Abstract
Objectives
Working memory impairment has been extensively studied in schizophrenia, but less is known about moderators of the impairment. Using the Consortium on the Genetics of Schizophrenia case-control study (COGS-2), we examined smoking status, types of antipsychotic medication, and history of substance as moderators for working memory impairment in schizophrenia.
Methods
From 5 sites, 1377 patients with schizophrenia or schizoaffective, depressed type and 1037 healthy controls completed the Letter-Number Span (LNS) Task. The LNS uses intermixed letter and digit stimuli that increase from 2 up to 8 stimuli. In the Forward condition, participants repeated the letters and numbers in the order they were presented. In the Reorder condition, participants repeated the digits in ascending order followed by letters in alphabetical order.
Results
Schizophrenia patients performed more poorly than controls, with a larger difference on Reorder than Forward conditions. Deficits were associated with symptoms, functional capacity, and functional outcome. Patients who smoked showed larger impairment than nonsmoking patients, primarily due to deficits on the Reorder condition. The impairing association of smoking was more pronounced among patients taking first-generation than those taking second-generation antipsychotic medications. Correlations between working memory and community functioning were stronger for nonsmokers. History of substance use did not moderate working memory impairment.
Conclusions
Results confirm the working memory impairment in schizophrenia, and indicate smoking status as an important moderator for these deficits. The greater impairment in smokers may reflect added burden of smoking on general health or that patients with greater deficits are more likely to smoke.
Keywords: schizophrenia, verbal working memory, letter-number span, moderators, smoking, antipsychotic medication
1. INTRODUCTION
Working memory is defined as an ability to maintain and manipulate the internal representation of a stimulus on-line (Baddeley, 1992). Working memory impairment is profound and enduring among schizophrenia patients (Lee and Park, 2005) and has been found in relatives of schizophrenia patients (Glahn et al., 2003) and individuals with schizotypal features (Mitropoulou et al., 2005; Smith et al., 2006). Thus, working memory impairment is suggested as a promising candidate for an endophenotype. Our previous phase of the Consortium on the Genetics of Schizophrenia (COGS) study showed the endophenotype validity of working memory in behavioral and heritability family studies (Greenwood et al., 2011; Greenwood et al., 2013; Horan et al., 2008). Using a large sample of participants from the COGS Phase 2 case-control study (COGS-2), this study examined potential moderators of verbal working memory impairment in schizophrenia.
The neurobiological mechanisms and components of working memory impairment (e.g., encoding as opposed to maintenance) are established, as well as its association with community functioning (Bittner et al., 2014; Coleman et al., 2012; Glahn et al., 2005; Green et al., 2008; Mayer et al., 2012). However, the effects of demographic and clinical features on working memory in schizophrenia are poorly understood. One consideration is the high rate of cigarette smoking in schizophrenia. Nicotine metabolites and other molecules in cigarette smoking interact with the dopaminergic system and increase release of dopamine in the mesolimbic system and prefrontal cortex (Brody et al., 2009; Marenco et al., 2004; Tsukada et al., 2005). Thus, schizophrenia patients might use nicotine as self-medication to ameliorate cognitive deficits or other clinical symptoms (e.g., Zammit et al., 2003).
Consistent with this hypothesis, several studies showed that nicotine administration reduced working memory impairment and other neurocognitive deficits in schizophrenia (Barr et al., 2008; George et al., 2002; Jacobsen et al., 2004; Sacco et al., 2005). However, most of these studies were conducted on smokers and examined effects before and after nicotine abstinence, raising a question as to whether the beneficial effects of nicotine were due to the reversal of smoking withdrawal-related working memory deficit. Indeed, acute nicotine administration worsened ketamine-induced working memory deficit in healthy individuals (D’Souza et al., 2012). Studies in non-clinical samples have shown either no effect or an impairing effect of chronic nicotine administration on working memory (Park et al., 2000; Wagner et al., 2013). A related consideration is that antipsychotic medications interact with nicotine receptors in the brain. Both first- and second-generation antipsychotic medications act as noncompetitive inhibitors (Grinevich et al., 2009) and it is possible that antipsychotic medication may negate any potential effect of nicotine on working memory (Addy and Levin, 2002). The interaction between antipsychotic medication and cigarette smoking on working memory in schizophrenia has yet to be examined.
Another factor to consider is comorbidity. Schizophrenia patients have higher rates of substance use than the general population (Hartz et al., 2014; Regier et al., 1990). In the general population, history of substance abuse is associated with poorer working memory (Ersche et al., 2006; Wareing et al., 2000). The studies on comorbid substance disorder and working memory in schizophrenia had relatively small samples and produced mixed findings: some studies found better performance of patients with a history of substance use (Schnell et al., 2009; Yucel et al., 2012), whereas others found no effect of past substance abuse (Donoghue et al., 2012; Thoma and Daum, 2008; Wojtalik and Barch, 2014). A recent meta-analysis (Donoghue et al., 2012) indicated that heterogeneity across studies makes it difficult to draw firm conclusions.
The aim of the current study is to investigate potential moderators of verbal working memory impairment in schizophrenia using the Letter-Number Span Task (LNS). With large samples of patients and controls from COGS-2, we asked two questions: 1) do the following moderators affect working memory impairment in schizophrenia: cigarette smoking, antipsychotic medication, the interaction between cigarette smoking and antipsychotic medication, and past substance abuse; and 2) do any of significant moderators identified modulate the relationship between working memory and community functioning in schizophrenia.
METHOD
More details about the recruitment, the selection criteria of participants and clinical assessment methods are available in the Supplemental and the introductory article for this theme (Swerdlow et al., submitted for this issue). The local institutional review boards of each site approved the study. All participants provided informed consent and were compensated for their participation
Procedures
The LNS (Gold et al., 1997; Wechsler, 1997) was administered as part of the COGS-2 research protocol. For the LNS task, the quality assurance (QA) site (University of California Los Angeles) performed QA checks periodically by verifying any score that was an outlier, defined as scores that were outside plus or minus 1.5 SD of the mean within each group. Every outlier score was then compared to the paper copy at the local site to confirm that it was accurate and valid (e.g., no data entry mistake, subjects understood the nature of the task, etc.).
The LNS task consisted of two conditions: the “Forward” and “Reorder” conditions. Both conditions employed a set of intermixed letters and digits that experimenters verbally presented at a rate of one per second. The number of characters (letters or digits) increased by one on each trial starting from the length of 2 stimuli, up to a maximum length of 8 stimuli. Each trial consisted of three sequences of the same length. In the Forward condition, participants were asked to repeat the letters and numbers in the same order as they were presented. In the Reorder condition, participants were asked to repeat the digits in ascending order first and then the letters in alphabetical order. Both conditions were discontinued if the participant failed all three sequences of the same length. The score for each condition was the total number of correctly recalled sequence (i.e., maximum score for each condition = 21).
Both patients and controls received the Global Assessment of Function Scale (GAF; Hall, 1995). Additional clinical assessment for patients included a modified versions of the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1984a) and Positive Symptoms (SAPS; Andreasen, 1984b), the Brief University of California San Diego Performance-based Skills Assessment (UPSA-B; Mausbach et al., 2007) as a measure of functional capacity, and the Role Functioning Scale (RFS) (RFS; McPheeters, 1984) for community functioning. The UPSA assess ability to perform everyday tasks necessary for independent community functioning (Twamley et al., 2002).
Statistical analysis
The main analytic tool for this study is the generalized linear model as it provides a unified statistical framework for univariate and repeated measures analysis of variance (ANOVA) and allows for covariate adjustment. To compare demographic and clinical characteristics, we conducted a series of univariate ANOVA with group and site as between-subject factors. To examine the patient-group differences on LNS, we conducted a series of repeated measures ANOVA in a step-wise way: first, a 2 by 2 repeated measures ANVOA with condition as within-subject factor and group as between-subject factor was performed. Second, to examine whether significant effects from this analysis can be explained by key demographic differences, we conducted a 2 by 2 repeated measures ANOVA with key demographics as covariates. Finally, we include site as additional covariate to explore whether site could explain any additional variance. To examine the potential moderators for working memory impairment in schizophrenia, we conducted a series of repeated measures ANOVA for each moderator variable, adjusted for covariates if needed. For any significant effect from ANOVA and repeated measures ANVOA, we also present Cohen’s f2 as a measure of effect size (Cohen, 1988). The Cohen’s f2 indicates the estimate of the amount of variances that can be explained by certain variables in multivariate models. f2 of 0.02 represents a small; f2 of 0.15 represents a moderate; and f2 of 0.35 indicates a large effect size. Bivariate correlation was examined between performance of LNS and measures on functional and clinical symptoms to examine the association between verbal working memory and functional outcome as well as clinical symptoms. Correlations between patients who currently smoke and those who never smoked were compared using Fishers r to z transformation.
RESULTS
Demographic and clinical characteristics of participants across 5 sites
2414 participants (1377 patients with schizophrenia or schizoaffective disorder, depressed type and 1037 controls) had performance data on the LNS; see Table 1 for demographic and clinical characteristics across the 5 sites. There were significant site differences and group differences on key demographic characteristics. For age, there were significant effects of site (F 4,2405 =40.54, p<.001, f2=0.06), group (F 4,2405 =266.39, p<.001, f2=0.10) and a significant site by group interaction (F 4,2405 =12.10, p<.001, f2=0.01). Overall patients were older than controls, with the differences significant within each site except Site 2. Similarly, for personal education, there were significant effects of site (F 4,2405 =5.33, p<.001, f2=0.006) and group (F 4,2405 =795.22, p<.001, f2=0.31) and a significant site by group interaction (F 4,2405 =12.33, p<.001, f2=0.01). Patients had lower personal education than controls. For parental education we also found significant effects of site (F 4,2205 =3.53, p<.01, f2=0.005) and group (F 4,2205 =144.76, p<.001, f2=0.06), as well as a significant site by group interaction (F 4,2205 =4.67, p<.01, f2=0.007). For racial distribution, 3 major races (i.e., Asian, African-American, Caucasian) were compared and there was a significant site effect when examining patients and controls separately (X2=172.16, p<.001, and X2=72.08, p<.001, respectively). For clinical characteristics of patients across sites, we found significant site effects for clinical symptoms on the SANS (F 4,1175 =278.87, p<.001, f2=0.95) and SAPS (F 4,1173 =17.48, p<.001, f2=0.05), as well the UPSA (F 4,1145 =10.84, p<.001, f2=0.03), SOF (F 4,1168 =27.48, p<.0001, f2=0.09), and RFS Total (F 4,820 =12.06, p<.001, f2=0.05). Across sites, patients also differed on smoking status (i.e., nonsmokers who never smoked and current smokers based on self-report) (X2=33.52, p<.001), past-mood disorders (X2=128.77, p<.001) and past substance disorders (X2=93.51, p<.001)
Table 1.
Demographic and clinical characteristics of schizophrenia patients and healthy controls
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Patients | Control | Patients | Control | Patients | Control | Patients | Control | Patients | |
| N | 222 | 351 | 209 | 259 | 195 | 269 | 195 | 256 | 216 | 242 |
| Age | 40.1 (12.7) | 46.6 (11.0) | 46.5 (8.2) | 48.4 (10.5) | 35.6 (12.3) | 45.8 (10.1) | 32.6 (12.5) | 43.8 (11.2) | 37.3 (14.6) | 46.3 (11.8) |
| % Female | 57.2 | 32.7 | 38.8 | 26.3 | 54.4 | 33.1 | 52.8 | 36.7 | 49.5 | 23.1 |
| Personal Edu. | 14.8 (2.1) | 12.3 (1.9) | 14.7 (1.6) | 12.8 (1.8) | 15.5 (2.3) | 11.9 (2.1) | 14.8 (2.3) | 12.5 (2.3) | 15.1 (2.4) | 13.1 (1.8) |
| Parental Edu. | 13.4 (3.1) | 12.5 (2.9) | 13.4 (2.6) | 12.3 (3.3) | 14.3 (3.2) | 11.8 (2.9) | 14.2 (2.8) | 12.5 (3.1) | 14.2 (3.0) | 12.8 (2.9) |
| % Hispanic | 19.4 | 18.5 | 15.3 | 15.8 | 10.3 | 22.7 | 6.7 | 4.7 | 6.5 | 5.4 |
| Race | ||||||||||
| Native American | 2 | 5 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 2 |
| Asia | 26 | 7 | 11 | 14 | 26 | 3 | 12 | 7 | 13 | 14 |
| Pacific Islander | 3 | 4 | 2 | 4 | 1 | 1 | 0 | 0 | 7 | 5 |
| African American | 38 | 64 | 55 | 100 | 67 | 149 | 68 | 172 | 17 | 52 |
| Caucasian | 112 | 192 | 129 | 118 | 92 | 99 | 100 | 63 | 155 | 128 |
| More than One | 41 | 79 | 11 | 22 | 8 | 10 | 15 | 13 | 23 | 41 |
| Not Reported | 0 | 0 | 1 | 0 | 1 | 6 | 0 | 0 | 0 | 0 |
| % Right handed | 84.7 | 88.4 | 83.7 | 89.2 | 89.2 | 85.5 | 89.2 | 84.8 | 83.8 | 82.2 |
| Smoking | ||||||||||
| Never:Past:Now | 177:23:22 | 107:41:203 | 174:8:27 | 102:16:141 | 171:1:23 | 106:11:152 | 178:0:17 | 122:1:133 | 190:0:26 | 140:1:101 |
| # cigarettes/day | 9.3 | 15.0 | 10.2 | 16.3 | 8.1 | 11.1 | 7.2 | 13.5 | 6.8 | 11.1 |
| % Past Mood Dis | 2.7 | 32.4 | 4.3 | 23.2 | 1.0 | 12.6 | 12.8 | 32.8 | 15.3 | 28.5 |
| % Past Sub Dis | 7.7 | 52.6 | 17.2 | 44.4 | 0.5 | 17.5 | 13.8 | 51.2 | 19.9 | 47.1 |
| GAF | 89.7 (7.6) | 40.6 (4.9) | 81.7 (8.3) | 45.6 (9.3) | 91.9 (5.4) | 48.9 (8.5) | 87.1 (6.1) | 43.8 (9.5) | 82.3 (7.1) | 40.5 (4.2) |
| Age of Onset | 22.5 (7.4) | 21.8 (7.7) | 22.1 (5.3) | 21.5 (6.2) | 23.1 (7.1) | |||||
| Global_SANS | 16.2 (4.0) | 9.2 (4.4) | 4.6 (3.7) | 10.4 (4.0) | 12.8 (3.7) | |||||
| Global_SAPS | 8.1 (4.1) | 6.1 (4.2) | 5.5 (3.1) | 7.5 (4.2) | 6.9 (3.6) | |||||
| UPSA Total | 71.8 (15.5) | 73.1 (14.1) | 65.7 (17.2) | 70.1 (15.3) | 73.2 (13.4) | |||||
| SOF Total | 45.8 (5.7) | 48.3 (5.9) | 46.1 (6.0) | 47.5 (6.0) | 42.9 (5.1) | |||||
| RFS Total | 14.3 (4.2) | 15.4 (4.6) | 15.0 (3.5) | 17.4 (4.5) | 15.4 (3.0) | |||||
| RFS Work | 1.6 (1.4) | 2.2 (1.5) | 3.5 (1.1) | 3.7 (1.6) | 3.2 (1.2) | |||||
| RFS INDEP | 5.0 (1.4) | 4.4 (1.3) | 3.7 (.9) | 5.0 (1.2) | 5.3 (1.2) | |||||
| RFS FAMILY | 4.3 (2.0) | 4.6 (1.8) | 3.9 (1.1) | 4.9 (1.5) | 4.2 (1.4) | |||||
| RFS SOCIALNET | 3.3 (1.9) | 4.1 (1.7) | 3.7 (1.0) | 3.7 (1.7) | 2.7 (1.3) | |||||
RFS Total: Site 1, N=242; Site 2, N=176; Site 3, N=220; Site 4, N=166; Site 5, N=161
GAF: GAF of the last month
Patient-control differences on LNS
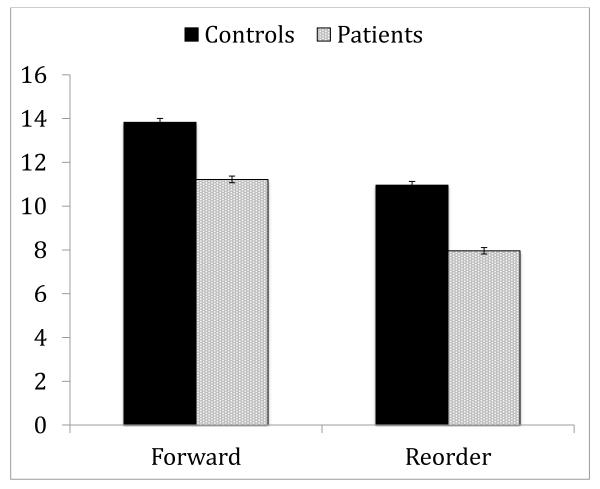
A 2 by 2 repeated measures ANOVA with condition as within-subject factor and group as between-subject factor showed a significant effects of condition (F 1,2408 =3595.73, p<.001, f2=1.49), group (F 1,2408 =731.21, p<.001, f2=0.30) and a significant condition by group interaction (F 1,2408 =14.11, p<.001, f2=0.005). Patients performed worse than controls and the group difference was more pronounced on Reorder (see Figure 1). The impairment of these patients on the Reorder condition (f2=0.29) was similar to the impairment seen previously in the COGS-1 samples (f2=0.22) (Horan et al., 2008)
Figure 1.
Performance of schizophrenia patients and controls on LNS
* Values are presented as mean ± 95% confidence interval of the mean.
Next, we examined whether demographics or sites could explain group differences on LNS by conducting a 2 by 2 repeated measures ANOVA with covariates. First, we included age and parental education as covariates and both were significant (age, F 1,2207 =55.89, p<.001, f2=0.02; parental education, F 1,2207 =100.81, p<.001, f2=0.04). Even with these covariates main effects and the interaction remained significant. Second, we included site as an additional covariate. The site effect was significant (F 1,2206 =27.51, p<.001, f2=0.01) but site did not interact with other factors. Thus, the patient-control difference and group by condition interaction on LNS were not explained by demographic differences between groups or across sites.
Moderator variables for verbal working memory within the schizophrenia sample
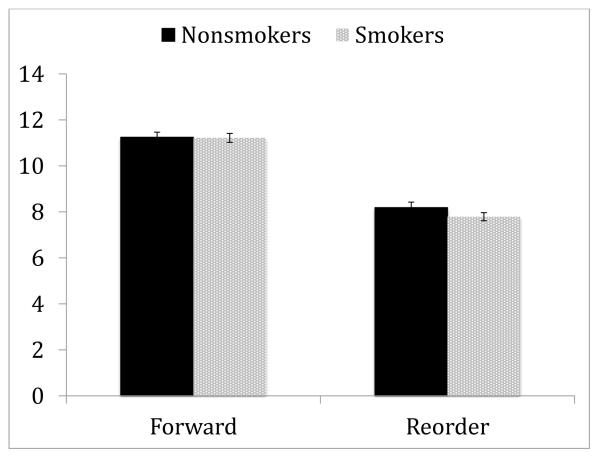
We examined whether smoking status moderates impaired performance of schizophrenia patients on LNS. Table 2 presents demographic and clinical characteristics of 577 nonsmokers (never smoked) and 730 smokers (currently smoking). A 2 by 2 repeated measures ANOVA with condition as within-subject factor and smoking status as between-subject factor (see Figure 2) showed a significant effect of condition (F 1,1301 =2375.27, p<.001, f2=1.825) and a significant condition by smoking status interaction (F 1,1306 =7.84, p<.01, f2=0.006). Post-hoc analyses showed that although the groups were comparable on Forward (F 1,1301 =.03, NS), nonsmokers performed better on the Reorder condition than smokers (F 1,1301 =7.20, p<.01, f2=0.005). To explore whether demographic differences (i.e., age) between groups explained the interaction, we conducted a 2 by 2 repeated measures ANOVA with age as a covariate. The main effect of age was significant (F 1,1300 =32.04, p<.001, f2=0.024) but no other effects were altered. We also examined the effect of age using age-matched subgroups of smokers and nonsmokers (577 nonsmokers and 715 smokers) and found that no effect was altered. Finally, when site was added as an additional covariate, no other effect was significant. Thus, the modulation of LNS performance by the smoking status was not explained by demographic differences between the two smoking groups or across site.
Table 2.
Demographic and clinical characteristics of nonsmoker schizophrenia patients and smoker schizophrenia patients across sites.
| Nonsmoker | Smokers | Statistics | |
|---|---|---|---|
| N | 577 | 730 | |
| Age | 45.6 (11.8) | 46.8 (10.1) | F 1,1306 =4.03, p<.05, f2=0.003 |
| % Female | 34.3 | 28.5 | X2=5.10, p<.02 |
| Personal Edu. | 13.1 (2.1) | 12.1 (1.9) | F 1,1306 =44.57, p<.001, f2=0.05 |
| Parental Edu. | 12.6 (3.3) | 12.3 (2.8) | F 1,1135 =2.38, NS |
| % Hispanic | 14.2 | 13.6 | |
| Race | X2=15.14, p<01 | ||
| Native American | 5 | 4 | |
| Asia | 27 | 16 | |
| Pacific Islander | 5 | 9 | |
| African American | 197 | 319 | |
| Caucasian | 266 | 301 | |
| More than One | 73 | 79 | |
| Not Reported | 4 | 2 | |
| % Right handed | 86.0 | 86.2 | X2=.14, NS |
| % Past Mood Dis | 26.9 | 24.9 | X2=2.25, NS |
| % Past Sub Dis | 27.4 | 53.4 | X2=89.76, p<.001 |
| GAF | 45.0 (8.5) | 42.8 (7.7) | F 1,1289 =23.02, p<.001, f2=0.017 |
| Age of Onset | 22.5 (7.0) | 22.0 (6.7) | F 1,1292 =1.92, NS |
| Global_SANS | 10.4 (5.3) | 11.2 (5.8) | F 1,1299 =6.19, p<.05, f2=0.004 |
| Global_SAPS | 6.3 (3.9) | 7.3 (4.1) | F 1,1291 =17.53, p<.001, f2=0.013 |
| UPSA Total | 71.8 (15.3) | 70.1 (15.5) | F 1,1266 =4.03=8, p<.05, f2=0.003 |
| SOF Total | 47.1 (6.2) | 45.3 (5.7) | F 1,1293 =23.39, p<.001, f2=0.021 |
| RFS Total | 16.5 (4.2) | 14.6 (3.9) | F 1,906 =50.34, p<.001, f2=0.055 |
| RFS Work | 3.2 (1.7) | 2.5 (1.5) | F 1,906 =32.72, p<.001, f2=0.036 |
| RFS INDEP | 4.9 (1.3) | 4.4 (1.3) | F 1,906 =37.87, p<.001, f2=0.041 |
| RFS FAMILY | 4.7 (1.5) | 4.1 (1.7) | F 1,906 =24.40, p<.001, f2=0.026 |
| RFS SOCIALNET | 3.6 (1.6) | 3.4 (1.5) | F 1,906 =4.16, p<.05, f2=0.004 |
RFS Total: Nonsmokers (N=393) and Current (N=514)
GAF: GAF of the last month
Figure 2.
The modulation of LNS performance by smoking status
* Values are presented as mean ± 95% confidence interval of the mean.
To examine antipsychotic medication as a potential moderator we compared performance of 4 subgroups of patients based on type of antipsychotic medication (105 patients taking first-generation antipsychotics, 993 patients taking second-generation antipsychotics, 130 patients taking both first- and second-generation antipsychotics, and 149 patients taking no antipsychotic medication). The supplementary Table shows demographic and clinical characteristics. A 2 by 2 repeated measures ANOVA with condition as within-subject factor and medication subgroup as between-subject factor found a significant effect of condition (F 1,1369 =1253.01, p<.001, f2=0.98) and a significant condition by medication interaction (F 1,1369 =4.61, p<.01, f2=0.010) (see Figure 3). All patients performed better on the Forward than Reorder conditions; 4 medication groups did not differ on the Forward, but patients taking no antipsychotic medication performed better on the Reorder than other medication groups.
Figure 3.
The modulation of LNS performance by antipsychotic medications
* Values are presented as mean ± 95% confidence interval of the mean.
To examine the interaction of antipsychotic medication and smoking status, we examined the effect of smoking status separately for patients who take first- and second-generation antipsychotic medications (N’s=103 and 946 respectively). For this analysis we excluded patients who were taking both types of medication. The patients taking first-generation antipsychotic medication included 41 nonsmokers and 62 smokers (see Figure 4a). The ANOVA showed a significant effect of condition (F 1,101 =155.33, p<.001, f2=1.537) and a significant condition by smoking status interaction (F 1,101 =6.15, p<.05, f2=0.060). Post-hoc analyses showed that, although both groups performed better on the Forward than Reorder conditions, the condition effect was more pronounced for smokers than nonsmokers. The second-generation antipsychotic group included 423 nonsmokers and 523 smokers (see Figure 4b). A 2 by 2 repeated measures ANOVA with condition as within-subject factor and smoking status as between-subject factor showed a significant effect of condition (F 1,944 =1762.61, p<.001, f2=1.867), but no other effect was significant. Both smokers and nonsmokers taking second-generation antipsychotic medications showed better performance on the Forward than Reorder conditions.
Figure 4.
The modulation of LNS performance by smoking status and antipsychotic medications
a) LNS performance by smoking status among patients taking first-generation antipsychotic medications; and b) LNS performance by smoking status among patients taking second-generation antipsychotic medication
* Values are presented as mean ± 95% confidence interval of the mean.
As another potential moderator, we examined the effect of history of substance use on LNS performance. There were 589 patients with a history of substance use and 784 without such a history. A 2 by 2 repeated measures ANOVA showed a significant effect of condition (F 1,1372 =2468.44, p<.001, f2=1.80), but no other effect.
Correlates of verbal working memory within the schizophrenia group
Table 3 shows association between LNS performance and clinical symptoms and functioning in the schizophrenia sample. Performance on Forward condition was negatively correlated with SANS global score and positively correlated with UPSA total, and RFS work, RFS Independent Living and RFS Total. Similarly, performance on Reorder condition was negatively correlated with SANS global score and SAPS global score and positively related to UPSA total and all scales of RFS except Social. Smoking status further moderated these associations. Performance on both conditions was more strongly associated with clinical symptoms and functioning in nonsmokers than smokers. For the Forward condition, these differences reach significance for the RFS total (p<.05). For the Reorder condition, there were significant differences in association with SANS global, RFS Work, RFS Family and RFS total between nonsmokers and current smokers (p’s <.05).
Table 3.
Association between performance on LNS and clinical symptoms and functional outcome
| Total sample of schizophrenia patients |
Nonsmoker | Smoker | ||||
|---|---|---|---|---|---|---|
| Forward | Reorder | Forward | Reorder | Forward | Reorder | |
| SANS Global | −.10** | −.09** | −.13** | −.15** | −.07* | −.04 |
| SAPS Global | −.03 | −.06* | −.02 | −.08* | −.06 | −.05 |
| UPSA | .29** | .39** | .32** | .44** | .28** | .35** |
| RFS | ||||||
| Work | .13** | .14** | .18** | .21** | .11* | .05 |
| Independent | .06* | .15** | .13** | .14** | .01 | .11* |
| Family | .04 | .08** | .12* | .15** | −.01 | .01 |
| Social | −.01 | .03 | .04 | .05 | −.02 | .01 |
| Total | .09** | .15** | .17** | .21** | .03 | .07 |
<.05
< .01
DISCUSSION
With a large sample of schizophrenia patients and controls, this study examined the role of smoking status, type of antipsychotic medication, and history of substance use as moderators for verbal working memory impairment in schizophrenia. COGS-2 included 5 sites and we observed significant variation on demographic and clinical characteristics across sites. However, these site differences did not explain our main findings. Overall, schizophrenia patients showed poorer performance than controls on LNS and the group difference was larger on Reorder than Forward condition, consistent with previous findings (Gold et al., 1997; Horan et al., 2008; Pukrop et al., 2003). Smoking status, but not history of substance use, moderated working memory performance. Patients who were currently smoking performed worse than nonsmoking patients, and this effect was more pronounced on the Reorder condition. Patients who take no antipsychotic medication showed better performance on the Reorder conditions compared to other medication groups. Smoking status also interacted with antipsychotic medication, such that the adverse effect of smoking on LNS Reorder performance was more pronounced among patients taking first-generation antipsychotic medications. Finally, better performance on LNS was associated with lower clinical symptoms and better indices of functioning in schizophrenia, but several of these associations were significantly weaker among patients who were smoking.
Previous studies on smoking and working memory in schizophrenia showed beneficial effects of smoking by assessing performance before and after smoking abstinence. Our study examined the effect of smoking status on performance rather than the acute effects of cigarette smoking. The impairing association of smoking status we found is consistent with findings in nonpsychiatric samples (Chamberlain et al., 2012; Jacobsen et al., 2005; Sabia et al., 2008), which showed detrimental effects of chronic smoking on cognitive function, including working memory. Our finding is also in line with recent studies using diffusion tensor imaging that showed lower white matter integrity in smoking compared to nonsmoking schizophrenia patients in several brain regions, including areas linked to working memory such as the frontal lobe (Cullen et al., 2012; Zhang et al., 2010). Thus, the beneficial effect of acute nicotine intake on cognition in schizophrenia seen in previous studies might be due to the reversal of withdrawal (Falcone et al., in press; Sacco et al., 2005). Indeed, smoking cessation impairs working memory (Jacobsen et al., 2004). The effect of abstinence is modulated by catechol-O-methyltransferase (Loughead et al., 2009), and variability in this gene has been shown to be associated with working memory in both healthy and clinical samples (Tan et al., 2007; Tunbridge et al., 2006).
This study also found that patients who did not take any antipsychotic medication performed better on the Reorder condition compared to patients who were taking antipsychotic medication. Although our finding is consistent with previous studies on the negative effect of antipsychotic medications on working memory (Castner et al., 2000; Reilly et al., 2006; Reilly et al., 2007), it should be noted that the COGS-2 cross-sectional design did not allow us to draw any firm conclusion about the casual relationship between antipsychotic medication and verbal working memory in schizophrenia. In other words, it is possible that better performance in non-medicated patients could be due to certain personal characteristics (e.g., not requiring medication) rather than the direct effect of antipsychotic medication per se (see Swerdlow et al., submitted for this issue about further discussion on antipsychotic medications and COGS endophenotypes). This study further showed that the negative effect of smoking on working memory was more pronounced among patients taking first-generation antipsychotic medications. There were not major demographic or clinical differences between patients taking first- and second-generation antipsychotic mediation. Thus, the poorer performance of smoking patients with first-generation antipsychotic medication cannot be explained by these features. Chronic cigarette smoking appears to decrease in D1-like receptors in the brain (Bruijnzeel and Markou, 2005). Both first- and second-generation antipsychotic medications may affect working memory, possibly due to down-regulation of D1 receptors and D2 receptor blockade (Castner et al., 2000; Reilly et al., 2006; Reilly et al., 2007) or an interaction of nicotine stimulation with dopamine hypofunction. Thus, chronic smoking along with antipsychotic treatments could contribute to impaired working memory performance of schizophrenia patients. Further studies will be needed to determine the mechanism for this effect and why it appears to be more pronounced in patients taking first-generation antipsychotic medications.
We also observed that better working memory performance was associated with higher indices of community functioning, which is consistent with previous findings on working memory as an important cognitive determinant of poor functioning in schizophrenia (Green et al., 2000; Harvey et al., 2011; Vesterager et al., 2012). Further, several of these associations were significantly weaker among smokers versus nonsmokers. Partly due to the association of cognition to daily functioning through functional capacity, there are considerable efforts to develop psychosocial and pharmacological interventions for cognitive deficits, including working memory impairment, in schizophrenia (Green et al., 2004; Nuechterlein et al., 2004). The pathway from working memory to functional outcome in schizophrenia may be moderated by smoking status.
In summary, in these large samples of patients and controls across 5 sites, verbal working memory impairment was clearly present and larger for patients who currently smoke. The effect of smoking status also interacted with types of antipsychotic medications: the impairing effect of smoking on Reorder was more pronounced among patients taking first-generation antipsychotics. History of substance use did not moderate LNS performance. Studies with more specific subtypes of working memory tasks instead of a relatively global measure such as the LNS will be able to investigate the underlying mechanisms through which these moderators affect working memory impairment and their clinically important associations to real world functioning. Future COGS studies will examine the genomic substrates of working memory and the relationship with other COGS-2 endophenotypes, similar to what we have done in COGS 1 (Seidman et al., submitted for this issue).
Supplementary Material
Acknowledgement
This study was supported by grants R01-MH065571, R01-MH065588, R01- MH065562, R01-MH065707, R01-MH065554, R01-MH065578, R01-MH065558, R01 MH86135, and K01-MH087889 from the National Institute of Mental Health.
Role of funding sources Other than providing support, the National Institute of Health does not have any further role in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors Dr. Lee completed all statistical analyses and wrote the first draft of this manuscript. Dr. Green conducted quality assurance for the LN measures across sites. Dr. Sprock conducted quality assurance for all data entered on the COGS2 website. All other authors participated in aspects of study design, including subjects recruitment, phenotyping, and validation of the clinical and endophenotype data. All authors were responsible for reviewing and approving the final version of the manuscript.
Conflict of interest Dr. Green has been a consultant to AbbVie, Biogen, DSP, EnVivo/Forum and Roche, and he is on the scientific advisory board of Mnemosyne. He has received research funds from Amgen. Dr. Lazzeroni is an inventor on a patent application filed by Stanford University on genetic polymorphisms associated with depression. Dr. Light has served as a consultant for Astellas, Forum, and Neuroverse. Dr. Nuechterlein has received unrelated research support from Janssen Scientific Affairs, Genentech, and Brain Plasticity, Inc., and has consulted to Genentech, Otsuka, Janssen, and Brain Plasticity, Inc. Dr. Swerdlow has been a consultant for Genco Sciences, Ltd. All other authors declare that they have no conflict of interest.
REFERENCES
- Addy N, Levin ED. Nicotine interactions with haloperidol, clozapine and risperidone and working memory function in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27(4):534–541. doi: 10.1016/S0893-133X(02)00327-5. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) The University of Iowa; Iowa City, IA: 1984a. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984b. [Google Scholar]
- Baddeley AD. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(3):480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Bittner RA, Linden DE, Roebroeck A, Hartling F, Rotarska-Jagiela A, Maurer K, Goebel R, Singer W, Haenschel C. The When and Where of Working Memory Dysfunction in Early-Onset Schizophrenia--A Functional Magnetic Resonance Imaging Study. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu050. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, Costello MR, Farahi J, Saxena S, Monterosso J, London ED. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(2):282–289. doi: 10.1038/npp.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Decreased sensitivity to the effects of dopamine D1-like, but not D2-like, receptor antagonism in the posterior hypothalamic region/anterior ventral tegmental area on brain reward function during chronic exposure to nicotine in rats. Brain Research. 2005;1058(1-2):91–100. doi: 10.1016/j.brainres.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Odlaug BL, Schreiber LR, Grant JE. Association between tobacco smoking and cognitive functioning in young adults. Am J Addict. 2012;21(Suppl 1):S14–19. doi: 10.1111/j.1521-0391.2012.00290.x. [DOI] [PubMed] [Google Scholar]
- Cohen JE. Statistical Power Analysis for the Behavioral Science. Lawrence Erlbaum Associates, Inc.; Hilsdale, HJ: 1988. [Google Scholar]
- Coleman MJ, Krastoshevsky O, Tu X, Mendell NR, Levy DL. The effects of perceptual encoding on the magnitude of object working memory impairment in schizophrenia. Schizophrenia Research. 2012;139(1-3):60–65. doi: 10.1016/j.schres.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Wallace S, Magnotta VA, Bockholt J, Ehrlich S, Gollub RL, Manoach DS, Ho BC, Clark VP, Lauriello J, Bustillo JR, Schulz SC, Andreasen NC, Calhoun VD, Lim KO, White T. Cigarette smoking and white matter microstructure in schizophrenia. Psychiatry research. 2012;201(2):152–158. doi: 10.1016/j.pscychresns.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Ahn K, Bhakta S, Elander J, Singh N, Nadim H, Jatlow P, Suckow RF, Pittman B, Ranganathan M. Nicotine fails to attenuate ketamine-induced cognitive deficits and negative and positive symptoms in humans: implications for schizophrenia. Biological Psychiatry. 2012;72(9):785–794. doi: 10.1016/j.biopsych.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Donoghue K, Mazzoncini R, Hart J, Zanelli J, Morgan C, Dazzan P, Morgan KD, Murray RM, Jones PB, Doody GA. The differential effect of illicit drug use on cognitive function in first-episode psychosis and healthy controls. Acta psychiatrica Scandinavica. 2012;125(5):400–411. doi: 10.1111/j.1600-0447.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31(5):1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Wileyto EP, Ruparel K, Gerraty RT, Laprate L, Detre JA, Gur R, Loughead J, Lerman C. Age-related differences in working memory deficits during nicotine withdrawal. Addict Biol. doi: 10.1111/adb.12051. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26(1):75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human Brain Mapping. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lonnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biological Psychiatry. 2003;53(7):624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Archives of General Psychiatry. 1997;54(2):159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophrenia bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RSE, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Kern RS, Baade LE, Fenton WS, Gold JM, Keefe RSE, Mesholam-Gately R, Seidman LJ, Stover E, Marder SR. Functional co- primary measures for clinical trials in schizophrenia: Results from the MATRICS psychometric and standardization study. American Journal of Psychiatry. 2008;165:221–228. doi: 10.1176/appi.ajp.2007.07010089. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R, Braff DL. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. The American journal of psychiatry. 2011;168(9):930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Swerdlow NR, Gur RE, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RC, Lazzeroni LC, Nuechterlein KH, Olincy A, Radant AD, Ray A, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Light GA, Braff DL. Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. The American journal of psychiatry. 2013;170(5):521–532. doi: 10.1176/appi.ajp.2012.12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinevich VP, Papke RL, Lippiello PM, Bencherif M. Atypical antipsychotics as noncompetitive inhibitors of alpha4beta2 and alpha7 neuronal nicotinic receptors. Neuropharmacology. 2009;57(2):183–191. doi: 10.1016/j.neuropharm.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36(3):267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Hartz SM, Pato CN, Medeiros H, Cavazos-Rehg P, Sobell JL, Knowles JA, Bierut LJ, Pato MT. Comorbidity of severe psychotic disorders with measures of substance use. JAMA Psychiatry. 2014;71(3):248–254. doi: 10.1001/jamapsychiatry.2013.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Raykov T, Twamley EW, Vella L, Heaton RK, Patterson TL. Validating the measurement of real-world functional outcomes: phase I results of the VALERO study. The American journal of psychiatry. 2011;168(11):1195–1201. doi: 10.1176/appi.ajp.2011.10121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Braff DL, Nuechterlein KH, Sugar CA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Green MF. Verbal working memory impairments in individuals with schizophrenia and their first-degree relatives: findings from the Consortium on the Genetics of Schizophrenia. Schizophrenia Research. 2008;103(1-3):218–228. doi: 10.1016/j.schres.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biological psychiatry. 2004;55(8):850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. Journal of abnormal psychology. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Molecular psychiatry. 2009;14(8):820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Carson RE, Berman KF, Herscovitch P, Weinberger DR. Nicotine- induced dopamine release in primates measured with [11C]raclopride PET. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(2):259–268. doi: 10.1038/sj.npp.1300287. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophrenia bulletin. 2007;33(6):1364–1372. doi: 10.1093/schbul/sbm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JS, Fukuda K, Vogel EK, Park S. Impaired contingent attentional capture predicts reduced working memory capacity in schizophrenia. PLoS ONE. 2012;7(11):e48586. doi: 10.1371/journal.pone.0048586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters HL. Statewide mental health outcome evaluation: a perspective of two southern states. Community Mental Health Journal. 1984;20(1):44–55. doi: 10.1007/BF00754103. [DOI] [PubMed] [Google Scholar]
- Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: importance of working memory. The American journal of psychiatry. 2005;162(10):1896–1903. doi: 10.1176/appi.ajp.162.10.1896. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Research. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Park S, Knopick C, McGurk S, Meltzer HY. Nicotine impairs spatial working memory while leaving spatial attention intact. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22(2):200–209. doi: 10.1016/S0893-133X(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Pukrop R, Matuschek E, Ruhrmann S, Brockhaus-Dumke A, Tendolkar I, Bertsch A, Klosterkotter J. Dimensions of working memory dysfunction in schizophrenia. Schizophrenia Research. 2003;62(3):259–268. doi: 10.1016/s0920-9964(02)00427-9. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA : the journal of the American Medical Association. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Archives of General Psychiatry. 2006;63(11):1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biological Psychiatry. 2007;62(7):818–821. doi: 10.1016/j.biopsych.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Sabia S, Marmot M, Dufouil C, Singh-Manoux A. Smoking history and cognitive function in middle age from the Whitehall II study. Archives of internal medicine. 2008;168(11):1165–1173. doi: 10.1001/archinte.168.11.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Archives of General Psychiatry. 2005;62(6):649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Schnell T, Koethe D, Daumann J, Gouzoulis-Mayfrank E. The role of cannabis in cognitive functioning of patients with schizophrenia. Psychopharmacology. 2009;205(1):45–52. doi: 10.1007/s00213-009-1512-9. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Hellemann GS, Nuechterlein KH, Braff DL, Greenwood TA, Cadenhead KS, Calkins ME, Freedman R, Gur RE, Gur RC, Lazzeroni LC, Light GA, Olincy A, Radant AD, Siever LJ, Silverman JM, Stone WS, Sprock J, Sugar C, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Green MF. Factor structure and heritability of endophenotypes in schizophrenia: Findings from the Consortium on the Genetics of Schizophrenai (COGS-1) submitted for this issue. [DOI] [PMC free article] [PubMed]
- Smith CW, Park S, Cornblatt B. Spatial working memory deficits in adolescents at clinical high risk for schizophrenia. Schizophrenia Research. 2006;81(2-3):211–215. doi: 10.1016/j.schres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Gur R, Braff DL. Consortium on the Genetics of Schizophrenia (COGS) Assessment of Endophenotypes for schizophrenia: An introduction to this special issue of schizophrenia research. Schizophrenia Research. doi: 10.1016/j.schres.2014.09.047. submitted for this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cerebral cortex. 2007;17(Suppl 1):i171–181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Thoma P, Daum I. Working memory and multi-tasking in paranoid schizophrenia with and without comorbid substance use disorder. Addiction. 2008;103(5):774–786. doi: 10.1111/j.1360-0443.2008.02156.x. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Miyasato K, Nishiyama S, Fukumoto D, Kakiuchi T, Domino EF. Nicotine normalizes increased prefrontal cortical dopamine D1 receptor binding and decreased working memory performance produced by repeated pretreatment with MK-801: a PET study in conscious monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30(12):2144–2153. doi: 10.1038/sj.npp.1300745. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biological psychiatry. 2006;60(2):141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Doshi RR, Nayak GV, Palmer BW, Golshan S, Heaton RK, Patterson TL, Jeste DV. Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. American Journal of Psychiatry. 2002;159(12):2013–2020. doi: 10.1176/appi.ajp.159.12.2013. [DOI] [PubMed] [Google Scholar]
- Vesterager L, Christensen TO, Olsen BB, Krarup G, Melau M, Forchhammer HB, Nordentoft M. Cognitive and clinical predictors of functional capacity in patients with first episode schizophrenia. Schizophrenia research. 2012;141(2-3):251–256. doi: 10.1016/j.schres.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Wagner M, Schulze-Rauschenbach S, Petrovsky N, Brinkmeyer J, von der Goltz C, Grunder G, Spreckelmeyer KN, Wienker T, Diaz-Lacava A, Mobascher A, Dahmen N, Clepce M, Thuerauf N, Kiefer F, de Millas JW, Gallinat J, Winterer G. Neurocognitive impairments in non-deprived smokers--results from a population-based multi-center study on smoking-related behavior. Addict Biol. 2013;18(4):752–761. doi: 10.1111/j.1369-1600.2011.00429.x. [DOI] [PubMed] [Google Scholar]
- Wareing M, Fisk JE, Murphy PN. Working memory deficits in current and previous users of MDMA (‘ecstasy’) Br J Psychol. 2000;91(Pt 2):181–188. doi: 10.1348/000712600161772. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd ed The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wojtalik JA, Barch DM. An FMRI study of the influence of a history of substance abuse on working memory-related brain activation in schizophrenia. Front Psychiatry. 2014;5:1. doi: 10.3389/fpsyt.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, Conus P, Takagi MJ, Fornito A, Wood SJ, McGorry PD, Pantelis C. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophrenia bulletin. 2012;38(2):316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingsson T, Lewis G. Investigating the association between cigarette smoking and schizophrenia in a cohort study. The American journal of psychiatry. 2003;160(12):2216–2221. doi: 10.1176/appi.ajp.160.12.2216. [DOI] [PubMed] [Google Scholar]
- Zhang X, Stein EA, Hong LE. Smoking and schizophrenia independently and additively reduce white matter integrity between striatum and frontal cortex. Biological Psychiatry. 2010;68(7):674–677. doi: 10.1016/j.biopsych.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.