Abstract
Objective
To determine the effect of weight gain on progression of early knee morphologic abnormalities using magnetic resonance imaging (MRI) in a longitudinal study over 48 months.
Design
We studied the right knee of 100 subjects from the Osteoarthritis Initiative, selecting subjects aged ≥ 45 with osteoarthritis risk factors who demonstrated weight gain (minimum 5% increase in body mass index, BMI, n=50) or no change in weight (BMI change < 2%, n=50), frequency matched for age, gender, and baseline BMI. Baseline and 48 month knee MRI studies were scored for lesions using a modified whole organ MRI score (WORMS). Logistic regression models were used to compare the differences between the two groups.
Results
The odds of worsening maximum cartilage (11.3, 95%, CI 3.5–51.4) and meniscal WORMS (4.5, 95% CI 1.4–17.3) were significantly greater in the weight gain group compared to the no change group, in addition to the odds of worsening cartilage defects at the patella and average meniscal WORMS (p<0.05). Odds of worsening average bone marrow edema pattern (BMEP) were significantly greater for the weight gain group compared to the no change cohort (p<0.05).
Conclusion
Our study demonstrated that weight gain is strongly associated with increased progression of cartilage degeneration in middle-aged individuals with risk factors for osteoarthritis.
Keywords: weight gain, cartilage degeneration, whole organ MRI score, osteoarthritis
Introduction
Osteoarthritis (OA) is the most common disease of joints and a leading cause of disability in the United States, affecting more than 27 million adults and over 35% of the population over the age of 65.[1, 2] The associated health care expenditures are enormous with costs of over $185 billion dollars annually and the incidence is rising, particularly as a function of both the aging population and the obesity epidemic.[3,4] First-line therapy for OA focuses on slowing disease progression and controlling symptoms with a combination approach that may include exercise, weight loss, physical therapy, nonsteroidal anti-inflammatory drugs, and/or intra-articular corticosteroid injections.[5] For many patients, however, total joint arthroplasty is eventually required despite faithful adherence to more conservative measures. The importance of prevention and disease modification is paramount.
Obesity is one of the main risk factors for OA. Previous studies have shown that there is a 60% lifetime risk of developing symptomatic knee OA in obese patients compared with 45% in the general population.[6] Excessive weight gain exacerbates joint degeneration by increasing joint loading, altering normal kinetics, and impairing regulatory pathways that maintain cartilage homeostasis.[4, 7] Weight-loss is an attractive, cost-effective measure to potentially slow and prevent the development of OA, but the precise relationship between weight loss and the pathophysiology of OA has not been well-established. Similarly, only a few studies have explored the specific detrimental effects of weight gain in terms of progression of irreversible morphologic abnormalities, particularly in articular cartilage.[7–9] Teichtahl et al, for example, demonstrated that in the subset of individuals with medial meniscal tears, 1% weight gain was associated with 0.2% increased loss of medial tibial cartilage volume, over the course of two years.
The diagnosis and clinical follow-up of patients with OA relies heavily on imaging with conventional radiography, which utilizes joint space as a surrogate for hyaline cartilage degeneration in the early stages of the disease.[10, 11] However, magnetic resonance imaging (MRI) has been shown in recent years to be more sensitive to subtle morphologic abnormalities within joints, and can provide direct high-resolution imaging of cartilage, meniscus, bone marrow and ligament degeneration, which represent the earliest structural changes in patients with OA.[12–16]
The Osteoarthritis Initiative (OAI) is a multicenter, longitudinal, prospective observational study of knee OA, sponsored by the National Institutes of Health that combines clinical, serologic, and joint imaging data obtained annually from 4,796 individuals between 45 and 79 years of age over a period of 8 years (online at http://www.oai.ucsf.edu). The purpose of this study was to examine the effect of weight gain on the progression of knee morphologic abnormalities in asymptomatic individuals aged 45 and older, with risk factors for OA, using serial 3T MRI performed over a period of 48 months as part of the OAI. We hypothesized that knees of individuals who gained weight would be more likely to have worsening of focal knee morphological abnormalities, particularly cartilage degradation, compared to those who did not gain weight.
Methods
Subjects
The study protocol, amendments, and informed consent documentation were approved by local institutional review boards. The study was compliant with the Health Insurance Portability and Accountability Act and all subjects provided informed consent.
Data used in the preparation of this manuscript was obtained from the publically available Osteoarthritis Initiative (OAI) database (http://www.oai.ucsf.edu/). The study population of the OAI includes subjects with symptomatic knee OA at baseline (progression cohort), those with no symptomatic knee OA but with risk factors for OA at baseline (incidence cohort), and normal controls. The specific OAI datasets used for this study were the baseline clinical dataset 0.2.2, baseline imaging datasets 0.E.1 and 0.C.2, the 48 month follow-up clinical dataset 6.2.1, and the 48 month follow-up imaging datasets 6.E.1.
We studied the right knee of 100 subjects from the OAI incidence cohort. The OAI performed scans of both knees in the subjects studied but time constraints prevented performing the full complement of sequences for both knees. A full complement was not obtained for the left knee. Right knees demonstrate a higher frequency of radiologic manifestations of osteoarthritis compared to left knees.[17] The right knee was chosen because the full imaging complement was available. Subjects in the OAI incidence cohort (n=3284) did not have symptomatic knee OA (defined as frequent symptoms and radiographic OA in the same knee) in either knee at baseline, but had at least one of the following OA risk factors at baseline: overweight or obesity, knee symptoms (“pain, aching, or stiffness in or around the knee” in the past 12 months), history of knee injury, history of knee surgery, family history of total knee replacement or Heberden nodes. Specific inclusion criteria for the present study included individuals with either a minimum 5% increase in BMI or less than 2% change in BMI over 48 months. Since there was minimal change in height over 48 months, changes in BMI represented changes in weight. We chose 5% as the minimum threshold for weight gain because recent studies have demonstrated that this amount of weight gain was associated with adverse effects on knee symptoms.[18] We hypothesized that early morphologic abnormalities seen on MR might also be seen with a 5% increase in weight. The 2% restriction was chosen for the no change group in order to be as restrictive as possible while establishing a cohort of subjects who met inclusion criteria and which was relatively comparable in size to those that met the weight gain inclusion criteria. There were 520 and 808 individuals in the incidence cohort who met the respective weight change criteria. Specific exclusion criteria included individuals with fluctuations in BMI over the 4 year period, i.e., individuals who did not consistently increase or maintain BMI over the 4 years. For example, for individuals with >5% weight gain between 0 and 48 months if there was a net weight loss between 0 and 12, 12 and 24, 24 and 36, or 36 and 48 months, they were excluded from the study. Similarly, for individuals with less than 2% change in weight over 48 months, if there was > 2% change in weight during annual follow-up intervals, those individuals were excluded. The two groups were denoted weight gain (WG) and no change (NC). A random sample of 50 individuals with 5% increase in BMI and available PASE scores was selected to compose a weight gain (WG) group. A sample of 50 individuals each with less than 2% change in BMI was selected and frequency matched to the WG group for age, gender, and baseline BMI.
Kellgren-Lawrence (KL) scores from knee radiographs at baseline were obtained from the OAI database for all subjects in our study and included in our analyses. Similarly, since physical activity may also be an independent risk factor for development of focal knee abnormalities we used data derived from the physical active score for the elderly (PASE) in our analyses. Subjects who did not have a minimum of four of five possible reported yearly PASE scores (baseline, 12 months, 24 months, 36 months, 48 months) were excluded.
Imaging
MR images for all subjects were obtained using four identical 3.0 Tesla (Siemens Magnetom Trio, Erlangen, Germany) scanners and quadrature transmit-receive coils (USA Instruments, Aurora, OH, USA) at four sites (The Ohio State University, Columbus, OH; University of Maryland, School of Medicine, Baltimore, MD; University of Pittsburgh, Pittsburgh, PA; and Memorial Hospital of Rhode Island, Pawtucket, RI). The following sequences were acquired and used for image analysis: sagittal 3D dual-echo in steady state (DESS) sequence (TR/TE = 16.3/4.7 ms, spatial resolution = 0.365mm × 0.456mm, slice thickness = 0.7mm, flip angle 25°, bandwidth 185 Hz/pixel), sagittal 2D intermediate-weighted (IW) fast spin-echo (FSE) sequence with fat saturation (TR/TE = 3200/30 ms, spatial resolution = 0.357mm × 0.511mm, slice thickness = 3.0mm, flip angle 180°, bandwidth 248 Hz/pixel), coronal 3D fast low angle shot (FLASH) sequence with selective water excitation (WE, TR/TE = 20/7.57 ms, spatial resolution = 0.313mm × 0.313mm, slice thickness = 1.5mm), and coronal 2D IW FSE sequence (TR/TE = 3700/29 ms, spatial resolution = 0.365mm × 0.456mm, slice thickness = 3.0mm, flip angle 12°, bandwidth 352 Hz/pixel). Detailed information about the sequences is available in the OAI MR protocol.[17]
Image Analysis
Baseline and 48 month follow-up MR images of the right knee were transferred to a picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ, USA). Baseline and follow-up images were reviewed side-by-side. Using the sequences listed above, the presence and grade of meniscal, cartilage and ligamentous lesions as well as bone marrow edema pattern (BMEP) were assessed using a modified whole organ MRI score (WORMS) as previously described and presented in more detail below.[20]
A randomised list of IDs for subjects meeting inclusion criteria were submitted by OAI staff to the study authors. Two radiologists at the local OAI site (M.B. with five years, L.N. with six years in musculoskeletal imaging, respectively) then analysed the MRI studies independently. In any cases where the modified WORMS gradings were not identical, a consensus reading was performed with a third, more experienced radiologist (T.M.L., 23 years of experience in musculoskeletal imaging). The radiologists were blinded to patient information while performing the WORMS grading.
WORMS Grading of the Knee
Meniscal morphology was assessed in six regions: the anterior, body, and posterior regions of the medial and lateral sides. “Intrasubstance abnormality” was added to the original WORMS classification to enable assessment of early degenerative disease. The grading scale ranged from 0 to 4: 0 = normal, 1 = intrasubstance abnormalities, 2 = non-displaced tear, 3 = displaced or complex tear, and 4 = complete destruction and maceration of the meniscus.
Cartilage lesions were scored on an eight-point scale: 0=normal cartilage, 1=normal thickness but increased or otherwise abnormal signal on fluid sensitive sequences; 2=partial-thickness focal defect <1 cm in greatest width; 2.5=full-thickness focal defect <1 cm in greatest width; 3=multiple areas of partial-thickness defects intermixed with areas of normal thickness, or a partial-thickness defect wider than 1 cm but <75% of the region; 4=diffuse (≥75% of the region) partial-thickness loss; 5=multiple areas of full-thickness loss or a full-thickness defect wider than 1 cm but <75% of the region; 6=diffuse (≥75% of the region) full-thickness loss. For clarity, a cartilage lesion was defined as any cartilage abnormality, WORMS grade 1 or higher and a cartilage defect was defined as any cartilage abnormality, WORMS grade 2 or higher. Bone marrow edema pattern was defined as poorly marginated areas of increased T2 signal intensity graded using a modified 4-point WORMS scale: 0, none; 1, diameter 0–5 mm; 2, 5–20 mm; 3, >20mm.
Cartilage pathology and presence of BMEP was assessed using a modified WORMS in which the number of anatomical compartments was reduced from 15 to 6: patella, trochlea, medial femoral condyle, medial tibia, lateral femoral condyle and lateral tibia (P, T, MFC, MT, LFC, LT) as previously described.[20–22] This modified WORMS was developed to more efficiently grade lesions in subjects with relatively mild pathology, which is expected in individuals without symptomatic OA. The reduction in anatomical compartments can potentially affect the number of grade 4 and grade 6 lesions, which were, however, expected to be rare in the OAI incidence cohort.[14, 21, 23, 24]
For each subject, compartmental average and overall maximum WORMS were calculated. Average WORMS was obtained at baseline and 48 month follow-up for cartilage compartments, menisci, and BMEP, along with the interval change. Similarly, the WORMS maximum score (WORMS max) was assigned to each knee at baseline and 48 month follow-up defined by the greatest WORMS score in any compartment for cartilage, menisci, and BMEP, as a measure of the global severity of knee lesions. The change in each respective WORMS maximum between baseline and follow-up was also calculated.
Statistical Analysis
The statistical analyses were performed with JMP software version 9- (SAS Institute, Cary, NC, USA) using a two-sided 0.05 level of significance. Descriptive statistics (i.e. mean age, BMI etc.) were calculated for each group; t-tests and Fisher’s exact tests were used to determine the differences between the subjects in the two groups at baseline.
The two groups were then compared with respect to increase over time (yes/no) in the primary outcomes of maximum cartilage, meniscus, and BMEP WORMS score using logistic regression models to calculate odds ratios and confidence intervals. These outcomes were chosen in order to increase sensitivity to global structural changes. Additional sensitivity analysis was conducted with respect to the cartilage and meniscus primary analyses to determine if there was any significant difference in the results when the interval development of signal abnormality (WORMS grade 1) was excluded from the regression models. Further exploratory analyses for hypothesis forming included comparisons of increase over time (yes/no) of individual cartilage compartment WORMS, average meniscal WORMS, and average BMEP worms using logistic regression models. Because there were no significant differences in any of the risk factors for OA incidence and progression, adjustments were only made for baseline BMI, average five-year PASE score, and three-level KL score (0–1, 2 or 3–4). When age or gender were tested as additional covariates, there was no significant change in the odds ratios (<10%) compared to the BMI, PASE, and KL model and therefore, to maintain statistical power, age and gender were not included as covariates. Similarly, splitting the KL groups did not significantly change the odds ratios and so they were grouped as 0–1, 2, or 3–4.
To assess the intra- and inter-reader reproducibility of the WORMS grading, 30 subjects were randomly selected and WORMS grading was performed by 2 readers (MB and LN) independently. Intra-class correlation coefficients were calculated to compare the exact WORMS score for meniscal and cartilage lesions in each compartment. The intra-class correlation coefficients for intra-observer agreement were 0.87 (0.804–0.932) for meniscus WORMS and 0.86 (0.801–0.928) for cartilage WORMS. Intra-class correlation coefficients for inter-observer agreement were 0.84 (0.771–0.911) for meniscus WORMS and 0.79 (0.72–0.868) for cartilage WORMS. These analyses demonstrate good WORMS grading reproducibility. The intra- and inter-reader reproducibility of WORMS grading by our group has also been validated in multiple prior studies.[21, 23, 25–27]
Results
Baseline Subject Characteristics and Focal Knee Lesions
Baseline characteristics of all subjects are described in Table 1. There were no significant differences in age, baseline BMI, gender, or any of the selected OA risk factors between the two groups. Table 1 also outlines the severity of morphological knee abnormalities across the two groups at baseline. At baseline, there was no significant difference in WORMS severity for baseline cartilage, meniscus, and BMEP lesions between the two groups (p>0.05).
Table 1.
Baseline demographics and WORMS scores of individuals with OA risk factors and either no change in weight or weight gain over 4 years.
| No Change (n=50) |
Weight Gain (n=50) |
p- value |
|
|---|---|---|---|
| Baseline Demographics/History | |||
| Age [years] ± SD | 59.1 ± 8.0 | 58.0 ± 8.3 | 0.75 |
| BMI [kg/m2] ± SD | 28.7 ± 4.8 | 28.1 ± 5.3 | 0.73 |
| Females | 33 (72%) | 34 (66%) | 1.00 |
| Knee symptoms in the past 12 months | 47 (94%) | 43 (86%) | 0.32 |
| History of knee injury | 21 (43%) | 23 (47%) | 0.84 |
| History of knee surgery | 11 (22%) | 10 (20%) | 1.00 |
| Family history of knee replacement surgery | 7 (15%) | 10 (20%) | 0.60 |
| Heberden nodes | 13 (26%) | 18 (37%) | 0.28 |
| Average five-year PASE score | 169 ± 75 | 175 ± 78 | 0.35 |
| Kellgren-Lawrence score (0–1) | 29 (58%) | 25 (50%) | 0.55 |
| Kellgren-Lawrence score (2) | 13 (26%) | 16 (32%) | 0.66 |
| Kellgren-Lawrence score (3–4) | 8 (16%) | 9 (18%) | 1.00 |
| Baseline Cartilage- Mean WORMS ± SD | 1.1 ± 1.7 | 1.6 ± 1.8 | 0.16 |
| Baseline Meniscus- Mean WORMS ± SD | 0.5 ± 1.1 | 0.7 ± 1.2 | 0.69 |
| Baseline BMEP- Mean WORMS ± SD | 0.4 ± 0.8 | 0.5 ± 0.9 | 0.54 |
Results are given as a percentage or mean ± standard deviation. A Fisher's exact test was used for categorical variables and a t-test for continuous variables.
Follow-up Subject Characteristics
Individuals in the NC group and WG group on average had a weight gain of 0.04% ± 0.95% (range −1.49% to 1.87%) and 15.2% ± 7.8% (range 5.98% to 40.08%), respectively. In the WG group, women gained slightly more weight on average than men (16.2% ± 7.3% compared to 13.2% ± 8.6%).
Longitudinal Analysis
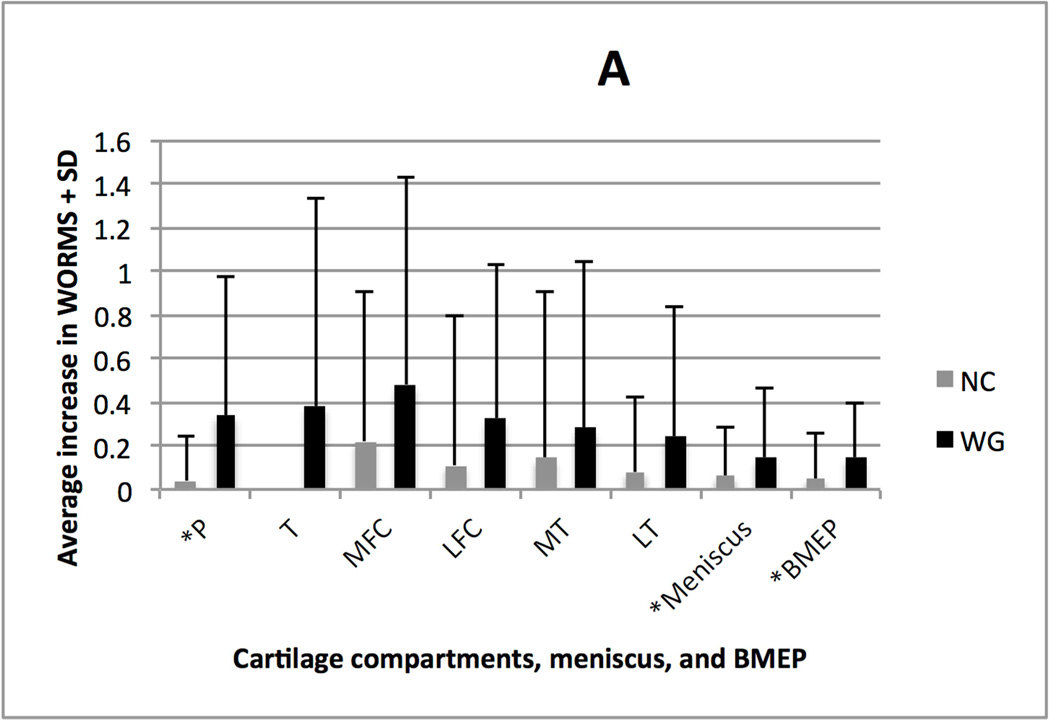
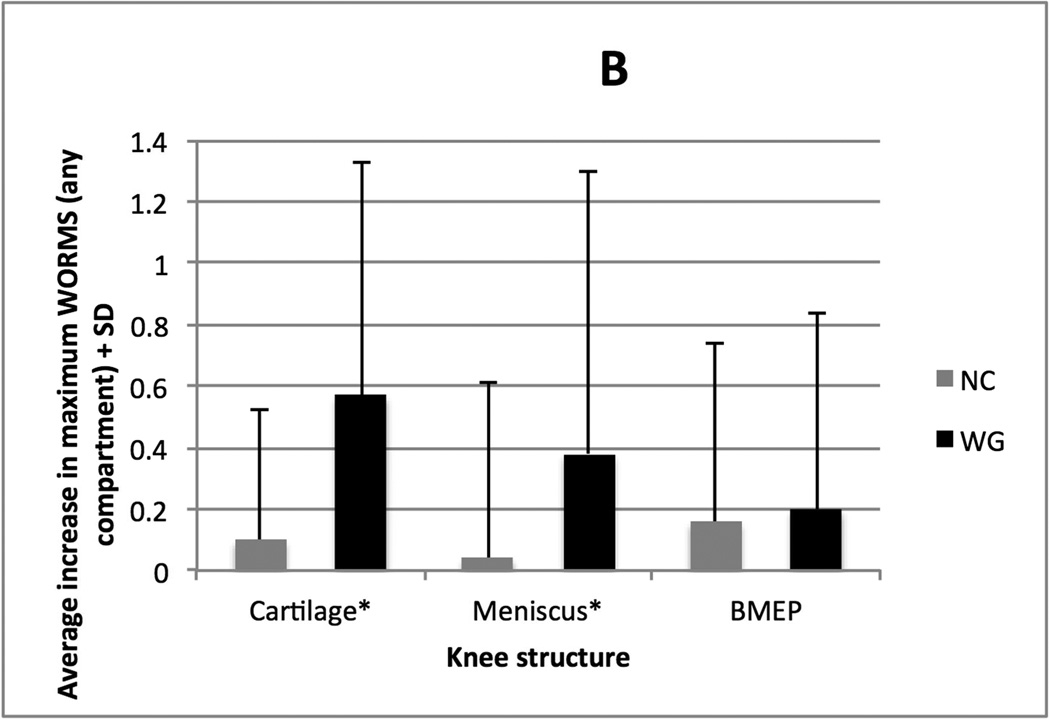
Figure 1A charts the average increase in maximum WORMS in any cartilage, meniscus, or BMEP compartment. Figure 1B charts the average progression of cartilage lesions from baseline to 48 month follow-up in the six compartments of the knee, in addition to the average progression in any single meniscal compartment and any single BMEP compartment, for the two groups. In the WG group, 34% (17/50) had the worsening of the WORMS score for menisci, 66% (33/50) for cartilage, and 52% (26/50) for BMEP. By comparison, in the NC group, 14% (7/50) had worsening of the WORMS for menisci, 14% (7/50) for cartilage, and 24% (12/50) for BMEP.
Figure 1. Longitudinal Analysis.
A. Average increase in maximum single compartment WORMS across all cartilage, meniscal, and BMEP compartments (+ one standard deviation). * indicates significant differences after logistic regression analysis (p<0.05).
B. Average increase in WORMS (+ one standard deviation) in each cartilage compartment, all meniscal compartments (averaged) and all BMEP compartments (averaged). * indicates significant differences after logistic regression analysis (p<0.05).
Table 2 summarizes the odds ratios and confidence intervals for the comparisons of cartilage, meniscal, and BMEP progression between the two groups.
Table 2.
Odds Ratios of progression of primary and exploratory analyses
| Change in WORMS | Weight Gain : No Change | p-value |
|---|---|---|
| Cartilage Max | 11.3 (3.5–51.4)* | <0.001 |
| Meniscus Max | 4.5 (1.4–17.3) | 0.016 |
| BMEP Max | 2.1 (0.7–6.3) | 0.174 |
| Cartilage P | 8.9 (2.2–60.0) | 0.006 |
| Cartilage T | ** | 0.996 |
| Cartilage MFC | 2.9 (0.8–12.1) | 0.110 |
| Cartilage LFC | 3.8 (1.1–18.2) | 0.057 |
| Cartilage MT | 3.9 (0.8–28.5) | 0.112 |
| Cartilage LT | 2.9 (0.8–14.4) | 0.134 |
| Meniscal Avg | 3.2 (1.2–9.3) | 0.023 |
| BMEP Avg | 3.2 (1.2–9.3) | 0.015 |
95% confidence intervals are in parentheses. Significant results (p<0.05) are bolded
Average increase in trochlear cartilage WORMS in the No Change group was equal to zero
With regard to the primary outcomes of increased maximum cartilage, meniscus, and BMEP WORMS, after controlling for baseline BMI, average five-year PASE score, and KL score, there was significantly increased odds of progression of cartilage WORMS max (OR: 11.3, 95% CI 3.5–51.4, p<0.001) and meniscus WORMS max (OR: 4.5, 95% CI 1.4–17.3, p=0.016) in the WG group compared to the NC group. However, there were no significant differences in change in maximum BMEP between the two cohorts. Sensitivity analyses with regard to the primary outcomes generated the same results (odds ratios, confidence intervals, and p-values) when the interval development of signal abnormality (WORMS grade 1) was excluded from the regression models. Only four total meniscal compartments in four individuals and six total cartilage compartments in six individuals demonstrated worsening as defined by development of signal abnormality.
Regarding the exploratory analyses, there were increased odds of progression at the patellar cartilage (OR: 8.9, 95% CI 2.2–60.0, p=0.006) in the WG group compared to the NC group (Figure 2). Additionally, the change in meniscal WORMS average paralleled the WORMS max result and also showed a significant difference (p=0.023). Finally, there were significantly elevated odds of worsening BMEP average in the WG group (OR: 3.2, 95% CI 1.2–9.3, p=0.015). The WG group demonstrated a higher average increase in WORMS max across any compartment for cartilage, menisci, and BMEP. This cohort also demonstrated a higher increase in average cartilage WORMS across each individual cartilage compartment, in addition to the average meniscal and BMEP compartments.
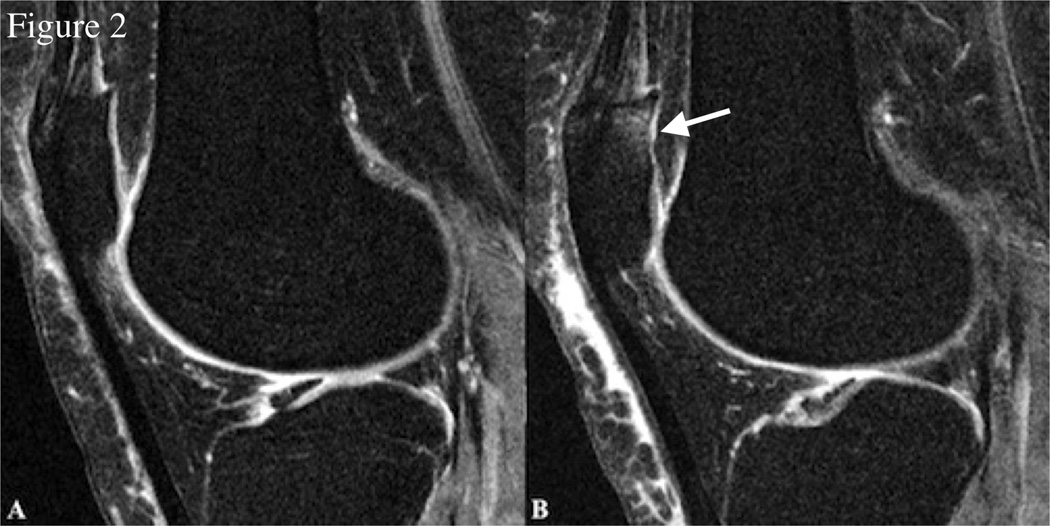
Figure 2.
Sagittal 3D dual-echo in steady state (DESS) sequence images of the right knee at baseline (A) and 48-month follow-up (B) in a representative individual with weight gain (approximately 5.7% increase in BMI from 33.1 to 35) over 48 months. There is interval development of a full thickness focal cartilage defect at the patella > 1 cm (WORMS grade 5) and adjacent increased bone marrow edema pattern (WORMS grade 2) (white arrow).
Discussion
We found that individuals with at least a five percent weight gain over four years had significantly higher progression of cartilage (global and compartment specific) and meniscal lesions compared to the control cohort with stable weight (less than two percent change). BMEP average was also significantly increased in the weight gain group compared to the no change group. Our study is the first to specifically correlate the progression of morphological abnormalities on knee MRI with at least four year change in weight.
While intuitively the link between obesity and OA[28–31] would also suggest an association with weight gain, there have been few studies examining this possible relationship.[32] Of note, Manninen et al demonstrated that the shift from normal to overweight between the ages of 20 and 30, 40, or 50 years, carried a higher risk for knee OA requiring arthroplasty than did persistent increased BMI.[8] More recently, Teichtahl et al showed that weight gain was associated with increased cartilage loss in adults with meniscal tears.[7] Our study similarly found that even after controlling for baseline BMI, physical activity, and KL score, patients with weight gain had significantly increased odds of progression of cartilage WORMS max, meniscal WORMS max, cartilage WORMS at the patella (and nearly significant at the lateral femoral condyle), average meniscal WORMS, and average BMEP, compared to individuals with no change in weight. Changes in dynamic joint loading are most likely pivotal. Basic science and clinical studies have demonstrated that abnormal loads can alter the composition and physical properties of articular cartilage.[33, 34] One recent study in particular noted that dynamic mechanical loading was associated with cartilage defects, suggesting that increased loading in the setting of weight gain plays a role in the pathological changes in articular cartilage.[35]
Excessive weight gain also appears to impair regulatory pathways that maintain cartilage homeostasis and increase the production of pro-inflammatory adipocytokines, which contribute to cartilage degeneration.[36] Additional serological and biochemical studies studies, however, are needed in order to precisely characterize how weight change affects these non-mechanical mechanisms of cartilage loss.
It is unclear why increased odds of cartilage degeneration in our weight gain group would not be seen in the medial, lateral, or patellofemoral compartments in a uniform fashion. Joint malalignment might play some role in this phenomenon. Felson et al, for example, have previously shown that the association of cartilage lesions with elevated BMI appears limited to regions of moderate malalignment.[37] However, others have shown that cartilage loss is one of the major drivers of malalignment, further complicating the issue.[38–40]
There are limitations to our study. We relied on a relatively small sample of 50 individuals in each of our groups compared to the total OAI incidence cohort size. The individuals were not randomized to weight gain versus no change in weight and comorbidities not incorporated into our model might have contributed to changes in patient weight. Additionally, we controlled for PASE values as part of our logistic regression analyses, which are limited by the subjective recall of the individuals in our study. Also of note, WORMS has limited sensitivity for subtle progression of lesions; for example, there were several patients in our cohorts who showed subtle increased cartilage defect size, but who did not have a change in WORMS score. Finally, because of relatively low rates of progression, we had relatively large 95% CIs, which limits the informative value of the specific calculated odds. Thus, the direction of the statistically significant odds ratios is likely more clinically meaningful than the specific number and associated confidence intervals reported.
In summary, our study demonstrated that weight gain was associated with increased progression of early degenerative changes of cartilage in middle-aged individuals with risk factors for OA but without initial clinical evidence of the disease. Given the enormous personal and societal burdens of OA, our findings emphasize the importance of public health initiatives aimed not only toward preventing obesity in overweight individuals, but also toward maintaining healthy normal weight.
Supplementary Material
Acknowledgments
This study was funded through National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grants U01-AR059507 and P50-AR060752. The study was made possible through support from the Osteoarthritis Initiative (OAI). The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
The authors would also like to extend their sincerest thanks to the entire Musculoskeletal Quantitative Research Group at UCSF for their support.
Role of the funding source
The study sponsors had no involvement in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; nor in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Bucknor had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Bucknor, Nardo, Joseph, Alizai, Srikhum, Nevitt, Lynch, McCulloch, Link.
Acquisition of data. Bucknor, Nardo, Alizai, Srikhum.
Analysis and interpretation of data. Bucknor, Nardo, Joseph, Alizai, Srikhum, Nevitt, Lynch, McCulloch, Link.
Competing interests: The authors have no competing interests.
References
- 1.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis and rheumatism. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis and rheumatism. 2009;60:3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 4.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 5.Ringdahl E, Pandit S. Treatment of knee osteoarthritis. American family physician. 2011;83:1287–1292. [PubMed] [Google Scholar]
- 6.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis and rheumatism. 2008;59:1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teichtahl AJ, Wluka AE, Wang Y, Strauss BJ, Proietto J, Dixon JB, et al. The longitudinal relationship between changes in body weight and changes in medial tibial cartilage, and pain among community-based adults with and without meniscal tears. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203210. [DOI] [PubMed] [Google Scholar]
- 8.Manninen P, Riihimaki H, Heliovaara M, Suomalainen O. Weight changes and the risk of knee osteoarthritis requiring arthroplasty. Annals of the rheumatic diseases. 2004;63:1434–1437. doi: 10.1136/ard.2003.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Current opinion in rheumatology. 2010;22:533–537. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi K, Shimada N, Moriyama M, Hayashi W, Tokoro T, Ohno-Matsui K. Two-year outcomes of intravitreal bevacizumab for choroidal neovascularization in Japanese patients with pathologic myopia. Retina. 2012;32:687–695. doi: 10.1097/IAE.0b013e3182278bae. [DOI] [PubMed] [Google Scholar]
- 11.Hunter DJ. Advanced imaging in osteoarthritis. Bulletin of the NYU hospital for joint diseases. 2008;66:251–260. [PubMed] [Google Scholar]
- 12.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226:373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 13.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T(1)rho and T(2) relaxation times predict progression of knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:69–76. doi: 10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls--data from the osteoarthritis initiative. Arthritis research & therapy. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar D, Schooler J, Zuo J, McCulloch CE, Nardo L, Link TM, et al. Trabecular bone structure and spatial differences in articular cartilage MR relaxation times in individuals with posterior horn medial meniscal tears. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:86–93. doi: 10.1016/j.joca.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jungmann PM, Li X, Nardo L, Subburaj K, Lin W, Ma CB, et al. Do cartilage repair procedures prevent degenerative meniscus changes?: longitudinal t1rho and morphological evaluation with 3.0-T MRI. The American journal of sports medicine. 2012;40:2700–2708. doi: 10.1177/0363546512461594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neame R, Zhang W, Deighton C, Doherty M, Doherty S, Lanyon P, et al. Distribution of radiographic osteoarthritis between the right and left hands, hips, and knees. Arthritis and rheumatology. 2004;50:1487–1494. doi: 10.1002/art.20162. [DOI] [PubMed] [Google Scholar]
- 18.Tanamas SK, Wluka AE, Davies-Tuck M, Wang Y, Strauss BJ, Proietto J, et al. Association of weight gain with incident knee pain, stiffness, and functional difficulties: a longitudinal study. Arthritis care & research. 2013;65:34–43. doi: 10.1002/acr.21745. [DOI] [PubMed] [Google Scholar]
- 19.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls - data from the osteoarthritis initiative. Arthritis research & therapy. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt MC, Lynch J, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects-data from the Osteoarthritis Initiative. Skeletal radiology. 2011 doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:1408–1416. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis care & research. 2012;64:248–255. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: Thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis care & research. 2013;65:23–33. doi: 10.1002/acr.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261:507–515. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: data from the osteoarthritis initiative. Journal of magnetic resonance imaging : JMRI. 2012;35:370–378. doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felson DT. Weight and osteoarthritis. The American journal of clinical nutrition. 1996;63:430S–432S. doi: 10.1093/ajcn/63.3.430. [DOI] [PubMed] [Google Scholar]
- 29.Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25:622–627. doi: 10.1038/sj.ijo.0801585. [DOI] [PubMed] [Google Scholar]
- 30.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis and rheumatism. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Annals of internal medicine. 2011;154:217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Wluka AE, Simpson JA, Giles GG, Graves SE, de Steiger RN, et al. Body weight at early and middle adulthood, weight gain and persistent overweight from early adulthood are predictors of the risk of total knee and hip replacement for osteoarthritis. Rheumatology. 2013 doi: 10.1093/rheumatology/kes419. [DOI] [PubMed] [Google Scholar]
- 33.Mundermann A, Dyrby CO, Andriacchi TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis and rheumatism. 2005;52:2835–2844. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- 34.Maly MR, Costigan PA, Olney SJ. Contribution of psychosocial and mechanical variables to physical performance measures in knee osteoarthritis. Physical therapy. 2005;85:1318–1328. [PubMed] [Google Scholar]
- 35.Creaby MW, Wang Y, Bennell KL, Hinman RS, Metcalf BR, Bowles KA, et al. Dynamic knee loading is related to cartilage defects and tibial plateau bone area in medial knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:1380–1385. doi: 10.1016/j.joca.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Annals of the rheumatic diseases. 2010;69:761–765. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 37.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis and rheumatism. 2004;50:3904–3909. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 38.Cooke TD, Scudamore A, Greer W. Varus knee osteoarthritis: whence the varus? The Journal of rheumatology. 2003;30:2521–2523. [PubMed] [Google Scholar]
- 39.Hunter DJ, Sharma L, Skaife T. Alignment and osteoarthritis of the knee. The Journal of bone and joint surgery. American volume. 2009;91(Suppl 1):85–89. doi: 10.2106/JBJS.H.01409. [DOI] [PubMed] [Google Scholar]
- 40.Hunter DJ, Zhang Y, Niu J, Tu X, Amin S, Goggins J, et al. Structural factors associated with malalignment in knee osteoarthritis: the Boston osteoarthritis knee study. The Journal of rheumatology. 2005;32:2192–2199. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.