0.09 |
| Less than lobectomy* | 13.7 | 1.7 | 110.6 | 0.01 |
|
| ||||
| Radiotherapy | ||||
| None (reference) | < 0.01 | |||
| Radioisotopes | 1.1 | 0.5 | 2.4 | 0.88 |
| XRT* | 8.9 | 3.1 | 25.1 | < 0.01 |
| Combined XRT and radioisotopes* | 8.1 | 2.1 | 31.4 | < 0.01 |
| Other | 2.9 | 0.3 | 24.6 | 0.34 |
Significant independent prognostic indicators
Table 5.
A Comparison of Clinical Features between Men and Women with PTC ≤ 2 cm as the First and Only Primary Tumors by Age Group, SEER 1998–2007.
| Patient Characteristics | Age (years) | Female (%) | Male (%) | P |
|---|---|---|---|---|
|
| ||||
| Tumor size | < 45 | 6,362 (49.6) | 1,182 (54.4) | < 0.01 |
| >1cm–2 cm | ≥45 | 5,055 (39.3) | 1,412 (41.3) | 0.03 |
|
| ||||
| Extrathyroidal extension | < 45 | 1,334 (10.5) | 314 (14.6) | < 0.01 |
| ≥45 | 1,422 (11.1) | 441 (13.1) | < 0.01 | |
|
| ||||
| Regional lymph node metastasis | < 45 | 2,645 (31.7) | 829 (52.9) | < 0.01 |
| ≥45 | 1,421 (17) | 785 (34.5) | < 0.01 | |
|
| ||||
| Distant metastasis | < 45 | 79 (0.8) | 31 (1.9) | < 0.01 |
| ≥45 | 127 (1.2) | 69 (2.4) | < 0.01 | |
|
| ||||
| Lobectomy, near total or total thyroidectomy | < 45 | 8,299 (84.5) | 1,438 (86.4) | 0.09 |
| ≥45 | 8,805 (80.8) | 2,305 (80.4) | 0.32 | |
|
| ||||
| Disease-specific mortality | < 45 | 10 (0.1) | 7 (0.3) | < 0.01 |
| ≥45 | 119 (0.9) | 49 (1.4) | < 0.01 | |
Discussion
Although most patients with thyroid cancer of follicular cell origin have excellent prognoses, TC-mortality cases have increased over the past decade, from 1,460 in 1994 to 2,800 in 2014,(1) as well as the number of patients diagnosed with PTC ≤ 2 cm. We focused our analysis on patients with TC-mortality and assessed the impact of PTC ≤ 2 cm on TC-mortality. We found that 12.3% of patients who died from thyroid cancer had primary PTC ≤ 2 cm. In addition, half of the patients with TC-mortality had common histologic subtypes of differentiated thyroid cancer, such as classic PTC, FVPTC, and FTC. A significantly higher rate of patients with PTC ≤ 2 cm was observed in the 2nd decade of this cohort, with a strong correlation between increasing thyroid cancer incidence and the increase of patients with PTC ≤ 2 cm consistent with previous findings that suggest earlier presentation (9). In addition, a decrease in the TC-mortality rate was observed, likely from the higher rate of small, low-risk thyroid cancer diagnosed in the more recent years. Similar to our previous study, which included thyroid cancer of follicular cell origin patients with all histology types and tumor sizes, (21) DSS in patients with PTC ≤ 2 cm was significantly shorter in men, in patients ≥ 45 years of age, and in cases with tumors 1–2 cm in size, extrathyroidal extension, and lymph node and distant metastases. However, gender and tumor size were not independent prognostic factors for TC-mortality in patients with PTC ≤ 2 cm. Age ≥45 years, extrathyroidal extension, lymph node and distant metastases, less than thyroid lobectomy, and XRT were independent prognostic factors.
Nonoperative management of thyroid cancer smaller than 1 cm. has been advocated by several investigators, including Ito and colleagues (13). In their cohort of over 1,200 patients with low-risk PTMC, 15% of patients required surgery due to tumor growth or patient preference. None of the patients developed distant metastasis or died from their disease (mean follow-up of 75 months). Unlike studies that advocated nonoperative management in patients with PTMC, our study evaluated the outcome of patients with PTC ≤ 2 cm. As growing numbers of patients with thyroid cancer are identified, most with small PTC, defining the important criteria for selecting patients for nonoperative management and for determining the resulting risk of TC-mortality are crucial. Thus, our findings are important for determining the factors associated with TC-mortality in these patients and in quantifying the possible adverse events associated with PTC ≤ 2 cm. In this study, we show that 12.3% of TC-mortality cases are attributable to small, PTC ≤ 2 cm. Because extrathyroidal extension and lymph node and distant metastases cannot accurately be assessed prior to surgery in most patients with low-risk small PTC, the only independent prognostic factor for TC-mortality that can be used reliably to risk stratify patients with PTC ≤ 2 cm is age ≥ 45 years. Although male gender was not an independent prognostic factor for TC-mortality in patients with PTC ≤ 2 cm in this study, men had higher rates of extrathyroidal extension, regional lymph node and distant metastases, and mortality compared to women, regardless of age. Therefore, patients ≥ 45 years of age or male patients of any age should undergo at least thyroid lobectomy as less than lobectomy was an independent prognostic factor for TC-mortality in patients with PTC ≤ 2 cm. Risk factors for overall survival in patients with PTMC included age ≥ 45 years, male gender, non-White race, lymph node metastasis, extrathyroidal extension, and distant metastasis. At least 2 of these risk factors were identified in 92% of patients who died from PTMC. (22) Although we observe a low TC-mortality rate in patients with PTMC who had no pathological evidence of extrathyroidal extension, lymph node metastasis and no clinically detectable distant metastasis which suggests that nonoperative management is reasonable, these features cannot be accurately assessed by radiographic imaging. Compared to incidentally found PTMC, non-incidental PTMC had significantly higher rates of multifocality, bilaterality, capsular invasion, and lymph node metastasis; thus, total thyroidectomy was recommended (23). In this cohort, we found a significantly higher rate of PTMC in the second decade. This may explain our finding of lower mortality rate in patients with PTC ≤ 2 cm diagnosed in the second decade of the cohort as patients diagnosed with PTMC are sometimes identified incidentally in patients undergoing thyroidectomy for other indications (24). The overall mortality in patients with thyroid cancer in our cohort by decade of diagnosis is comparable to other series from the same decade (25, 26).
The limitation of the SEER database is that most of the patients underwent surgical intervention, and the small number of patients who did not undergo surgery were older and had more advanced disease; (27) therefore, the outcome of nonoperative management cannot be compared. However, the significant number of TC-mortality accounted for by PTC ≤ 2 cm is even more compelling given that these patients were treated. The SEER database lacks some clinical and pathological data, such as disease recurrence, multicentricity, and the presence of lymphocytic thyroiditis, which may affect the outcome as these could be important risk factors for TC-mortality in patients with small PTC. In addition, treatments for patients with similar disease and staging vary among different institutions and or regions contributing to the SEER database. Despite these limitations, SEER data provide a broad insight regarding the disease outcome at a population level with adequate statistical power that most individual cohorts cannot achieve.
In summary, our study demonstrates that 12.3% of TC-mortality occurred in patients with PTC ≤ 2 cm who had a thyroidectomy. Given the rise in the incidence of small thyroid cancer, nonoperative management for this growing population should be used with caution. Patients ≥ 45 years of age or men of any age with PTC ≤ 2 cm should receive at least thyroid lobectomy.
Acknowledgments
Funding sources:
The research activities performed in this manuscript were supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
The research activities performed in this manuscript were supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Financial disclosures:
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 3.The American Cancer Society. Cancer facts and figures. 2013 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf.
- 4.Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, et al. [accessed October, 2011];SEER Cancer Statistics Review, 1975–2004. 2007 Available from URL: http://seer.cancer.gov/csr/1975_2004/
- 5.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79(3):564–73. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 7.Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, et al. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1092–100. doi: 10.1158/1055-9965.EPI-08-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S. 1985–1995 [see commetns] Cancer. 1998;83(12):2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Jama. 2006;295(18):2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 10.Malandrino P, Pellegriti G, Attard M, Violi MA, Giordano C, Sciacca L, et al. Papillary thyroid microcarcinomas: a comparative study of the characteristics and risk factors at presentation in two cancer registries. J Clin Endocrinol Metab. 2013;98(4):1427–34. doi: 10.1210/jc.2012-3728. [DOI] [PubMed] [Google Scholar]
- 11.Reddy RM, Grigsby PW, Moley JF, Hall BL. Lymph node metastases in differentiated thyroid cancer under 2 cm. Surgery. 2006;140(6):1050–4. doi: 10.1016/j.surg.2006.08.010. discussion 1054–5. [DOI] [PubMed] [Google Scholar]
- 12.Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254(4):653–60. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27–34. doi: 10.1089/thy.2013.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubitz CC, Kong CY, McMahon PM, Daniels GH, Chen Y, Economopoulos KP, et al. Annual financial impact of well-differentiated thyroid cancer care in the United States. Cancer. 2014;120(9):1345–52. doi: 10.1002/cncr.28562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute, U.S. National Institutes of Health. Surveillance Epidemiology and End Results: ICD-O-3 Coding Materials. 2012;2012 Available at In. [Google Scholar]
- 16.Office of Management and Budget. Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. Vol. 2003 Washington, DC: 2003. Available at In. [Google Scholar]
- 17.Mann HB, Whiteney DR. On a test of whether one of two random variables is stochastically larger than the other. The Annals of Mathematical Statistics. 1947;18:50–60. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–81. [Google Scholar]
- 19.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–70. [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 21.Nilubol N, Zhang L, Kebebew E. Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid. 2013;23(6):695–702. doi: 10.1089/thy.2012.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated?: an analysis of 18,445 cases. Ann Surg. 2011;254(4):653–60. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 23.Vasileiadis I, Karatzas T, Vasileiadis D, Kapetanakis S, Charitoudis G, Karakostas E, et al. Clinical and pathological characteristics of incidental and nonincidental papillary thyroid microcarcinoma in 339 patients. Head Neck. 2014;36(4):564–70. doi: 10.1002/hed.23333. [DOI] [PubMed] [Google Scholar]
- 24.Karatzas T, Vasileiadis I, Charitoudis G, Karakostas E, Tseleni-Balafouta S, Kouraklis G. Bilateral versus unilateral papillary thyroid microcarcinoma: predictive factors and associated histopathological findings following total thyroidectomy. Hormones (Athens) 2013;12(4):529–36. doi: 10.14310/horm.2002.1441. [DOI] [PubMed] [Google Scholar]
- 25.Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82(11):3553–62. doi: 10.1210/jcem.82.11.4373. [DOI] [PubMed] [Google Scholar]
- 26.Sciuto R, Romano L, Rea S, Marandino F, Sperduti I, Maini CL. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann Oncol. 2009;20(10):1728–35. doi: 10.1093/annonc/mdp050. [DOI] [PubMed] [Google Scholar]
- 27.Sanabria A, Dominguez LC, Vega V, Osorio C. Prognosis of patients with thyroid cancer who do not undergo surgical treatment: a SEER database analysis. Clin Transl Oncol. 2011;13(9):692–6. doi: 10.1007/s12094-011-0716-8. [DOI] [PubMed] [Google Scholar]


