Abstract
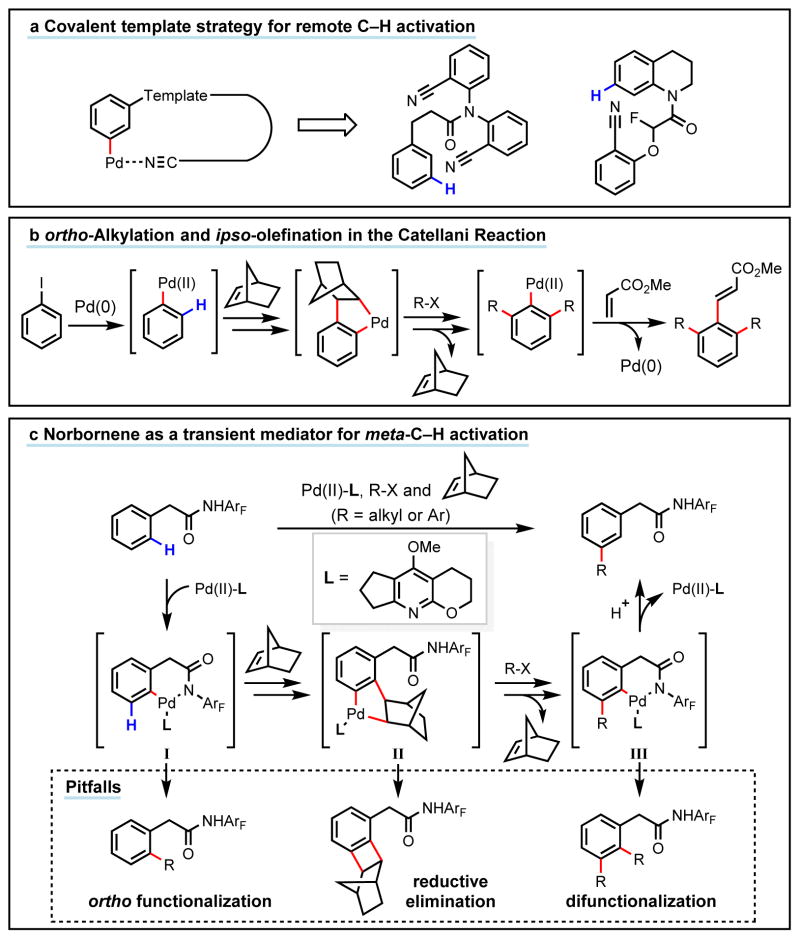
Achieving site selectivity in C–H functionalization reactions is a significant challenge, especially when the target C–H bond is distant from existing functional groups.1–5 Coordination of a functional group to a metal catalyst is often a key driving force and control element in many important reactions including asymmetric hydrogenation,6 epoxidation7, 8, and lithiation9. Exploitation of this effect has led to the development of a broad range of directed C–H activation reactions.10–14 However, such C–H activation methods are limited to proximal C–H bonds, which are spatially and geometrically accessible from the directing functional group. Development of meta-selective C–H functionalizations remains a significant challenge.1–5,15–17 We recently developed a U- shaped template that can be used to overcome this constraint and have shown that it can be used to selectively activate remote meta-C–H bonds.1, 2 While this approach has proven applicable for a diverse set of substrates and catalytic transformations,3–5 the need for a covalently attached complex template is a significant drawback for synthetic applications. In this manuscript, we report an alternative approach, one that employs norbornene as a transient mediator to achieve meta-selective C–H activation with a simple and common ortho-directing group. The use of a newly developed pyridine-based ligand is crucial for relaying the palladium catalyst to the meta position by norbornene following initial ortho- C–H activation. Thus, this catalytic reaction demonstrates the feasibility of switching ortho-selectivity to meta-selectivity in C–H activation of the same substrate by catalyst control.
Inspired by the unique reactivity of norbornene in palladium-catalyzed reactions discovered by Catellani (Fig. 1b),18–22 we hypothesized that ortho-palladacycle I (Fig. 1c) could react with norbornene to provide an intermediate that can undergo activation of the meta-C–H bond (intermediate II). Reaction of palladacycle II with a coupling partner would form a new C–C or C–heteroatom bond and subsequent β-carbon elimination of norbornene followed by protodemetalation of the aryl-palladium bond would regenerate the palladium catalyst. As such, this reaction pathway would provide a new approach to achieve catalytic meta-selective C–H activation using standard ortho-directing groups with norbornene as a transient mediator. Herein we report the realization of this concept through the design of a pyridine-based ligand that suppresses several undesired reaction pathways and promotes meta-selective alkylation and - arylation of phenylacetic acid derivatives. We anticipate that this approach will enable many previously reported ortho-C–H activation reactions to be achieved with high levels of meta- selectivity.
Figure 1. Design of a new approach for meta-C–H activation.
a, Previously reported remote meta-C–H activation directed by a U-shaped template. b, The norbornene-mediated Catellani reaction functionalizes ortho-positions of aryl iodides. c, The development of a tailor-made ligand that switches the ortho-selectivity to meta-selectivity using norbornene as a transient mediator (this work). ArF, 4-(CF3)C6F4. The activated C–H bonds (blue) and the newly formed bonds (red) are highlighted.
At the outset, we were cognizant that our proposed meta-C–H activation with norbornene as a transient mediator would face several key challenges (Fig. 1c). First, it was unclear if 1,2- migratory insertion with norbornene could occur from a cyclopalladated intermediate (I) and whether this pathway would be faster than the competitive reaction of the palladacycle with a terminal electrophile. In a recent report from the Bach’s group, the C2-selective C–H alkylation of indoles via the initial aminopalladation of norbornene with the indolyl N-H bonds is inspiring.23, 24 Second, the resultant intermediate II could undergo reductive elimination to form a benzocyclobutene thereby preventing the desired meta-C–H functionalization (Fig. 1c).25 Third, rather than undergoing protodemetalation, intermediate III could be further functionalized to afford the undesired ortho, meta-difunctionalized products.
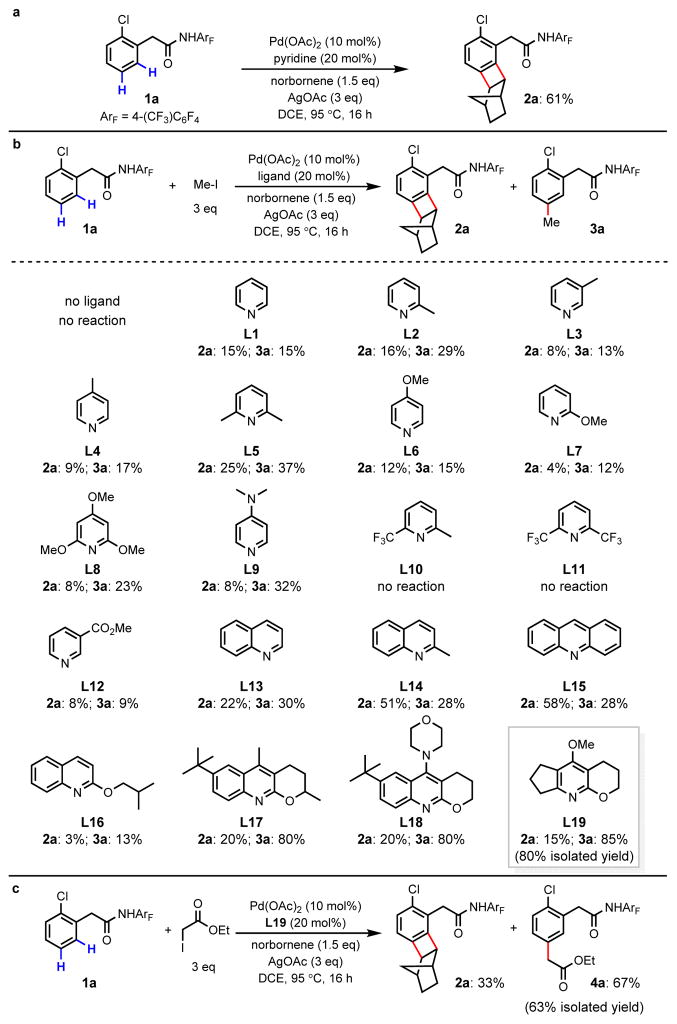
We initiated our investigation with a phenylacetic acid–derived N-2,3,5,6-tetrafluoro-4- trifluoromethylphenyl amide substrate. This auxiliary has been proven effective in the ortho-C– H activation of C(sp2)–H bonds by Pd(II) catalysts.12 To probe the feasibility of each step in the proposed catalytic cycle, we first treated substrate 1a with Pd(OAc)2 and norbenene under various conditions in an attempt to identify conditions for 1,2-migratory insertion without success (see supplementary information). Although, ortho-C–H activation of 1a does not require ligands,26 our recent finding that 9-methylacridine promotes directed ortho-C–H activation27 prompted us to search for pyridine-type ligands that would facilitate the 1,2-migratory insertion. We found that the addition of pyridine as a ligand enables 1,2-migratory insertion of norbornene into the putative ortho-palladacycle intermediate. Subsequent meta-C–H palladation and reductive elimination presumably then took place to give benzocyclobutene 2a in 61% yield (Fig. 2a). Notably, formation of 2a was not observed in the absence of pyridine ligand.
Figure 2. Discovery of a ligand that enables meta-C–H alkylation using norbornene as a transient mediator.
a, Pyridine promotes Pd-catalyzed reaction of phenylacetic amide with norbornene to afford benzocyclobutene via a Catellani pathway. DCE, 1,2-dichloroethane. b, A modified pyridine-type ligand enables selective meta-C–H methylation. Yields are determined by crude 1H NMR analysis. c, meta-C–H alkylation with ethyl iodoacetate.
Having established the first step in the proposed catalytic cycle (1,2-migratory insertion), we next sought to identify conditions to prevent benzocyclobutene formation and promote meta- functionalization with an electrophile. Encouragingly, in the presence of methyl iodide, the desired meta-methylated product 3a was obtained in 15% yield, and formation of 2a was reduced to 15% yield (Fig. 2b, L1). Further screening of simple pyridine ligands revealed that alkyl substitution at the 2-position improved the yield of meta-methylation (L2 and L5, 29% and 37% yield, respectively). We further found that pyridine ligands with electron-donating substituents lead to improved product selectivity (L6–L9) while electron-deficient pyridine ligands provided both poor selectivity and reactivity (L10–L12). Prompted by our recent progress in developing quinoline-based ligands for C–H activation,28, 29 we subsequently examined this class of ligands. Although the use of 2-methylquinoline (L14) and acridine (L15) as ligands led to increased reactivity, formation of benzocyclobutene 2a was favored. Since electron-donating substituents enhance selectivity for the meta-methylation pathway, we tested several quinoline ligands containing alkoxy groups at the 2-position. Although 2-(isobutoxy)quinoline (L16) is an ineffective ligand, the use of ligand L17, containing a 2-alkoxy group with a constrained conformation in a fused ring, leads to full conversion, affording 3a in 80% yield. Installation of a highly electron-donating amine substituent on a similar ligand L18 did not improve the selectivity. Ligand L19, containing a fused cyclopentane ring, further improves the selectivity for meta-methylation, providing 3a in 85% yield. Selective meta-alkylation with ethyl iodoacetate is also achieved under optimized conditions to give the desired product 4a in 67% yield (Fig. 2c).
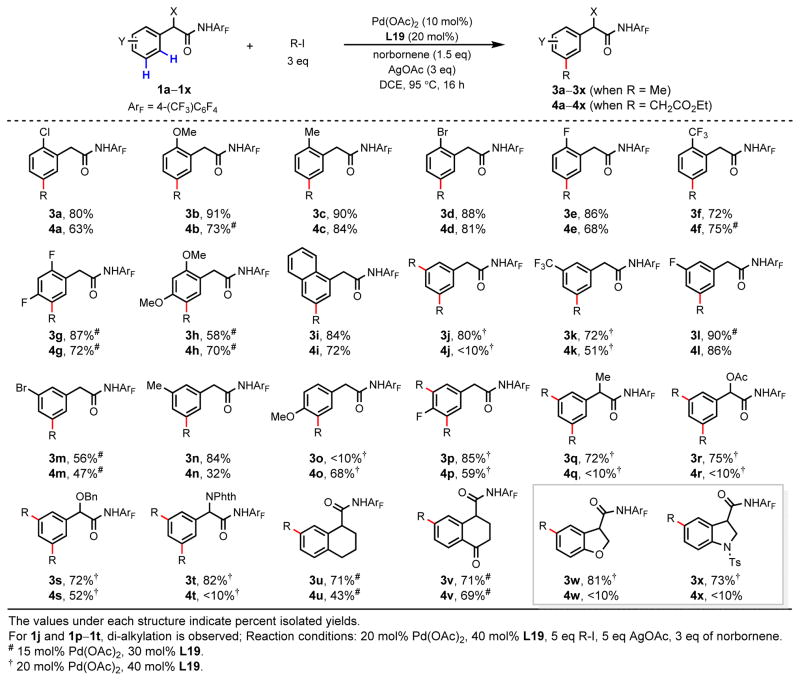
The scope of this norbornene-mediated meta-alkylation was investigated using methyl iodide (3a–3x) and ethyl iodoacetate (4a–4x) as coupling partners (Fig. 3). Electron-donating and - withdrawing ortho-substituents are tolerated in this transformation, providing moderate to excellent yields (3a–3h and 4a–4h). An ortho-bromide substituent is also compatible with this protocol (3d and 4d). Alkylation of 1-naphthylacetic amide 1i occurs exclusively at the 3- position, rather than the 8-position (3i and 4i). When an unsubstituted amide substrate 1j is used, methylation occurs at both meta positions to provide dimethylated product 3j in 80% yield. Coupling of ethyl iodoacetate with this substrate, however, mainly produces the benzocyclobutene byproduct; the yield of the desired dialkylated product 4j was <10%. This result demonstrates the subtle reactivity differences between these two alkylating reagents. Substrates bearing electron-donating or -withdrawing meta-substituents are also reactive with this protocol (3k–3n and 4k–4n). Methylation of the more sterically hindered p-MeO-substituted substrate 1o is sluggish and poor yielding, providing 3o in <10% yield. Interestingly, mono- alkylation of 1o with ethyl iodoacetate affords 4o in 68% yield. Substrate containing the less steric hindered p-F-group reacted well with both coupling partners to afford di-alkylated products 3p and 4p in 85% and 59% yields, respectively. Methylation reaction was found to proceed effectively with a broad range of α-substituents, including methyl, acetoxyl, benzyloxy and phthaloyl-protected amino groups to give the di-methylated products in good yields (3q–3t). Thus, this transformation enables the direct meta-functionalization of key biologically-active building-blocks, such as mandelic acid (3r and 3s) and phenylglycine (3t). In contrast, ethoxycarbonylmethylation reaction is effective only for 1s, containing a benzyloxy group at the benzylic position (4s). Notably, both alkylation reactions are compatible with tetrahydronapthalene (1u) and tetralone (1v) substrates, despite the amide directing groups being out of plane with the target C(aryl)–H bonds. We were pleased to observe that meta-methylation is effective for the heterocyclic dihydrobenzofuran- and indoline-containing substrates 1w and 1x. However, ethoxycarbonylmethylation of these heterocycles provides only trace product.
Figure 3. meta-C–H alkylation of phenylacetic amides.
R-I, methyl iodide or ethyl iodoacetate; X, Y, generic substituents; Ac, acetyl; Bn, benzyl; Phth, phthaloyl; Ts, 4-toluenesulfonyl.
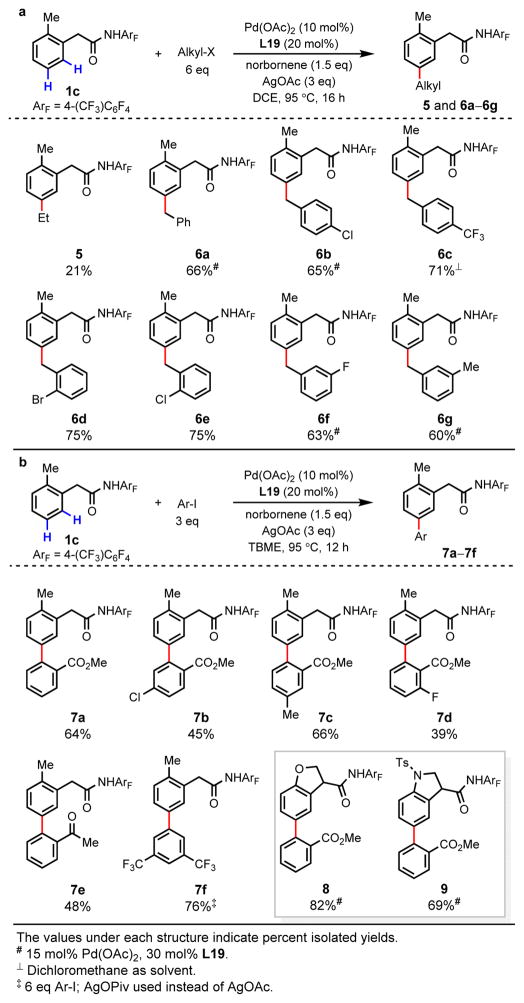
We subsequently examined the generality of this meta-C–H functionalization strategy by investigating additional organohalide coupling partners (Fig. 4). Ethylation with ethyl iodide under standard conditions leads to exclusive formation of the undesired benzocyclobutene byproduct. Increasing the loading of ethyl iodide (6 eq) affords meta-ethylated product 5 in 21% yield, with the benzocyclobutene still formed as the major product. We were pleased to find that meta-alkylation with benzyl bromide, a more reactive electrophile, proceeds smoothly to afford 6a in 66% yield. Both electron-donating and -withdrawing substituents on the ortho-, meta- and para- positions of the benzyl bromide coupling partner are tolerated, providing meta-benzylated products 6b–6g in 60–75% yield.
Figure 4. Scope of organohalide coupling partners.
a, meta-C–H Alkylation with alkyl halides. Alkyl-X, iodoethane or benzyl bromides. b, meta-C–H arylation with aryl iodides. TBME, t- butyl methyl ether.
Finally, we examined whether this approach for meta-C–H functionalization could be adapted to achieve meta-C–H arylation, thereby providing a method for the synthesis of biaryl compounds. Gratifyingly, arylation with methyl 2-iodobenzoate in t-butyl methyl ether (TBME) provides the meta-arylated product 7a in 64% yield. The use of methyl 3-iodobenzoate or methyl 4-iodobenzoate, however, results in the formation of the benzocyclobutene adduct as the major product. These experiments suggest that the presence of an ortho-coordinating group is required to promote oxidative addition of the aryl halide, consistent with previous studies.19, 30 As such, we examined other substituted 2-iodobenzoates and were able to obtain the desired products in moderate yields (7b–7d). Similarly, 2′-iodoacetophenone is a competent coupling partner, affording 7e in 48% yield. Arylation with highly reactive, electron-deficient 3,5- bis(trifluoromethyl)iodobenzene is also effective, providing the meta-arylated product 7f in 76% yield. The successful meta-arylation of substrates containing dihydrobenzofuran (8, 82% yield) and indoline (9, 69% yield) groups with methyl 2-iodobenzoate is especially noteworthy in terms of demonstrating late-stage diversification of heterocycles with possible applications in medicinal chemistry. We anticipate that further development of the ancillary pyridine ligand will broaden the scope of alkyl and aryl halides in this reaction, ultimately leading to a broadly useful method for meta-C–H functionalization using common ortho-directing groups and norbornene as a transient mediator.
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, 1R01 GM102265) for their financial support. L.-Z.F. is a visiting scholar from School of Pharmacy, Xinxiang Medical University and is sponsored by the China Scholarship Council. We thank J. Spangler and A. Homs for constructive suggestions.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions X.-C.W. and R.-Y.Z. developed the reactions. W.G. and S.L. synthesized the ligands. L.-Z.F. examined the substrate scope. K.M.E. performed preliminary studies. J.-Q.Y. conceived this concept and prepared this manuscript with feedback from X.-C.W.
The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article.
References
- 1.Leow D, Li G, Mei TS, Yu JQ. Activation of remote meta-C–H bonds assisted by an end-on template. Nature. 2012;486:518–522. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang RY, Li G, Yu JQ. Conformation-induced remote meta-C–H activation of amines. Nature. 2014;507:215–220. doi: 10.1038/nature12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Lee H, Tan KL. Meta-selective C–H functionalization using a nitrile-based directing group and cleavable Si-tether. J Am Chem Soc. 2013;135:18778–18781. doi: 10.1021/ja4107034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan L, Dastbaravardeh N, Li G, Yu JQ. Cross-coupling of remote meta-C–H bonds directed by a U-shaped template. J Am Chem Soc. 2013;135:18056–18059. doi: 10.1021/ja410760f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, et al. Pd(II)-catalyzed meta-C–H olefination, arylation, and acetoxylation of indolines using a U-shaped template. J Am Chem Soc. 2014;136:10807–10813. doi: 10.1021/ja505737x. [DOI] [PubMed] [Google Scholar]
- 6.Brown JM. Selectivity and mechanism in catalytic asymmetric synthesis. Chem Soc Rev. 1993;22:25–41. [Google Scholar]
- 7.Johnson RA, Sharpless KB. In: Catalytic Asymmetric Synthesis. 2. Ojima I, editor. John Wiley & Sons, Inc; Hoboken: 2005. pp. 231–280. [Google Scholar]
- 8.Li Z, Yamamoto H. Hydroxamic acids in asymmetric synthesis. Acc Chem Res. 2013;46:506–518. doi: 10.1021/ar300216r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartung CG, Snieckus V. In: Modern Arene Chemistry. Astruc D, editor. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2004. pp. 330–367. [Google Scholar]
- 10.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daugulis O, Do HQ, Shabashov D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc Chem Res. 2009;42:1074–1086. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engle KM, Mei TS, Wasa M, Yu JQ. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc Chem Res. 2012;45:788– 802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colby DA, Bergman RG, Ellman JA. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem Rev. 2010;110:624–655. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wencel-Delord J, Dröge T, Liu F, Glorius F. Towards mild metal-catalyzed C–H bond activation. Chem Soc Rev. 2011;40:4740–4761. doi: 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]
- 15.Saidi O, et al. Ruthenium-catalyzed meta-sulfonation of 2-phenylpyridines. J Am Chem Soc. 2011;133:19298–19301. doi: 10.1021/ja208286b. [DOI] [PubMed] [Google Scholar]
- 16.Duong HA, Gilligan RE, Cooke ML, Phipps RJ, Gaunt MJ. Copper(II)- catalyzed meta-selective direct arylation of α-aryl carbonyl compounds. Angew Chem Int Ed. 2011;50:463–466. doi: 10.1002/anie.201004704. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann N, Ackermann L. meta-Selective C–H bond alkylation with secondary alkyl halides. J Am Chem Soc. 2013;135:5877–5884. doi: 10.1021/ja401466y. [DOI] [PubMed] [Google Scholar]
- 18.Catellani M, Frignani F, Rangoni A. A complex catalytic cycle leading to a regioselective synthesis of o, o′-disubstituted vinylarenes. Angew Chem Int Ed. 1997;36:119– 122. [Google Scholar]
- 19.Faccini F, Motti E, Catellani M. A new reaction sequence involving palladium- catalyzed unsymmetrical aryl coupling. J Am Chem Soc. 2004;126:78–79. doi: 10.1021/ja039043g. [DOI] [PubMed] [Google Scholar]
- 20.Martins A, Mariampillai B, Lautens M. Synthesis in the key of Catellani: norbornene-mediated ortho C–H functionalization. Top Curr Chem. 2010;292:1–33. doi: 10.1007/128_2009_13. [DOI] [PubMed] [Google Scholar]
- 21.Cárdenas DJ, Martín-Matute B, Echavarren AM. Aryl transfer between Pd(II) centers or Pd(IV) intermediates in Pd-catalyzed domino reactions. J Am Chem Soc. 2006;128:5033–5040. doi: 10.1021/ja056661j. [DOI] [PubMed] [Google Scholar]
- 22.Dong Z, Dong G. Ortho vs Ipso: Site-selective Pd and norbornene-catalyzed arene C– H amination using aryl halides. J Am Chem Soc. 2013;135:18350–18353. doi: 10.1021/ja410823e. [DOI] [PubMed] [Google Scholar]
- 23.Jiao L, Bach T. Palladium-catalyzed direct 2-alkylation of indoles by norbornene- mediated regioselective cascade C–H activation. J Am Chem Soc. 2011;133:12990–12993. doi: 10.1021/ja2055066. [DOI] [PubMed] [Google Scholar]
- 24.Jiao L, Herdtweck E, Bach T. Pd(II)-catalyzed regioselective 2-alkylation of indoles via a norbornene-mediated C–H activation: mechanism and applications. J Am Chem Soc. 2012;134:14563–14572. doi: 10.1021/ja3058138. [DOI] [PubMed] [Google Scholar]
- 25.Catellani M, Ferioli L. An improved synthesis of 1,4-cis, exo-hexa- or tetrahydromethano- or -ethanobiphenylene derivatives catalyzed by palladium complexes. Synthesis. 1996:769–772. [Google Scholar]
- 26.Wang XC, et al. Pd(II)-catalyzed C H iodination using molecular I2 as the sole oxidant. J Am Chem Soc. 2013;135:10326–10329. doi: 10.1021/ja4055492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu RY, He J, Wang XC, Yu JQ. Ligand-promoted alkylation of C(sp3)–H and C(sp2)–H bonds. J Am Chem Soc. 2014;136:13194–13197. doi: 10.1021/ja508165a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J, et al. Ligand-controlled C(sp3)–H arylation and olefination in synthesis of unnatural chiral α–amino acids. Science. 2014;343:1216–1220. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Chen G, Feng CG, Gong W, Yu JQ. Ligand-enabled γ-C–H olefination and carbonylation: construction of β-quaternary carbon centers. J Am Chem Soc. 2014;136:5267–5270. doi: 10.1021/ja501689j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins A, Candito DA, Lautens M. Palladium-catalyzed reductive ortho-arylation: evidence for the decomposition of 1,2-dimethoxyethane and subsequent arylpalladium(II) reduction. Org Lett. 2010;12:5186–5188. doi: 10.1021/ol1019037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.