Abstract
Evolutionary theories of aging posit that greater reproductive effort causes somatic decline given a fundamental trade-off between investing energy in reproduction and repair. Few studies in high fertility human populations support this hypothesis, and problems of phenotypic correlation can obscure the expected trade-off between reproduction and somatic condition. This cross-sectional study investigates whether greater reproductive effort is associated with reduced calcaneal bone mineral density (BMD) among female Tsimane forager-farmers of lowland Bolivia. We also investigate whether female Tsimane BMD values are lower than sex- and age-matched US reference values, despite the fact that Tsimane engage in higher physical activity levels that can increase mechanical loading. To measure calcaneal BMD, quantitative ultrasonography was performed on 130 women (mean ± SD age = 36.6 ± 15.7, range = 15 – 75) that were recruited regardless of past or current reproductive status. Anthropometric and demographic data were collected during routine medical exams. As predicted, higher parity, short inter-birth interval, and earlier age at first birth are associated with reduced BMD among Tsimane women after adjusting for potential confounders. Population-level differences are apparent prior to the onset of reproduction, and age-related decline in BMD is greater among Tsimane compared to American women. Greater cumulative reproductive burden may lower calcaneal BMD individually and jointly with other lifestyle and heritable factors. Fitness impacts of kin transfers in adulthood may determine the value of investments in bone remodeling, and thus affect selection on age-profiles of bone mineral loss.
Keywords: Disposable soma, maternal depletion, quantitative ultrasonography, calcaneus, Tsimane
All adult organisms face a fundamental trade-off between investing energy in reproduction and somatic repair. The “disposable soma” theory proposes that natural selection optimizes levels of somatic repair below that required for extensive longevity due to prioritized fitness gains of earlier investments in reproduction (Kirkwood and Rose 1991). Controlled experiments among non-human animal models demonstrate expected trade-offs between reproduction and longevity (Kirkwood and Austad 2000). Among humans, some studies support the hypothesis that greater reproductive effort is associated with greater mortality (Westendorp and Kirkwood 1998) and somatic decline (Miller 2010; Tracer 1991; Tracer 2002), as indicated by reduced anthropometric status (e.g. weight, BMI, skinfold, micro-nutrient stores). Other studies find no associations (Hurt et al. 2006; Tracer 2002) or find that greater reproductive effort is associated with greater longevity (Lycett et al. 2000) or favorable somatic condition (Adair and Popkin 1992). As is common in life history studies lacking experimental designs, problems of self-selection or phenotypic correlation can obscure the expected trade-off between reproduction and somatic repair.
Unlike other mineralized tissues such as tooth enamel, bone can be repaired and constant remodeling is necessary to maintain its strength and rigidity. Aside from facilitating locomotion, protecting and supporting vital organs, and producing blood cells in marrow, bone stores calcium and other essential minerals. Bone is a mineral source for the competing demands of maternal somatic repair (DiGirolamo et al. 2012) and fetal bone accretion or lactation (Prentice 2003). Because mineral allocations to somatic repair and reproduction draw from the same general bone mineral reservoir, direct metabolic trade-offs should, in principle, manifest in bone. In developed nations the combination of low fertility, reduced lactation duration, longer birth spacing, energy-rich diets including mineral and vitamin supplements, sedentary lifestyle, and reduced pathogen burden may relax energetic constraints that might otherwise reduce bone mineral density (BMD). Although in developed nations, a large fraction of older adults with bone fractures die in the following year, and many more experience significant functional limitations (e.g. Brauer et al. 2009). To date, only one study has examined maternal bone reserves in relation to reproduction in an energy-limited, natural fertility society (hereafter small-scale society) where reproduction is expected to influence somatic condition. Calcaneal BMD among Shuar forager-horticulturalist women of Amazonian Ecuador is not consistently associated with reproductive effort, although small sample size of women who have completed reproduction precludes investigation of longer-term trade-offs (Madimenos et al. 2012).
Here we investigate whether greater reproductive effort is associated with reduced BMD among 130 women aged 15–75 in a small-scale population, the Tsimane of lowland Bolivia. Tsimane women rarely use contraception and invest considerable energy in reproduction relative to women in low fertility societies of the developed world. Tsimane total fertility rate is 9 births per woman, mean inter-birth interval (IBI) is 30 months, breastfeeding is on-demand, and mean weaning age is 19 months (Mcallister et al. 2012; Veile et al. 2014). Tsimane also experience high pathogen burden and lack public health infrastructure, which leads to greater immune activation throughout life (Blackwell et al. 2011; Vasunilashorn et al. 2010, 2011). In addition, Tsimane regularly experience risk of food shortfalls associated with a mixed foraging and horticultural economy (Gurven et al. 2012; Stieglitz et al. 2014). Together, these factors are expected to constrain women’s ability to maintain or improve skeletal health following repeated reproductive bouts that are closely spaced (cf. Jelliffe and Maddocks 1964). On the other hand Tsimane engage in subsistence activities of moderate intensity throughout life, often without footwear, and are less sedentary than women in developed nations (Gurven et al. 2013), which may improve skeletal health due to periosteal responses to mechanical loading (e.g. Lieberman et al. 2010; Mayhew et al. 2005).
Bone metabolism and reproduction
Major bone-forming minerals include calcium, phosphorus, magnesium and zinc. To meet greater mineral demands for pregnancy and lactation, maternal physiology responds by mobilizing mineral from bone, increasing efficiency of intestinal mineral absorption, and/or increasing renal mineral conservation (Prentice 2003; Zapata et al. 2004). Indicators of bone formation and resorption in serum and urine are elevated during pregnancy and lactation, suggesting that skeletal mineral is being mobilized and restored. During pregnancy, fetal mineral accretion accelerates in mid-gestation, with calcium accretion averaging roughly 200 mg/day during the third trimester (Forbes 1976). In principle, during pregnancy maternal BMD could either decrease due to greater mineral demands, increase due to higher levels of calciotropic hormones and greater bone-loading associated with weight gain (particularly in the third trimester), or remain unchanged relative to pre-pregnancy BMD (Sowers 1996). Early stages of pregnancy may be characterized by bone resorption followed by later stages of bone formation (Black et al. 2000), but there is considerable variability in maternal skeletal response to pregnancy across and within populations, and across skeletal sites. Most longitudinal studies demonstrate either a decrease in BMD or no significant change at one or multiple skeletal sites from before pregnancy to shortly after birth (reviewed in Olausson et al. 2008).
Whereas the relationship between BMD loss and pregnancy is inconsistent across and within populations, there is stronger evidence that extended lactation leads to lower BMD. Human breast milk contains a significant amount of calcium (roughly 200 – 400 mg/day is secreted into breast milk), and maternal BMD temporarily declines during lactation (Olausson et al. 2008; Prentice et al. 1995). Lactation-induced BMD reductions are pronounced at skeletal sites rich in trabecular bone, which is more porous, metabolically active and prone to bone loss than cortical bone (Prentice 2003). The magnitude of the initial reduction and subsequent restorative capacity partly depends on the intensity and duration of lactation. Among healthy American women, studies utilizing dual-energy x-ray absorptiometry (DXA) indicate that extended lactation (≥6 months) is associated with BMD losses of about 5% at the lumbar spine, with restoration of BMD to pre-pregnancy values by 12 months post-partum (Sowers et al. 1993). In contrast, DXA studies of rural Gambian women, who have high fertility, closely spaced births, on-demand breastfeeding for approximately two years, and low calcium intake, indicate incomplete restoration of lumbar spine BMD to pre-pregnancy values by 12 months post-partum (Jarjou et al. 2010). Complete BMD restoration among Gambian women may occur later in lactation or after weaning (Sawo et al. 2013).
Studies of parity-specific effects on maternal BMD do not reveal a consistent pattern. Several studies report a negative, sometimes non-linear association between parity and BMD (Allali et al. 2007; Ghannam et al. 1999; Gur et al. 2003; Saadi et al. 2003), others a positive association (Cure-Cure et al. 2002; Streeten et al. 2005), and others no association (Kojima et al. 2002). Again, self-selection or phenotypic correlation can obscure expected trade-offs, for example, if nulliparous women are used as a baseline against which parous women are compared. Parity is strongly correlated with age, especially in high fertility populations, and the relative importance of parity and age in affecting BMD remains unclear. Recent cross-sectional studies of changes to bone tissue over the lifespan have utilized quantitative computed tomography (qCT) to estimate volumetric BMD (vBMD, expressed in grams per centimeter3) and examine bone micro-architecture (e.g. trabecular thickness, cortical porosity) in three dimensions, but only in developed nations with low fertility (e.g. Khosla 2013). For American women aged 20+, trabecular vBMD peaks in early adulthood and undergoes accelerated loss around menopause, as calciotropic hormone concentrations decline. Key micro-structural changes include loss of trabecular number and greater trabecular separation, which compromises structural integrity and increases skeletal fragility and fracture risk. Overall, American women experience decreases in trabecular vBMD of 55% at central skeletal sites (e.g. lumbar spine, femoral neck), and 24% at peripheral sites (e.g. distal radius, tibia) from ages 20 – 90. Few studies have examined age- and parity-specific effects on maternal BMD in energy-limited high fertility societies (using qCT or other technology), and so population-level comparisons of bone mineral accrual and loss throughout adulthood across diverse environments are scarce.
While qCT or DXA are preferred methods for BMD assessment, they are costly, non-portable, and may be invasive for field populations. As a viable and validated alternative, quantitative ultrasonography (qUS) provides a portable, field-friendly, non-invasive means of evaluating peripheral bone properties (Baroncelli 2008; Madimenos et al. 2012; Njeh et al. 1997). In this study, evaluation of bone is based on calcaneal (heel) qUS measurement, and we report estimated calcaneal BMD (see Methods). BMD explains roughly 75% of the variance in bone strength (i.e. the ability to withstand an applied load); the remaining variance may be due to state of bone remodeling, micro-architecture and other factors (Njeh et al. 1997). The calcaneus is the first of the seven tarsal bones to begin ossification (in the 4th – 7th intrauterine month) (Platzer 2009), and when fully ossified by the early 20s is the most massive tarsal bone. The calcaneus is six-sided, irregularly shaped, and it articulates superiorly with the talus, which is the primary contact between the bones of the foot and leg. The calcaneus helps transmit weight and thrust from the tibia, and serves as a lever for the attachment of the calf muscles via the tendo calcaneus, the strongest tendon in the human body. This lever action is present in other hominoids, and while the principal trabecular orientation of the calcaneus appears to be conserved, trabecular structure of the calcaneus of the genus Homo is more anistropic and less dense compared to that of Pan, Gorilla and Pongo (Maga et al. 2006). The human calcaneus is over 90% trabecular bone by volume. Correlations between mineral content of the calcaneus and other skeletal sites rich in trabecular bone such as the lumbar spine, femoral neck, and Ward’s triangle range from 0.71–0.77 among Western women aged 20–86 (Vogel et al. 1988). Given its trabecular structure and accessibility for densitometry, the calcaneus is a useful skeletal site for assessing maternal bone reserves in relation to reproduction in small-scale societies.
Study goals
In this cross-sectional study we document age-related change in Tsimane female calcaneal BMD in adulthood, including age of peak BMD, age at which BMD loss accelerates, and the magnitude of BMD loss with age. We test whether higher parity (P1), short IBI (P2), and early age at first birth (P3) are associated with reduced calcaneal BMD after adjusting for potential confounders including age, anthropometric status and current reproductive status (e.g. pregnant, lactating). To examine whether self-selection into maternity affects results, we conduct separate analyses including and omitting nulliparous women as a reference group. We examine whether parity-specific effects on BMD are stronger among post-menopausal women relative to pre-menopausal women, which is expected if fitness returns from investments in bone remodeling diminish as reproduction ceases and offspring dependency load declines. Lastly, we test whether Tsimane women show reduced calcaneal BMD relative to age-matched American women (P4), despite the fact that Tsimane engage in higher physical activity levels that can increase mechanical loading. Aside from greater cumulative reproductive burden, other factors (e.g. reduced calcium intake, greater immune activation) may directly or indirectly contribute to lower BMD. If so, then population-level differences should be apparent prior to the onset of reproduction. We explore whether the magnitude of age-related decline in BMD is greater for Tsimane relative to US women, which could result from cumulative effects of reproduction and other factors. As a preliminary attempt to document prevalence of osteopenia (low bone mass) and osteoporosis among Tsimane women, we compare Tsimane BMD to young adult reference values (Tsimane and US) based on World Health Organization (WHO) criteria.
METHODS
Study population
Tsimane forager-horticulturalists of lowland Bolivia (pop. ~ 15,000) are semi-sedentary and live in 90+ villages, nearly all of which lack running water and electricity. Tsimane diet consists of cultigens grown in small swiddens (66% of calories; mostly rice, plantains, sweet manioc and corn), lean meat from hunting (17%), freshwater fish (7%), and fruits and nuts gathered from the forest (6%) (Martin et al. 2012). Market foods (e.g. pasta, sugar) and domesticated animals (e.g. chicken, pig) each provide 2% of the daily calories, and eggs account for <0.5% of calories. Relative to Western dietary standards calcium intake is low (estimated ~320 mg/day, unpublished data). Few Tsimane raise cattle (<5% of families), most cattle owners maintain small herds (<3 head) and do not process milk for consumption. Despite a lean diet and high fertility with on-demand breastfeeding, Tsimane breast-milk concentration of long-chain polyunsaturated fatty acids is high relative to Western women, and in cross-sectional analyses does not decline with parity or age (Martin et al. 2012).
Women’s physical activity level (PAL) is in the “moderate to active” range (PAL = 1.73–1.85) and remains constant throughout adulthood (Gurven et al. 2013). Older women (aged 50+) report muscular-skeletal pain in 45% of annual medical exams conducted by Tsimane Health and Life History Project (THLHP) physicians (n = 999 exams). Women are more likely than age-matched men to report pain at several appendicular and axial sites, and women demonstrate worse balance, coordination and walking endurance (Stieglitz et al. In press). Older women also report being in worse overall health than age-matched men. In the present sample 15% of older women (4/26) reported fracturing a bone (chosh, or toc) in the past five years; in all but one case a physician was not visited for confirmation or treatment. The four incidents included: 1) a fractured clavicle from a tree fall while harvesting cacao, 2) a fractured rib from falling while carrying a heavy load of leaves for roof construction, 3) a fractured rib from physical abuse by a husband, and 4) a fractured wrist from falling while running away from inebriated men during a community party.
Participants
One hundred and thirty women participated in this study. Mean age ± SD is 36.6 ± 15.7 (range = 15 – 75). No participant reported ever using hormonal contraception or vitamin or mineral supplements. Women were recruited regardless of past or current reproductive status to obtain a representative sample, examine whether self-selection into maternity affects BMD, and explore cross-sectional variability in BMD associated with phases of the reproductive cycle. The sample consists of 20 nulliparous and 110 parous women (86 pre-menopausal, 44 post-menopausal). Women were categorized as post-menopausal if they reported during THLHP medical exams not having experienced a menstrual cycle in the past year, and were neither pregnant nor lactating at the time of the study. Among pre-menopausal women 57 were cycling, 19 lactating, and 10 pregnant at the time of the study.
For all protocols institutional (UNM and UCSB) IRB approval was granted, as was informed consent at three levels: 1) Tsimane government that oversees research projects, 2) village leadership, and 3) study participants.
Calcaneal ultrasound
qUS is commonly used for research and diagnostic purposes (Baroncelli 2008). Diagnostic sensitivity of calcaneal BMD from qUS in the prediction of hip fracture has been shown in large prospective studies to be similar to that of hip BMD measured with DXA (Njeh et al. 1997). Correlations of BMD measured with qUS and DXA range from 0.28 – 0.86; this variance may be attributed to differences in skeletal sites measured, ultrasound machines used, or to the fact that ultrasound velocity may be dependent on aspects of bone other than mineral density (see next paragraph). While DXA tests are preferred before administering clinical treatment for osteoporosis, qUS is often used in remote settings without DXA access. BMD measurements of the right heel were obtained using a gel-based Sahara Clinical Bone Sonometer (Hologic, Waltham, MA). Ultrasound transducers are mounted on a motorized caliper that enables direct contact with the heel through elastomer pads and a coupling gel. One transducer serves as a transmitter and the other as a receiver. Foot, ankle and leg positions are fixed by a device extending from the foot to the shin.
BMD (expressed in g/cm2) is an estimate of bone mineral content per surface area. Micro-architectural properties of bone alter the shape, intensity and speed of ultrasound waves passing through bone. Attenuation of ultrasound waves through bone occurs by a reduction in wave amplitude and results in loss of energy. In trabecular bone the major attenuation mechanism is scattering (i.e. redistribution of energy in one or more directions), whereas in cortical bone the major mechanism is absorption (i.e. dissipation of energy by conversion to heat). The Sahara sonometer generates multiple measures including speed of sound (SoS, expressed in meters per second), which reflects ultrasound wave velocity through the calcaneus for a given heel width. Another measurement is broadband ultrasound attenuation (BUA, expressed in decibels per megahertz). Bone attenuates high frequency sound waves more than low frequency waves, and BUA reflects ultrasound wave attenuation through the calcaneus in a frequency range (0.2–0.6 MHz) where wave attenuation is linearly associated with frequency. BUA is the slope of the linear regression of wave attenuation versus frequency within this range; the slope is less steep for osteoporotic bone (Njeh et al. 1997). For both SoS and BUA, lower values indicate reduced bone mineral status. BMD is estimated from a linear combination of SoS and BUA: calcaneal BMD = 0.002592*(SoS + BUA) – 3.687 (Frost et al. 2000).
Instrumental quality control scans of a phantom provided by the manufacturer with known SoS and BUA values were performed daily. Short-term reproducibility of BMD measurements was assessed by duplicate scans (morning, afternoon) of 15 healthy volunteers (THLHP employees including men and non-pregnant, non-lactating women). The mean coefficient of variation was 2.6%. One sonometer was used throughout the study and measurements were taken by two operators. No systematic differences in BMD measurements were found across operators or over time.
Anthropometrics and demography
Height and weight were measured during THLHP medical exams using a Seca stadiometer (Road Rod 214) and Tanita scale (BF680). The scale uses a method of bioelectrical impedance analysis to estimate percent body fat. Using weight and percent body fat we calculated fat mass (weight*percent body fat) and fat-free mass (weight – fat mass).
Reproductive histories were elicited in the Tsimane language among adults aged 15+ by Gurven, Stieglitz and Tsimane research assistants. Birth years were assigned based on a combination of methods including using known ages from written records, relative age lists, dated events, photo comparisons of people with known ages, and cross-validation of information from independent interviews of kin (Gurven et al. 2007). Each method provides an independent estimate of age, and when estimates yielded a date of birth within a three-year range, the average was generally used. Individuals for whom reliable ages could not be ascertained are not included in analyses. The outcome of each pregnancy reported during reproductive histories was recorded as either ending in a live birth or terminating pre-term. Whether miscarriages (including stillbirths) are included or omitted from parity counts does not affect results, and results reported here reflect only live births. IBI refers to the number of months between live births for women with ≥2 live births.
Data analysis
Unpaired t tests, Mann-Whitney U tests, chi-square tests and one-way ANOVA were used to compare demographics, anthropometrics and BMD by reproductive status (i.e. pre- vs. post-menopausal, nulliparous vs. parous, or cycling vs. lactating vs. pregnant). Ordinary least squares (OLS) regression was used to model associations between demographics, anthropometrics and BMD. BMD age trends were assessed using a combination of linear, loess and piecewise linear regressions. A second-order age term was included in OLS regressions because BMD is not expected to decrease linearly with age. Piecewise regression was used to establish the break-point at which rate of decline in BMD accelerates (Muggeo 2003). Linear and loess regressions were used to cross-check results of piecewise regression. BMD is reported as a raw value or as a percentage of the adult maximum. Unstandardized and standardized regression coefficients are reported. Continuous and categorical measures of reproductive parameters (i.e. parity, mean IBI, age at first birth) were used to explore linear and non-linear associations with BMD. For comparisons between Tsimane and American women we calculated two T-scores for each Tsimane woman, using either a Tsimane or American young adult reference group (WHO 1994). T-scores represent the difference in one’s result from the mean in a young adult (aged 20–29) population, expressed in SD units. T-scores were calculated as follows: T = (P-YA)/SDYA, where P is one’s estimated calcaneal BMD, YA is the young adult reference group mean BMD, and SDYA is the standard deviation of the young adult mean. The WHO defines osteopenia as a T-score between −1.0 and −2.5, and osteoporosis as a T-score below −2.5.
RESULTS
Sample characteristics for pre- and post-menopausal Tsimane women are shown in Table 1. All nulliparous women were < age 25 at the time of study (71% were < age 20). Most nulliparous women were unmarried and continued residing in their natal homes.
Table 1.
Sample characteristics for pre- and post-menopausal Tsimane women
| Pre-menopausal (n = 86) |
Post-menopausal (n = 44) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Min. | Max. | Mean | SD | Min. | Max. |
| Age (years) | 27.3 | 8.5 | 15 | 44 | 54.7** | 9.7 | 41 | 75 |
| Height (cm) | 150.8 | 5.0 | 139.6 | 169.8 | 149.7 | 3.9 | 142.5 | 160.0 |
| Weight (kg) | 55.2 | 9.3 | 31.9 | 90.1 | 53.2 | 8.9 | 33.0 | 74.5 |
| Body fat (%) | 27.0 | 7.1 | 11.2 | 40.1 | 27.8 | 7.2 | 11.7 | 43.3 |
| Fat mass (kg) | 15.6 | 6.2 | 5.2 | 33.0 | 15.3 | 6.0 | 4.7 | 32.3 |
| Fat-free mass (kg) | 40.5 | 4.3 | 32.8 | 57.1 | 37.8** | 4.1 | 27.8 | 47.1 |
| Nulliparous (1=yes, 0=no) | 0.23 | ----- | ----- | ----- | 0.00** | ----- | ----- | ----- |
| Cycling (1=yes, 0=no) | 0.66 | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
| Lactating (1=yes, 0=no) | 0.22 | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
| Pregnant (1=yes, 0=no) | 0.12 | ----- | ----- | ----- | ----- | ----- | ----- | ----- |
| Number of births | 3.6 | 3.3 | 0 | 13 | 9.7** | 3.7 | 1 | 15 |
| Mean IBI (months) | 30.9 | 11.9 | 11.8 | 76.0 | 30.4 | 9.9 | 10.5 | 56.0 |
| Age at first birth (years) | 18.0 | 2.5 | 14 | 26 | 18.5 | 3.1 | 14 | 27 |
| Estimated calcaneal BMD (g/cm2) | 0.46 | 0.08 | 0.32 | 0.71 | 0.40** | 0.09 | 0.24 | 0.63 |
p < 0.01 compared to pre-menopausal women (t-test, Mann-Whitney U test, or χ2 test)
Abbreviations: IBI: inter-birth interval; BMD: bone mineral density
Tsimane calcaneal BMD by age
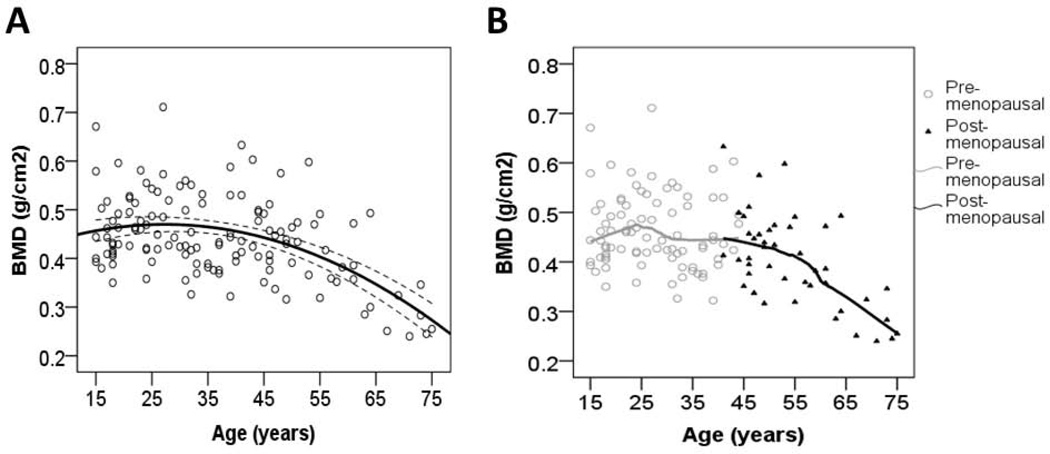
BMD changes non-linearly with age (Unstd. BAge = 0.005, p = 0.024, BAge2 = −0.0001, p = 0.001, adj. R2 = 0.25, n = 130) (Fig. 1A). In this fitted model, 97% of the maximum adult female BMD is attained by age 15, and maximum BMD is attained by age 27. BMD remains at 94% of the adult maximum by age 45, and pronounced decline with age is evident by age 53 (i.e. the break-point from piecewise linear regression). Among post-menopausal women age-related decline in BMD is linear (Unstd. BAge = −0.006 g/cm2, Std. β = −0.629, p < 0.001, n = 44) (Fig. 1B), amounting to an annual loss of 1.5% of the post-menopausal mean. By age 75 BMD is at 58% of the adult maximum.
Fig. 1.
Estimated calcaneal BMD by age and menopausal status (n = 130). Shown is a quadratic fit with 95% CI (1A), and a LOESS fit (1B).
BMD, anthropometrics, and reproductive status
BMD is more strongly associated with weight (Std. βWeight = 0.11, 95% CI: −0.05 – 0.27, p = 0.172, controlling for age and age2, n = 130) than with height (Std. βHeight = 0.002, 95% CI: −0.15 – 0.16, p = 0.978). BMD is not associated with percent body fat (Std. β% body fat = 0.11, 95% CI: −0.05 – 0.28, p = 0.182), but is associated with fat mass (Std. βFat mass = 0.15, 95% CI: −0.02 – 0.31, p = 0.077) and fat-free mass (Std. βFat-free mass = 0.16, 95% CI: −0.01 – 0.33, p = 0.071). In subsequent analyses of BMD we control for age and weight.
Nulliparous vs. parous women
On average nulliparous women are younger than parous women (18.2 vs. 39.9 years, t = −13.94, p < 0.001, n = 130), and lighter (50.0 vs. 55.4 kg, t = −2.46, p = 0.015), although not after controlling for age. Mean BMD is not significantly different between nulliparous and parous women using either the full sample (0.47 vs. 0.44 g/cm2, t = 1.427, p = 0.156) or only women aged 15–24 (0.47 vs. 0.46 g/cm2, t = 0.206, p = 0.838, n = 38).
Cycling vs. lactating vs. pregnant women
Mean age is not different among cycling (27.0 years, n = 57), lactating (26.6, n = 19), and pregnant women (30.3, n = 10) (F = 0.712, p = 0.494). Differences in mean weight across the three groups are modest (F = 1.453, p = 0.240), perhaps because 6/10 pregnant women were in their first trimester (mean = 3.2 months, range = 2 – 5). Post-hoc tests indicate greater mean weight for pregnant women (58.7 kg, 95% CI: 52.3 – 65.1) compared to lactating (52.6 kg, 95% CI: 49.3 – 56.9, p = 0.098) but not cycling women (55.4 kg, 95% CI: 52.8 – 58.1, p = 0.306), and no difference between lactating and cycling women (p = 0.260). Only one lactating woman had an infant <6 months of age at the time of study (mean age of youngest child = 12.5 months, range = 3 – 24), and so mothers engaging in more intensive breastfeeding are under-represented. No significant differences were found across the three groups in percent body fat, fat mass, or fat-free mass.
Despite modest differences in age and body composition, mean BMD is different across the three groups (F = 3.784, p = 0.027) (Fig. S1). Mean BMD is higher among pregnant women (0.52 g/cm2, 95% CI: 0.45 – 0.59) compared to lactating (0.44 g/cm2, 95% CI: 0.41 – 0.47, p = 0.008) and cycling women (0.46 g/cm2, 95% CI: 0.44 – 0.48, p = 0.021); there is no difference between lactating and cycling women (p = 0.342). In subsequent analyses of BMD we include lactating and pregnant dummy variables to control for potential confounding effects of current reproductive status among pre-menopausal women.
BMD by parity
Pre-menopausal women
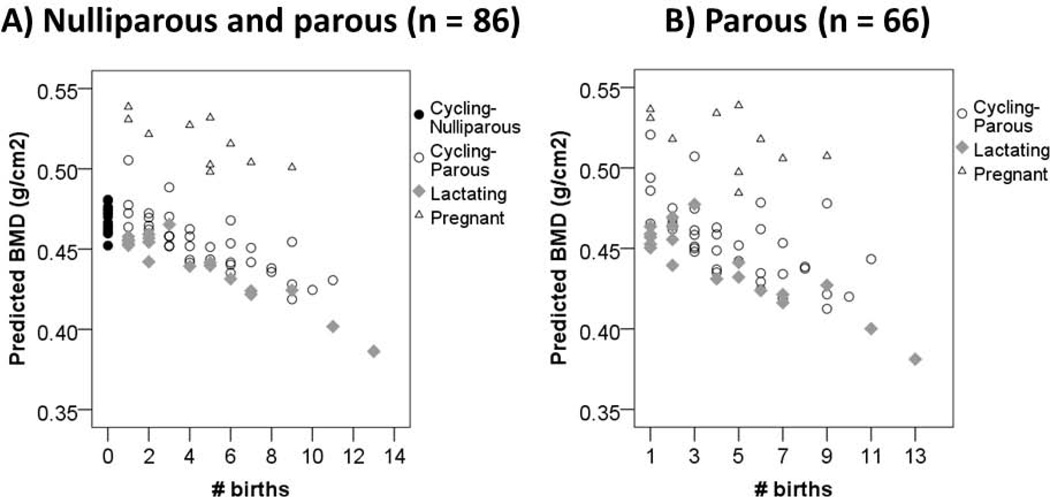
BMD is inversely associated with number of births in the pooled sample of nulliparous and parous women (Unstd. B# births = −0.010 g/cm2, 95% CI: −0.019 – 0.0002, p = 0.057, controlling for age, weight, and lactating and pregnant dummies, n = 86) (Table 2). This association strengthens in the parous-only sample (Unstd. B# births = −0.012 g/cm2, 95% CI: −0.022 – −0.001, p = 0.031, n = 66), amounting to a BMD loss of 2.6% (95% CI: −4.8% to −0.44%) of the pre-menopausal parous mean per additional birth. In this model age is not a significant predictor (Unstd. BAge = 0.002 g/cm2, p = 0.32). Figure 2 shows predicted BMD by number of births and reproductive status for pooled (2A) and parous-only (2B) samples. Substituting a categorical parity measure among the parous-only sample indicates substantial BMD decline after the 5th birth (Fig. S2).
Table 2.
Unstandardized (standardized) coefficients from OLS regressions of paritya on estimated calcaneal BMD (g/cm2) for pre- and post-menopausal Tsimane women.
| Pre-menopausalb |
Post-menopausalc | ||
|---|---|---|---|
| Parity measure | Nulliparous + Parous |
Parous only |
|
| Continuous | |||
| # births | −0.010t (−0.42) | −0.012* (−0.45) | −0.004 (−0.14) |
| Categorical | |||
| 10+ births | −0.074 (−0.87) | −0.086 (−1.00) | −0.097* (−1.05) |
| 6–9 births | −0.069 (−0.80) | −0.071t (−0.83) | −0.094t (−1.01) |
| 3–5 births | −0.021 (−0.24) | −0.016 (−0.19) | −0.123* (−1.32) |
| 1–2 births | −0.004 (−0.05) | (ref.) | (ref.) |
| 0 births | (ref.) | ||
| N=86 | N=66 | N=44 | |
p ≤ 0.10
p ≤ 0.05
Continuous and categorical measures of parity were added in separate models to examine linear and non-linear associations.
Controlling for age, weight, and dummy variables indicating whether lactating and pregnant at BMD measurement
Controlling for age and weight (no nulliparous post-menopausal women were sampled)
Fig. 2.
Estimated calcaneal BMD by number of births and reproductive status among pre-menopausal Tsimane women. Predicted values are obtained from OLS regression including age, weight, and lactating and pregnant dummies as controls. Figure 2A includes the pooled sample of nulliparous and parous women; Figure 2B includes parous women only.
Post-menopausal women
If we model the association between parity and BMD using a categorical parity measure, BMD of women with 1–2 births is 1.05 SD’s higher than BMD of women with 10+ births (p = 0.034; Table 2, Fig. S2). In this model each SD increase in age is associated with a 0.55 SD decrease in BMD (Unstd. BAge = −0.005 g/cm2, p<0.001). BMD is inversely associated with number of births although the association is not significant (Unstd. B# births = −0.004 g/cm2, 95% CI: −0.009 – 0.002, p = 0.239; Table 2, Fig. S3).
BMD by mean IBI and age at first birth
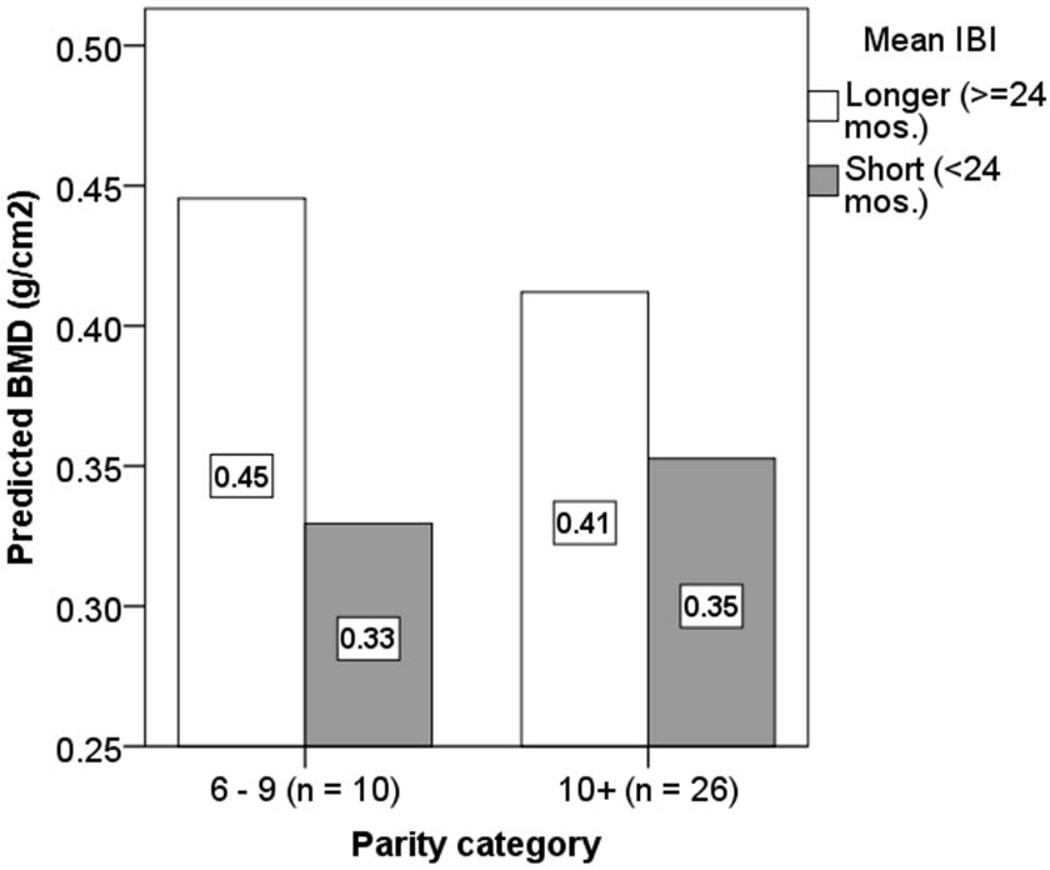
We limit analyses to post-menopausal women to test whether BMD is positively associated with mean IBI1. BMD is positively associated with IBI after controlling for age and weight (Table 3: Model A) (Fig. S4). This association remains (Std. βMean IBI = 0.27, 95% CI: −0.042 – 0.587, p = 0.087) after also controlling for parity, which is a significant predictor (Table 3: Model B). BMD among women with longer IBI’s (>24 mos.) is about 20% higher than among women with short IBI’s (Table 3: Models C-D) (Fig. 3).
Table 3.
Unstandardized (standardized) coefficients from OLS regressions of mean IBI and age at first birtha on estimated calcaneal BMD (g/cm2) for multiparous post-menopausal Tsimane women (n = 41).
| Continuous IBI measure | Categorical IBI measure | ||||
|---|---|---|---|---|---|
| Model A | Model B | Model C | Model D | Model E | |
| Parameter | Unstd. B (Std. β) | Unstd. B (Std. β) | Unstd. B (Std. β) | Unstd. B (Std. β) | Unstd. B (Std. β) |
| Age (years) | −0.006** (−0.63) | −0.006** (−0.61) | −0.006** (−0.61) | −0.006** (−0.59) | −0.005** (−0.54) |
| Weight (kg) | 0.002 (0.18) | 0.001 (0.07) | 0.002 (0.18) | 0.001 (0.07) | 0.002 (0.17) |
| Parity category | |||||
| 10+ births | −0.113 (−1.22) | −0.157* (−1.70) | −0.133* (−1.43) | ||
| 6–9 births | −0.127 (−1.37) | −0.167* (−1.80) | −0.134* (−1.45) | ||
| 3–5 births | −0.183* (−1.97) | −0.208** (−2.24) | −0.191** (−2.06) | ||
| 2 births | (ref.) | (ref.) | (ref.) | ||
| Mean IBI (mos.) | 0.002* (0.25) | 0.003t (0.27) | |||
| Mean IBI ≥24 mos. (vs. < 24 mos.) | 0.077** (0.83) | 0.078** (0.84) | 0.071** (0.77) | ||
| Age at first birth ≥16 (vs. < age 16) |
0.061* (0.66) | ||||
| Adjusted R2 | 0.43 | 0.47 | 0.50 | 0.56 | 0.63 |
p ≤ 0.10
p ≤ 0.05
p ≤ 0.01
Continuous and categorical measures were added in separate models to examine linear and non-linear associations.
Fig. 3.
Estimated calcaneal BMD by parity and mean IBI among post-menopausal Tsimane women. Predicted values are obtained from OLS regression (using parameters in Table 3: Model D). Other parity categories are omitted because women with short mean IBI’s are not represented.
BMD among women who initiated reproduction later (≥ age 16) is 15% higher than among women who initiated reproduction earlier after controlling for age, weight, parity, and mean IBI (Table 3: Model E). We find no significant interaction effects of early age at first birth and parity, of early age at first birth and mean IBI, or of mean IBI and parity on BMD. Age at first birth (continuous or categorical) is not associated with BMD among pre-menopausal women in uni- or multivariate analyses (not shown).
Comparison of Tsimane BMD to American reference values
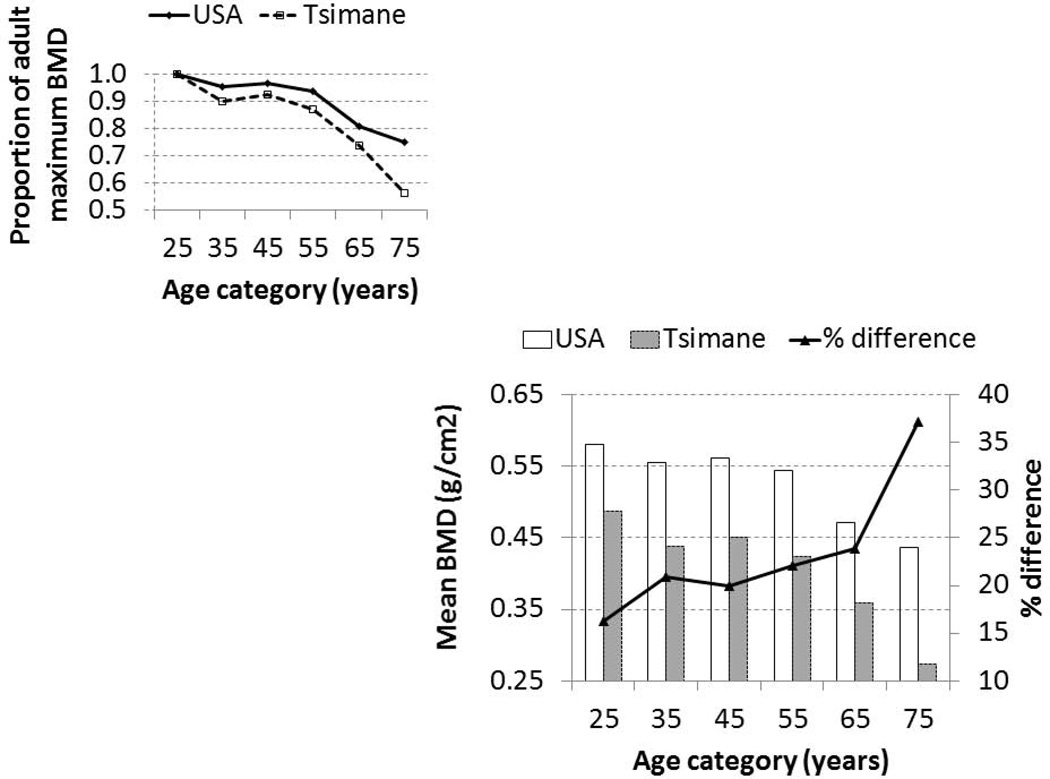
Figure 4 shows BMD by age among Tsimane and American women. On average Tsimane BMD is 16% lower in the 20s, 20–24% lower from the 30s to the 60s, and 37% lower by the 70s. Whereas by the 70s Tsimane BMD falls below 60% of the adult maximum, American BMD remains at 75%. Because anthropometric data on the American reference sample are not available, it is difficult to determine the extent to which population-level differences in BMD are due to differences in body weight or other body compositional characteristics. However, the magnitude of the population-level differences remains very similar throughout adulthood after adjusting Tsimane BMD for weight (not shown).
Fig. 4.
Estimated calcaneal BMD and proportion of the adult maximum (upper left) by age among Tsimane and American women, as measured by the Sahara Clinical Bone Sonometer. US reference values are provided by the manufacturer based on measurements of 2,208 participants across nine geographical regions. Mean BMD per decade is provided by the manufacturer at decade midpoints. Women below age 20 and above 80 are omitted since comparative data are not available.
Using the Tsimane female young adult reference mean ± SD (0.487 ± 0.072 g/cm2), mean T-scores are osteopenic by the 60s, although a substantial percentage of women (39%) are osteopenic in the 30s (Table 4). Using the American female young adult reference mean ± SD (0.581 ± 0.112 g/cm2), mean T-scores are osteopenic in the 30s, and 58% of all Tsimane are osteopenic (compared to 31% using the Tsimane young adult reference mean). Each reference group yields an identical prevalence of osteoporosis for women aged 20+ (6.5%, n = 107) and women aged 60+ (53.8%, n = 13).
Table 4.
Tsimane T-scorea (mean and proportions osteopenic, osteoporotic) by age using Tsimane and American female young adult reference groups.
| Young adult reference group |
||||||||
|---|---|---|---|---|---|---|---|---|
| Tsimane |
American |
|||||||
| Proportion of women |
Proportion of women |
|||||||
| Age category (n) | Mean T-score |
T-score ≥ −1 Normalb |
−1 < T-score < −2.5 Osteopenic |
T-score ≤ −2.5 Osteoporotic |
Mean T-score |
T-score ≥ −1 Normal |
−1 < T-score < −2.5 Osteopenic |
T-score ≤ −2.5 Osteoporotic |
| 20–29 (27) | −0.01 | 0.93 | 0.07 | 0 | −0.84 | 0.56 | 0.44 | 0 |
| 30–39 (28) | −0.67 | 0.61 | 0.39 | 0 | −1.27 | 0.29 | 0.71 | 0 |
| 40–49 (26) | −0.51 | 0.62 | 0.38 | 0 | −1.17 | 0.35 | 0.65 | 0 |
| 50–59 (13) | −0.87 | 0.54 | 0.46 | 0 | −1.40 | 0.31 | 0.69 | 0 |
| 60–69 (8) | −1.79 | 0.25 | 0.38 | 0.38d | −1.99 | 0.25 | 0.38 | 0.38d |
| 70–79c (5) | −2.96 | 0 | 0.20 | 0.80 | −2.74 | 0 | 0.20 | 0.80 |
| Total (107) | −0.68 | 0.63 | 0.31 | 0.07d | −1.28 | 0.36 | 0.58 | 0.07d |
See Methods for T-score definition.
The WHO defines osteopenia (low bone mass) as a T-score between −1.0 and −2.5, and osteoporosis as a T-score ≤ −2.5. T-scores ≥ −1 are classified as normal.
Tsimane women aged 75–79 are not represented (max. age = 75)
Total ≠ 1 due to rounding error.
DISCUSSION
As predicted by the disposable soma theory, greater reproductive effort among Tsimane women is associated with reduced calcaneal BMD, an indicator of somatic decline. We find a decline in BMD of 1 SD for both pre- and post-menopausal women with 10+ births compared to women with 1–2 births after controlling for potential confounders (Table 2; Fig. S2). Short IBI is also associated with reduced BMD among women who have completed fertility (Table 3), perhaps indicating incomplete cycles of bone resorption and formation during reproductive years, and long-term effects on bone mineral status. In addition, early age at first birth is associated with reduced BMD, suggesting a trade-off between early fertility and subsequent somatic condition (cf. Madimenos et al. 2012). Our results do not support the possibility that greater reproductive effort is protective against subsequent bone loss, as has been suggested in developed nations with low fertility (e.g. Chantry et al. 2004). Instead, our results support a negative trade-off between fertility and somatic condition that is predicted by multiple evolutionary theories of aging (Hamilton 1966; Kirkwood and Austad 2000; Medawar 1952; Williams 1957). In response to high daily variance in availability of the nutrient-dense resources upon which humans have relied over evolutionary history (Gurven et al. 2012; Hill and Kintigh 2009; Kaplan et al. 2007), mobilization of maternal skeletal mineral stores during lactation may have guaranteed sufficient milk production to support infant growth. While maternal regulatory mechanisms may largely compensate for acute BMD losses during reproductive years (e.g. by retaining excess mineral in circulation to facilitate storage), accelerated bone mineral loss after menopause that is mediated by declining calciotropic hormone levels appears to be a robust feature of human aging in diverse socio-ecologies (e.g. Aspray et al. 1996). Age-related bone loss is also apparent among free-ranging female chimpanzees (Morbeck et al. 2002), who lack menopause and have longer birth spacing than human foragers.
Among pre-menopausal women self-selection into maternity does not strongly affect our results. No nulliparous post-menopausal women were sampled in this study, and so we are unable to determine whether such self-selection affects results at older ages. In high fertility societies nulliparity may be associated with other factors contributing to reduced BMD (e.g. sex steroid deficiency), and nulliparous women may be inappropriate controls for studies examining trade-offs between reproduction and somatic condition (Cure-Cure et al. 2002). Nulliparous women and those with 4+ births in cohort studies show higher mortality risks than women with low fertility (Hurt et al. 2006).
We find attainment of near-maximal BMD by age 15, roughly when regular ovarian cycling begins, and continued increases in BMD into the late 20s, as fertility and offspring dependency load increases. Tsimane BMD remains >90% of the maximum into the late 40s, suggesting tightly linked processes of bone resorption and formation during the reproductive years. Accelerated BMD decline begins in the early to mid-50s, as the number of highly dependent offspring approaches zero (Gurven and Walker 2006). By the early 70s, when few highly dependent grand-offspring remain, female BMD is 60–70% of the 15 year-old value and rate of BMD decline is marked. Some of these age-related changes are consistent with those found among women in developed nations, where at least 90% of peak bone mass (i.e. the highest level of bone mass achieved from growth) is acquired by late adolescence, and mineral acquisition continues into the late 20s (Bachrach 2001; Bailey et al. 1996; Matkovic et al. 1994). Recent cross-sectional and longitudinal qCT studies of trabecular vBMD at multiple sites (e.g. distal radius, tibia, lumbar spine) among American women aged 20+ suggest that while bone losses can begin as early as the third decade, losses accelerate around the age of menopause with changes in mineral regulating hormones (e.g. estrogen, progesterone) (Devlin 2011; Khosla 2013).
Despite the general similarities across populations, Tsimane women lose more BMD with age overall than American women at a peripheral skeletal site rich in trabecular bone (Fig. 4). Once peak calcaneal BMD is achieved in the late 20s Tsimane women lose 42% by age 75, compared to 25% among age-matched American women. These differences are apparent despite the fact that Tsimane engage in physically intensive subsistence work throughout life, and are less sedentary than American women (Gurven et al. 2013). Maintenance of structural integrity can result from periosteal response to mechanical loading (Lieberman et al. 2010; Mayhew et al. 2005). For example, bone can respond to greater force by increasing mass and thus reducing the strain generated by a force. Physically intensive subsistence work throughout adult life can increase mechanical strain on weight-bearing bones, and may prevent even greater age-related decline in BMD among Tsimane (cf. Sayers et al. 2011).
Aside from greater cumulative reproductive burden, other factors likely contribute to low BMD among Tsimane, as population-level differences are apparent prior to the onset of reproduction (Fig. 4; Table 4). Tsimane women in their 20s, both nulliparous and parous, show lower calcaneal BMD than age-matched American women, and this difference persists even after adjusting Tsimane BMD for weight, height and parity (not shown). Similarly, rural Gambian women aged 18 – 30 have lower radial bone mineral content than age-matched British women as assessed by single-photon absorptiometry (Prentice et al. 1991). Using X-rays, Himes et al. (1975) found reduced metacarpal cortex among rural Guatemalan pre-school children compared to American pre-schoolers, even after adjusting for body size (also see Walker et al. 1970). Together these findings suggest important contributions of genetic and environmental factors in affecting peak bone mass and subsequent bone loss. Twin and family studies indicate that 50 – 85% of the variance in peak BMD is genetically determined, and have identified multiple potential heritable determinants of bone strength (e.g. skeletal geometry, bone turnover rate, age at menarche) (Ralston and Uitterlinden 2010; Towne et al. 2005). Bone accrual and strength are likely determined by effects of polymorphisms in multiple genes, and their interactions with multi-level factors affecting bone metabolism (e.g. nutrient intake, excretion and absorption efficiency, physical activity level, mechanical loading, hormonal status) (e.g. Bachrach 2001; Ellison 1982).
One possibility is that greater immune activation in populations with greater pathogen exposure may lead to reduced BMD. Despite widespread recognition that morbidity inhibits somatic growth, effects of pathogen burden and immune activation on human bone metabolism, especially during periods of rapid mineral accretion, have not been well-characterized in free-living populations (but see May et al. 1993; Munday et al. 2006). Among Tsimane, high pathogen load (due to viruses, bacteria, parasites) increases immune activation throughout life (Blackwell et al. 2011; Gurven et al. 2008; Vasunilashorn et al. 2010), increasing biomarkers of inflammation (e.g. C-reactive protein, interleukin-6) that have been shown to stimulate osteoclastic bone resorption and inhibit osteoblast function in humans and other species (Ginaldi et al. 2005; Manolagas and Jilka 1995; Schett et al. 2006). Gastro-intestinal parasites are also common among Tsimane (Blackwell et al. 2013), and may directly impact mineral absorption efficiency (cf. Lunn 2000), with potential downstream consequences for bone mineralization. Perhaps there are also gene variants among Tsimane that in combination with their lifestyle favor reduced peak bone mass, in spite of high physical activity levels. For example, high IL-6 gene transcription contributes to lean body mass (Wernstedt et al. 2004) and potentially reduced bone mass. A promoter SNP (position −174) underlying IL-6 production is monomorphic among Tsimane (Vasunilashorn et al. 2011), suggesting a potential mechanism linking genetic history, high infectious burden, reduced body mass, and reduced BMD. Future studies will examine these possibilities to understand why, throughout life, Tsimane female BMD is noticeably lower than that of Ecuadorian Shuar females, who similarly engage in a foraging-horticultural lifestyle but also experience greater market access, reduced pathogen burden, and have lower fertility and higher BMI (Madimenos et al. 2012).
Dietary factors also likely contribute to variation in bone mineral status across and within populations, although we do not directly consider such factors in the present study. Controlled calcium supplementation trials, conducted mostly among healthy youth in developed nations, consistently demonstrate gains in bone mineral accrual rates, although responses to supplementation can vary by baseline energetic status, age, and skeletal site (Bachrach 2001). Indian pre-schoolers aged 3 – 5 accustomed to low calcium intake (~ 200 mg/day) but with adequate protein and calorie intake maintain positive calcium balance (Begum and Pereira 1969; also see Prentice and Bates 1993). Further suggesting a link between macro-nutrient intake and bone mineral status, among Senegalese children 9 – 24 months old, severe protein-energy malnutrition is associated with reduced serum osteocalcin, a marker of bone formation (Ndiaye et al. 1995). Tsimane rarely experience such severe malnutrition as occurs in kwashiorkor or marasmus, but low energetic surplus may interact with high immune activation to reduce BMD, despite high physical activity levels and compensatory mechanisms that regulate mineral balance.
This study has several limitations. The design is cross-sectional, which limits our ability to document age-related change in BMD, or establish that greater reproductive effort causes lower BMD. Another limitation is that we use qUS to assess BMD rather than DXA, which is preferred for diagnostic purposes, or qCT which provides three-dimensional estimates of trabecular and cortical vBMD. We therefore cannot examine micro-architectural bone properties (e.g. trabecular separation, cortical porosity) which can affect fracture risk even after adjusting for BMD. In addition, we only measure one peripheral skeletal site rich in trabecular bone, and we lack data on central sites (e.g. lumbar spine, hip) and sites rich in cortical bone.
To conclude, this study examines calcaneal BMD in a representative sample of 130 Tsimane women aged 15–75. We find that greater reproductive effort is associated with somatic decline, particularly after menopause as bone resorption outpaces formation. Fitness impacts of kin transfers in adulthood may also influence the value of investments in bone remodeling, and affect selection on age-profiles of bone mineral loss. Human food sharing and pooled energy budgets due to intergenerational transfers inflate personal energy budgets and permit somatic investment that otherwise would not be possible by relying on one’s own efforts (Gurven et al. 2012). Processes of bone resorption and formation are strongly connected during the reproductive years, but weaken in older adulthood as reproduction ceases and downward inter-generational transfers subside. Future studies that standardize methods (e.g. techniques to measure bone, skeletal sites and bone properties measured, age ranges, adjustment for confounders) are needed for valid species- and population-level comparisons of trabecular and cortical bone losses across the lifespan. Studies of skeletal tissue in vivo in small-scale societies may reveal novel insights into bone metabolism and micro-architecture.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gandhi Yetish, Matthew Schwartz, and THLHP personnel for assistance with data collection and coding. Funding was provided by the National Institutes of Health/National Institute on Aging (R01AG024119).
Footnotes
Pre-menopausal women are omitted from analyses as their mean IBI is censored. In addition, the sub-sample of pre-menopausal women with short IBI’s (≤24 months) may not be representative. On average pre-menopausal women with short IBI’s are 2% shorter and 8% lighter than women with longer IBI’s, whereas among post-menopausal women this difference is not apparent. Moreover, of 97 multiparous study participants, the only women to have lost at least one young child (< age 4) since the THLHP’s inception were two pre-menopausal women with short IBI’s. While, as expected, post-menopausal women with short IBI’s have higher completed fertility than women with longer IBI’s (12.2 vs. 9.4 births, t = 2.465, p = 0.018), for pre-menopausal women there is no significant difference (short IBI = 6.3 births vs. longer IBI = 5.1 births, t = 1.236, p = 0.158). Together these sample characteristics might indicate unmeasured factors affecting variation in BMD, and to minimize potential confounders we omit pre-menopausal women from analyses.
LITERATURE CITED
- Adair LS, Popkin BM. Prolonged lactation contributes to depletion of maternal energy reserves in Filipino women. The Journal of nutrition. 1992;122(8):1643–1655. doi: 10.1093/jn/122.8.1643. [DOI] [PubMed] [Google Scholar]
- Allali F, Maaroufi H, Aichaoui SE, Khazani H, Saoud B, Benyahya B, Abouqal R, Hajjaj-Hassouni N. Influence of parity on bone mineral density and peripheral fracture risk in Moroccan postmenopausal women. Maturitas. 2007;57(4):392–398. doi: 10.1016/j.maturitas.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Aspray TJ, Prentice A, Cole TJ, Sawo Y, Reeve J, Francis RM. Low bone mineral content is common but osteoporotic fractures are rare in elderly rural Gambian women. J Bone Miner Res. 1996;11(7):1019–1025. doi: 10.1002/jbmr.5650110720. [DOI] [PubMed] [Google Scholar]
- Bachrach LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. 2001;12(1):22–28. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- Bailey DA, Faulkner RA, McKay HA. Growth, physical activity, and bone mineral acquisition. Exerc Sport Sci Rev. 1996;24:233–266. [PubMed] [Google Scholar]
- Baroncelli GI. Quantitative ultrasound methods to assess bone mineral status in children: technical characteristics, performance, and clinical application. Pediatric research. 2008;63(3):220–228. doi: 10.1203/PDR.0b013e318163a286. [DOI] [PubMed] [Google Scholar]
- Begum A, Pereira S. Calcium balance studies on children accustomed to low calcium intakes. British Journal of Nutrition. 1969;23(4):905–911. doi: 10.1079/bjn19690101. [DOI] [PubMed] [Google Scholar]
- Black A, Topping J, Durham B, Farquharson R, Fraser W. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. Journal of Bone and Mineral Research. 2000;15(3):557–563. doi: 10.1359/jbmr.2000.15.3.557. [DOI] [PubMed] [Google Scholar]
- Blackwell A, Gurven M, Sugiyama L, Madimenos F, Liebert M, Martin M, Kaplan H, Snodgrass J. Evidence for a peak shift in a humoral response to helminths: Age profiles of IgE in the Shuar of Ecuador, the Tsimane of Bolivia, and the U.S. NHANES. PLoS Neglected Tropical Diseases. 2011;5(6):e1218. doi: 10.1371/journal.pntd.0001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Martin M, Kaplan H, Gurven M. Antagonism between two intestinal parasites in humans: the importance of co-infection for infection risk and recovery dynamics. Proceedings of the Royal Society B: Biological Sciences. 2013;280(1769):20131671. doi: 10.1098/rspb.2013.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the united states. JAMA. 2009;302(14):1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantry CJ, Auinger P, Byrd RS. Lactation among adolescent mothers and subsequent bone mineral density. Archives of Pediatrics & Adolescent Medicine. 2004;158(7):650–656. doi: 10.1001/archpedi.158.7.650. [DOI] [PubMed] [Google Scholar]
- Cure-Cure C, Cure-Ramírez P, Teran E, Lopez-Jaramillo P. Bone-mass peak in multiparity and reduced risk of bone-fractures in menopause. International journal of gynecology & obstetrics. 2002;76(3):285–291. doi: 10.1016/s0020-7292(01)00583-5. [DOI] [PubMed] [Google Scholar]
- Devlin MJ. Estrogen, exercise, and the skeleton. Evolutionary Anthropology: Issues, News, and Reviews. 2011;20(2):54–61. doi: 10.1002/evan.20299. [DOI] [PubMed] [Google Scholar]
- DiGirolamo DJ, Clemens TL, Kousteni S. The skeleton as an endocrine organ. Nature Reviews Rheumatology. 2012;8(11):674–683. doi: 10.1038/nrrheum.2012.157. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Skeletal growth, fatness and menarcheal age: a comparison of two hypotheses. Hum Biol. 1982;54(2):269–281. [PubMed] [Google Scholar]
- Forbes GB. Calcium accumulation by the human fetus. Pediatrics. 1976;57(6):976–977. [PubMed] [Google Scholar]
- Frost M, Blake G, Fogelman I. Can the WHO criteria for diagnosing osteoporosis be applied to calcaneal quantitative ultrasound? Osteoporosis International. 2000;11(4):321–330. doi: 10.1007/s001980070121. [DOI] [PubMed] [Google Scholar]
- Ghannam N, Hammami M, Bakheet S, Khan B. Bone mineral density of the spine and femur in healthy Saudi females: relation to vitamin D status, pregnancy, and lactation. Calcified tissue international. 1999;65(1):23–28. doi: 10.1007/s002239900652. [DOI] [PubMed] [Google Scholar]
- Ginaldi L, Di Benedetto M, De Martinis M. Osteoporosis, inflammation and ageing. Immunity & Ageing. 2005;2(1):14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur A, Nas K, Cevik R, Sarac AJ, Ataoglu S, Karakoc M. Influence of number of pregnancies on bone mineral density in postmenopausal women of different age groups. Journal of bone and mineral metabolism. 2003;21(4):234–241. doi: 10.1007/s00774-003-0415-9. [DOI] [PubMed] [Google Scholar]
- Gurven M, Jaeggi AV, Kaplan H, Cummings D. Physical Activity and Modernization among Bolivian Amerindians. Plos One. 2013;8(1):e55679. doi: 10.1371/journal.pone.0055679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Finch C, Crimmins EM. Aging and inflammation in two epidemiological worlds. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63(2):196–199. doi: 10.1093/gerona/63.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Zelada Supa A. Mortality experience of Tsimane Amerindians of Bolivia: Regional variation and temporal trends. American Journal of Human Biology. 2007;19:376–398. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- Gurven M, Stieglitz J, Hooper PL, Gomes C, Kaplan H. From the womb to the tomb: The role of transfers in shaping the evolved human life history. Experimental Gerontology. 2012;47:807–813. doi: 10.1016/j.exger.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Walker R. Energetic demand of multiple dependents and the evolution of slow human growth. Proceedings of the Royal Society Series B. 2006;273:835–841. doi: 10.1098/rspb.2005.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD. The moulding of senescence by natural selection. Journal of Theoretical Biology. 1966;12(1):12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Hill K, Kintigh K. Can anthropologists distinguish good and poor hunters? Implications for hunting hypotheses, sharing conventions, and cultural transmission. Current Anthropology. 2009;50(3):369–378. [Google Scholar]
- Himes J, Martorell R, Habicht J, Yarbrough C, Malina R, Klein R. Patterns of cortical bone growth in moderately malnourished preschool children. Human Biology. 1975:337–350. [PubMed] [Google Scholar]
- Hurt LS, Ronsmans C, Thomas SL. The effect of number of births on women's mortality: systematic review of the evidence for women who have completed their childbearing. Population studies. 2006;60(1):55–71. doi: 10.1080/00324720500436011. [DOI] [PubMed] [Google Scholar]
- Jarjou LM, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake. The American journal of clinical nutrition:ajcn. 2010:29217. doi: 10.3945/ajcn.2010.29217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelliffe DB, Maddocks I. Notes on ecologic malnutrition in the New Guinea Highlands. Clinical Pediatrics. 1964;3(7):432–438. doi: 10.1177/000992286400300710. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Gurven M, Lancaster J. Brain evolution and the human adaptive complex: An ecological and social theory. In: Gangestad S, Simpson J, editors. The Evolution of Mind: Fundamental Questions and Controversies. New York: Guilford Press; 2007. pp. 269–279. [Google Scholar]
- Khosla S. Pathogenesis of age-related bone loss in humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68(10):1226–1235. doi: 10.1093/gerona/gls163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408(6809):233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1991;332(1262):15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Kojima N, Douchi T, Kosha S, Nagata Y. Cross-sectional study of the effects of parturition and lactation on bone mineral density later in life. Maturitas. 2002;41(3):203–209. doi: 10.1016/s0378-5122(01)00296-1. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Venkadesan M, Werbel WA, Daoud AI, D’Andrea S, Davis IS, Mang’Eni RO, Pitsiladis Y. Foot strike patterns and collision forces in habitually barefoot versus shod runners. Nature. 2010;463(7280):531–535. doi: 10.1038/nature08723. [DOI] [PubMed] [Google Scholar]
- Lunn PG. The impact of infection and nutrition on gut function and growth in childhood. Proc Nutr Soc. 2000;59(1):147–154. doi: 10.1017/s0029665100000173. [DOI] [PubMed] [Google Scholar]
- Lycett JE, Dunbar R, Voland E. Longevity and the costs of reproduction in a historical human population. Proceedings of the Royal Society of London Series B: Biological Sciences. 2000;267(1438):31–35. doi: 10.1098/rspb.2000.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madimenos FC, Snodgrass JJ, Liebert MA, Cepon TJ, Sugiyama LS. Reproductive effects on skeletal health in Shuar women of Amazonian Ecuador: A life history perspective. American Journal of Human Biology. 2012;24(6):841–852. doi: 10.1002/ajhb.22329. [DOI] [PubMed] [Google Scholar]
- Maga M, Kappelman J, Ryan TM, Ketcham RA. Preliminary observations on the calcaneal trabecular microarchitecture of extant large-bodied hominoids. American Journal of Physical Anthropology. 2006;129(3):410–417. doi: 10.1002/ajpa.20276. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332(5):305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- Martin MA, Lassek WD, Gaulin SJ, Evans RW, Woo JG, Geraghty SR, Davidson BS, Morrow AL, Kaplan HS, Gurven MD. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Maternal & child nutrition. 2012;8(3):404–418. doi: 10.1111/j.1740-8709.2012.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovic V, Jelic T, Wardlaw GM, Ilich JZ, Goel PK, Wright JK, Andon MB, Smith KT, Heaney RP. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93(2):799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May RL, Goodman AH, Meindl RS. Response of bone and enamel formation to nutritional supplementation and morbidity among malnourished Guatemalan children. American Journal of Physical Anthropology. 1993;92(1):37–51. doi: 10.1002/ajpa.1330920104. [DOI] [PubMed] [Google Scholar]
- Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J. Relation between age, femoral neck cortical stability, and hip fracture risk. The Lancet. 2005;366(9480):129–135. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- Mcallister L, Gurven M, Kaplan H, Stieglitz J. Why do women have more children than they want? Understanding differences in women's ideal and actual family size in a natural fertility population. American Journal of Human Biology. 2012;24(6):786–799. doi: 10.1002/ajhb.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar PB. An Unsolved Problem in Biology. London: Lewis; 1952. [Google Scholar]
- Miller EM. Maternal hemoglobin depletion in a settled Northern Kenyan pastoral population. American Journal of Human Biology. 2010;22(6):768–774. doi: 10.1002/ajhb.21078. [DOI] [PubMed] [Google Scholar]
- Morbeck M, Galloway A, Sumner D. Getting old at Gombe: skeletal aging in wild-ranging chimpanzees. Interdisciplinary Topics in Gerontology: Aging in Nonhuman Primates. J. Erwin and P. Hof. Basel, Karger. 2002;31:48–62. [Google Scholar]
- Muggeo VM. Estimating regression models with unknown break-points. Statistics in medicine. 2003;22(19):3055–3071. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- Munday K, Ginty F, Fulford A, Bates CJ. Relationships between biochemical bone turnover markers, season, and inflammatory status indices in prepubertal Gambian boys. Calcif Tissue Int. 2006;79(1):15–21. doi: 10.1007/s00223-005-0276-4. [DOI] [PubMed] [Google Scholar]
- Ndiaye B, Lemonnier D, Sall M, Prudhon C, Diaham B, Zeghoud F, Guillozo H, Leite N, Wade S. Serum osteocalcin regulation in protein-energy malnourished children. Pediatric Research. 1995;37(5):606–610. doi: 10.1203/00006450-199505000-00008. [DOI] [PubMed] [Google Scholar]
- Njeh C, Boivin C, Langton C. The role of ultrasound in the assessment of osteoporosis: a review. Osteoporosis International. 1997;7(1):7–22. doi: 10.1007/BF01623454. [DOI] [PubMed] [Google Scholar]
- Olausson H, Laskey MA, Goldberg GR, Prentice A. Changes in bone mineral status and bone size during pregnancy and the influences of body weight and calcium intake. The American journal of clinical nutrition. 2008;88(4):1032–1039. doi: 10.1093/ajcn/88.4.1032. [DOI] [PubMed] [Google Scholar]
- Platzer W. Color Atlas of Human Anatomy, Locomotor System. Vol. 1. Stuttgart, New York: Thieme; 2009. [Google Scholar]
- Prentice A. Micronutrients and the bone mineral content of the mother, fetus and newborn. The Journal of nutrition. 2003;133(5):1693S–1699S. doi: 10.1093/jn/133.5.1693S. [DOI] [PubMed] [Google Scholar]
- Prentice A, Bates C. An appraisal of the adequacy of dietary mineral intakes in developing countries for bone growth and development in children. Nutrition Research Reviews. 1993;6:51–69. doi: 10.1079/NRR19930006. [DOI] [PubMed] [Google Scholar]
- Prentice A, Jarjou L, Cole TJ, Stirling DM, Dibba B, Fairweather-Tait S. Calcium requirements of lactating Gambian mothers: effects of a calcium supplement on breast-milk calcium concentration, maternal bone mineral content, and urinary calcium excretion. The American journal of clinical nutrition. 1995;62(1):58–67. doi: 10.1093/ajcn/62.1.58. [DOI] [PubMed] [Google Scholar]
- Prentice A, Shaw J, Laskey M, Cole TJ, Fraser DR. Bone mineral content of British and rural Gambian women aged 18–80+ years. Bone and mineral. 1991;12(3):201–214. doi: 10.1016/0169-6009(91)90033-v. [DOI] [PubMed] [Google Scholar]
- Ralston SH, Uitterlinden AG. Genetics of osteoporosis. Endocr Rev. 2010;31(5):629–662. doi: 10.1210/er.2009-0044. [DOI] [PubMed] [Google Scholar]
- Saadi HF, Reed RL, Carter AO, Dunn EV, Qazaq H, Al-Suhaili A. Quantitative ultrasound of the calcaneus in Arabian women: relation to anthropometric and lifestyle factors. Maturitas. 2003;44(3):215–223. doi: 10.1016/s0378-5122(02)00339-0. [DOI] [PubMed] [Google Scholar]
- Sawo Y, Jarjou L, Goldberg G, Laskey M, Prentice A. Bone mineral changes after lactation in Gambian women accustomed to a low calcium intake. European journal of clinical nutrition. 2013;67(11):1142–1146. doi: 10.1038/ejcn.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers A, Mattocks C, Deere K, Ness A, Riddoch C, Tobias JH. Habitual levels of vigorous, but not moderate or light, physical activity is positively related to cortical bone mass in adolescents. Journal of Clinical Endocrinology and Metabolism. 2011;96(5):E793–E802. doi: 10.1210/jc.2010-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G, Kiechl S, Weger S, Pederiva A, Mayr A, Petrangeli M, Oberhollenzer F, Lorenzini R, Redlich K, Axmann R, et al. High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch Intern Med. 2006;166(22):2495–2501. doi: 10.1001/archinte.166.22.2495. [DOI] [PubMed] [Google Scholar]
- Sowers M. Pregnancy and lactation as risk factors for subsequent bone loss and osteoporosis. Journal of Bone and Mineral Research. 1996;11(8):1052–1060. doi: 10.1002/jbmr.5650110803. [DOI] [PubMed] [Google Scholar]
- Sowers M, Corton G, Shapiro B, Jannausch ML, Crutchfield M, Smith ML, Randolph JF, Hollis B. Changes in bone density with lactation. JAMA. 1993;269(24):3130–3135. [PubMed] [Google Scholar]
- Stieglitz J, Jaeggi A, Blackwell A, Trumble B, Gurven M, Kaplan H. Work to live and live to work: Productivity, transfers, and psychological well-being in adulthood and old age. In: Weinstein M, Lane M, editors. Advances in Biodemography: Cross-Species Comparisons of Social Environments and Social Behaviors, and their Effects on Health and Longevity. Washington DC: National Academies Press; 2014. pp. 197–221. [Google Scholar]
- Stieglitz J, Kaplan H, Schniter E, von Rueden C, Gurven M. Reduced functional status and social conflict increase risk of depression in later adulthood among Bolivian forager-farmers. Journal of Gerontology: Social Sciences. doi: 10.1093/geronb/gbu080. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeten EA, Ryan KA, McBride DJ, Pollin TI, Shuldiner AR, Mitchell BD. The relationship between parity and bone mineral density in women characterized by a homogeneous lifestyle and high parity. The Journal of Clinical Endocrinology & Metabolism. 2005;90(8):4536–4541. doi: 10.1210/jc.2004-1924. [DOI] [PubMed] [Google Scholar]
- Towne B, Czerwinski SA, Demerath EW, Blangero J, Roche AF, Siervogel RM. Heritability of age at menarche in girls from the Fels Longitudinal Study. American Journal of Physical Anthropology. 2005;128(1):210–219. doi: 10.1002/ajpa.20106. [DOI] [PubMed] [Google Scholar]
- Tracer DP. Fertility-related changes in maternal body composition among the au of Papua New Guinea. American Journal of Physical Anthropology. 1991;85(4):393–405. doi: 10.1002/ajpa.1330850404. [DOI] [PubMed] [Google Scholar]
- Tracer DP. Somatic versus reproductive energy allocation in Papua New Guinea: Life history theory and public health policy. American Journal of Human Biology. 2002;14(5):621–626. doi: 10.1002/ajhb.10073. [DOI] [PubMed] [Google Scholar]
- Vasunilashorn S, Crimmins EM, Kim JK, Winking J, Gurven M, Kaplan H, Finch CE. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. American Journal of Human Biology. 2010;22(6):731–740. doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasunilashorn S, Finch CE, Crimmins EM, Vikman SA, Stieglitz J, Gurven M, Kaplan H, Allayee H. Inflammatory gene variants in the Tsimane, an indigenous Bolivian population with a high infectious load. Biodemography and Social Biology. 2011;57(1):33–52. doi: 10.1080/19485565.2011.564475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veile A, Martin M, McAllister L, Gurven M. Modernization is associated with intensive breastfeeding patterns in the Bolivian Amazon. Social Science & Medicine. 2014;100:148–158. doi: 10.1016/j.socscimed.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JM, Wasnich RD, Ross PD. The clinical relevance of calcaneus bone mineral measurements: a review. Bone Miner. 1988;5(1):35–58. doi: 10.1016/0169-6009(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Walker AR, Walker BF, Richardson BD, Christ HH. Cortical thickness of bone in underprivileged populations. Am J Clin Nutr. 1970;23(3):244–245. doi: 10.1093/ajcn/23.3.244. [DOI] [PubMed] [Google Scholar]
- Wernstedt I, Eriksson AL, Berndtsson A, Hoffstedt J, Skrtic S, Hedner T, Hulten LM, Wiklund O, Ohlsson C, Jansson JO. A common polymorphism in the interleukin-6 gene promoter is associated with overweight. Int J Obes Relat Metab Disord. 2004;28(10):1272–1279. doi: 10.1038/sj.ijo.0802763. [DOI] [PubMed] [Google Scholar]
- Westendorp RG, Kirkwood TB. Human longevity at the cost of reproductive success. Nature. 1998;396(6713):743–746. doi: 10.1038/25519. [DOI] [PubMed] [Google Scholar]
- WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Geneva: WHO Press; 1994. [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Zapata CLV, Donangelo CM, Woodhouse LR, Abrams SA, Spencer EM, King JC. Calcium homeostasis during pregnancy and lactation in Brazilian women with low calcium intakes: a longitudinal study. The American journal of clinical nutrition. 2004;80(2):417–422. doi: 10.1093/ajcn/80.2.417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.