Abstract
Background
Long-term survivors of pediatric cancer are at risk for life-threatening late effects of their cancer. Previous studies have shown excesses in long-term mortality within high-risk groups defined by demographic and treatment characteristics.
Methods
To investigate conditional survival in a pediatric cancer population, we performed an analysis of conditional survival in the original Childhood Cancer Survivor Study (CCSS) cohort and the Surveillance, Epidemiology and End Results (SEER) database registry. The overall probability of death for patients in 5 years and 10 years after they survived 5, 10, 15, and 20 years since cancer diagnosis, and cause-specific death in 10 years for 5-year survivors were estimated using the cumulative incidence method.
Results
Among CCSS and SEER patients who were alive 5 years post cancer diagnosis, within each diagnosis group at least 92% are alive in the subsequent 5 years, except leukemia patients of whom only 88% of 5-year survivors remain alive in the subsequent 5 years. The probability of all-cause mortality in the next 10 years on patients who survived at least 5 years after diagnosis, was 8.8% in CCSS and 10.6% in SEER, approximately three quarter of which were due to neoplasms as causes of death.
Conclusion
The risk of death of pediatric cancer survivors in 10 years can vary between diagnosis groups by at most 12% even up to 20 years post diagnosis. This information is clinically important in counseling patients on their conditional survival, particularly when survivors are seen in long-term follow-up.
Keywords: pediatric cancer, survivors, conditional survival, cause-specific mortality, cohort study
Introduction
Improvements in therapy and supportive care for children diagnosed with cancer have increased the overall 5-year survival rate from 45% for patients diagnosed in the mid-1970s to over 80% [1]. Estimates place the number of childhood cancer survivors in the U.S. at 420,000 at the end of 2013 [2]. Moreover, it is estimated that the number of years of life saved by the successful treatment of childhood cancer exceeds all other cancer in the United States (US) [1]. When compared to adults, children have more potential life-years ahead of them, thus the quality of survivorship becomes even more important. Long-term survivors of childhood cancer are at risk for serious, disabling, life-threatening or fatal treatment-related late effects including second malignancies, cardiac and vascular abnormalities, and pulmonary complications [2–7]. Previous studies of childhood cancer survivors have shown excesses in long-term mortality and have characterized high-risk groups defined by demographic and treatment characteristics [8–11].
Conditional survival is the likelihood of continued survival for a specified interval of time after having already survived for a specific time interval after cancer diagnosis [12–16]. This measurement of survival is clinically relevant because the likelihood of survival changes with increasing duration of follow-up from initial cancer diagnosis. As an example, the risk of recurrence and disease-related mortality is higher in the immediate period after diagnosis, whereas the risk of non-cancer related death and treatment-related death often increases with time [13–15]. In this instance, conditional survival is particularly useful for evaluation of childhood malignancies with a relatively high initial rate of cancer-related mortality that diminishes with time [15].
In contrast to the pediatric oncology literature, conditional survival is increasingly being applied in assessment of adult cancers through the use of large population databases such as the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER), as well as selected clinical trials [13–20]. To investigate conditional survival in the pediatric cancer population, we performed an analysis in two large and commonly cited populations of childhood cancer patients, the original Childhood Cancer Survivor Study (CCSS) cohort and the SEER-9 database. The CCSS cohort includes 5-year survivors of selected childhood cancers diagnosed and treated at 26 collaborating academic institutions. SEER collects and publishes cancer incidence and survival data from population-based cancer registries within the US. The objective of this analysis is to estimate conditional survival over time for pediatric cancer survivors starting with 5-year survivors for all causes and cause-specific deaths and determine the effect of primary diagnosis and institution (CCSS)/geographic location (SEER) on conditional survival. These data will provide a framework by which clinicians can counsel survivors and their families on survival with time.
Methods
CCSS Cohort
The CCSS is a multi-institutional retrospective cohort study of individuals with cancer before the age of 21 and survived for five or more years. Details of the study design, methods, and cohort characteristics have been previously reported [21]. In brief, eligibility criteria for CCSS are: a) diagnosis of leukemia, CNS malignancies (all histologies), Hodgkin lymphoma, non-Hodgkin lymphoma, malignant kidney tumor, neuroblastoma, soft tissue sarcoma, or bone tumor; b) diagnosis and initial treatment at one of the 26 collaborating CCSS institutions; c) diagnosis date between January 1, 1970 and December 31, 1986; d) age less than 21 years at diagnosis; e) survival of five years from diagnosis. The CCSS protocol and contact documents were reviewed and approved by the Human Subjects Committee at each participating institution.
CCSS data include self-report questionnaires and medical record abstraction of detailed treatment data from the treating institution [22, 23]. The initial baseline questionnaire was administered beginning in 1994 and was updated periodically with follow-up questionnaires through 2009. Demographics, including race and ethnicity were obtained on the baseline survey. Characteristics of the original cancer diagnosis were abstracted from the medical records at the treating institution.
Causes of Death in CCSS
Methods for ascertaining and categorizing deaths within the CCCS cohort have been described previously [8, 9]. In brief, to determine vital status of all eligible individuals, as of December 31, 2007, a National Death Index (NDI) search was requested for deaths occurring during the time period 1979–2007. For those who died in the US, cause of death information was provided by the NDI, using the International Classification of Disease (ICD-9 & ICD-10) classification [24]. For deaths occurred in 1975–78 (i.e., the years not covered by the NDI), copies of the death certificates were requested from all states where deaths had occurred. Death certificate data were not available for survivors who were Canadian residents at the time of death, thus they were excluded from the analyses. Within CCSS, there were 2,287 individuals who had been reported to have died by December 31, 2007.
SEER Registry
The SEER Program of the National Cancer Institute (NCI) is a source of information on cancer incidence and survival in the United States [25]. Between 1975 and 1986, the SEER registries were limited to nine regions (SEER-9): Atlanta, Connecticut, Detroit, San Francisco-Oakland, Seattle-Puget Sound, and the entire states of Hawaii, Iowa, New Mexico and Utah. The cancer diagnoses were restricted to those included in the CCSS cohort. Mortality data reported by SEER are provided by the National Center for Health Statistics. For the analyses reported here, the most recent follow-up year for mortality data was 2007.
Data Analysis
To select the same calendar years of diagnosis as SEER, CCSS survivors whose childhood cancer diagnosis occurred between 1975–86 were included. Demographic and clinical characteristics of survivors in the CCSS and the patients in SEER who survived at least 5 years since the original cancer diagnosis were tabulated. Patients with missing demographic or clinical characteristics, except race, were not included in the analysis. We compared demographic and clinical characteristics of the CCSS and SEER 5-year survivors using the Chi-square test. Conditional survival curves were estimated for CCSS and SEER separately with the Kaplan-Meier estimator, conditioned on having survived 5, 10, 15, and 20 years, for all survivors and by cancer diagnoses. Conditional probabilities of cause-specific death in 10 years for 5-year survivors (by 15 years since cancer diagnosis) and their 95% confidence interval were estimated by the cumulative incidence method, treating death due to any other causes as competing risk events. Causes of death were categorized into five groups: neoplastic (ICD-9 codes 140-239 & ICD-10 codes C00-C97, D00-D48); infectious (ICD-9 codes 001-018, 020-037, 039-088, 090-139, 461, 480-487, 541 & ICD-10 codes A00-A08, A15-A33,A40-B99,J09-J18); cardiac/vascular (ICD-9 codes 390-398, 401-404, 410-438, 440-448, 451-453, 456-459 & ICD-10 codes I00-I13, I20-I51, I60-I78,); external (ICD-9 codes 800-999 & ICD-10 codes U01-U03, V00-X59, X60-Y09, Y35, Y85-Y87, Y89); and the others. The permutation test was used to compare CCSS and SEER conditional probabilities of cause-specific death within 15 years of cancer diagnosis.
Five year probability of death conditioned on surviving 5 years, 10 years, 15, years and 20 years since cancer diagnosis and their 95% confidence interval were estimated by the cumulative incidence method, treating death as all cause of death for all survivors and for survivors of specific childhood cancer diagnoses. Graphs were produced using R, version 2.15.2 [26]. All the other statistical analyses were performed using SAS version 9.3 (SAS Institute).
Results
Study population characteristics
A total of 16,602 survivors in the CCSS cohort and 6,576 survivors in SEER registry met the eligibility criteria for this analysis. Demographic and clinical characteristics of survivors are shown in Table 1. The CCSS and SEER survivors were similar with respect to treatment era and sex, but different with respect to cancer diagnosis group, age at diagnosis, and possibly race/ethnicity (a proportion of CCSS survivors did not report their race/ethnicity). The median age at diagnosis among the 5-year survivors was 6.8 years in the CCCS cohort, compared to 11.0 years among the SEER survivors.
Table 1.
Characteristics of pediatric cancer patients who survived 5+ years post diagnosis
| CCSS | SEER | p-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Total 5-year survivors | 16,602 | 6,576 | |||
| Death status for those with survival/censor time 5+ years | |||||
| Alive | 14315 | 5389 | 5389 | 82.0 | |
| Dead | 2287 | 1187 | 1187 | 18.0 | |
| Sex | 0.01 | ||||
| Female | 7411 | 3056 | 3056 | 46.5 | |
| Male | 9191 | 3520 | 3520 | 53.5 | |
| Race/Ethnicity | |||||
| White, Non-Hispanic | 10094 | 37 | 37 | 0.6 | <0.001 |
| Black, Non-Hispanic | 591 | 5610 | 5610 | 85.3 | |
| Other | 1058 | 553 | 553 | 8.4 | |
| missing | 4859 | 376 | 376 | 5.7 | |
| Diagnosis group | <0.001 | ||||
| Leukemia | |||||
| Acute lymphoblastic | 4983 | 1329 | 1329 | 20.2 | |
| Acute myeloid leukemia | 438 | 131 | 131 | 2.0 | |
| Other leukemia | 188 | 99 | 99 | 1.5 | |
| Lymphoma | |||||
| Hodgkin lymphoma | 1999 | 1244 | 1244 | 18.9 | |
| Non-Hodgkin lymphoma | 1273 | 475 | 475 | 7.2 | |
| Bone Tumor | |||||
| Ewing sarcoma | 484 | 125 | 125 | 1.9 | |
| Osteosarcoma | 899 | 192 | 192 | 2.9 | |
| Other bone tumors | 82 | 47 | 47 | 0.7 | |
| Central Nervous System | |||||
| Astrocytomas | 1488 | 837 | 837 | 12.7 | |
| Medulloblastoma, PNET* | 481 | 190 | 190 | 2.9 | |
| Other CNS tumors | 374 | 505 | 505 | 7.7 | |
| Kidney tumors | 1413 | 408 | 408 | 6.2 | |
| Neuroblastoma | 1101 | 345 | 345 | 5.3 | |
| Soft tissue sarcoma | 1399 | 649 | 649 | 9.9 | |
| Age at diagnosis | <0.001 | ||||
| 0–4 | 6714 | 1902 | 1902 | 28.9 | |
| 5–9 | 3715 | 1189 | 1189 | 18.1 | |
| 10–14 | 3318 | 1157 | 1157 | 17.6 | |
| 15–20 | 2855 | 2328 | 2328 | 35.4 | |
| Treatment Era | <0.001 | ||||
| 1975–1980 | 7364 | 3059 | 3059 | 46.5 | |
| 1981–1986 | 9238 | 3517 | 3517 | 53.5 | |
Primitive Neuroectodermal Tumor
Conditional survival in CCSS and SEER
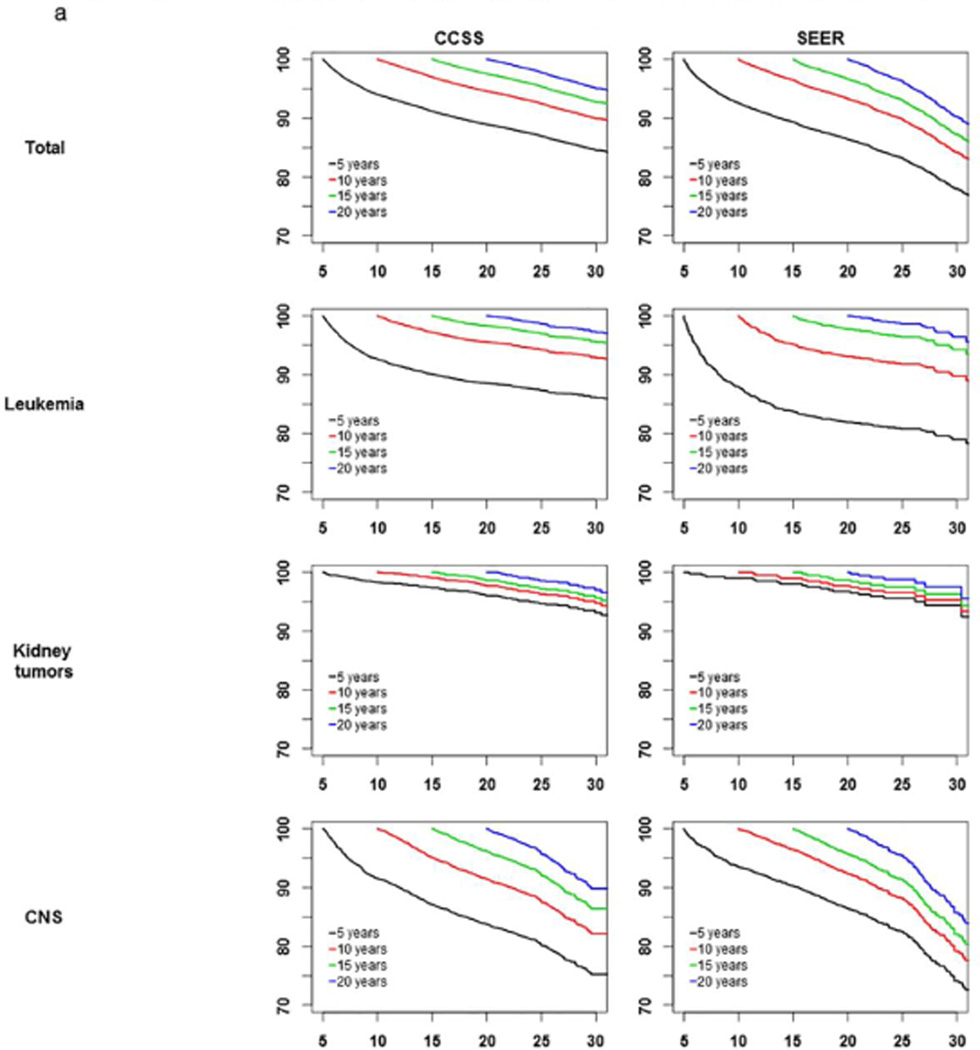
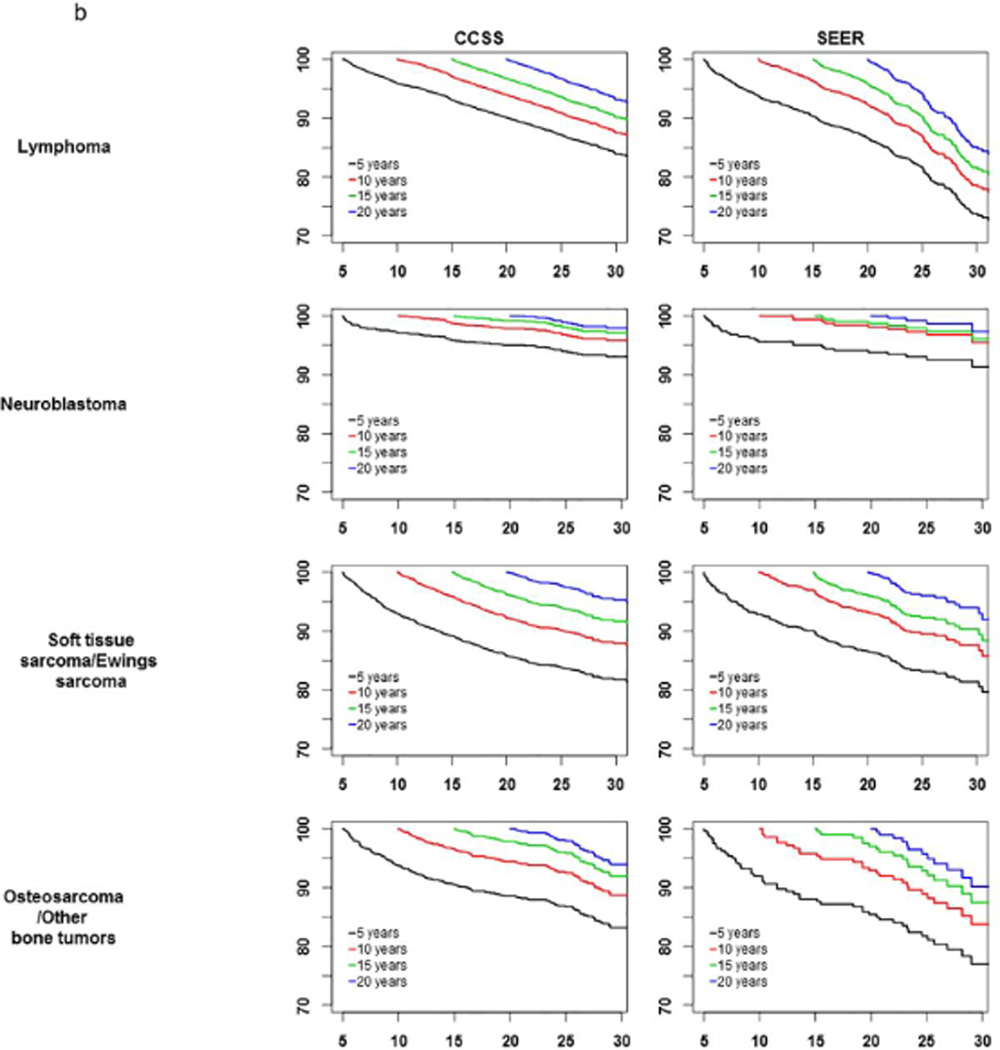
Conditional survival curves are shown in Figure 1 for all patients and by primary diagnosis, conditioned on 5, 10, 15, and 20 years of survival since diagnosis. Among all CCSS patients who were alive at least 5 years up to 20 years since cancer diagnosis, at least 91.6% of them within each diagnosis group were alive in the subsequent 5 years. Similarly, within SEER, at least 91.9% of all patients within each diagnosis group who survived at least 5 years up to 20 years after cancer diagnosis were alive in the subsequent 5 years, except leukemia patients of whom only 88% of 5-year survivors remain alive in the subsequent 5 years.
Figure 1.
a-b: Survival curves conditioned on 5-, 10-, 15-, 20-years survival after cancer diagnosis.
In both CCSS and SEER cohorts, in survival curves conditioned on 10-, 15-, and 20-year survival were similar and largely independent of their survival time since cancer diagnosis. For kidney tumors, neuroblastoma, and lymphoma patients, survival curves were parallel for 5-, 10-, 15- and 20-year survivors, meaning the chance of being alive for the next period(s) or time(s) was similar regardless of the number of years survived since 5-years after cancer diagnosis.
Probability of death in the next 10 years for 5-year survivors in CCSS and SEER
Tables 2A and Table 2B show the overall and diagnosis-specific probability of all cause and cause-specific death by 15 years. The probability of death by 15 years since diagnosis for patients who have survived 5 years since cancer diagnosis was 8.8% in CCSS and 10.6% in SEER. This probability of death in 10 years varied greatly by diagnosis: in CCSS, 12.9% among CNS survivors was the highest, followed by 10.8% among soft tissue/Ewing sarcoma survivors, 9.9% among leukemia survivors, and 9.5% among osteosarcoma/other bone tumor survivors. Lymphoma (6.9%), neuroblastoma (4.2%), and kidney tumors (2.6%) survivors had substantially lower conditional probabilities of death. In SEER, the highest probability of death was 16.0% among leukemia followed by 11.4% among osteosarcoma/other bone tumor survivors, 10.0% among lymphoma survivors, 9.7% among CNS survivors, and 9.2% among soft tissue/Ewing sarcoma survivors. Neuroblastoma (4.8%) and kidney tumor (2.2%) survivors had substantially lower conditional probabilities of death in the next 10 years for those who survived 5 years since cancer diagnosis. The majority of deaths for 5-year survivors in the next 10 years (5–15 years from diagnosis) were due to “neoplasms”.
Table 2.
| A. The probability of death in the next 10 years of CCSS patients who survived 5 years after cancer diagnosis by cause of death and cancer type | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis Group | Survival probability | All cause of death | Cause-specific death* |
|||||
| Neoplasms | Infectious | |||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Total | 91.2 | (90.8, 91.6) | 8.8 | (8.4, 9.2) | 6.3 | (6.0, 6.7) | 0.3 | (0.2, 0.4) |
| Leukemia | 90.1 | (89.3, 90.9) | 9.9 | (9.1, 10.7) | 7.4 | (6.7, 8.1) | 0.4 | (0.2, 0.5) |
| Kidney tumors | 97.4 | (96.5, 98.2) | 2.6 | (1.8, 3.5) | 1.6 | (0.9, 3.9) | 0 | - |
| CNS | 87.1 | (85.8, 88.5) | 12.9 | (11.5, 14.2) | 9.5 | (8.3, 10.7) | 0.3 | (0.1, 0.5) |
| Lymphoma | 93.1 | (92.3, 94.0) | 6.9 | (6.0, 7.7) | 4.4 | (3.7, 5.1) | 0.3 | (0.1, 0.5) |
| Neuroblastoma | 95.8 | (94.6, 97.0) | 4.2 | (3.0, 5.4) | 2.9 | (1.9, 3.9) | 0.1 | (0, 0.3) |
| Soft tissue sarcoma/ Ewing sarcoma | 89.2 | (87.8, 90.6) | 10.8 | (9.4, 12.2) | 8.1 | (6.8, 9.3) | 0.2 | (0, 0.3) |
| Osteosarcoma/Other bone tumors | 90.5 | (88.7, 92.4) | 9.5 | (7.6, 11.3) | 6.6 | (5.1, 8.2) | 0.4 | (0, 0.8) |
| Diagnosis Group | Cause-specific death* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cardiac/Vascular | External | Other | Unknown | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Total | 0.3 | (0.2, 0.4) | 0.5 | (0.4. 0.6) | 0.4 | (0.3. 0.5) | 1.0 | (0.9, 1.1) |
| Leukemia | 0.1 | (0, 0.2) | 0.3 | (0.2, 0.5) | 0.4 | (0.3, 0.6) | 1.3 | (1.0, 1.6) |
| Kidney tumors | 0.4 | (0.1, 0.8) | 0.3 | (0, 0.6) | 0.3 | (0, 0.6) | 0.1 | (0, 0.2) |
| CNS | 0.2 | (0,0.3) | 0.6 | (0.3, 0.9) | 0.6 | (0.3, 0.9) | 1.8 | (1.3, 2.3) |
| Lymphoma | 0.7 | (0.4, 1.0) | 0.6 | (0.3, 0.8) | 0.4 | (0.2, 0.6) | 0.6 | (0.3, 0.8) |
| Neuroblastoma | 0.2 | (0, 0.4) | 0.4 | (0, 0.7) | 0.2 | (0, 0.4) | 0.5 | (0, 0.9) |
| Soft tissue sarcoma/ Ewing sarcoma | 0.5 | (0.2, 0.8) | 0.5 | (0.2, 0.9) | 0.5 | (0.2, 0.9) | 1.1 | (0.6, 1.5) |
| Osteosarcoma/Other bone tumors | 0.4 | (0, 0.8) | 0.8 | (0.3, 1.4) | 0.3 | (0, 0.7) | 0.9 | (0.3, 1.5) |
| B. The probability of death in the next 10 years of SEER patients who survived 5 years after cancer diagnosis by cause of death and cancer type | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis Group | Survival probability | All cause of death | Cause-specific death* |
|||||
| Neoplasms | Infectious | |||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Total | 89.4 | (88.6, 90.3) | 10.6 | (9.7, 11.4) | 8.1 | (7.4, 8.7) | 0.3 | (0.2, 0.4) |
| Leukemia | 83.7 | (81.9, 85.6) | 16.3 | (14.4, 18.1) | 13.3 | (11.6, 15.0) | 0.4 | (0.1, 0.8) |
| Kidney tumors | 98.0 | (96.7, 99.4) | 2.0 | (0.6, 3.3) | 1.5 | (0.3, 2.7) | 0 | - |
| CNS | 90.3 | (88.8, 91.7) | 9.7 | (8.3, 11.2) | 7.6 | (6.3, 9.0) | 0.2 | (0, 0.4) |
| Lymphoma | 90.3 | (88.9, 91.7) | 9.7 | (8.3, 11.1) | 6.3 | (5.1, 7.4) | 0.3 | (0.1, 0.6) |
| Neuroblastoma | 95.0 | (92.7, 97.3) | 5.0 | (2.7, 7.3) | 3.8 | (1.8, 5.8) | 0 | - |
| Soft tissue sarcoma/ Ewing sarcoma | 90.1 | (87.9, 92.2) | 9.9 | (7.8, 12.1) | 7.7 | (5.8, 9.6) | 0.1 | (0, 0.4) |
| Osteosarcoma/Other bone tumors | 88.1 | (83.9, 92.2) | 11.9 | (7.8, 16.1) | 9.0 | (5.3, 12.6) | 1.3 | (0, 2.7) |
| Diagnosis Group | Cause-specific death* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cardiac/Vascular | External | Other | Unknown | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Total | 0.4 | (0.3, 0.6) | 0.5 | (0.3, 0.7) | 0.5 | (0.3, 0.6) | 0.8 | (0.6, 1.0) |
| Leukemia | 0.3 | (0, 0.5) | 0.5 | (0.1, 0.8) | 0.7 | (0.2, 1.1) | 1.2 | (0.6, 1.7) |
| Kidney tumors | 0 | - | 0.3 | (0, 0.7) | 0 | - | 0.2 | (0, 0.7) |
| CNS | 0.2 | (0, 0.4) | 0.4 | (0.1, 0.7) | 0.7 | (0.3, 1.2) | 0.6 | (0.2, 1.0) |
| Lymphoma | 1.0 | (0.5, 1.5) | 0.9 | (0.5, 1.4) | 0.3 | (0, 0.5) | 0.8 | (0.4, 1.3) |
| Neuroblastoma | 0.6 | (0, 1.4) | 0 | - | 0 | - | 0.6 | (0, 1.4) |
| Soft tissue sarcoma/ Ewing sarcoma | 0.4 | (0, 0.8) | 0.4 | (0, 0.8) | 0.4 | (0, 0.8) | 0.9 | (0.2, 1.6) |
| Osteosarcoma/Other bone tumors | 0 | - | 0.4 | (0, 1.2) | 0.9 | (0, 2.0) | 0.4 | (0, 1.3) |
Five year mortality of CCSS patients conditioned on surviving 5-, 10- years, 15- and 20-years after cancer diagnosis
Table 3A shows the risk of death in the subsequent 5-years overall and by diagnosis for 5-, 10-, 15- and 20-year survivors of pediatric cancer. For patients with kidney tumors who had survived at least 5 years, the subsequent 5-year mortality was less than 2% for 5- 10-, 15- and 20-year survivors with little change in mortality across time periods. The risk of mortality in next 5 years for 5-year survivors of leukemia was much higher than their risk of mortality having survived 10 years since cancer diagnosis. A similar pattern was seen for patients with a history of neuroblastoma, soft tissue sarcoma, Ewing sarcoma, osteosarcoma, other bone tumors, and CNS tumors. The 5 year risk of death for lymphoma patients was highest in 5-year and 20-year survivors, the differences in risk of death did not vary more than 2% across patients’ years since cancer diagnosis.
Table 3.
5-year mortality of CCSS and SEER patients when they survived 5 years, 10 years, 15 years and 20 years after cancer diagnosis
| Diagnosis Groups | CCSS | SEER |
|---|---|---|
| % Death (95% CI) | % Death (95% CI) | |
| Total Patients | ||
| 5–10 years | 5.96 (5.92, 6.01) | 7.35 (7.22, 7.48) |
| 10–15 years | 3.03 (2.96, 3.10) | 3.34 (3.12, 3.55) |
| 15–20 years | 2.47 (2.39, 2.55) | 3.31 (3.08, 3.54) |
| 20+ years | 3.04 (2.97, 3.12) | 5.97 (5.79, 6.15) |
| Leukemia | ||
| 5–10 years | 7.31 (7.19, 7.44) | 10.96 (10.58, 11.34) |
| 10–15 years | 2.81 (2.59, 3.03) | 4.55 (3.85, 5.26) |
| 15–20 years | 1.70 (1.41, 2.00) | 2.01 (0.86, 3.15) |
| 20+ years | 1.67 (1.37, 1.97) | 1.94 (0.71, 3.17) |
| Kidney tumors | ||
| 5–10 years | 1.70 (0.64, 2.75) | 1.25 (0, 6.71) |
| 10–15 years | 0.94 (0, 2.39) | 1.00 (0, 5.87) |
| 15–20 years | 1.38 (0.18, 2.58) | 1.28 (0, 5.67) |
| 20+ years | 1.77 (0.69, 2.85) | 1.88 (0, 5.68) |
| CNS | ||
| 5–10 years | 8.41 (8.13, 8.68) | 6.40 (5.91 6.89) |
| 10–15 years | 4.89 (4.49, 5.30) | 3.46 (2.72, 4.19) |
| 15–20 years | 3.82 (3.34, 4.30) | 4.22 (3.53, 4.91) |
| 20+ years | 5.45 (5.03, 5.87) | 8.02 (7.49, 8.54) |
| Lymphoma | ||
| 5–10 years | 4.10 (3.81, 4.39) | 6.17 (5.72, 6.61) |
| 10–15 years | 2.90 (2.54, 3.26) | 3.64 (3.01, 4.27) |
| 15–20 years | 3.25 (2.90, 3.60) | 4.09 (3.46, 4.72) |
| 20+ years | 4.61 (4.31, 4.92) | 9.38 (8.95, 9.80) |
| Neuroblastoma | ||
| 5–10 years | 2.91 (1.88, 3.94) | 4.34 (1.68, 7.01) |
| 10–15 years | 1.31 (0, 2.90) | 0.62 (0, 8.33) |
| 15–20 years | 0.85 (0, 2.86) | 1.27 (0, 6.77) |
| 20+ years | 1.43 (0, 2.99) | 1.35 (0, 6.99) |
| Soft tissue sarcoma/Ewing sarcoma | ||
| 5–10 years | 7.01 (6.63, 7.39) | 7.11 (6.19, 8.02) |
| 10–15 years | 4.11 (3.57, 4.65) | 2.97 (1.39, 4.55) |
| 15–20 years | 3.81 (3.23, 4.40) | 3.88 (2.42, 5.34) |
| 20+ years | 3.22 (2.55, 3.89) | 4.44 (3.00, 5.89) |
| Osteosarcoma/Other bone tumors | ||
| 5–10 years | 6.22 (5.44, 6.99) | 7.95 (5.16, 10.74) |
| 10–15 years | 3.48 (2.36, 4.60) | 4.23 (0, 8.61) |
| 15–20 years | 2.14 (0.65, 3.63) | 2.94 (0, 8.46) |
| 20+ years | 3.68 (2.53, 4.84) | 6.70 (2.93, 10.47) |
Five year mortality of SEER patients conditioned on surviving 5-, 10-, 15-, and 20-years after cancer diagnosis
Table 3B shows the mortality in the next 5 years for each diagnosis group and overall for patients in SEER who survived 5-, 10-, and 15-years since cancer diagnosis. Comparable to the CCSS data, the mortality of kidney tumors patients who survived at least 5 years since cancer diagnosis was less than 2 % across all time periods of follow up and their change in mortality across all time periods was less than 1%. Also similar to CCSS the 5-year mortality for leukemia, neuroblastoma, soft tissue sarcoma, and Ewing sarcoma patients was much higher for 5-year survivors versus those who had survived 10-years or more. The 5 year mortality of Osteosarcoma patients and Other bone tumors patients were similar when they were five years since cancer diagnosis and when they were 20 years since cancer diagnosis. Unlike other cancer groups, the 5 year mortality of CNS patients and lymphoma patients were the highest after they survived 20 years since cancer diagnosis and the difference in 5 year mortality across years since cancer diagnosis in these two cancer group patients can be more than 5 percent.
Discussion
Investigation of long-term outcomes using conditional survival can provide useful information for pediatric cancer survivors, family members, and healthcare providers. To our knowledge there is very limited data looking at conditional survival in a cohort of pediatric cancer survivors. The CCSS and SEER databases provided us the opportunity to investigate conditional survival in two large cohorts of survivors of childhood cancer. For the overwhelming majority of diagnoses, the current data demonstrate subsequent five year survival to be ≥ 92% when conditioning on having survived 5, 10, 15, and 20 years from initial diagnosis.
The current study allowed for a more detailed analysis of conditional survival than the previous analyses of the CCSS, where overall mortality of 5-year childhood cancer survivors was found to be increased when compared to the general US population. We originally reported on late mortality in 2001 with an update in 2008 [8, 9]. In these earlier analyses of late mortality, there was a decrease in the standardized mortality rate (SMR) with increasing survival time after diagnosis. All-cause 30-year cumulative mortality was 18.1% (95% CI = 17.3 to 18.9) for 5-year survivors, 12.4% (95% CI = 10.3 to 11.6) for 10-year survivors, 9.5% (95% CI = 8.7 to 10.3) for 15-year survivors, and 7.0% (95% CI = 6.3 to 7.8) for 20-year survivors [9]. Within the CCSS cohort, 20-year conditional survival after surviving 5 years is over 90% for all diagnoses except CNS tumors. Of particular interest in our previous mortality analysis, there were several diagnoses (acute lymphoblastic leukemia, neuroblastoma, kidney tumors) whose 20-year conditional survival exceeded 95%, which were comparable to expected survival curves from the US general population [8].
Bleyer et al. used the SEER database to determine the conditional survival of 15- to 39-year olds diagnosed with cancer during 1975–2000 compared with younger (< 15 years of age) and older patients (≥ 40 years of age) [27]. It was found that although adolescents and young adults (AYAs 15–39 year olds) had a better prognosis at diagnosis, their probability of survival thereafter did not increase as rapidly as younger and older patients, particularly for relative survival. In fact, AYAs had a lower conditional survival improvement than any other age group, including infants and the elderly. In the SEER data, we found a similar pattern with individuals diagnosed with lymphoma whose 20 year conditional survival rates were the lowest among all diagnoses. The explanation for this is not known, but may be due to the occurrence of life-threatening late effects (i.e., subsequent cancer, cardiac disease) reported in 20+ year survivors of Hodgkin lymphoma [5, 28].
When looking at the results of this analysis, it is important to highlight differences in the two cohorts, which may account for some of the discordance seen in these analyses. First, the SEER survivor population is older, with 36% of this cohort being between the ages of 15–20 at diagnosis (vs. 17% in CCSS), and only 29% being diagnosed between 0–4 (vs. 40% in CCSS). Second, the frequency of diagnoses differs, with more leukemia survivors seen at CCSS institutions (43% vs. 24% in SEER), and less CNS tumor (14% vs. 23% at SEER sites) and fewer lymphoma patients seen at CCSS (20% vs. 27% at SEER sites). These differences may be indicative of possible policies for pediatric institutions that restrict patients over the age of 18 years or referral patterns by healthcare providers who may be more likely to refer younger patients to a children’s hospital, and adolescents to adult facilities, or comfort level within adult facilities to see younger patients. In addition, analysis of CCSS to SEER data may serve as a surrogate comparison of patients likely treated in an academic/pediatric cooperative group setting (i.e., Children’s Oncology Group) to patients from a large population-based dataset (SEER) which is more likely to contain information on patients treated at non-academic and adult institutions. Likewise, the CCSS cohort may be more likely to contain patients treated on clinical trials versus the SEER database.
There are several limitations to this analysis. First, a large proportion of SEER patients and CCSS survivors were missing race/ethnicity and we could not adjust for it completely, limiting our ability to look at conditional survival comparisons among these sub-groups. Second, diagnosis staging data was not available on either cohort, limiting our ability to control for this in our analysis. It would be of interest in future analyses to be able to look at other possible risk factors, such as socioeconomic status, to understand the differences seen in these two populations. Lastly, it is possible that there may be misclassification regarding cause of death in both cohorts. However, since both cohorts utilize US vital records, we can assume that the bias would be similar in both cohorts.
Clinicians are often faced with questions from patients and parents about prognosis, including when they can be considered to be cured. The information typically available to clinicians is survival curves created from the cancer diagnosis. This, however, only describes what happens to patients starting from the beginning (diagnosis). The conditional survival data presented here allows clinicians a new way of thinking about long-term follow-up, namely, after having survived certain numbers of years, what will happen. The CCSS itself is an example of conditional survival analysis in that patients were required to have survived 5 years in order to participate in this cohort study. Most reports from the CCSS detail what happens to patients after they have already survived the first 5 years from cancer diagnosis. This current study starts to take the next step towards allowing clinicians to give more individualized prognosis when they see patients in long-term follow-up. At each visit, patients and parents will be able to receive updated information about the future that is based on the time points they have already achieved.
Conclusion
Overall, conditional survival is high in 5-year survivors of pediatric cancer, however there still remains a risk for death in these survivors even up to 20 years post diagnosis. The similarities in the trends seen in both cohorts allow clinicians a new way of thinking about long-term follow-up. In addition, this measurement of survival is clinically relevant because it more accurately reflects future patient outcomes, taking into account a patient’s time since initial cancer diagnosis. For healthcare providers, as with survivors and their families, this information is clinically important and warrants continued follow-up and ongoing medical care in these long-term survivors.
Acknowledgments
This work was supported by NCI grant CA 55727
Footnotes
Conflicts of interest: None
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]
- 2.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Hudson MM. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin. 2004 Jul-Aug;54(4):208–236. doi: 10.3322/canjclin.54.4.208. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz CL, Hobbie WL, Constine LS, Ruccione KS. In: Survivors of Childhood and Adolescent Cancer. 2nd ed. C L, editor. Mosby: St. Louis MO; 2005. [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006 Oct 12;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014 Apr 20;32(12):1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mertens AC, Yasui Y, Neglia J, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19(13):3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 9.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong GT, 1, Pan Z, Ness KK, Srivastava D, Robison LL. Temporal trends in cause-specific late mortality among 5-year survivors of childhood cancer. J Clin Oncol. 2010 Mar 1;28(7):1224–1231. doi: 10.1200/JCO.2009.24.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer. 1999;85(2):485–491. [PubMed] [Google Scholar]
- 13.Hwang SL, Yang YH, Lieu AS, et al. The conditional survival statistics for survivors with primary supratentorial astrocytic tumors. J Neurooncol. 2000;50(3):257–264. doi: 10.1023/a:1006484220764. [DOI] [PubMed] [Google Scholar]
- 14.Meng L, Maskarinec G, Lee J. Ethnicity and conditional breast cancer survival in Hawaii. J Clin Epidemiol. 1997;50(11):1289–1296. doi: 10.1016/s0895-4356(97)00183-2. [DOI] [PubMed] [Google Scholar]
- 15.Moller MB, Pedersen NT, Christensen BE. Conditional survival of patients with diffuse large B-cell lymphoma. Cancer. 2006;106(10):2165–2170. doi: 10.1002/cncr.21877. [DOI] [PubMed] [Google Scholar]
- 16.Skuladottir H, Olsen JH. Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol. 2003;21(16):3035–3040. doi: 10.1200/JCO.2003.04.521. [DOI] [PubMed] [Google Scholar]
- 17.Henson DE, Ries LA, Carriaga MT. Conditional survival of 56,268 patients with breast cancer. Cancer. 1995;76(2):237–242. doi: 10.1002/1097-0142(19950715)76:2<237::aid-cncr2820760213>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: surveillance, epidemiology, and end results data. Cancer. 2001;92(8):2211–2219. doi: 10.1002/1097-0142(20011015)92:8<2211::aid-cncr1565>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Lin CL, Lieu AS, Lee KS, et al. The conditional probabilities of survival in patients with anaplastic astrocytoma or glioblastoma multiforme. Surg Neurol. 2003;60(5):402–406. doi: 10.1016/s0090-3019(03)00322-7. discussion 406. [DOI] [PubMed] [Google Scholar]
- 20.Merrill RM, Henson DE, Ries LA. Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon. Dis Colon Rectum. 1998;41(9):1097–1106. doi: 10.1007/BF02239430. [DOI] [PubMed] [Google Scholar]
- 21.Robison LL, Mertens AC, Boice JD, et al. Study Design and Cohort Characteristics of the Childhood Cancer Survivor Study: A Multi-Institutional Collaborative Project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 22.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, based on the recommendations of the Ninth Revision Conference, 1975. Geneva: World Health Organization; 1977. [Google Scholar]
- 25.The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. http://seer.cancer.gov/
- 26.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: 2012. ISBN 3-900051-07-0, URL http://www.R-project.org/. [Google Scholar]
- 27.Bleyer A, Choi M, Fuller CD, et al. Relative lack of conditional survival improvement in young adults with cancer. Semin Oncol. 2009;36(5):460–467. doi: 10.1053/j.seminoncol.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P, Stovall M, Robison LL, Hudson MM. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117(6):1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]