Abstract
Background
In the pig-to-nonimmunosuppressed baboon artery patch model, a graft from an α1,3-galactosyltransferase gene-knockout pig transgenic for human CD46 (GTKO/CD46) induces a significant adaptive immune response (elicited anti-pig antibody response, increase in T cell proliferation on MLR, cellular infiltration of the graft), which is effectively prevented by anti-CD154mAb-based therapy.
Methods
As anti-CD154mAb is currently not clinically applicable, we evaluated whether it could be replaced by CD28/B7 pathway blockade or by blockade of both pathways (using belatacept+anti-CD40mAb [2C10R4]). We further investigated whether a patch from a GTKO/CD46 pig with a mutant human MHC class II transactivator (CIITA-DN) gene would allow reduction in the immunosuppressive therapy administered.
Results
When grafts from GTKO/CD46 pigs were transplanted with blockade of both pathways, a minimal or insignificant adaptive response was documented. When a GTKO/CD46/CIITA-DN graft was transplanted, but no immunosuppressive therapy was administered, a marked adaptive response was documented. In the presence of CD28/B7 pathway blockade (abatacept or belatacept), there was a weak adaptive response that was diminished when compared with that to a GTKO/CD46 graft. Blockade of both pathways prevented an adaptive response.
Conclusion
Although expression of the mutant MHC CIITA-DN gene was associated with a reduced adaptive immune response when immunosuppressive therapy was inadequate, when blockade of both the CD40/CD154 and CD28/B7 pathways was present, the response even to a GTKO/CD46 graft was suppressed. This was confirmed after GTKO/CD46 heart transplantation in baboons.
Keywords: Anti-CD40 monoclonal antibody, Artery patch, Costimulation blockade, CTLA4-Ig, Pig, Xenotransplantation
INTRODUCTION
The introduction of genetically-engineered pigs, e.g., α1,3-galactosyltransferase gene-knockout (GTKO) [1,2] or transgenic for a human complement-regulatory protein (CD55, CD46, CD59) [3–8], has contributed to a significant increase in the survival of pig organs transplanted into nonhuman primates. Progress has also been made in understanding the mechanisms of the innate and adaptive (T cell-dependent) immune responses, and by the introduction of novel immunosuppressive agents, e.g., those that inhibit costimulation [9,10].
Our interest has been directed to two topics – (i) costimulation blockade as a form of immunosuppression [9,11–16], and (ii) genetic manipulation of the pig to provide protection from the primate adaptive immune response [17,18].
Immunosuppressive therapy (IS) based on blockade of the CD40/CD154 pathway by an anti-CD154 monoclonal antibody (mAb) prevents the adaptive response to allografts and xenografts, contributing to prolonged graft survival [9,11–14,16,19–27]. However, clinical use of anti-CD154mAbs has currently been abandoned due to thrombotic complications [9,12,16,28–31].
However, the CD40/CD154 interaction remains a promising target, and various CD40-specific antibodies have been shown to extend kidney and islet allograft survival in nonhuman primates without thromboembolic phenomena [32–41]. CD28/B7 costimulatory pathway blockade has also been tested in clinical trials of organ allotransplantation with encouraging results [42–44].
Pig heart transplantation in nonhuman primates is time-consuming, expensive, and associated with complications, e.g., thrombotic microangiopathy, consumptive coagulopathy. We therefore developed a simple artery patch transplantation procedure in the pig-to-baboon model that successfully exposes the baboon to sufficient pig antigens to induce an adaptive immune response in the absence of coagulation dysfunction [16], and therefore allows us to determine the efficacy of IS in preventing this response. When no or inadequate IS is administered, a GTKO pig xenograft induces significant T cell proliferative and elicited IgM and IgG anti-pig responses, accompanied by cellular infiltration in the graft [9]. An anti-CD154mAb-based, but not a CTLA4-Ig (abatacept)-based, regimen prevented this response [16].
An alternative or adjunctive approach is to transplant cells, tissues, or organs from pigs that have been genetically-engineered to provide some protection from the primate adaptive immune response. To date, this approach has included the production of (i) pigs expressing a costimulation-blockade agent, e.g., CTLA4-Ig, either constitutively [17,45] or in specific cells [46–50], or (ii) pigs transgenic for a human mutant MHC class II transactivator gene (CIITA-DN pigs), which reduces swine leukocyte antigen class II expression both when the cell is quiescent and when activated [18,51].
We have now explored (i) the efficacy of a patch graft from a CIITA-DN pig to reduce the adaptive response, and (ii) the ability of CD28/B7 pathway blockade or blockade of both pathways to prevent this response. An anti-CD40mAb was not tested alone as our in vitro studies suggested that it was less successful than blockade of the CD28/B7 pathway alone (Hara H, et al. manuscript in preparation). To our knowledge, these represent the first in vivo studies utilizing pigs with the CIITA-DN genetic modification in a nonhuman primate transplantation model.
MATERIALS AND METHODS
Animals
Baboons (Papio species, n=20; Division of Animal Resources, Oklahoma University Health Sciences Center, Oklahoma City, OK), 3–4 years-old, weighing 6–9kg and of known AB blood type, were recipients of pig artery patch (n=16) or heterotopic heart (n=4) grafts. GTKO/CD46/CIITA-DN, GTKO/CD46, or GTKO pigs of blood group O (nonA), weighing 30–80kg (Revivicor, Blacksburg, VA), generated by nuclear transfer/embryo transfer from modified fibroblasts from Large White/Landrace/Duroc cross-breed pigs [1,18,52], served as sources of carotid artery patches or hearts. Some pigs provided two or more patch grafts. All pigs were tested to confirm (i) absence of Gal expression and (ii) expression of CD46 and CIITA-DN in the vascular endothelium of the aorta (18,53).
GTKO/CD46 pig cells have been demonstrated to provide considerable protection against the primate humoral immune response [53,54] and CIITA-DN pig cells have been shown to protect from the primate adaptive response (18).
In vitro assays were carried out on cells from GTKO/CD46/CIITA-DN pigs to demonstrate the efficacy of the mutant human MHC class II transactivator gene.
All animal care was in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985). Protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Surgical procedures
Anesthesia in pigs and baboons, and intravascular catheter placements in baboons have been described previously [55], as has the surgical technique of pig artery patch transplantation in baboons [16]. A 2×1cm patch was sutured into the anterior wall of the abdominal aorta as an onlay graft. The ischemic period was 2–3h in all cases. The technique of heterotopic heart transplantation has also been described previously (9,13,14,55).
Experimental groups (Table 1)
Table 1.
Details of Group 1 and 2 Experiments
| Baboon # | Immunosuppressive Regimen | Elective Graft Survival (days) | IS Variations* |
|---|---|---|---|
| Group 1 (n=8; all received grafts from GTKO [Gp.1A] or GTKO/CD46 [Gp.1B] pigs) (a) | |||
| 7608 | 1A | 14* | CVF, MMF |
| 18408 | 1A | 28 | CVF, MMF |
| 18208 | 1A | 28 | CVF, MMF |
| 18608 | 1A | 28 | CVF, MMF |
| 5412 | 1B | 28 | CVF, MMF |
| 5912 | 1B | 28 | Rapamycin |
| 12812 | 1B | 48 | |
| 12912 | 1B | 48 | Rapamycin |
|
| |||
| Group 2 (n=8; all received grafts from GTKO/CD46/CIITA pigs) (b, c) | |||
| 19310 | No IS | 28 | |
| 15911 | 2A | 28 | CVF, MMF |
| 16011 | 2A | 21* | CVF, MMF |
| 12612 | 2A | 48 | Rapamycin |
| 12712 | 2A | 48 | Rapamycin |
| 5812 | 2A | 48 | Rapamycin |
| 16111 | 2B | 28 | CVF, MMF |
| 16211 | 2B | 28 | CVF, MMF |
|
| |||
| Group3 (n=4; all received grafts from GTKO/CD46 pigs) (c) | |||
| 5512 | 3 | 99 | Rapamycin;Pneumonia; |
| 5712 | 3 | 130 | Tacrolimus;Graft failure; |
| 17913 | 3 | 124 | Rapamycin;Graft failure |
| 18013 | 3 | 118 | Rapamycin;Graft failure |
Regimen 1A: ATG, CVF, MP, CTLA4-Ig (abatacept), MMF
Regimen 2A: ATG (+/−CVF), MP, CTLA4-Ig (abatacept or belatacept), MMF or rapamycin
Regimens 1B and 2B, and 3: ATG (+/−CVF), MP, anti-CD40mAb (2C10R4), CTLA4-Ig (belatacept), MMF, rapamycin, or tacrolimus
Euthanized after bleeding from a disrupted intra-arterial catheter
The studies were divided into three groups, based on the nature of the graft (artery patch [n=16] or heart [n=4]) and graft-source pig (GTKO or GTKO/CD46 or GTKO/CD46/CIITA-DN). In Group 1 (n=8), baboons received patch grafts from GTKO (n=4) or GTKO/CD46 (n=4) pigs, and in Group 2 (n=8) from GTKO/CD46/CIITA-DN pigs.
Group 1 and 2 baboons were euthanized at the end of the experiment (at 28 or 48 days). Group 3 baboons (n=4) received heart grafts from GTKO/CD46 pigs.
Immunosuppressive and supportive therapy (Table 2)
Table 2.
Details of immunosuppressive and supportive therapy
| Dose | Duration | |
|---|---|---|
| Induction therapy | ||
|
| ||
| Thymoglobulin (ATG) * (Genzyme, Cambridge, MA) | 1–10mg/kg i.v. | days −3, −1 * |
|
| ||
| Cobra venom factor (Complement Technology, Tyler, Texas) | 100 units/day i.v. | days −1, 0, 1 |
|
| ||
| Methylprednisolone (Pfizer, New York, NY) | 5mg/kg i.v. | before each dose of ATG, and on days −1, 0 |
|
| ||
| Maintenance costimulation blockade | ||
|
| ||
| Abatacept (BMC, Princeton, NJ) | 25mg/kg i.v. | days −1, 0, 2, 4, 7,10,14, then every 14 days |
| or | ||
| Belatacept (BMC, Princeton, NJ) | 20mg/kg i.v. | days −1, 0, 4, 7, 14, then every 14days |
|
| ||
| Anti-CD40mAb (NIH NHP Resource Center, Boston, MA) | 25mg/kg i.v. | days −1, 0a, 4, 7, 10a, 14, then every 7 days |
|
| ||
| Maintenance pharmacologic immunosuppressive and supportive therapy | ||
|
| ||
| MMF (Genentech, South San Francisco, CA) | 25–110mg/kg/day | continuous i.v. infusion from day −3 (to maintain a constant blood level of 3–5μg/mL) |
|
| ||
| Rapamycin (LC, Laboratories, Woburn, MA) | 0.01mg/kg i.m. x2/day | to maintain a blood trough level of 10–15ng/mL |
|
| ||
| Tacrolimus (LC, Laboratories, Woburn, MA) | 0.05–0.03mg/kg i.m. x2/day | to maintain a blood trough level of 10–15ng/mL |
|
| ||
| Methylprednisolone (Astellas, Deerfield, IL) | 5mg/kg i.v. | tapering to 0.25mg/kg/day i.m. |
Day-1 dose of ATG was given only if needed to reduce lymphocyte count <500×103/mm3.
Additional injection for heart baboons
Additional pharmacologic therapy
Famotidine 0.25mg/kg i.v. (administered when an intravascular catheter was present)
Ganciclovir 5mg/kg i.v. (administered when an intravascular catheter was present)
Valganciclovir 15mg/kg/day p.o.
The anti-CD40mAb (2C10R4) was obtained from Dr Keith Reimann at the NIH NHP Resource Center, Boston, MA. and the CTLA4-Ig (abatacept or belatacept) was purchased (Table 2). 2C10R4 is prepared against rhesus monkey cells [56], but has a significant effect against baboon cells [10]. The doses of the costimulation-blockade agents were based on previous studies by others and ourselves [16,43,56–59].
Baboons in Group 1 (that received grafts from GTKO or GTKO/CD46 pigs) received either a CTLA4-Ig (abatacept)-based IS regimen (Group 1A, n=4) or combined CD28/B7 and CD40/CD154 pathway blockade with belatacept and anti-CD40mAb (Group 1B, n=4).
To determine the role of the CIITA-DN mutation, one baboon in Group 2 (all of which received grafts from GTKO/CD46/CIITA-DN pigs) received no IS (as a control). Five baboons received CD28/B7 pathway blockade (Group 2A, n=5), that was either abatacept-based (n=2) or belatacept-based (n=3). (As we could detect no difference in the effect of these two agents in the doses used, we report them as a single subgroup.) Two baboons (Group 2B) received combined CD28/B7 and CD40/CD154 pathway blockade with belatacept and anti-CD40mAb (the same regimen as in Group 1B).
All baboons in Group 3 (heart transplants, n=4) received hearts from GTKO/CD46 pigs and IS based on blockade of both pathways, i.e., belatacept+anti-CD40mAb (as in Groups 1B and 2B). However, tapering low-dose methlprednisolone was added to the regimen (Table 2).
To ensure that hyperacute rejection did not occur, in the early part of this study 9 baboons received cobra venom factor, but this was later considered unnecessary. The decision not to include maintenance corticosteroids in the regimen was based on the observations of Yamada et al [60,61]. Rapamycin replaced mycophenolate mofetil (MMF) in some experiments simply because we have found it necessary to administer MMF by a continuous i.v. infusion (as many baboons will not take it orally), whereas rapamycin can be administered by i.m. injection [62] (Table 1). (We have not observed any difference in response whether MMF or rapamycin has been administered.)
Anticoagulation
No postoperative heparin was administered to the baboons in any group.
Pig aortic endothelial cell (pAEC) culture
pAEC were cultured as previously described [53]. Activation of subconfluent pAEC was carried out by co-culture with recombinant porcine interferon-γ (IFN-γ, 50ng/mL: Serotec, Raleigh, NC) for adequate periods. Surface expression of SLA class I (clone JM1E3, Serotec) and class II (clone 2E9/13, Serotec) antigens on pAEC was detected by LSR II flow cytometer (Beckton Dickinson, Franklin Lakes, NJ), as previously described [18].
Measurement of anti-pig nonGal IgM and IgG antibodies
Anti-pig (nonGal) antibody levels were measured by flow cytometry using GTKO/CD46 pAEC as target cells, as previously described [16]. Briefly, 20μl baboon sera, which had been heat-inactivated for 30min at 56°C, were incubated with 0.1–0.2×106 pAEC for 30min at 4°C. pAEC were washed and incubated for 30min at 4°C with secondary antibodies (1:20) - anti-human FITC-IgM (μ-chain-specific) and FITC-IgG (γ-chain-specific) (Invitrogen, Carlsbad, CA). Negative controls were obtained by incubating the target cells with secondary anti-human antibodies only (and no serum). Binding of IgM and IgG was assessed using relative mean fluorescence intensity (MFI), which was calculated by dividing the MFI value for each sample by the negative control. Data acquisition was performed with a LSR II flow cytometer (BD), and data were analyzed using Flowjo software. Antibody levels in all samples from a single baboon were measured on the same occasion.
CFSE-Mixed lymphocyte reaction (MLR)
CFSE-MLR was carried out before transplantation and between days 21–28 or 41–48 after transplantation, as previously described [54,63]. Recipient baboon and donor pig peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood and labeled with CFSE (Molecular Probes, Eugene, OR) at a final concentration of 5μM. Responder PBMC (2×106 cells/ml) from baboon recipients were co-cultured with irradiated (2,800cGy) PBMC prepared as stimulator cells (2×106 cells/ml) from the autologous baboon or donor pig. The responder:stimulator ratio was 1:1. After 6 days culture, cells were harvested for staining with phycoerythrin (PE)-Cy7-conjugated CD4 (clone SK3) and PE-conjugated CD8 (clone RPA-T8) mAbs (BD Pharmingen, San Diego, CA) together with a Pacific Blue-conjugated CD3e (clone SP34-2) mAb (BD Pharmingen). Flow cytometry was performed using LSR II flow (BD Bioscience, San Jose, CA). Percentage of T cell proliferation was analyzed by FlowJo software (Tree Star, Ashland, OR).
Monitoring of baboon CD4+ or CD8+ T cells and CD21+ B cells
Blood samples were collected before any therapy, and on days −1, 4, 7, 14, 21, 28 +/− 42 for flow cytometric analysis. FITC-conjugated mouse anti-human CD3ε (clone SP34), PEcy7-conjugated anti-CD4 (clone SK3), PE-conjugated anti-CD8 (clone RPA-T8), and APC-conjugated anti-CD21 (clone B-ly4) antibodies were obtained from BD Pharmingen. CD21 was used as a B cell marker because baboons in other studies (not included here) were administered anti-CD20 mAb. Percentages of CD3+CD4+, CD3+CD8+ T cells, and CD21+ B cells were determined by flow cytometry. Data acquisition was performed with a LSR II flowcytometer, and data were analyzed using Flowjo software.
Histopathology and immunohistopathology of pig grafts
The pig artery patch xenografts (with surrounding baboon aortic tissue) were excised at euthanasia. The central part of the graft, i.e., avoiding the areas around the suture lines, was used for histological examination. At necropsy in baboons with heart xenografts, both ventricles, both atria, and donor aorta and pulmonary artery were examined.
For conventional histology, tissues were fixed in 10% formalin, embedded in paraffin, and sections were stained with hematoxylin and eosin (H&E) +/− trichrome. For immunohistochemistry studies, cryosections (4μm) were stained by using primary mAbs to demonstrate antibody and complement deposition (IgM, IgG, C3), as previously described [9,16,64,65].
Statistical analysis
Data for the in vitro studies are presented as mean ± SEM. Significance of differences was determined by paired Student’s t-test. Statistical analysis was performed using social sciences software GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Values of p<0.05 were considered statistically significant. In some cases, the small number of in vivo studies in each group precluded statistical comparisons.
RESULTS
IN VITRO STUDIES
Effect of the CIITA-DN modification
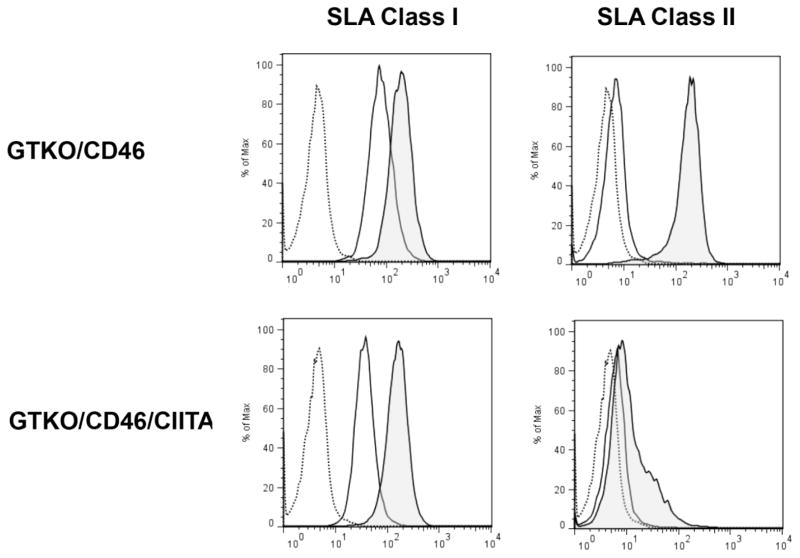
Human CIITA-DN suppresses the expression of SLA class II on pAEC (Figure 1)
Figure 1. Mutant human CIITA-DN suppresses the expression of SLA class II on pAEC.
Expression of SLA class II on GTKO/CD46/CIITA-DN pAECs was compared with that on GTKO/CD46 pAECs. The pAECs were activated with porcine interferon-γ (pIFN-γ; 50ng/ml) for 48h. After activation of pAECs from both GTKO/CD46 and GTKO/CD46/CIITA-DN pigs, expression of SLA class I was increased. A small increase in SLA class II expression was observed on GTKO/CD46/CIITA-DN pAECs after activation, but there was a marked increased expression of SLA class II on GTKO/CD46 pAECs. Isotype control (dotted line), before activation (solid line), and after activation (shaded).
Cultured CIITA-DN pAEC were activated with pIFN-γ for 48h. The expression of SLA class II on pAEC was strongly suppressed. In contrast, the expression of SLA class I on pAEC was up-regulated similarly in GTKO/CD46/CIITA-DN and GTKO/CD46 pAEC after activation with pIFN-γ.
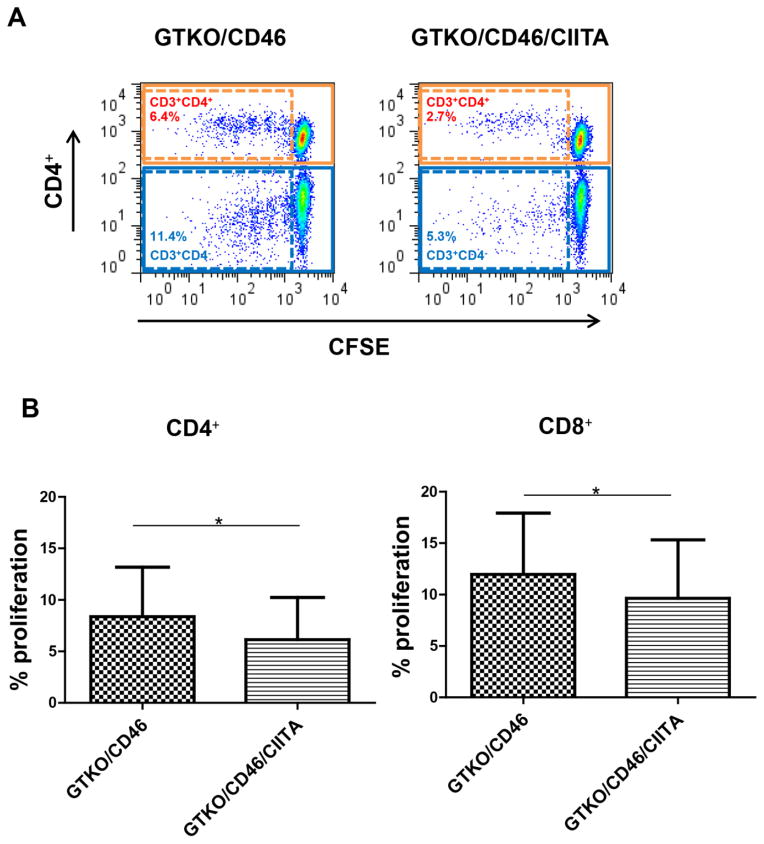
Reduction of baboon T cell response to pPBMC by suppression of SLA class II expression (Figure 2)
Figure 2. Reduction of baboon T cell response to pPBMC associated with suppression of SLA class II expression.
(A) Flow cytometry of the CD4+ and CD8+ responses in representative experiments (% refers to percentage proliferation). There was reduced CD4+ and CD8+ T cell proliferation to GTKO/CD46/CIITA-DN pPBMC. (B) Mean percentage proliferation of CD4+ and CD8+ cells. There were significantly lower baboon CD4+ and CD8+ T cell responses to GTKO/CD46/CIITA-DN pPBMC compared with those to GTKO/CD46 pPBMC (*p<0.05, respectively). The proliferative responses of baboon CD4+ and CD8+ T cells to autologous PBMC were negligible (not shown). We conclude that the reduced proliferation of CD8+ cells was secondary to reduction in CD4+ T cell help.
The baboon CD4+ and CD8+T cell responses to GTKO/CD46/CIITA-DN pPBMC were significantly lower than to GTKO/CD46 pPBMC (p<0.05). We suggest that the CD8+T cell response was reduced because the CD4+ cells no longer provided help.
IN VIVO STUDIES
Results in Groups 1 and 2: Baboons with artery patch grafts
Recipient baboon survival and clinical complications (Table 1)
All 16 baboons remained healthy and active throughout the periods of follow-up. There were no complications from the surgical procedure or IS. Follow-up was electively for either 28 (n=9) or 48 (n=5) days, except in two cases when the baboon was euthanized on day 14 or 21 (following bleeding from an intra-arterial catheter) (Table 1). In later cases, follow-up was extended from 28 to 48 days to ensure that a gradual or late elicited anti-pig antibody response was not being missed.
Immunologic monitoring
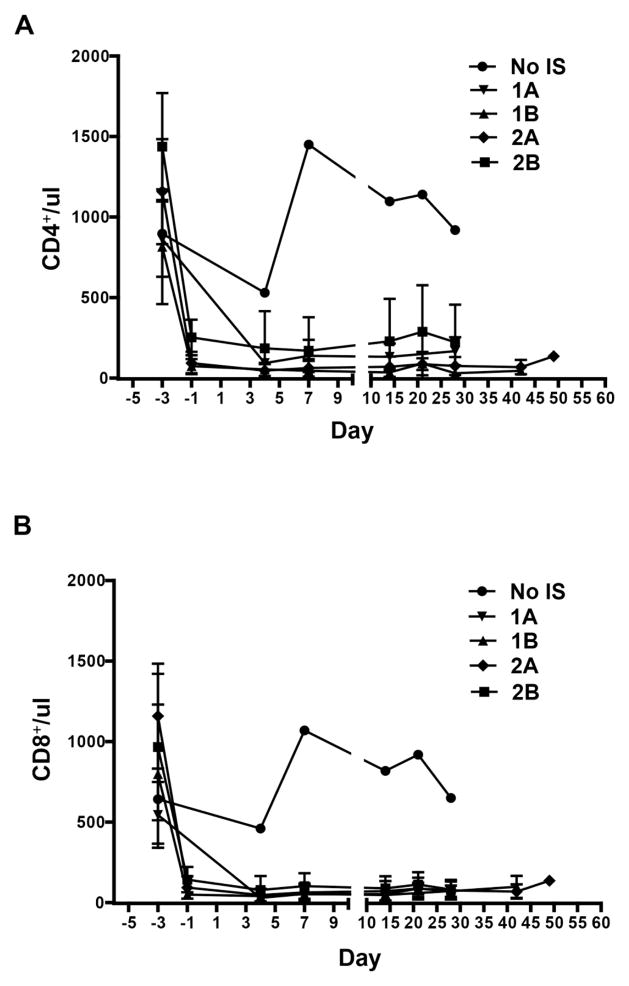
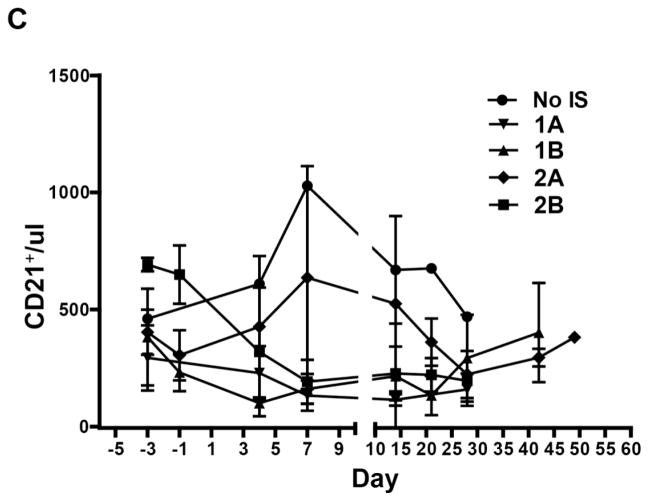
T cell (CD4+, CD8+) and B cell (CD21+) kinetics in baboon recipients (Figure 3)
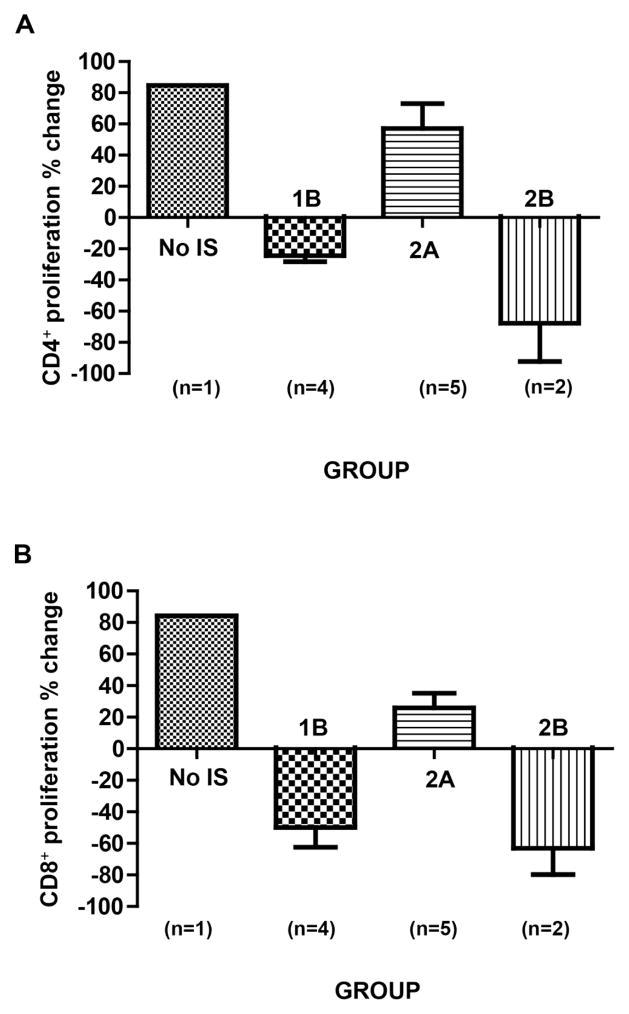
Figure 3. The mean numbers of CD4+, CD8+ T cells, and CD21+ B cells in GTKO or GTKO/CD46 or GTKO/CD46/CIITA-DN pig artery patch recipients.
Depletion of CD4+ (A) and CD8+ (B) T cells in blood of recipient baboons was observed after ATG, but was not seen when no IS was administered. Some depletion of CD21+ B cells (C) was observed in some baboons (which we are unable to explain). The mean numbers of CD4+, CD8+, and CD21+ cells in baboons in each group are shown with standard error (SE).
In all baboons (with all regimens), after the administration of anti-thymocyte globulin on day −3, a profound depletion of T cells (CD3+, CD4+, CD8+) was obtained, and was maintained throughout the course of the experiment, irrespective of differences in regimen or maintenance therapy (Figures 3A,B). CD3+T cell counts were generally maintained <500/ul, with CD4+ and CD8+ cell numbers frequently less than half of this throughout the period of follow-up. Some depletion of CD21+ cells occurred in Groups 1B and 2B (Figure 3C) (which was unexpected and which we are unable to explain).
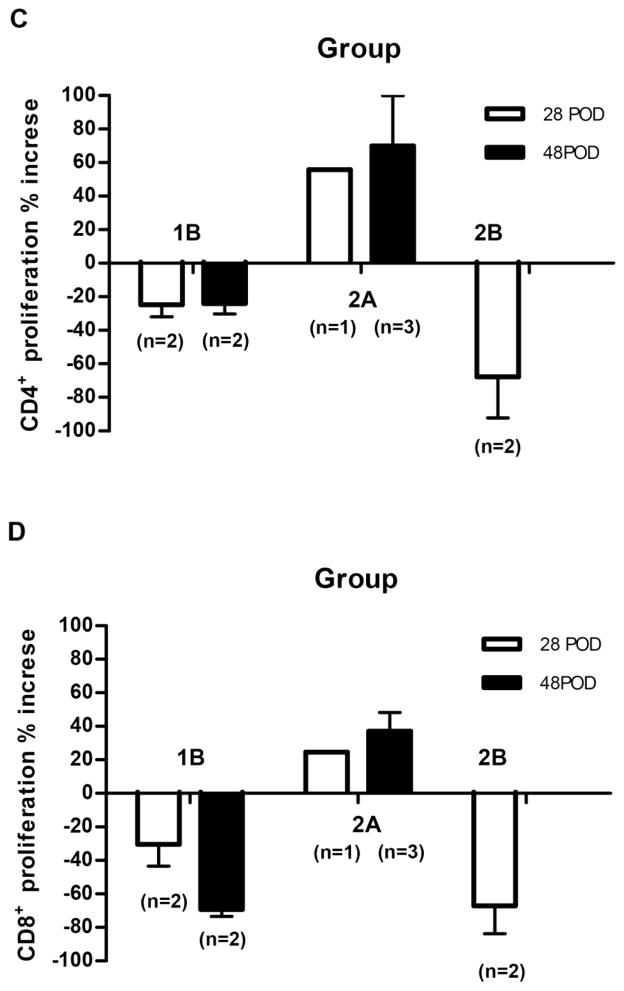
Recipient baboon T cell proliferation in response to pig nonGal antigens (Figure 4)
Figure 4. Recipient baboon mean cellular responses to GTKO or GTKO/CD46 or GTKO/CD46/CIITA-DN pig artery patch grafts *.
When a CTLA4-Ig (abatacept or belatacept)-based regimen was administered (Group 2A), CD4+ (A) and CD8+ (B) T cell proliferative responses were seen on MLR, as well as in the single baboon in Group 1 that received no IS. It was only when anti-CD40mAb+belatacept were administered together that no T cell proliferative response was documented (Groups 1B and 2B). The CD4+ and CD8+ T cellular proliferative responses were less to a GTKO/CD46/CIITA-DN graft (Group 2B) than to a GTKO/CD46 graft (Group 1B) (statistical analyses were not carried out as numbers too small).
The assays were carried out pre-transplantation (pre-Tx) and during the last week of each study (post-Tx) (see Table 1). We could not detect any relationship between the result of the MLR and the day of the assay, i.e., day 21–28 or 48 (C and D). (*We do not show the MLR data in Group 1A because we used a different method of measuring the MLR in Group 1A from that in other groups.
The single baboon that received no IS developed an approximate 80% increase in CD4+ and CD8+ proliferative responses (Figure 4A,B). Baboons receiving a regimen based on CD28 pathway blockade alone (Group 2A) developed a weaker proliferative response. In contrast, a regimen based on blockade of both CD40/CD154 and CD28/B7 pathways (Groups 1B and 2B) prevented a T cell proliferative response. When comparable IS was administered (e.g., Groups 1B vs 2B), the response to a GTKO/CD46/CIITA-DN pig graft appeared to be reduced compared to that to a GTKO/CD46 graft (Figure 4A,B). No statistical analysis was carried out because of the small numbers in each group. We observed no significant difference in the response whether the MLR was carried out on days 21–28 or 48 (Figure 4C,D).
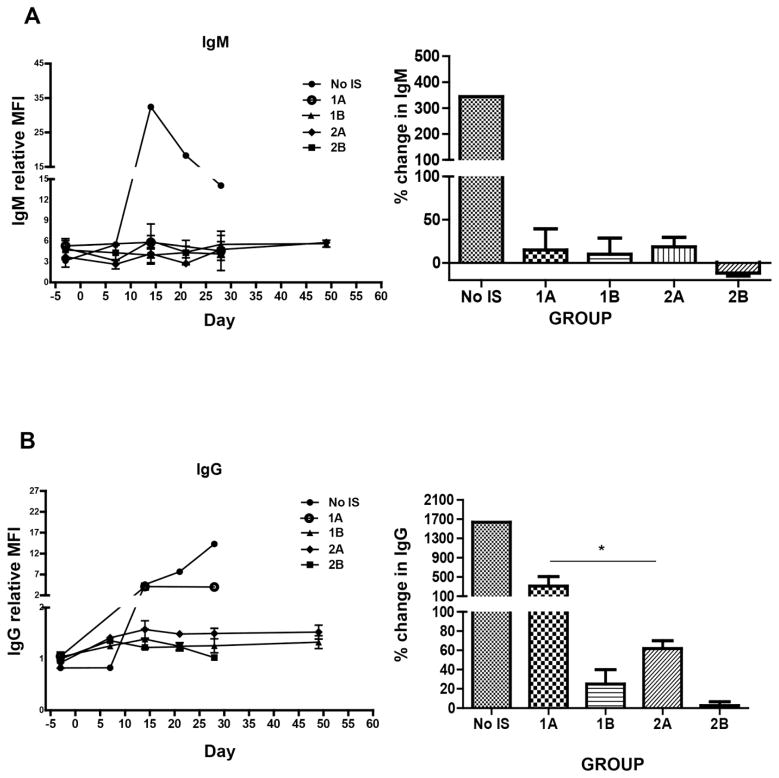
Recipient baboon elicited antibody responses (Figure 5)
Figure 5. Recipient baboon mean IgM (A) and IgG (B) antibody responses to GTKO or GTKO/CD46 or GTKO/CD46/CIITA-DN pig artery patch grafts.
Left panel shows the mean IgM and IgG antibody responses throughout the course of each experiment. Right panel shows the percentage change in IgM and IgG antibody responses at the end of each experiment. The single baboon with no IS developed anti-pig IgM (A) and IgG (B) antibody responses. In abatacept- or belatacept-based regimens (Groups 1A and 2A), the antibody response was greatly attenuated, though there remained a modest IgG response; the IgG response in Group 2A was significantly reduced when compared with that in Group 1A (p<0.05), suggesting a beneficial effect of the CIITA-DN mutation. With the anti-CD40mAb+belatacept-based regimen, an elicited IgG antibody response was minimal or absent (Groups 1B or 2B, respectively),
The single baboon that received no IS developed all of the features of an adaptive immune response, including a 3.5-fold increase in anti-nonGal IgM (Figure 5A) and a 16-fold increase in anti-nonGal IgG (Figure 5B). When the CD28/B7 pathway alone was blocked by CTLA4-Ig (Groups 1A vs 2A), the responses were greatly attenuated, though this was more obvious in the presence of a GTKO/CD46/CIITA-DN graft (Figure 5B). Indeed, the IgG response to the grafts expressing the CIITA-DN mutation (Group 2A) was significantly reduced compared to that to the GTKO grafts (Group 1A) (p<0.05), suggesting a beneficial effect of the mutation. In baboons in which blockade of both pathways was effective (Groups 1B vs 2B), no difference could be seen in anti-pig IgG response (Figure 5B).
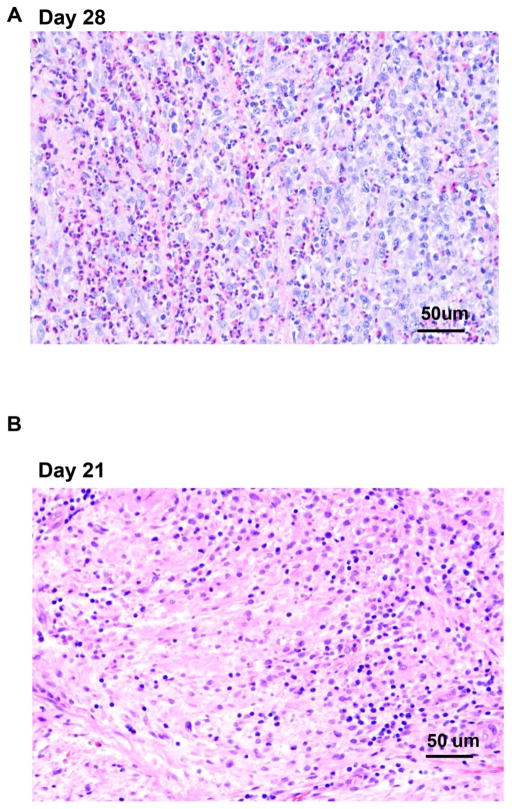
Histopathology of pig patch grafts at euthanasia (Figure 6)
Figure 6. Histopathology of GTKO or GTKO/CD46 or GTKO/CD46/CIITA-DN pig artery patch grafts at euthanasia.
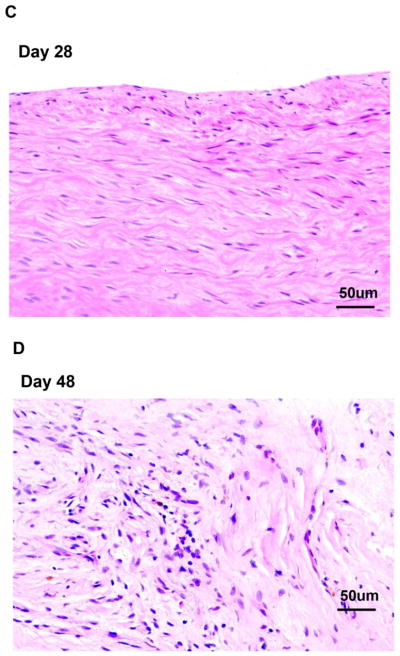
(A) At euthanasia on day 28, the GTKO/CD46/CIITA-DN pig artery patch graft in the baboon with no IS showed a massive full-thickness infiltrate (eosinophilic, lymphocytic, and monocytic). (B) The pig grafts in Groups 1A and 2A showed a mild infiltration with mixed inflammatory cells (lymphocytes and monocytes). Cellular infiltration in the GTKO/CD46/CIITA-DN graft was absent in Group 2B (day 28) (C), and minimal in Group 1B (day 28 or 48) (in which a GTKO/CD46 graft was transplanted) (D). No obvious differences were observed in the grafts, whether the experiments were terminated on day 28 or 48. (Magnification x20)
The graft in the baboon receiving no IS showed a massive full-thickness eosinophilic, lymphocytic, and monocytic infiltrate, particularly at the adventitial-medial junction (Figure 6A). Group 1 grafts were intact, but with several scattered eosinophils, although features of inflammation were rare (Figure 6D). In Group 2A, the grafts showed a mild increase in thickness of the subintima and media with a mixed inflammatory cellular infiltrate (lymphocytic and monocytic) (Figure 6B). In Group 2B, the histologic appearance of the graft was normal with a lack of significant inflammation (Figure 6C). In summary, cellular infiltration of the grafts was minimal and less in Groups 1B and 2B than in Groups 1A and 2A. We did not observe any platelet adhesion/aggregation or thrombus formation on the endothelial surface of any graft either macroscopically or microscopically.
Antibody and complement deposition
Immunofluorescence indicated weak-to-moderate deposition of IgM, IgG, and complement in all grafts, with weakest deposition in Group 2B (not shown).
Results in Group 3: Baboons with heart grafts
In order to determine whether the results documented from the artery patch transplants truly reflected those that would be seen after heart transplantation, we carried out 4 experiments using GTKO/CD46 pigs as donors (as these were more readily available than GTKO/CD46/CIITA-DN pigs at the time) and the IS regimen based on blockade of both pathways, i.e., belatacept+anti-CD40mAb.
Recipient baboon survival and clinical complications (Table 1)
The heart grafts in the 4 baboons in Group 3 could be followed for approximately 4 months (Table 1). The experiment was terminated in one baboon (with a functioning graft) that developed a cytomegalovirus infection. The other baboons were euthanized following graft failure.
Immunologic monitoring
T cell (CD4+, CD8+) and B cell (CD21+) kinetics in baboon recipients (Supplementary Figure 1)
In all baboons, after the administration of anti-thymocyte globulin on day −3, a profound depletion of T cells (CD4+, CD8+) was observed, and was maintained for approximately one week, after which there was some slow recovery (Supplementary Figure 1). However, CD4+ and CD8+ cell numbers frequently remained <250 cells/μl throughout the period of follow-up, even beyond 4m. There was no substantial change in CD21+B cell numbers.
Recipient baboon T cell proliferation in response to pig nonGal antigens
Post-transplant, proliferation of CD4+ or CD8+ cells against GTKO/CD46 pig cells was greatly reduced (by 52% and 63%, respectively) (not shown).
Recipient baboon elicited antibody responses (Supplementary Figure 2)
There was no increase in IgM in any of the baboons (Supplementary Figure 2A). IgG sensitization to nonGal antigens expressed on the pAECs did not occur (Supplementary Figure 2B).
Histopathology of pig heart grafts at euthanasia (Supplementary Figure 3)
There were no features suggestive of acute cellular rejection (graft infiltrating cells). Patchy, focally extensive areas of fibrosis (scarring) were seen in the hearts at the time of euthanasia (Supplementary Figure 3). However, thrombosed or recanalizing vessels were not apparent, casting doubt on the cause of the observed changes.
Antibody and complement deposition
Immunofluorescence indicated weak IgM, IgG, and complement deposition in all grafts (not shown). No attempt was made to determine expression of the transgenes in the explanted hearts.
DISCUSSION
One of the current challenges in organ xenotransplantation is to develop immunosuppressive regimens that protect against T cell-mediated rejection. However, it is difficult to test these without the confusing issue of coagulation dysregulation. The pig-to-baboon artery patch model offers this possibility.
To summarize the present study, (i) when IS was inadequate, e.g., with blockade of the CD28/B7 pathway alone, the T cell response to a CIITA-DN graft was reduced, but (ii) even in the presence of a CIITA-DN graft, blockade of the CD28/B7 pathway alone appeared inadequate in that it did not completely prevent a T cell-dependent response, whereas (iii) combined blockade of the CD40/CD154 and CD28/B7 pathways successfully prevented this response whether a GTKO/CD46 graft expressed the CIITA-DN mutation or not. This observation made in the artery patch model was also seen in the heterotopic heart transplant model, suggesting that the artery patch model is potentially valuable for the preliminary screening of an IS regimen.
Like many studies in nonhuman primate models, the present study is limited by the relatively small number of experiments in each group. In addition, there were some minor variations in the IS regimens. Nevertheless, we believe that some tentative conclusions can be drawn from the observations we have made.
In our established pig-to-baboon artery patch model, by exposing the recipient to sufficient pig antigen for an essential period of time to induce an adaptive immune response, we can monitor innate and adaptive immune responses to pig xenografts [16]. Using GTKO pigs as sources of grafts, our initial studies in this model indicated that (i) a patch graft in a non-immunosuppressed baboon was sufficient to induce an adaptive immune response (manifest by a proliferative response on MLR, an elicited anti-pig IgM and IgG antibody response, and intense T cell infiltration of the graft with some B cells, neutrophils and macrophages); and (ii) an anti-CD154mAb-based regimen prevented all of the features of the adaptive response [16].
In the present study, even when the graft was taken from a GTKO/CD46/CIITA-DN pig, a regimen based on blockade of the CD28/B7 pathway was not totally successful, though the response was reduced. In contrast, when belatacept was combined with an anti-CD40mAb, the regimen prevented all features of a response.
We did not test an anti-CD40mAb-based regimen in the absence of belatacept. Our in vitro studies indicated that belatacept had a stronger suppressive effect on MLR (the inhibitory effect on baboon T cell proliferation induced by pig ECs) than anti-CD40mAb (Hara H, et al, manuscript in preparation), and so we did not put a priority on testing this latter agent in vivo. Lee et al reported an in vitro study that demonstrated that belatacept was a more powerful suppressive agent than anti-CD154mAb [66], which is clearly not the case in vivo. However, in the present study, the anti-CD40mAb (2C10R4) was prepared against rhesus monkeys rather than baboons, and this may be important. Furthermore, Mohiuddin et al have demonstrated prolonged graft survival of pig hearts in baboons receiving high doses of this agent in the absence of belatacept [67]; anti-CD40mAb alone at high dosage (50mg/kg) therefore appears to be sufficient.
The T cell proliferative response, the IgM and IgG antibody responses, and the extent of cellular infiltration of the graft were all marginally greater when baboons received grafts from GTKO/CD46 pigs than from pigs additionally transgenic for CIITA-DN, but the heart transplant studies indicated that this advantage may not be clinically important in regard to the immediate T cell response. However, although we emphasize that we currently have no data to support such a conclusion, we tentatively suggest that, through reducing the effects of graft endothelial activation by inhibiting upregulation of SLA class II, CIITA-DN might prove beneficial in inhibiting the development of graft vasculopathy (chronic rejection). We have not yet determined whether the pig endothelial cells of the graft are replaced to any extent by baboon endothelial cells. However, even if this is the case, the model is sufficient to stimulate an immune response.
In our in vitro studies, no increase in SLA class II expression was observed on GTKO/CD46/CIITA-DN pAECs after activation, though there was increased expression of SLA class II on GTKO/CD46 pAECs. This confirms earlier work by us [18] and others [68]. The impact of donor MHC class II expression (on graft vascular endothelium and passenger leukocytes) has been demonstrated in the mouse allotransplantation model [69]. Donor-derived passenger leukocytes, including antigen-presenting cells, migrate into host lymphoid tissues after transplantation. These antigen-presenting cells express MHC class II and stimulate host CD4+T cells and initiate graft rejection through the direct pathway [70,71]. Therefore, CIITA-DN pigs are likely to be most beneficial in reducing the direct route of xenoantigen presentation.
Although it might be anticipated that the adaptive immune response to a pig organ might be greater than to an artery patch, we tested the combined anti-CD40mAb-belatacept-based regimen in the pig-to-baboon heart transplantation model with follow-up for up to 18 weeks and demonstrated it to prevent an adaptive response as successfully as in the artery patch model.
Importantly, although we have documented the thrombotic effect of an anti-CD154mAb in both organ and artery patch transplantation models [16,30], we have not observed any thrombosis associated with anti-CD40mAb therapy in either model (Iwase H. et al, manuscript submitted). Furthermore, this outcome was achieved in the absence of a continuous heparin infusion, which we have always felt to be necessary when administering an anti-CD154mAb-based regimen [9,16,30].
In conclusion, this study would suggest that, in the absence of anti-CD154mAb therapy, blockade of both the CD28/B7 and CD40/CD154 pathways prevents a primate T cell response to transplanted GTKO/CD46 or GTKO/CD46/CIITA-DN pig tissues and organs. Furthermore, although, when effective IS was administered, the effect of the CIITA-DN genetic modification was minimal, it may allow some reduction in the intensity of the immunosuppressive regimen in the long-term.
Supplementary Material
Highlights.
In the presence of a GTKO/CD46 pig artery patch graft, blockade of the CD28/B7 pathway reduced, but did not completely prevent, an adaptive immune response in baboons, whereas blockade of both the CD28/B7 and CD154/CD40 pathways did.
The transplantation of a graft from a GTKO/CD46 pig additionally transgenic for a mutant SLA class II transactivator (CIITA-DN) marginally reduced the in vivo adaptive immune response in baboons.
Combined blockade of the CD28/B7 and CD154/CD40 pathways prevented an adaptive immune response to GTKO/CD46 pig heart grafts, with follow-up for >4 months.
Acknowledgments
Burcin Ekser, MD, was a recipient of NIH NIAID T32 AI 074490 training grant. Mohamed Ezzelarab, MD, was supported in part by the Joseph A. Patrick Fellowship of the Thomas E. Starzl Transplantation Institute. 2C10R4 was kindly provided by Keith Reimann of the NIH NHP Resource Center, Boston, MA. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants #U19 AI090959-01, #U01 AI068642, and # R21 A1074844, and # 1PO1 HL107152, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Blacksburg, VA. The baboons used in the study were from the Oklahoma University Health Sciences Center, Baboon Research Resources, which is supported by the Office of the Director, NIH, under Award Number P40OD010431 and P40OD010988. The authors thank Yuko Miyagawa and Dirk J van der Windt for technical help in performing in vitro assay.
Abbreviations
- AEC
aortic endothelial cells
- AHXR
acute humoral xenograft rejection
- CIITA-DN
MHC Class II transactivator dominant-negative gene mutant
- GTKO
α1,3-galactosyltransferase gene-knockout
- IS
immunosuppressive therapy
- mAb
monoclonal antibody
- MMF
mycophenolate mofetil
- p
pig
- PBMC
peripheral blood mononuclear cells
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
Carol Phelps and David Ayares are employees of Revivicor. No other author has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science (New York, NY) 2003;299:411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335–40. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DK, Koren E, Oriol R. Genetically engineered pigs. Lancet. 1993;342:682–3. doi: 10.1016/0140-6736(93)91791-j. [DOI] [PubMed] [Google Scholar]
- 5.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1995;1:964–6. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 6.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–83. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DK, Dorling A, Pierson RN, 3rd, Rees M, Seebach J, Yazer M, et al. Alpha1,3-galactosyltransferase gene-knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84:1–7. doi: 10.1097/01.tp.0000260427.75804.f2. [DOI] [PubMed] [Google Scholar]
- 8.Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–83. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 9.Ezzelarab M, Garcia B, Azimzadeh A, Sun H, Lin CC, Hara H, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–12. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper DK, Satyananda V, Ekser B, Van der Windt DJ, Hara H, Ezzelarab M, et al. Progress in pig-to-nonhuman primate transplantation models (1998–2013): a comprehensive review of the literature. Xenotransplantation. 2014;21:397–419. doi: 10.1111/xen.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buhler L, Awwad M, Basker M, Gojo S, Watts A, Treter S, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 12.Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Tseng YL, Houser S, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–72. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 14.Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80:1493–500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 15.Ekser B, Long C, Kumar G, Hara H, Dons EM, Thacker J, et al. Heart transplantation using genetically-engineered pigs: can CTLA4-lg replace anti-CD154mAb in the imunosuppressive regimen? Xenotransplantation. 2011;18:284. (Abstract 332) [Google Scholar]
- 16.Ezzelarab MB, Ekser B, Echeverri G, Hara H, Ezzelarab C, Long C, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–32. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phelps CJ, Ball SF, Vaught TD, Vance AM, Mendicino M, Monahan JA, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–85. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 18.Hara H, Witt W, Crossley T, Long C, Isse K, Fan L, et al. Human dominant-negative class II transactivator transgenic pigs - effect on the human anti-pig T-cell immune response and immune status. Immunology. 2013;140:39–46. doi: 10.1111/imm.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci U S A. 1999;96:8132–7. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–93. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 21.Wu G, Pfeiffer S, Schroder C, Zhang T, Nguyen BN, Lea W, et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation. 2005;12:197–208. doi: 10.1111/j.1399-3089.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 23.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–3. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 24.Cardona K, Milas Z, Strobert E, Cano J, Jiang W, Safley SA, et al. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant. 2007;7:2260–8. doi: 10.1111/j.1600-6143.2007.01933.x. [DOI] [PubMed] [Google Scholar]
- 25.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229:294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9:2716–26. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 27.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF, Jr, Thomas ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12:763–71. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 29.Kirk AD, Knechtle SJ, Sollinger HW. Preliminary results of the use of humanized anti-CD154 in human renal allotransplantation. Am J Transplant. 2001;1(Suppl 1) (Abstract) [Google Scholar]
- 30.Knosalla C, Gollackner B, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism revisted. Transplantation. 2002;74:416–7. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 31.Knosalla C, Gollackner B, Buhler L, Mueller NJ, Houser S, Mauiyyedi S, et al. Correlation of biochemical and hematological changes with graft failure following pig heart and kidney transplantation in baboons. Am J Transplant. 2003;3:1510–9. doi: 10.1046/j.1600-6135.2003.00258.x. [DOI] [PubMed] [Google Scholar]
- 32.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 33.Pearson TC, Trambley J, Odom K, Anderson DC, Cowan S, Bray R, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74:933–40. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 34.Haanstra KG, Ringers J, Sick EA, Ramdien-Murli S, Kuhn EM, Boon L, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75:637–43. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 35.Adams AB, Shirasugi N, Jones TR, Durham MM, Strobert EA, Cowan S, et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. Journal of immunology (Baltimore, Md: 1950) 2005;174:542–50. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 36.Haanstra KG, Sick EA, Ringers J, Wubben JA, Kuhn EM, Boon L, et al. Costimulation blockade followed by a 12-week period of cyclosporine A facilitates prolonged drug-free survival of rhesus monkey kidney allografts. Transplantation. 2005;79:1623–6. doi: 10.1097/01.tp.0000158426.64631.ed. [DOI] [PubMed] [Google Scholar]
- 37.Aoyagi T, Yamashita K, Suzuki T, Uno M, Goto R, Taniguchi M, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009;9:1732–41. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 38.Thompson P, Cardona K, Russell M, Badell IR, Shaffer V, Korbutt G, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011;11:947–57. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badell IR, Russell MC, Cardona K, Shaffer VO, Turner AP, Avila JG, et al. CTLA4Ig prevents alloantibody formation following nonhuman primate islet transplantation using the CD40-specific antibody 3A8. Am J Transplant. 2012;12:1918–23. doi: 10.1111/j.1600-6143.2012.04029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Badell IR, Thompson PW, Turner AP, Russell MC, Avila JG, Cano JA, et al. Nondepleting anti-CD40-based therapy prolongs allograft survival in nonhuman primates. Am J Transplant. 2012;12:126–35. doi: 10.1111/j.1600-6143.2011.03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe M, Yamashita K, Suzuki T, Kamachi H, Kuraya D, Koshizuka Y, et al. ASKP1240, a fully human anti-CD40 monoclonal antibody, prolongs pancreatic islet allograft survival in nonhuman primates. Am J Transplant. 2013;13:1976–88. doi: 10.1111/ajt.12330. [DOI] [PubMed] [Google Scholar]
- 42.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–33. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 43.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–53. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 44.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–46. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 45.Koshika T, Phelps C, Fang J, Lee SE, Fujita M, Ayares D, et al. Relative efficiency of porcine and human cytotoxic T-lymphocyte antigen 4 immunoglobulin in inhibiting human CD4+ T-cell responses co-stimulated by porcine and human B7 molecules. Immunology. 2011;134:386–97. doi: 10.1111/j.1365-2567.2011.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin C, Plat M, Nerriere-Daguin V, Coulon F, Uzbekova S, Venturi E, et al. Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Res. 2005;14:373–84. doi: 10.1007/s11248-004-7268-4. [DOI] [PubMed] [Google Scholar]
- 47.Phelps C, Vaught T, Ball S, Mendicino M, Walters A, Monahan J, et al. Multi-transgenic pigs designed for xenoislet transplants. Xenotransplantation. 2009;16:374. (Abstract) [Google Scholar]
- 48.Leveque X, Cozzi E, Naveilhan P, Neveu I. Intracerebral xenotransplantation: recent findings and perspectives for local immunosuppression. Current opinion in organ transplantation. 2011;16:190–4. doi: 10.1097/MOT.0b013e32834494b5. [DOI] [PubMed] [Google Scholar]
- 49.Ayares D, Vaught T, Ball S, Ramsoondar J, Monahan J, Mendicino M, et al. Islet-specific expression of TFPI, CD39, and CTLA4lg in transgenic pigs deisgned for xenoislet transplantation. Xenotransplantation. 2011;18:269. (Abstract 120) [Google Scholar]
- 50.Wijkstrom M, Iwase H, Hara H, Ekser B, Van der Windt DJ, Long C. Glucose metabolism in pigs expressing human genes under an insulin promoter. Xenotransplantation. 2014 doi: 10.1111/xen.12145. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yun S, Gustafsson K, Fabre JW. Suppression of human anti-porcine T-cell immune responses by major histocompatibility complex class II transactivator constructs lacking the amino terminal domain. Transplantation. 1998;66:103–11. doi: 10.1097/00007890-199807150-00016. [DOI] [PubMed] [Google Scholar]
- 52.Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–5. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 53.Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–74. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 54.Hara H, Koike N, Long C, Piluek J, Roh DS, SundarRaj N, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Investigative ophthalmology & visual science. 2011;52:5278–86. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooper DK, Ye Y, Niekrasz M. Heart transplantation in primates. In: Cramer DV, Podesta LG, Makowka L, editors. Handbook of Animal Models in Transplantation Research. Boca Raton: CRC Press; 1994. pp. 173–200. [Google Scholar]
- 56.Lowe M, Badell IR, Thompson P, Martin B, Leopardi F, Strobert E, et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12:2079–87. doi: 10.1111/j.1600-6143.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–94. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Page A, Srinivasan S, Singh K, Russell M, Hamby K, Deane T, et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. Am J Transplant. 2012;12:115–25. doi: 10.1111/j.1600-6143.2011.03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lowe MC, Badell IR, Turner AP, Thompson PW, Leopardi FV, Strobert EA, et al. Belatacept and sirolimus prolong nonhuman primate islet allograft survival: adverse consequences of concomitant alefacept therapy. Am J Transplant. 2013;13:312–9. doi: 10.1111/j.1600-6143.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–4. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 61.Griesemer AD, Hirakata A, Shimizu A, Moran S, Tena A, Iwaki H, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9:2669–78. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant. 2013;13:1989–2005. doi: 10.1111/ajt.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin YJ, Hara H, Tai HC, Long C, Tokita D, Yeh P, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–62. doi: 10.1097/TP.0b013e318188acb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–8. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu A, Hisashi Y, Kuwaki K, Tseng YL, Dor FJ, Houser SL, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172:1471–81. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee RS, Yamada K, Womer KL, Pillsbury EP, Allison KS, Marolewski AE, et al. Blockade of CD28-B7, but not CD40-CD154, prevents costimulation of allogeneic porcine and xenogeneic human anti-porcine T cell responses. Journal of immunology (Baltimore, Md: 1950) 2000;164:3434–44. doi: 10.4049/jimmunol.164.6.3434. [DOI] [PubMed] [Google Scholar]
- 67.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML, 3rd, Lewis BG, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–9. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Quinn G, Bower R, Dos-Santos Cruz G, Giovino M, Xu Y, Patience C, et al. Structural and functional characteristics of a dominant-negative isoform of porcine MHC class II transactivator. Eur J Immunogenet. 2003;30:259–70. doi: 10.1046/j.1365-2370.2003.00397.x. [DOI] [PubMed] [Google Scholar]
- 69.Qian S, Fu F, Li Y, Lu L, Rao AS, Starzl TE, et al. Impact of donor MHC class I or class II antigen deficiency on first- and second-set rejection of mouse heart or liver allografts. Immunology. 1996;88:124–9. doi: 10.1046/j.1365-2567.1996.d01-633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faustman DL, Steinman RM, Gebel HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci U S A. 1984;81:3864–8. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–14. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.