Abstract
Although extracellular signal-regulated kinase (ERK) ½ has been shown for its necessity for a variety of the Raf/MEK/ERK pathway signaling, its sufficiency in mediating the pathway signaling has not been firmly established. In an effort to address this, we evaluated previously reported ERK2 mutants that exhibit enhanced activity of autophosphorylation of TEY sites in the activation loop for their ability to induce growth arrest and differentiation in LNCaP and PC12 cells. Here, we demonstrate that expression of ERK2-L73P/S151D, containing Lys73Pro and Ser151Asp replacements that synergistically promote ERK autophosphorylation, is sufficient to induce growth arrest and differentiation whereas ERK2-I84A and ERK2-R65S/D319N are not as effective. When compared to the constitutively active MEK1-ΔN3/S218E/S222D, expression of ERK2-L73P/S151D could only mildly increase ERK kinase activity in cells, as determined by the ERK substrates, p90RSK and ELK1. Nevertheless, ERK2-L73P/S151D expression effectively induced downregulation of androgen receptor, Rb and E2F1, and upregulation of p16INK4A and p21CIP1, which were accompanied by cell cycle arrest and morphological differentiation, in LNCaP cells and neurite-like processing in PC12 cells. These effects and TEY site phosphorylation of ERK2-L73P/S151D were abrogated upon introducing the active site-disabling Lys52Arg mutation, confirming its sufficiency in this signaling. Moreover, introduction of the mutations (producing Asp316/319Ala or Asp319Asn) that impair the common docking site/D-domain-based physical interaction of ERK did not significantly affect the ERK2-L73P/S151D signaling, suggesting that ERK2 can mediate growth arrest and differentiation independently of the conventional ERK-target interaction mechanism. Our study presents a convincing example of ERK sufficiency for Raf/MEK/ERK signaling.
Keywords: Raf, MEK, ERK/MAPK, growth arrest, differentiation
INTRODUCTION
The Raf/MEK/extracellular signal-regulated kinase (ERK) pathway is a highly specific three-layered kinase pathway that consists of the Ser/Thr kinase Raf (i.e., A-Raf, B-Raf, or C-Raf/Raf-1), the highly homologous dual-specificity kinases MEK1/MAP2K1 and MEK2/MAP2K2 (collectively referred to as MEK1/2), and the ubiquitously expressed Ser/Thr kinase ERK1 and ERK2 (collectively referred to as ERK1/2). Upon activation by Raf, MEK1/2 sequentially phosphorylate ERK1/2 at the Tyr and Thr residues in the TEY site of their activation loop, which induces activation conformational changes [1–3]. ERK1/2 then activate/inactivate various targets, including transcription factors, other kinases, phosphatases, cytoskeletal proteins, scaffolds, receptors and signaling components. A typical characteristic of the mitogen-activated protein kinase pathways is the high specificity between MEK and ERK, which is due to the strikingly high affinity between MEK and ERK relative to a typical enzyme-substrate interaction [4]. Accordingly, ERK1/2 are expected to serve as the focal point of the Raf/MEK/ERK pathway and to account for most, if not all, effects mediated by MEK1/2. Nevertheless, sufficiency of ERK1/2 activation for pathway signaling has not been clearly determined because, unlike MEK1/2 or most other kinases, the TEY site of ERK1/2 cannot be replaced by phosphomimetic amino acids to generate a constitutively active mutant [5].
ERK1/2 can autophosphorylate the TEY site, predominantly the Tyr residue, via an intramolecular reaction, which is constrained at low levels in cells to prevent MEK1/2-independent auto-activation of ERK [6–8]. Recent studies have demonstrated that the rates of ERK2 autophosphorylation can be increased by several synergistic mutations, including the combination of Lys73Pro and Ser151Asp (L73P/S151D) which facilitates hydrogen bonding interactions between the phosphoryl acceptor and catalytic nucleophile, the Ile84Ala (I84A) mutation on the gate keeper residue, and the combination of Arg65Ser and the sevenmaker mutation (R65S/D319N) which facilitates autophosphorylation and phosphatase insensitivity [9–11]. Autoactivation rendered by these mutations increased kinase activity of ERK2 about 50-fold in vitro, which is substantially lower than the levels of MEK1/2-mediated ERK1/2 activation; MEK1/2 can increase ERK1/2 activity over 1,000-fold. Nevertheless, these mutants require careful evaluation in different contexts of Raf/MEK/ERK signaling because different magnitude of pathway activity can induce different physiological outputs [12–15].
Although mainly known for its role in mediating cell cycle progression and survival (reviewed in [16]), the Raf/MEK/ERK pathway can also mediate cell cycle arrest and differentiation (reviewed in [17–19]). Anti-proliferative Raf/MEK/ERK signaling has significance in different physiological settings, including early development, neuronal differentiation, and tumor response to chemotherapy. This growth inhibitory signaling has also been demonstrated in many different cell line models. For example, constitutively active Raf or MEK could sufficiently induce G0/G1 phase cell cycle arrest in the human prostate tumor line LNCaP [20–23] and neurite-like processing in the rat pheochromocytoma line PC12, a model for neuronal differentiation [24, 25]. Using these models, we have evaluated the auto-activating ERK mutants for their ability to mediate growth arrest and differentiation. In this study, we demonstrate that ectopic expression of the ERK mutant containing L73P/S151D replacement (ERK2-L73P/S151D) can sufficiently induce growth arrest in LNCaP and differentiation in PC12 although ERK2 mutants containing I84A or R63S/D319N are not effective. We then examine the effects of a few known domain/motif mutations on ERK2-L73P/S151D signaling and whether upstream signals or the downstream effector ELK1 is required for ERK2-L73P/S151D signaling. This study provides strong evidence that ERK activation is sufficient for the Raf/MEK/ERK pathway to mediate growth arrest and differentiation signaling.
RESULTS
ERK2-L73P/S151D undergoes autophosphorylation more efficiently than ERK2-I84A and ERK2-R65S/D319N in LNCaP cells
We previously demonstrated that the basal levels of MEK/ERK activity in LNCaP cells are substantially lower than those detected in other cell types, including primary normal human diploid fibroblasts [21]. Because the auto-activating ERK mutants can still be phosphorylated by MEK1/2, we expected that this characteristic of LNCaP could help evaluating auto-activating ERK2 mutants by minimizing the interference of upstream activators of ERK1/2.
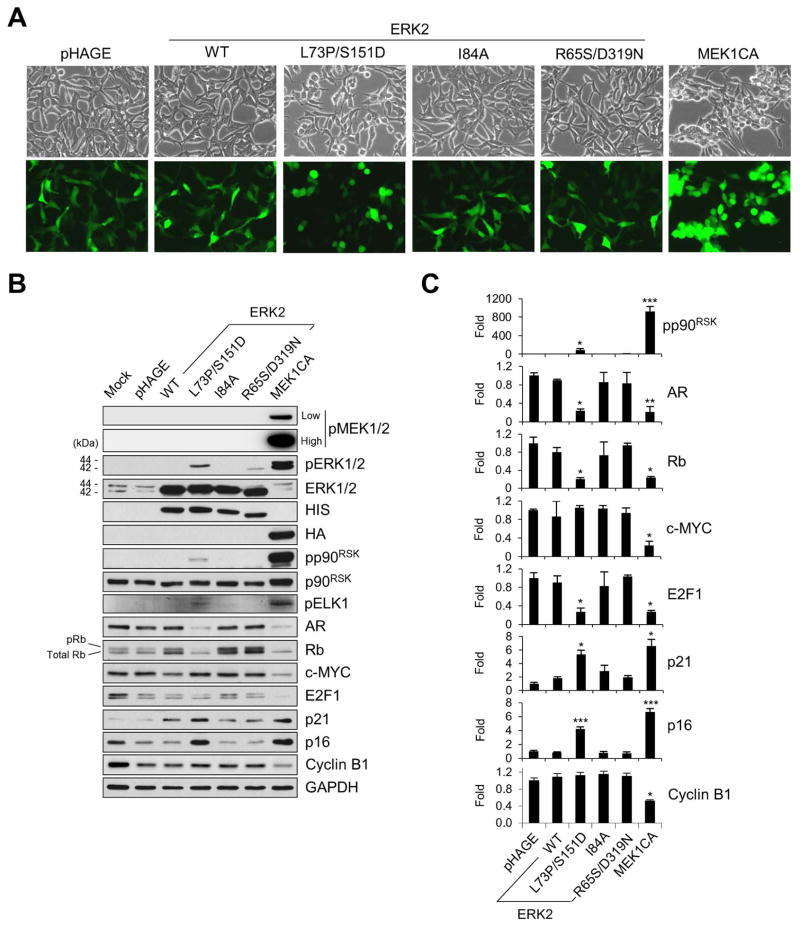
To determine the ability of ERK2-L73P/S151D, ERK2-I84A, and ERK2-R65S/D319N to induce growth arrest signaling, LNCaP cells were transduced for 48 hours with the lentivirus expressing each of the mutants at higher than 90% infection efficiency (Fig. 1A). These ERK mutants were expressed in LNCaP cells at similar levels, as determined by Western blot analyses of their N-terminal HIS tag as well as total ERK1/2 (Fig. 1B). Under these conditions, Western blot signal detected by an antibody specific to phosphorylated TEY sites of ERK1/2 (Thr202/Tyr204 of ERK1 and Thr183/Tyr185 of ERK2) was significantly increased in cells expressing ERK2-L73P/S151D, although the signal intensity was substantially lower than that detected in cells expressing a constitutively active MEK1 that harbors ΔN3/S218E/S222D mutations (MEK1CA). In contrast, no obvious sign of ERK1/2 phosphorylation was detected in cells expressing wild type ERK2 or ERK2-I84A mutant while a very weak signal was detected in cells expressing ERK2-R65S/D319N. In cells infected with these ERK mutants, no significant phosphorylation signals for endogenous ERK1/2 or MEK1/2 were detected (Fig. 1B), suggesting that phosphorylation of ERK2-L73P/S151D and ERK2-R65S/D319N was mainly driven by autophophorylation. These results suggest that, among the three ERK mutants, ERK2-L73P/S151D can most efficiently undergo autophosphorylation in cells.
FIG. 1. Evaluation of autophosphorylating ERK2 mutants for growth inhibitory signaling in LNCaP cells.
LNCaP cells were infected with lentivirus expressing wild type ERK2, ERK2-L73P/S151D, ERK2-I84A, ERK2-R65S/D319N, and the constitutively active MEK1 (MEK1CA) for 2 days. pHAGE is the control empty virus. (A) Cells were observed for morphological changes. GFP expression indicates similar infection efficiency. (B) Total cell lysates were analyzed by Western blotting for expression of phosphorylated MEK1/2 (pMEK1/2), phosphorylated ERK1/2 (pERK1/2), phosphorylated ELK1 (pELK1), phosphorylated p90RSK (pp90RSK), p90RSK, AR, Rb, c-MYC, E2F1, p21CIP1 (p21), p16INK4A (p16), and cyclin B1. Higher Rb bands on the blot indicate phosphorylated Rb (pRb). HIS and HA are the N-terminal tag of the exogenous ERK2 and MEK1 constructs, respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is the control for equal protein loading. (C) Densitometry of Western blot data in B. Data (means ± SE) are from a representative experiment performed in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t-Test). Experiments were repeated at least 3 times with similar results.
Expression of ERK2-L73P/S151D mildly increases net kinase activity of ERK1/2 in LNCaP cells
We next determined kinase activity of these ERK2 mutants in LNCaP cells by measuring phosphorylation levels of p90RSK, a bona fide readout of in vivo ERK1/2 kinase activity [26], and ELK1, a transcription factor which stimulates gene expression via the serum response element (SRE) upon phosphorylation by ERK1/2 in the nucleus [27].
We found that ERK2-L73P/S151D expression mildly but significantly increased p90RSK phosphorylation in LNCaP cells, although this increase was substantially lower than the levels induced by MEK1CA (Fig. 1B). According to our densitometry data, ERK2-L73P/S151D expression induced p90RSK phosphorylation about 10-fold less effectively than MEK1CA expression (Fig. 1C). ERK2-L73P/S151D expression also mildly increased ELK1 phosphorylation (Fig. 1B). Consistent with this, ERK2-L73P/S151D expression increased activity of the SRE-luciferase reporter about 2-fold, which was less than half of the levels induced by MEK1CA expression (data shown below in Fig. 2E). In contrast, overexpression of wild type ERK2, ERK2-I84A, or ERK2-R65S/D319N did not significantly affect these surrogate makers of ERK activity (Fig. 1A to 1C). These data indicate that ERK2-L73P/S151D, but not ERK2-I84A or ERK2-R65S/D319N, can increase ERK kinase activity in cell, albeit mildly and less effectively than MEK1CA.
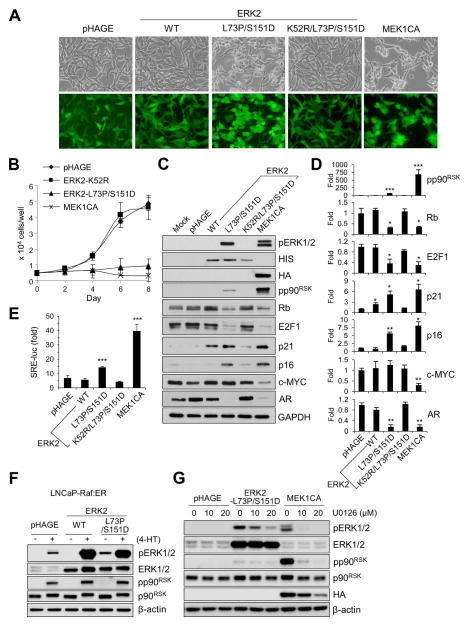
FIG. 2. ERK2-L73P/S151D can sufficiently induce growth inhibitory signaling in LNCaP cells in a manner dependent on its active site.
(A to D) LNCaP cells were infected with lentivirus expressing wild type ERK2, ERK2-L73P/S151D, ERK2-K52R/L73P/S151D (kinase-dead ERK2- L73P/S151D), and MEK1CA. (A) Cells were observed for morphological changes at post-infection day 2. (B) Cell growth was monitored for 8 days by cell counting. (C) Total cell lysates harvested at post-infection day 2 were analyzed by Western blotting for expression of the indicated proteins. (D) Densitometry of Western blot data in C. (E) LNCaP cells transfected with pTAL-Luc vector harboring SRE were infected with the indicated lentiviruses for 2 days before luciferase reporter assay. (F) LNCaP-Raf:ER cells infected by lentiviral pHAGE expressing wild type ERK2 or ERK2-L73P/S151D were treated with 1 μM 4-hydroxytamoxifen (4-HT) for 24 hours before analyzing total cell lysates by Western blotting. (G) LNCaP cells infected by lentiviral pHAGE expressing ERK2-L73P/S151D or MEK1CA were treated with different doses of U0126 for 24 hours before analyzing total cell lysates by Western blotting. Data (means ± SE) are from a representative experiment performed in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t-Test).
Expression of ERK2-L73P/S151D is sufficient to induce growth arrest signaling in LNCaP cells
Intriguingly, despite its relatively low kinase activity, ERK2-L73P/S151D could substantially affect LNCaP cell proliferation. ERK2-L73P/S151D overexpression induced significant downregulation of androgen receptor (AR), phosphorylated Rb, and E2F1, but significant upregulation of p16INK4A and p21CIP1 (Fig. 1B; densitometry in Fig. 1C). Unphosphorylated Rb sequesters E2F1, a key transcription factor promoting S-phase cell cycle progression, while p16INK4A and p21CIP1 stimulates this event by inhibiting cyclin-dependent kinases [28]. Downregulation of AR, a pivotal nuclear transcription factor for prostate epithelium [28], is a key mechanism that triggers these changes in LNCaP cells upon Raf/MEK/ERK activation [20]. Notably, ERK2-L73P/S151D induced these effects as effectively as MEK1CA, although it did not downregulate c-MYC and cyclin B1 levels as opposed to MEK1CA (Fig. 1B; densitometry in Fig. 1C). c-MYC is another critical transcription factor that mediates G1 to S and G2 to M phase cell cycle progression [29] while cyclin B1 regulates G2/M phase transition [30]. In contrast, wild type ERK2 or other ERK2 mutants could not induce similar effects although they could mildly increase p21CIP1 levels (Fig. 1B and 1C).
Indeed, ERK2-L73P/S151D induced similar morphological changes as induced by MEK1CA (Fig. 1A and 2A), and strongly suppressed LNCaP cell proliferation (Fig. 2B). This was accompanied by significantly decreased S phase and increased G0/G1 phase cell population in the culture, as determined at post-infection day 4 (Table 1). These effects of ERK2-L73P/S151D expression were similar to the effects of MEK1CA expression, strongly supporting the role of ERK1/2 as the bona fide effectors of MEK1/2. All these effects of ERK2-L73P/S151D were specific to its ability to undergo autophosphorylation. When Lys52 of ERK2-L73P/S151D, the key residue for phosphoryl transfer, was switched with Arg to introduce “kinase-dead” mutation, the ERK mutant could no longer undergo autophosphorylation (Fig. 2C), increase p90RSK phosphorylation (Fig. 2C; densitometry in Fig. 2D) or SRE-luciferase reporter activity (Fig. 2E), or induce those aforementioned effects on morphology (Fig. 2A), growth (Fig. 2B), and the surrogate markers for growth arrest (Fig. 2C and 2D) in LNCaP cells.
Table 1.
Cell cycle analysis of LNCaP cells expressing ERK2 and MEK1 mutants
|
% of cells in phase
|
||||
|---|---|---|---|---|
| G0/G1 | S | G2/M | SubG1 | |
| pHAGE | 71.3 ± 0.26 | 7.70 ± 0.50 | 20.8 ± 0.38 | 0.17 ± 0.05 |
| ERK2-K52R | 68.4 ± 0.63 | 6.42 ± 0.72 | 24.9 ± 1.45 | 0.29 ± 0.14 |
| ERK2-L73P/S151D | 76.3 ± 0.50 | 3.04 ± 0.21 | 20.1 ± 0.26 | 0.46 ± 0.24 |
| ERK2-L73P/S151D/D319N | 75.0 ± 0.75 | 4.18 ± 0.33 | 20.4 ± 0.60 | 0.42 ± 0.16 |
| MEK1CA | 76.3 ± 1.85 | 2.15 ± 0.21 | 21.1 ± 1.93 | 0.28 ± 0.15 |
LNCaP cells were infected with pHAGE, pHAGE-ERK2-K52R, pHAGE-ERK2-L73P/S151D, pHAGE-ERK2-L73P/S151D/D319N, or pHAGE-MEK1CA virus for 4 days. The values shown are percentages of cells in each phase of the cell cycle. Data (means ± SE) are from a representative experiment performed in triplicate. P values for S phase relative to pHAGE and ERK2-K52R are 4.222E-5 and 1.273E-3 for ERK2-L73P/S151D and ERK2-L73P/S151D/D319N, respectively (One-way ANOVA).
Of note, ERK2-L73P/S151D could be phosphorylated and, in turn, increase p90RSK phosphorylation as efficiently as wild type ERK2 in LNCaP cells upon activation of the tamoxifen-inducible ΔRaf-1:ER (Fig. 2F), indicating its intact responsiveness to upstream signals. Therefore, we determined to what extent TEY phosphorylation of ERK2-L73P/S151D was affected by basal MEK1/2 activity in LNCaP cells using the MEK1/2-specific inhibitor U0126. Indeed, when compared at equal doses, U0126 did not inhibit ERK2-L73P/S151D phosphorylation as effectively as MEK1CA-induced phosphorylation of endogenous ERK1/2 (Fig. 2G). These data further support the auto-activating capability of ERK2-L73P/S151D in cells.
We also evaluated ERK2-L73P/S151D using an adenoviral expression system and observed identical effects as demonstrated by the lentivirally expressed ERK2-L73P/S151D (data not shown), ruling out any potential bias due to an expression system. These data demonstrate that ERK2-L73P/S151D can mimic most, albeit not entire, growth inhibitory effects of MEK1CA in LNCaP cells.
Introduction of the sevenmaker mutation does not increase the activity of ERK2-L73P/S151D in LNCaP cells
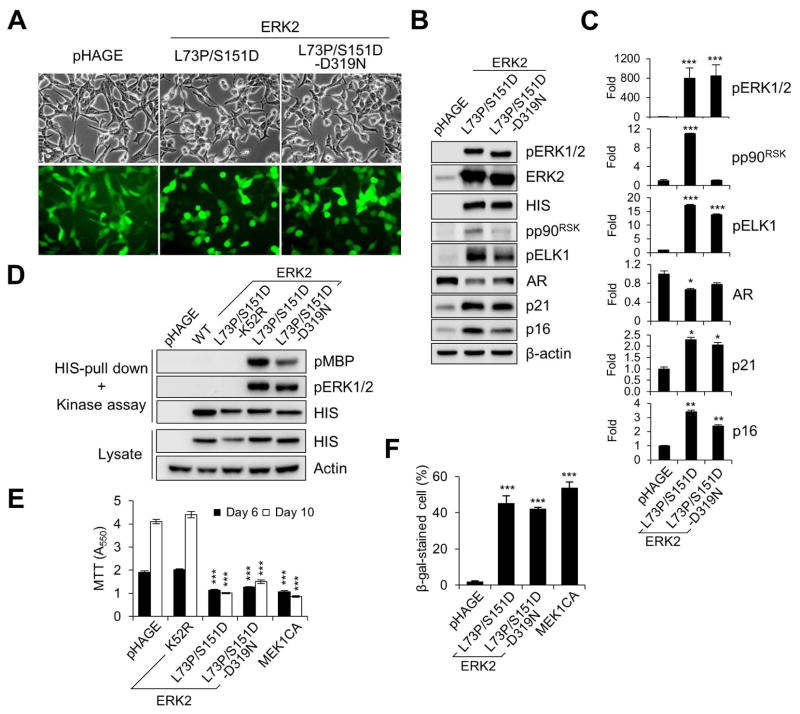
The sevenmaker gain-of-function mutation elevates ERK kinase activity by disrupting ERK interaction with its specific phosphatases [31, 32]. Introduction of this mutation (producing D319N) also increased kinase activity of bacterially expressed ERK2-L73P/S151D although it did not increase ERK2-L73P/S151D-induced c-fos luciferase reporter activity in HEK293 cells [9]. We determined whether the sevenmaker mutation could increase the activity of ERK2-L73P/S151D in LNCaP cells. When LNCaP cells were transduced with lentiviral ERK2-L73P/S151D and ERK2-L73P/S151D/D319N at similar infection efficiency (Fig. 3A), these ERK2 mutants were overexpressed at similar levels, as determined by Western blot detection of their N-terminal HIS tags (Fig. 3B). Unexpectedly, introduction of D319N did not increase phosphorylation of ERK2-L73P/S151D (Fig. 3B; densitometry in Fig. 3C) or its kinase activity, as determined by an in vitro kinase assay of the ERK mutants purified from LNCaP cells using myelin basic protein as the substrate (Fig. 3D). ERK2-L73P/S151D/D319N exhibited significantly decreased ability to phosphorylate p90RSK (Fig. 3B and 3C) while it induced similar or slightly reduced levels of morphological changes (Fig. 3A), ELK1 phosphorylation, downregulation of AR, and upregulation of p21CIP1 and p16INK4A (Fig. 3B and 3C), growth inhibition (Fig. 3E), and cell cycle arrest (Table 1). When expressed in LNCaP cells for prolonged periods, both ERK2-L73P/S151D and ERK2-L73P/S151D/D319N induced expression of senescence associated β-galactosidase activity at similar levels (Fig. 3F), which is a marker suggesting cellular senescence triggered upon aberrant activation of the MEK/ERK pathway [33]. Together, these data indicate that the sevenmaker mutation did not increase the activity of ERK2-L73P/S151D or its ability to mediate growth inhibitory signaling in LNCaP cells.
FIG. 3. Effects of sevenmaker mutation on ERK2-L73P/S151D signaling.
LNCaP cells were infected with lentivirus expressing wild type ERK2, ERK2-L73P/S151D, ERK2-L73P/S151D/D319N, ERK2-K52R/L73P/S151D, and MEK1CA. (A) Cells were observed for morphological changes at post-infection day 2. (B) Total cell lysates harvested at post-infection day 2 were analyzed by Western blotting for expression of the indicated proteins. (C) Densitometry of Western blot data in B. (D) In vitro kinase assay of HIS-tagged ERK2 isolated from cells. Kinase assay reaction and whole cell lysate were analyzed by Western blotting. Phosphorylation of the kinase substrate MBP (pMBP) indicates ERK catalytic activity. (E) Cell growth was monitored at post-infection days 6 and10 by MTT assay. (F) Senescence-associated acidic β-galactosidase activity was assayed at post-infection day 10. Data (means ± SE) are from a representative experiment performed in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t-Test).
ERK2-L73P/S151D can also induce PC12 differentiation
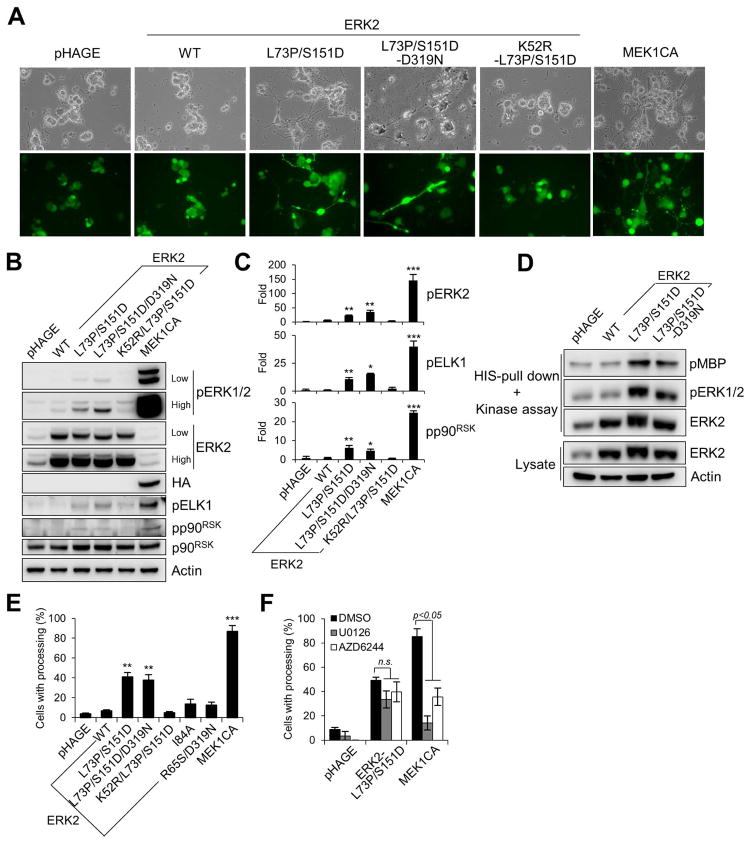
Neurite-like processing has been used as a key marker for PC12 differentiation induced by neuronal growth factor, for which the MEK/ERK pathway was an important effector [24, 25]. To investigate whether ERK2-L73P/S151D expression could be sufficient for PC12 differentiation, PC12 cells were transduced at a high efficiency by different lentiviral ERK2 constructs (Fig. 4A). Analysis of total cell lysates indicated that ERK2-L73P/S151D expression could mildly increase Western blot signals specific to phosphorylated TEY sites of ERK1/2, although the signal intensity was much lower than that induced by MEK1CA (Fig. 4B; densitometry in Fig. 4C). Unlike in LNCaP cells, phosphorylation of wild type ERK2 was more prominently detected in PC12 cells, possibly indicating the presence of higher basal MEK1/2 activity in the cell line. Nevertheless, when these exogenous ERK proteins were purified from PC12 cells and were analyzed by the in vitro kinase assay, ERK2-L73P/S151D exhibited higher levels of activation loop phosphorylation and kinase activity than wild type ERK2 (Fig. 4D).
FIG. 4. ERK2-L73P/S151D can sufficiently induce neurite-like processing in PC12 cells.
(A to E) PC12 cells were infected for 7 days with lentiviral wild type ERK2, ERK2-L73P/S151D, ERK2-L73P/S151D/D319N, ERK2-K52R/L73P/S151D, and MEK1CA. (A) Cells were observed for morphological changes. GFP expression indicates similar infection efficiency. (B) Total cell lysates were analyzed by Western blotting for expression of the indicated proteins. (C) Densitometry of Western blot signals for pERK2, pELK1, and pp90RSK in B. (D) In vitro kinase assay of HIS-tagged ERK2 isolated from cells. Kinase assay reaction and whole cell lysate were analyzed by Western blotting. Phosphorylation of the kinase substrate MBP (pMBP) indicates ERK catalytic activity. (E) Scores of neurite-like processing at post-infection day 7. (F) PC12 cells were infected for 7 days with lentiviral ERK2-L73P/S151D and MEK1CA in the presence of 10 μM U0126 or 40 nM AZD6244 before scoring neurite-like processing. Data (means ± SE) are from a representative experiment performed in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant (Student’s t-Test). Experiments were repeated at least 3 times with similar results.
In PC12 cells, ERK2-L73P/S151D expression increased phosphorylation of p90RSK and ELK1, albeit mild and substantially lower than the levels induced by MEK1CA (Fig. 4B; densitometry in Fig. 4C). Indeed, PC12 cells expressing ERK2-L73P/S151D developed neurite-like processing although the efficiency was about two-fold lower than cells expressing MEK1CA (Fig. 4E). However, the wild type ERK2 or the kinase-dead version of ERK2-L73P/S151D did not induce neurite-like processing while ERK2-I84A and ERK2-R65S/D319N induced the differentiation much less effectively than ERK2-L73P/S151D (Fig. 4E). Meanwhile, the sevenmaker mutation (D319N) did not significantly increase the activity of ERK2-L73P/S151D (Fig. 4B to 4D) or its ability to induce PC12 differentiation (Fig. 4A and 4E). Moreover, the MEK1/2-specific inhibitor U0126 and AZD6244 did not inhibit ERK2-L73P/S151D-induced PC12 differentiation as effectively as MEK1CA-induced differentiation (Fig. 4F). These data demonstrate that ERK2-L73P/S151D can sufficiently induce PC12 differentiation and suggest that ERK2-L73P/S151D functions in PC12 cells in a similar manner as seen in LNCaP cells.
D-domain/common docking site interaction is not necessary for ERK2-L73P/S151D to mediate LNCaP growth arrest and PC12 differentiation
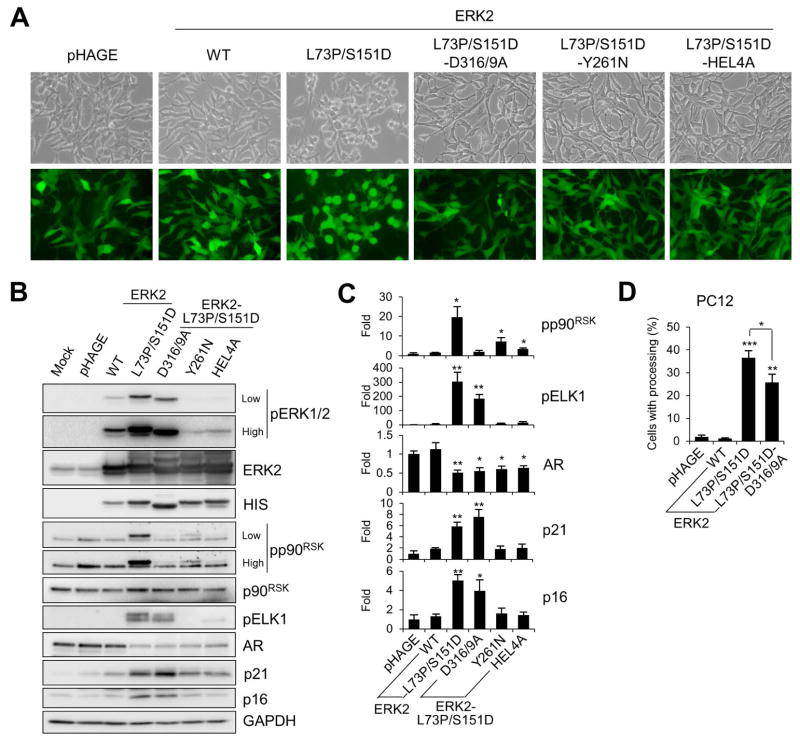
The specificity of ERK1/2 signaling is conferred by its specific physical interaction or subcellular localization. We investigated whether growth inhibitory effects of ERK2-L73P/S151D could be affected by any of the known mutations that hinder physical interactions or subcellular localization of EKR2, i.e., D316/9A, Y261N, and H176E/L4A, which impair D-domain/common docking site interaction, FXFP domain interaction, and ERK nuclear localization, respectively [34–36].
When LNCaP cells were transduced at similar efficiencies with the lentivirus that expresses ERK2-L73P/S151D containing D316/9A, Y261N, or H176E/L4A mutation (Fig. 5A), these ERK2 mutants were expressed at similar levels (Fig. 5B). We found that D316/9A mutation did not significantly affect autophosphorylation of ERK2-L73P/S151D whereas Y261N and H176E/L4A mutations substantially reduced the ability of ERK2-L73P/S151D to undergo autophosphorylation (Fig. 5B). Since this suggested that Y261N and H176E/L4A mutations, but not D316/9A, inactivated ERK2-L73P/S151D, further analysis was focused on D316/9A mutation. It was previously demonstrated that p90RSK interacts with ERK1/2 via its D-domain [37] whereas ELK1 interacts with ERK1/2 via its FXFP motif [38]. Consistent with this, D316/9A mutation inhibited ERK2-L73P/S151D-mediated p90RSK phosphorylation, but not ELK1 phosphorylation (Fig. 5B; densitometry in Fig. 5C), suggesting that D316/9A mutation specifically inhibited ERK2-L73P/S151D function mediated via the D-domain/common docking site interaction. Intriguingly, D316/9A mutation disabled the ability of ERK2-L73P/S151D to induce morphological changes in LNCaP cells (Fig. 5A). Nevertheless, D316/9A mutation did not significantly affect the ability of ERK2-L73P/S151D to downregulate AR or to upregulate p16INK4A and p21CIP1 in the cell line (Fig. 5B; densitometry in Fig. 5C). In PC12 cells, D316/9A mutation only mildly decreased the ability of ERK2-L73P/S151D to induce neurite-like processing (Fig. 5D). These data suggest that the D-domain/common docking site interaction is not necessary for ERK2-L73P/S151D to mediate LNCaP growth arrest and PC12 differentiation although it is necessary for ERK2-L73P/S151D-induced morphological changes in LNCaP cells.
FIG. 5. Effects of domain/motif-specific mutations on ERK2-L73P/S151D signaling.
(A to C) LNCaP cells were infected with lentivirus expressing wild type ERK2, ERK2-L73P/S151D, ERK2-L73P/S151D/D316/9A, ERK2-L73P/S151D/Y261N, and ERK2-L73P/S151D/HEL4A for 2 days. (A) Cells were observed for morphological changes. GFP expression indicates similar infection efficiency. (B) Total cell lysates harvested at post-infection day 2 were analyzed by Western blotting for expression of the indicated proteins. (C) Densitometry of Western blot data of B. (D) Scores of neurite-like processing in PC12 cells infected with lentiviral wild type ERK2, ERK2-L73P/S151D, and ERK2-L73P/S151D/D316/9A for 7 days. Data (means ± SE) are from a representative experiment performed in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t-Test). Experiments were repeated at least 3 times with similar results.
ELK1 is required for ERK2-L73P/S151D to mediate PC12 differentiation, but not LNCaP growth arrest
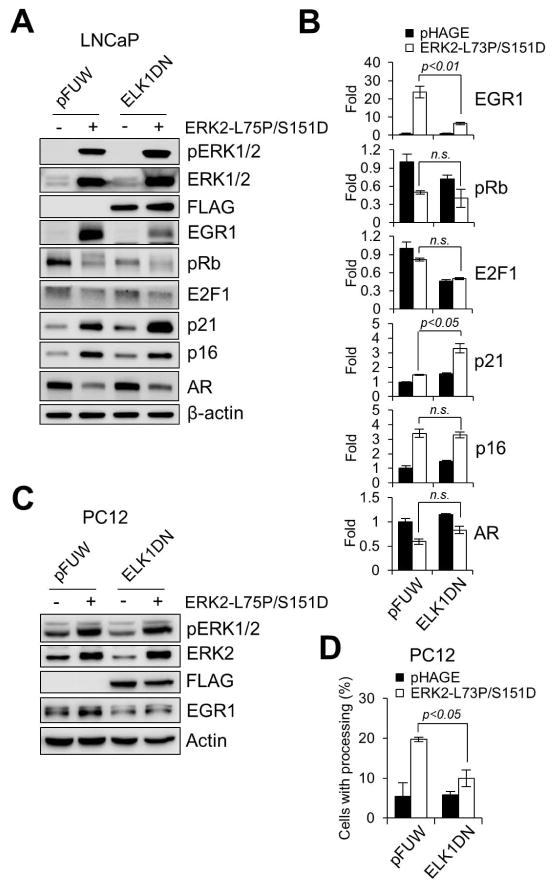
Due to the inability of ERK2-L73P/S151D/Y261N to undergo autophosphorylation, the significance of FXFP-based interaction could not be determined. We thus determined whether ELK1, a key effector interacting with ERK1/2 through the FXFP motif [38], was required for the effects of ERK2-L73P/S151D in LNCaP and PC12 cells using the dominant negative ELK1 construct, REST/Elk-1ΔC [39].
As predicted form the known EGR1 regulation by ELK1 in response to ERK1/2 activation [40], overexpression of REST/Elk-1ΔC substantially inhibited ERK2-L73P/S151D-induced expression of the transcription factor EGR1 in LNCaP cells (Fig. 6A; densitometry in Fig. 6B). Nevertheless, ERK2-L73P/S151D still induced downregulation of phosphorylated Rb and AR and upregulation of p16INK4A and p21CIP1 (Fig. 6A and 6B), suggesting that ELK1 activation is not necessary for ERK2-L73P/S151D to mediate growth arrest signaling in LNCaP cells. In contrast, REST/Elk-1ΔC overexpression significantly inhibited both EGR1 expression and neurite-like processing induced by ERK2-L73P/S151D in PC12 cells (Fig. 6C and 6D), suggesting that ELK1 is necessary for the PC12 differentiation. These data demonstrate that ERK2-L73P/S151D can utilize different downstream effectors in a context-dependent manner.
FIG. 6. Effects of dominant negative ELK1 on ERK2-L73P/S151D signaling.
LNCaP and PC12 cells were coinfected with lentiviruses expressing ERK2-L73P/S151D and the dominant negative ELK1, REST/Elk-1ΔC (ELK1DN). Empty pHAGE and pFUW are the controls for ERK2-L73P/S151D and ELK1DN, respectively. (A) Total lysates of LNCaP cells infected for 2 days were analyzed by Western blotting. (B) Densitometry of Western blot data in A. (C) Total lysates of PC12 cells infected for 3 days were analyzed by Western blotting. (D) Scores of neurite-like processing from PC12 cells at post-infection day 5. Data (means ± SE) are from a representative experiment performed in triplicate. P values were determined by Student’s t-Test. n.s., not significant.
DISCUSSION
A number of evidence has demonstrated the pivotal role of ERK1/2 as the focal point of the Raf/MEK/ERK pathway. For example, it has been consistently demonstrated that ERK1/2 is necessary for the pathway signaling in a variety of biological contexts including cell proliferation [41–43] as well as growth arrest [21, 44]. Nevertheless, the evidence for sufficiency of ERK1/2 activation for Raf/MEK/ERK signaling has been limited. Although a seminary study demonstrated that a constitutively active MEK-ERK chimera can sufficiently induce neurite-like processing of PC12 cells and NIH3T3 transformation [8], MEK-dependent nature of the reagent limits determination of ERK sufficiency in the signaling. Our demonstration of the ability of ERK2-L73P/S151D to reproduce most, although not entire, growth inhibitory effects of MEK1CA in LNCaP and PC12 cells provides strong evidence in support of the sufficiency of ERK1/2 activation for pathway signaling.
Although known as Ser/Thr kinase, ERK2 can autophosphorylate its Tyr185 but not Thr183 [6–8]. Tyr185 is proximal to the phosphoryl transfer residue Lys52 in the active site whereas Thr183 faces away from the active site, which may account for Tyr185 autophosphorylation [2]. While Tyr185 phosphorylation is critical in inducing active-conformational change of ERK, subsequent Thr183 phosphorylation disrupts hydrogen bonding around Asp334, thus locking the kinase into the active conformation [45]. Therefore, phosphorylation of both residues is important to achieve maximal ERK activity in cells [2, 8, 45]. As previously addressed [9], low catalytic activity of ERK2-L73P/S151D adheres to this context because it is mainly in mono-phosphorylated state. Nevertheless, despite its low activity, our data demonstrate that ERK2-L73P/S151D can sufficiently induce growth arrest and differentiation, proposing that high magnitude pathway activation is not necessary for induction of those physiological outputs. Intriguingly, a recent study proposed that mono-phosphorylated ERK2, on Thr of the TEY site, has significance under certain pathophysiological context [46]. Therefore, it may be possible that mono- versus di-phosphorylation status also determines the specificity of EKR1/2 signaling.
Of note, we previously demonstrated that active site-, but not activation loop-, disabled ERK mutants could selectively restore growth inhibitory signaling in response to Raf/MEK activation in LNCaP cells in which ERK1/2 were partially depleted [21]. These restored responses did not include Raf/MEK-induced c-MYC downregulation, suggesting that the regulation may require high catalytic activity of ERK1/2. Since ERK2-L73P/S151D could not induce c-MYC downregulation, these two results are consistent in that they propose an involvement of multiple thresholds of ERK activity in growth inhibitory signaling. Because generation of a kinase-dead ERK2 mutant in active conformation is currently not possible, there is a limit in addressing whether ERK2-L73P/S151D mediates its effects via its low but sustained kinase activity or its physical structure in active conformation independently of its kinase activity. Nevertheless, our observations further elaborate the general notion that depending upon the magnitude and the duration of activation, the Raf/MEK/ERK pathway can mediate a variety of physiological outputs [12–15].
The diversity of signaling outputs relative to the simplicity of ERK1/2 substrate signature (S/T-P) has led to the identification of different domain/motifs specific to the physical interaction between ERK1/2 and their substrates, regulators, and scaffolds [12, 14]. The D-domain/common docking site interaction is a major mechanism in this regard. The D316/9A mutation that inhibits these interactions did not affect the ability of ERK2-L73P/S151D to mediate LNCaP growth arrest and PC12 differentiation signaling, although it disabled the ability of ERK2-L73P/S151D to mediate p90RSK phosphorylation and morphology changes of LNCaP cells. Similar effects were observed with D319N, which also alters the common docking site of ERK2. Therefore, it appears that the D-domain/common docking site interaction is not necessary for relaying ERK1/2 signaling toward growth arrest in LNCaP and differentiation in PC12 cells. Since the D-domain/common docking site interaction is required for ERK1/2 interaction with MEK1/2 [47], these data also support the notion that ERK2-L73P/S151D activity was not mainly driven by basal MEK1/2 activity in our model cell lines, which is corroborated by the effects of MEK1/2 inhibitors.
Although ERK2-L73P/S151D was also evaluated in different studies, its sufficiency for ERK signaling was not clearly demonstrated [48, 49]. Different cell types maintain different basal MEK1/2 activity to which ERK2-L73P/S151D can respond. Accordingly, the degree of basal MEK1/2 activity should be considered for the evaluation of ERK2-L73P/S151D effect in a cell type. Based upon our observation that autophosphorylated ERK2-L73P/S151D can sufficiently mediate most, although not entire, growth inhibitory effects of MEK1CA in LNCaP and PC12 cells, we conclude that ERK1/2 activation is sufficient for the Raf/MEK/ERK pathway to mediate growth arrest and differentiation signaling. Our established system may provide a platform to further address the underlying molecular mechanisms.
MATERIALS AND METHODS
Cell culture, growth curve, senescence-associated β-galactosidase staining, and neurite-like processing assay
The human prostate carcinoma line LNCaP (ATCC) was maintained in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 U of penicillin and 100 μg of streptomycin per ml. PC12 (ATCC) was maintained in Dulbeco’s Modified Eagles Medium (Invitrogen) supplemented with 10% horse serum and 5% fetal bovine serum. The LNCaP line stably expressing ΔRaf-1:ER (LNCaP-Raf:ER) was previously described [21]. For cell growth curve, LNCaP cells were seeded in 24-well plates (Corning, Corning, NY) at a density of 5 × 103 cells per well. Cell proliferation was monitored by counting cell numbers every 2 days using hemocytometer or by the colorimetric 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide assay, as described previously [23]. Senescence-associated acidic β-galactosidase was stained using the SA β-gal staining kit (Sigma, St. Louis, MO) according to the manufacturer’s instructions. To score neurite-like processing, PC12 cells were seeded in 24-well plates at a density of 1 × 103 cells per well and were infected with virus for 72 h. Formation of neurite-like processing was determined as previously described [24, 25].
Plasmids and recombinant viruses
Genes encoding rat wild type ERK2, ERK2-L73P/S151D, and ERK2-I84A (obtained from N. Ahn, HHMI) were subcloned into EcoRI/XbaI sites of pBluescript SK (−). To generate ERK2-R65S/D319N, rat wild type ERK2 in pBluescript were serially mutagenized using CAGACCTACTGTCAGAGTACCCTGAGAGAG and CTCTCTCAGGGTACTCTGACAGTAGGTCTG (for R65S), and then GCAGTATTATGACCCAAGTAATGAGCCCATTGCTGAAGC and GCTTCAGCAATGGGCTCATTACTTGGGTCATAATACTGC (for D319N). To generate ERK2-K52R/L73P/S151D and ERK2-L73P/S151D/D316A/D319A, ERK2-L73P/S151D in pBluescript was mutagenized using GTTCGAGTTGCTATCCGGAAAATCAGTCCTTTTG and CAAAAGGACTGATTTTCCGGATAGCAACTCGAAC (for K52R), and TGGAGCAGTATTATGCCCCAAGTGCTGAGCCCATTGCTG and CAGCAATGGGCTCAGCACTTGGGGCATAATACTGCTCCA (for D316A/D319A), respectively. To generate ERK2-L73P/S151D-HEL4A, MluI/XbaI-cut fragment of pBluscript-GFP-ERK2-L73P/S151D was switched with the corresponding gene fragment of ERK2-L4A in pCMV vector (obtained from M. Cobb, UT Southwestern). The resulting pBluscript-GFP-ERK2-L73P/S151D/L4A was then mutagenized to introduce H176E using GTTGCAGATCCAGACGAGGATCATACAGGGTTC and GAACCCTGTATGATCCTCGTCTGGATCTGCAAC. These ERK2 mutants in pBluescript were then ligated to XhoI/XbaI site of pHAGE-GFP to generate lentiviral constructs. To generate pHAGE-ERK2-L73P/S151D/Y261N, MluI/XbaI-cut fragment of pHAGE-GFP-ERK2-L73P/S151D was switched with the corresponding gene fragment of ERK2-Y261N in pCMV (obtained from M. Cobb, UT Southwestern). pHAGE-ERK2-L73P/S151D/D319N was generated by mutagenizing pHAGE-ERK2-L73P/S151D with GCAGTATTATGACCCAAGTAATGAGCCCATTGCTGAAGC AND GCTTCAGCAATGGGCTCATTACTTGGGTCATAATACTGC. Generation of lentiviral pHAGE-MEK1CA expressing MEK1-R4F (ΔN3/S218E/S222D) was previously described [21]. QuikChange Site-Directed Mutagenesis Kit (Stratagene, CA) was used for mutagenesis. pFUW-REST/Elk-1ΔC was obtained from G. Thiel (Univ. Saarand Medical Center, Germany). REST/Elk-1ΔC is a fusion protein that consist of ELK1 lacking the activation domain, but retaining the DNA- and serum response factor-binding domains, and the transcriptional repression domain of REST [40].
Viral infection
Lentivirus was produced by co-transfecting 293T cells with the lentiviral expression vector pHAGE and packaging vectors, as previously described [21]. The resulting supernatant was collected after 48–72 h. For infection, lentiviral supernatant was mixed with polybrene (Sigma) at 8 μg/ml. Viral titers were determined by infecting recipient cells with serially diluted viral supernatants and scoring cells expressing GFP at 48 h post-infection. Cells were switched into fresh culture medium on the following day of infection.
Cell cycle analysis
Cell cycle profile was analyzed as previously described [21]. Briefly, cells were washed with ice-cold 0.2% bovine serum albumin in phosphate-buffered saline, resuspended in 250 mM sucrose/40 mM citrate buffer (pH 7.6) containing 0.5% DMSO. Nuclei were prepared, stained with propidium iodide, and analyzed by LSR II flow cytometer (Becton Dickinson, Franklin Lakes, N.J.) with a gate that selects single nuclei within a normal size range. The cell cycle parameters from 10,000 gated nuclei were determined, and subsequent analysis was conducted using FCS Express software (De Novo Software, Los Angeles, CA).
Luciferase reporter assay of serum responsive element (SRE)
LNCaP cells were cotransfected with the pTAL-Luc vector harboring SRE (Clontech, Mountain View, CA) and pRL-TK (Promega, Madison, WI) using Lipofectamine 2000 (Invitrogen). Cells were then seeded in triplicate in 24-well plates and infected with lentivirus. Cell lysates harvested at post-infection day 2 were analyzed for fire fly and renilla luciferase activity using Dual-Glo™ Luciferase Assay System (Promega) according to the manufacturer’s instruction.
In vitro kinase assay
Cells infected with lentiviral ERK2 constructs were lysed in 50 mM Tris (pH 7.5) containing 150 mM NaCl and 1% NP-40. HIS-tagged ERK2 was purified from 200 μg cell lysates using Ni-sepharose (GE Healthcare Bio-Sciences, Piscataway, NJ). Kinase activity of HIS-ERK2 was determined using the MAP kinase assay kit (Millipore, Billerica, MA). Briefly, HIS-ERK2 was incubated in the assay buffer containing Mg2+, ATP and myelin basic protein at 30°C for 30 min. Phosphorylated myelin basic protein was then detected by Western blotting.
Immunoblot analysis
Cells harvested at various times were lysed in 62.5 mM Tris (pH 6.8)-2% SDS mixed with the protease inhibitor cocktail (Sigma) that contains 4-(2-aminoethyl) benzenesulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, and aprotinin, and briefly sonicated before determining the protein concentration using the BCA reagent (Pierce, Rockford, IL). 50 μg of protein was resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane filter (Millipore, Billerica, MA), and stained with Fast Green reagent (Fisher Scientific, Pittsburgh, PA). Membrane filters were then blocked in 0.1 M Tris (pH 7.5)-0.9% NaCl-0.05% Tween 20 with 5% nonfat dry milk, and incubated with appropriate antibodies. Antibodies were diluted as follows: phospho-MEK1/2 (Ser217/221 for MEK1 and Ser222/226 for MEK2), 1:2,500; phospho-ERK1/2 (Thr202/Tyr204 for ERK1 and Thr183/Tyr185 for ERK2), 1:2,500; ERK1/2, 1:2,500; phospho-ERK1/2 (Thr202/Tyr204), 1:2,500; p90RSK, 1:2,500; phospho-p90RSK (Thr359/Ser363), 1:2,500; phspho-ELK1 (Ser383), 1:2,000; AR, 1:2,500; GAPDH, 1:5,000 (Cell Signaling, Boston, MA); E2F1, 1:1,000; c-MYC, 1:1,000; PARP, 1:1,000 (Thermo Fisher Scientific, Waltham, MA); p21CIP1, 1:1,000; cyclin b1, 1:2,000; HA, 1:2,500; HIS, 1:2,500 (Santa Cruz Biotech, Santa Cruz, CA); Rb, 1:1,000; p16INK4A, 1:2,000 (BD Bioscience, San Jose, CA). The Supersignal West Pico and Femto chemiluminescence kits (Pierce) were used for visualization of the signal. For densitometry, immunoblots were scanned and analyzed using Image Lab (BioRad, Hercules, CA).
Acknowledgments
We thank Natalie Ahn and Melanie Cobb for MEK and ERK genes, Gerald Thiel for pFUW-REST/Elk-1ΔC, Richard Mulligan for lentiviral pHAGE vector, and Cas Moeling for technical assistance. This work was supported by the National Cancer Institute (R01CA138441) and American Cancer Society (RSGM-10-189-01-TBE) to J.I.P.
Abbreviations
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- MEK
mitogen-activated protein kinase kinase
Footnotes
AUTHOR CONTRIBUTIONS
JP designed and supervised the study. SH, PW, SY, and JP performed experiments and analyzed the data. PW and JP prepared the manuscript.
References
- 1.Xiao Y, Lee T, Latham MP, Warner LR, Tanimoto A, Pardi A, Ahn NG. Phosphorylation releases constraints to domain motion in ERK2. Proc Natl Acad Sci U S A. 2014;111:2506–2511. doi: 10.1073/pnas.1318899111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang F, Strand A, Robbins D, Cobb MH, Goldsmith EJ. Atomic structure of the MAP kinase ERK2 at 2.3 A resolution. Nature. 1994;367:704–711. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]
- 3.Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90:859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda M, Gotoh Y, Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. Embo J. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Askari N, Diskin R, Avitzour M, Yaakov G, Livnah O, Engelberg D. MAP-quest: could we produce constitutively active variants of MAP kinases? Mol Cell Endocrinol. 2006;252:231–240. doi: 10.1016/j.mce.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Seger R, Ahn NG, Boulton TG, Yancopoulos GD, Panayotatos N, Radziejewska E, Ericsson L, Bratlien RL, Cobb MH, Krebs EG. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991;88:6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossomando AJ, Wu J, Michel H, Shabanowitz J, Hunt DF, Weber MJ, Sturgill TW. Identification of Tyr-185 as the site of tyrosine autophosphorylation of recombinant mitogen-activated protein kinase p42mapk. Proc Natl Acad Sci U S A. 1992;89:5779–5783. doi: 10.1073/pnas.89.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- 9.Emrick MA, Hoofnagle AN, Miller AS, Ten Eyck LF, Ahn NG. Constitutive activation of extracellular signal-regulated kinase 2 by synergistic point mutations. J Biol Chem. 2001;276:46469–46479. doi: 10.1074/jbc.M107708200. [DOI] [PubMed] [Google Scholar]
- 10.Emrick MA, Lee T, Starkey PJ, Mumby MC, Resing KA, Ahn NG. The gatekeeper residue controls autoactivation of ERK2 via a pathway of intramolecular connectivity. Proc Natl Acad Sci U S A. 2006;103:18101–18106. doi: 10.1073/pnas.0608849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin-Salomon V, Kogan K, Ahn NG, Livnah O, Engelberg D. Isolation of intrinsically active (MEK- independent) variants of the ERK family of map kinases. J Biol Chem. 2008;283:34500–34510. doi: 10.1074/jbc.M806443200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Wortzel I, Seger R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 16.Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Park JI. Growth arrest signaling of the Raf/MEK/ERK pathway in cancer. Front Biol (Beijing) 2014;9:95–103. doi: 10.1007/s11515-014-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 20.Hong SK, Kim JH, Lin MF, Park JI. The Raf/MEK/extracellular signal-regulated kinase 1/2 pathway can mediate growth inhibitory and differentiation signaling via androgen receptor downregulation in prostate cancer cells. Exp Cell Res. 2011;317:2671–2682. doi: 10.1016/j.yexcr.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong SK, Yoon S, Moelling C, Arthan D, Park JI. Noncatalytic function of ERK1/2 can promote Raf/MEK/ERK-mediated growth arrest signaling. J Biol Chem. 2009;284:33006–33018. doi: 10.1074/jbc.M109.012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravi RK, McMahon M, Yangang Z, Williams JR, Dillehay LE, Nelkin BD, Mabry M. Raf-1-induced cell cycle arrest in LNCaP human prostate cancer cells. J Cell Biochem. 1999;72:458–469. doi: 10.1002/(sici)1097-4644(19990315)72:4<458::aid-jcb2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Wu PK, Hong SK, Veeranki S, Karkhanis M, Starenki D, Plaza JA, Park JI. A Mortalin/HSPA9-Mediated Switch in Tumor-Suppressive Signaling of Raf/MEK/Extracellular Signal-Regulated Kinase. Mol Cell Biol. 2013;33:4051–4067. doi: 10.1128/MCB.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 25.Wood KW, Qi H, D’Arcangelo G, Armstrong RC, Roberts TM, Halegoua S. The cytoplasmic raf oncogene induces a neuronal phenotype in PC12 cells: a potential role for cellular raf kinases in neuronal growth factor signal transduction. Proc Natl Acad Sci U S A. 1993;90:5016–5020. doi: 10.1073/pnas.90.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazar DF, Wiese RJ, Brady MJ, Mastick CC, Waters SB, Yamauchi K, Pessin JE, Cuatrecasas P, Saltiel AR. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- 27.Kortenjann M, Thomae O, Shaw PE. Inhibition of v-raf-dependent c-fos expression and transformation by a kinase-defective mutant of the mitogen-activated protein kinase Erk2. Mol Cell Biol. 1994;14:4815–4824. doi: 10.1128/mcb.14.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal. 2008;6:e001. doi: 10.1621/nrs.06001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dang CV, O’Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–264. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Takizawa CG, Morgan DO. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr Opin Cell Biol. 2000;12:658–665. doi: 10.1016/s0955-0674(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 31.Bott CM, Thorneycroft SG, Marshall CJ. The sevenmaker gain-of-function mutation in p42 MAP kinase leads to enhanced signalling and reduced sensitivity to dual specificity phosphatase action. FEBS Lett. 1994;352:201–205. doi: 10.1016/0014-5793(94)00958-9. [DOI] [PubMed] [Google Scholar]
- 32.Chu Y, Solski PA, Khosravi-Far R, Der CJ, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–6501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 33.Deschenes-Simard X, Gaumont-Leclerc MF, Bourdeau V, Lessard F, Moiseeva O, Forest V, Igelmann S, Mallette FA, Saba-El-Leil MK, Meloche S, Saad F, Mes-Masson AM, Ferbeyre G. Tumor suppressor activity of the ERK/MAPK pathway by promoting selective protein degradation. Genes Dev. 2013;27:900–915. doi: 10.1101/gad.203984.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitehurst AW, Robinson FL, Moore MS, Cobb MH. The death effector domain protein PEA-15 prevents nuclear entry of ERK2 by inhibiting required interactions. J Biol Chem. 2004;279:12840–12847. doi: 10.1074/jbc.M310031200. [DOI] [PubMed] [Google Scholar]
- 35.Yazicioglu MN, Goad DL, Ranganathan A, Whitehurst AW, Goldsmith EJ, Cobb MH. Mutations in ERK2 binding sites affect nuclear entry. J Biol Chem. 2007;282:28759–28767. doi: 10.1074/jbc.M703460200. [DOI] [PubMed] [Google Scholar]
- 36.Lidke DS, Huang F, Post JN, Rieger B, Wilsbacher J, Thomas JL, Pouyssegur J, Jovin TM, Lenormand P. ERK nuclear translocation is dimerization-independent but controlled by the rate of phosphorylation. J Biol Chem. 2010;285:3092–3102. doi: 10.1074/jbc.M109.064972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gavin AC, Nebreda AR. A MAP kinase docking site is required for phosphorylation and activation of p90(rsk)/MAPKAP kinase-1. Curr Biol. 1999;9:281–284. doi: 10.1016/s0960-9822(99)80120-1. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 1999;13:163–175. [PMC free article] [PubMed] [Google Scholar]
- 39.Stefano L, Al Sarraj J, Rossler OG, Vinson C, Thiel G. Up-regulation of tyrosine hydroxylase gene transcription by tetradecanoylphorbol acetate is mediated by the transcription factors Ets-like protein-1 (Elk-1) and Egr-1. J Neurochem. 2006;97:92–104. doi: 10.1111/j.1471-4159.2006.03749.x. [DOI] [PubMed] [Google Scholar]
- 40.Mayer SI, Thiel G. Calcium influx into MIN6 insulinoma cells induces expression of Egr-1 involving extracellular signal-regulated protein kinase and the transcription factors Elk-1 and CREB. Eur J Cell Biol. 2009;88:19–33. doi: 10.1016/j.ejcb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Lefloch R, Pouyssegur J, Lenormand P. Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels. Mol Cell Biol. 2008;28:511–527. doi: 10.1128/MCB.00800-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vantaggiato C, Formentini I, Bondanza A, Bonini C, Naldini L, Brambilla R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J Biol. 2006;5:14. doi: 10.1186/jbiol38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voisin L, Julien C, Duhamel S, Gopalbhai K, Claveau I, Saba-El-Leil MK, Rodrigue-Gervais IG, Gaboury L, Lamarre D, Basik M, Meloche S. Activation of MEK1 or MEK2 isoform is sufficient to fully transform intestinal epithelial cells and induce the formation of metastatic tumors. BMC Cancer. 2008;8:337. doi: 10.1186/1471-2407-8-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guegan JP, Ezan F, Gailhouste L, Langouet S, Baffet G. MEK1/2 overactivation can promote growth arrest by mediating ERK1/2-dependent phosphorylation of p70S6K. J Cell Physiol. 2014;229:903–915. doi: 10.1002/jcp.24521. [DOI] [PubMed] [Google Scholar]
- 45.Barr D, Oashi T, Burkhard K, Lucius S, Samadani R, Zhang J, Shapiro P, MacKerell AD, van der Vaart A. Importance of domain closure for the autoactivation of ERK2. Biochemistry. 2011;50:8038–8048. doi: 10.1021/bi200503a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan AC, Deb-Basu D, Orban MW, Gotlib JR, Natkunam Y, O’Neill R, Padua RA, Xu L, Taketa D, Shirer AE, Beer S, Yee AX, Voehringer DW, Felsher DW. Nanofluidic proteomic assay for serial analysis of oncoprotein activation in clinical specimens. Nat Med. 2009;15:566–571. doi: 10.1038/nm.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanoue T, Nishida E. Molecular recognitions in the MAP kinase cascades. Cell Signal. 2003;15:455–462. doi: 10.1016/s0898-6568(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 48.Gaumont-Leclerc MF, Mukhopadhyay UK, Goumard S, Ferbeyre G. PEA-15 is inhibited by adenovirus E1A and plays a role in ERK nuclear export and Ras-induced senescence. J Biol Chem. 2004;279:46802–46809. doi: 10.1074/jbc.M403893200. [DOI] [PubMed] [Google Scholar]
- 49.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]