Abstract
Introduction
Peri-implantitis has a prevalence of 11-47%, involves destruction of peri-implant bone, and may lead to implant loss. A detailed understanding of the pathogenesis of peri-implantitis is lacking. The objective of this study was to develop a murine model of experimental peri-implantitis.
Materials and Methods
Machined, smooth surface screw-shaped titanium implants were placed in the healed alveolar bone of the left maxillary molars of C57BL/6J male mice, eight weeks after tooth extraction. Peri-implantitis was induced by securing silk ligatures around the head of the implant fixtures. Implant survival and peri-implant bone levels were analyzed by micro-computerized tomography (micro-CT) scans and histology twelve weeks after ligature placement.
Results
Implant survival was 60% (6/10) for implants with ligatures and 100% (8/8) for controls. Micro-CT revealed significantly greater bone loss around the implants that received ligatures and that survived as compared to controls. The radiographic findings were confirmed via histology and toluidine blue staining.
Conclusions
This study describes a murine model of experimental peri-implantitis around screw-shaped titanium implants placed in the edentulous alveolar bone. This model should be a useful tool to dissect pathogenic mechanisms of peri-implantitis and evaluate potential treatment interventions.
Keywords: peri-implantitis, dental implant, animal model, bone loss
Introduction
Peri-implantitis is an inflammatory condition of the soft tissues and bone around dental implants which leads to bone destruction and eventually to implant loss (1). With a prevalence of 11%-47%, peri-implantitis poses a significant clinical problem given the cumulative number of implants delivered overtime (2, 3). No effective treatment protocols for peri-implantitis exist, partly due to incomplete knowledge of the pathogenic mechanisms of this disease.
Peri-implantitis shares some common features with periodontitis (4). Fixtures with peri-implantitis present with mucosal inflammation, increased pocket depth and clinical attachment and radiographic bone loss. Both diseases are initiated by the collection of the bacterial biofilm (5). The bacterial composition of the biofilm that develops in pockets around implants is mostly Gram-negative and the microbiota is similar to that associated with periodontitis (6). However, more recent studies demonstrated that, even though periodontitis and peri-implantitis are initiated by bacteria, there are significant differences in bacterial composition between the two diseases (7). At the host response level, the inflammatory infiltrate present in peri-implantitis lesions shares many common cellular components with periodontitis such as the presence of plasma cells, lymphocytes and macrophages (8). On the other hand, peri-implantitis lesions present with distinct characteristics from those associated with periodontitis because they often occur around the circumference of the fixture, as opposed to surface-specific lesions observed around teeth with periodontitis (9). Histopathologically, the inflammatory infiltrate in peri-implantitis penetrates deep and beyond the soft tissues surrounding the pocket, often spreading to bone and its trabecular spaces (10). The connective tissue around implant fixtures is loosely organized, which facilitates migration of the inflammatory infiltrate (11-14). Little biochemical and molecular information exists about the possible role of specific pro-inflammatory and other mediators in the pathogenesis of peri-implantitis (15-18).
The understanding of peri-implantitis could be facilitated by the development of a simple, easily reproducible and inexpensive animal model that parallels the clinical scenario. Mice constitute an attractive option not only because they fit the criteria described above, but also because of the availability of genetically manipulated strains, which are invaluable tools in dissecting the mechanisms of diseases. Only one murine model for peri-implantitis has been described (19) thus far; in that model, implants were however placed in the center of the palate and not in the alveolar bone.
The purpose of this study was to develop a ligature-induced murine model of peri-implantitis. The hypothesis to be tested in this study is that peri-implantitis can be induced around dental implant fixtures installed in post-extraction, healed maxillary edentulous ridges of mice.
Materials and Methods
Mice
Eighteen four-week-old C57BL/6J (The Jackson Laboratories, Bar Harbor, ME) male mice were used according to the guidelines of the Chancellor's Animal Research Committee of the University of California, Los Angeles. In addition, the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines for the execution and submission of studies in animals were followed (20). Mice were fed a soft diet (Bio Serve®, Frenchtown, NJ) ad libitum for the duration of the experiment. Eight animals/implants were included in the control group and 10 animals/implants in the experimental group (ligature, peri-implantitis). At the end of the experimental period, there were eight remaining implants in the control group and six in the experimental group. No other adverse events were observed in the animals other than loss of implant osseointegration. All the procedures performed in the mice including tooth extraction, implant placement and ligature placement were performed under 10× magnification (Leica S6D Stereozoom, Chicago, IL).
Induction of Peri-Implantitis
Mice had their maxillary left first, second, and third molars extracted under inhalation anesthesia with 3% isoflurane and allowed to heal for eight weeks. Extractions were performed by tooth elevation with a #5 dental explorer (G. Hartzell and Son, Concord, CA). Mice were given oral antibiotics diluted in the drinking water for four weeks (sulfamethoxazole and trimethoprim, UPS; 850 μ g/170 μ g per mL).
Machined, smooth surface screw-shaped implants were fabricated from 6AL4V titanium rods (D. P. Machining Inc., La Verne, CA). The threaded surface of the implants was 1 mm long and 0.5 mm in diameter. Fixtures were placed eight weeks after teeth were extracted (Fig. 1). Animals were anesthetized with 3% isoflurane and a mesio-distal incision was made in keratinized tissue using a 12D blade in the area corresponding to the previously present teeth using the right maxillary molars as spatial reference. After elevation of buccal and palatal full thickness flaps with a #5 dental explorer, osteotomy was performed with a 0.3 mm diameter carbide micro hand drill (BIG Kaiser Precision Tooling Inc., Hoffman Estates, IL) mounted on a manual handle that was activated by rotation. The osteotomy sites were approximately 1 mm deep into the healed extraction sockets. Titanium implants (one per animal) were self-tapped in the region of the first/second maxillary left molars using a clock-wise screwing motion. Implants were allowed to heal for four weeks, during which time mice were given antibiotics and fed as described above.
Figure 1. Research design.
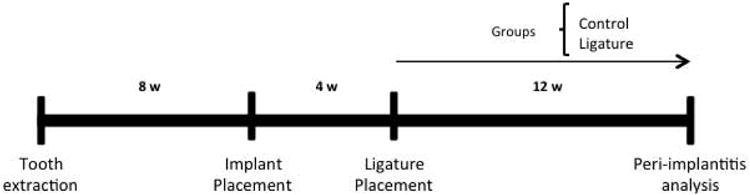
Schematic diagram depicting timing of the experimental design.
After four weeks, osseointegration was evaluated by applying bucco-lingual wiggling forces to the fixtures with two dental explores on anesthetized animals and observing implant movement under 10× magnification. Once implants were clinically determined to be osseointegrated, 6-0 silk ligatures (P.B.N. Medicals, Stenløse, Demark) were tied around each fixture immediately apical to the implant head in animals in the experimental group. Implants in the control group did not receive ligatures. Control and peri-implantitis animals were randomly selected by the toss of a coin. Twelve weeks after ligature placement, maxillae were harvested using a digital optical microscope (Keyence® VHX-1000, Osaka, Japan), fixed in 4% paraformaldehyde for 48h, and stored in 70% ethanol.
Micro-Computerized Tomography Analysis
Mouse maxillae were imaged using micro-computerized tomography (micro-CT) (Model 1172, SkyScan, Kontich, Belgium) at 10 μ m resolution as described by Kang et al. (21). Linear measurements were performed using Dolphin software.
In order to quantify bone loss after the induction of peri-implantitis, samples were oriented such that the head and the shaft of the implant were perpendicular to each other in the sagittal and coronal planes. Measurements were taken from points where the implant head met the shaft and vertically to the alveolar bone on the mesial, distal, buccal, and palatal areas; measurements were averaged to generate a mean bone level value for each fixture. All measurements were performed by a blinded examiner.
Histology
For the descriptive histological assessment of implant osseointegration and bone levels, undecalcified maxillae were embedded in methyl methacrylate (22). Sagittal or coronal sections were obtained with a Buehler Isomet Low Speed Saw (Lake Bluff, IL) at 200 μ m thickness. Sections were ground to a final thickness of 20 μ m with the Exakt Grinding System (Lake Bluff, IL) (23) and stained with toluidine blue. Sections were then digitized using the Aperio Image Scope model V11.1.2.752 (Vista, CA).
Statistical Analysis
Bone level measurements in each group were averaged (mean ± standard error of the mean) and the data between groups compared using Students' t-tests (GraphPad, GraphPad Software, Inc. La Jolla, CA).
Results
Induction of Peri-Implantitis
Our clinical and histological observations of the healing of extraction sockets in mice revealed that the ideal time for implant placement after tooth extraction is at eight weeks. Our previous studies also showed that implant survival after the initial osseointegration period was 86% (data not shown). Four weeks after implant placement, a ligature was tied below the head of the implant for twelve weeks in the implants that demonstrated clinical osseointegration. Implant survival was 60% (6/10) in the experimental animals versus 100% (8/8) in the control group (Table 1).
Table 1.
Implant survival rate twelve weeks after peri-implantitis induction.
| Implants at Lig Placement | Implants Remaining | Percent Implant Survival | |
|---|---|---|---|
| No Ligature | 8 | 8 | 100% |
| Ligature | 10 | 6 | 60% |
Among the surviving implants, the experimental group presented with more gingival swelling than controls (Fig. 2A). Micro-CT analysis revealed the alveolar bone crest position to be considerably more apical in the experimental group when compared to controls (Fig. 2B). The average distance between the apical surface of the implant head and the bone was 0.422 ± 0.019 mm in the experimental group as compared to 0.226 ± 0.016 mm in the control group at sixteen weeks after placement. The difference between the two groups was statistically significant. Because four implants in the experimental group were not present at sacrifice, an assumption was made that they exfoliated due to the peri-implantitis process that reached the apex of the fixtures.
Figure 2. Peri-implantitis Development.
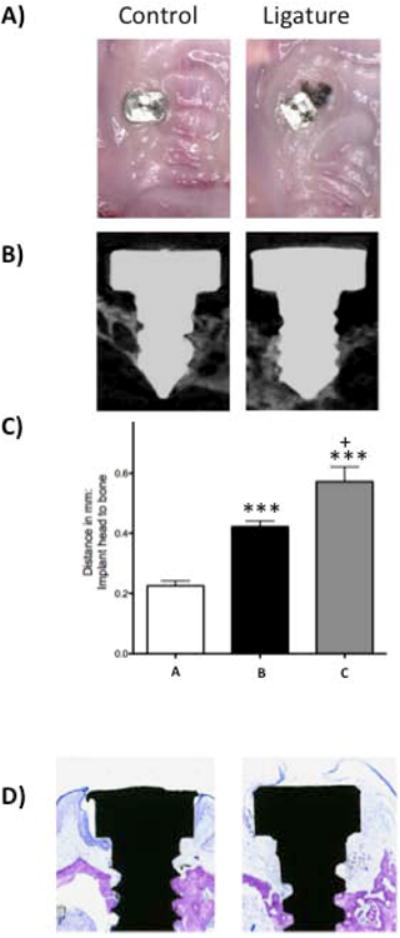
A) Representative clinical images of the control (left) and experimental (right) groups at twelve weeks after ligature placement. B) Representative sagittal micro-CT images of control (left) and experimental (right) groups. Notice the reduced alveolar bone level on the experimental specimen. C) Graph representing the average distance of the implant head to the alveolar bone. Data are mean ± standard error of the mean. Left bar (a) corresponds to the control group, middle bar (b) corresponds to the experimental group excluding lost implants and right bar (c) corresponds to the experimental group including the lost implants. ***p<0.001 comparing control (a) (n≥8) and experimental groups (b and c) (n≥6, and n≥10, respectively). + p<0.05 comparing groups b and c. D) Representative histological sample stained with toluidine blue twelve weeks after ligature placement in the control (left) and experimental group (right). Notice reduced alveolar bone level and supra-crestal inflammatory infiltrate on experimental specimen.
For purposes of data analysis, the four experimental animals with lost implants were also combined with those in the experimental group and 1 mm of bone loss assigned to the fixtures. Mean bone loss in the experimental group (including the lost fixtures) was 0.579 ± 0.0490 mm as compared to 0.4221 ± 0.019 mm in the experimental group (excluding the lost fixtures); the difference was statistically significant (Fig. 2C). Fig. 2D shows histology of control and experimental sites twelve weeks after ligature placement; bone level is more apical in the experimental specimen, which also presented with a dense inflammatory infiltrate in the area immediately coronal to the alveolar bone.
Discussion
The results of this study confirm that machined titanium implants with smooth surfaces can be successfully placed in healed post-extraction molar areas of mice. Additionally, these data suggest that murine peri-implantitis can be induced around osseointegrated fixtures by installation of silk ligatures. The process of murine molar extraction, alveolar ridge healing, implant osseointegration and development of peri-implantitis herein described share several features with the same sequence of events in humans. Thus, our murine model resembles human dental implant biology in a way that is closer than the model previously proposed. The main difference between our model and the one described by Becker et al. is that theirs included placement of the implant in the central palatal bone and not in an area previously occupied by a tooth, which does not fully mimic implants placed in humans (19).
Osseointegration was 82% in this study (data not shown), which is lower than the 96% observed in humans (24), but still within an acceptable range from which analogies could be drawn to the healing process in humans. It is likely that the most common reason for early implant failure was related to variability in our ability to determine if fixtures presented with primary stability at the time of placement. Because of the model's reduced size and the fact that implant installation was delivered without a standardized torque device, there were unavoidable variations in determining the endpoint for fixture insertion. Implant primary stability was evaluated by attempting to detect bucco-palatal movement of the fixture when wiggling it between two periodontal probes, which may also not allow for detection of lack of stabilization that is subtler to tactile means.
Four of the mice in the experimental (peri-implantitis) group did not present with the implant fixture at the end of the study. All mice in the control group had their implants at twelve weeks after peri-implantitis induction via ligature placement. It is not possible to precisely determine the cause of fixture loss in four of the experimental mice or the point in time when they occurred. It is likely, however, that the reason why implants were not present in these mice was because the ligature-induced peri-implantitis process resulted in progressive bone loss to the point that implants became mobile and exfoliated on their own. This suggested outcome of the non-accounted implants is based on the findings associated with the six mice in the experimental group where the implant fixtures were present at the end of the study and in which significant peri-implant bone loss was observed.
Placement of ligatures around the osseointegrated implant fixtures resulted in clinical, radiographic and histological findings that are consistent with those of peri-implantitis in humans and other experimental animal models (3, 8, 10). The six specimens of the experimental group that retained the implant fixtures by the end of the study presented with clinical inflammation, alveolar bone loss and a supra-alveolar soft tissue infiltrate that was rich in inflammatory cells. In contrast, mice in the control group presented with clinical, radiographic and histological features similar to those observed in mice that were sacrificed at eight weeks following implant installation. The bone level around implants in the experimental group was markedly different from those in the control group and statistically significant. This strongly suggests that the installation of silk ligatures and its associated inflammatory process were responsible for the peri-implant bone loss. The rate of bone loss around implants placed in our murine model should be characterized in greater detail so that experimental end-points may be determined at a stage before implants are exfoliated. In the future, the processes of osseointegration and peri-implantitis development could be observed by periodic micro-CT examinations of the same animals at different time points within the experimental period by high-resolution in vivo micro-CT scanners. As these devices become more accessible to researchers, they will allow the quantification of longitudinal bone loss in peri-implantitis.
The characterization of the inflammatory process and mechanisms of bone destruction associated with this murine model at the cellular and molecular levels is the subject of current investigation by our research group. At this point, any comments about the basic phenomena associated with the observed clinical process would be speculative in nature.
Animal models are essential for understanding disease initiation and progression because they avoid limitations associated with human studies, such as the ability to control the environment and to replicate experimental conditions. A dog model has been used to study peri-implantitis and shares similarities with humans, but presents with limitations in terms of dissecting the molecular mechanisms involved in disease initiation and progression due to the limited availability of molecular and genetic tools (10, 11, 14, 25). Currently, there are large databases available consisting of mouse genetic profiles and phenotypic characteristics that allow reasonable extrapolations from mice to humans (26-28). We elected to utilize the ligature model because it is the most widely accepted periodontitis rodent model (29). In addition, some authors consider this model as the most representative of human periodontitis (30). The methodology employed in this study is easily transferable to a rat model, which would provide with yet another animal model to validate comparisons made between peri-implant disease in rodents and humans.
Human peri-implantitis is a highly prevalent problem (2, 3) that will tend to increase because of the growing number of dental implants that are placed in patients. It is estimated that over 500,000 implants are currently placed per year in the United States only, and many more around the world. This poses considerable quality of life and financial burdens to society, given that the pathogenesis of peri-implantitis is poorly understood and proven treatment options essentially non-existent. Current treatment options for peri-implantitis include non-surgical and resective/reconstructive surgical therapies (31), but the actual efficacy of these treatment methods has not been determined. Therefore, understanding the pathogenesis of peri-implantitis in a systematic manner will allow for the identification of procedural and/or pharmacological treatment approaches for this condition and the murine model should be a valuable instrument in enlightening these paths.
In conclusion, we successfully developed a peri-implantitis murine model. This model should allow future dissection of the pathologic pathways involved in peri-implantitis and aid in developing preventive and therapeutic approaches for peri-implantitis.
Acknowledgments
This work was supported by a seed grant provided by UCLA School of Dentistry. FP was supported by the NIH/NIDCR supplement DE 019465-S1. SH was supported by the NIH/NIDCR T90 DE022734-01. AB, JP and AA were supported by Science Without Borders CNPq - Brazil. We would like to thank Dr. Renata Pereira, from the Bone Histomorphometry Core Facility at the UCLA School of Medicine, for her assistance with the undecalcified histologic sections. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63–76. doi: 10.1111/j.1600-0757.1998.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 2.Fransson C, Lekholm U, Jemt T, Berglundh T. Prevalence of subjects with progressive bone loss at implants. Clin Oral Implants Res. 2005;16:440–446. doi: 10.1111/j.1600-0501.2005.01137.x. [DOI] [PubMed] [Google Scholar]
- 3.Roos-Jansaker AM, Lindahl C, Renvert H, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. J Clin Periodontol. 2006;33:290–295. doi: 10.1111/j.1600-051X.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- 4.Mombelli A, Marxer M, Gaberthuel T, Grunder U, Lang NP. The microbiota of osseointegrated implants in patients with a history of periodontal disease. J Clin Periodontol. 1995;22:124–130. doi: 10.1111/j.1600-051x.1995.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 5.Lindhe J, Meyle J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:282–285. doi: 10.1111/j.1600-051X.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- 6.Leonhardt A, Renvert S, Dahlen G. Microbial findings at failing implants. Clin Oral Implants Res. 1999;10:339–345. doi: 10.1034/j.1600-0501.1999.100501.x. [DOI] [PubMed] [Google Scholar]
- 7.Dabdoub SM, Tsigarida AA, Kumar PS. Patient-specific analysis of periodontal and peri-implant microbiomes. J Dent Res. 2013;92:168S–175S. doi: 10.1177/0022034513504950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piattelli A, Scarano A, Piattelli M. Histologic observations on 230 retrieved dental implants: 8 years experience (1989-1996) J Periodontol. 1998;69:178–184. doi: 10.1902/jop.1998.69.2.178. [DOI] [PubMed] [Google Scholar]
- 9.Lang NP, Berglundh T. Periimplant diseases: where are we now?--Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38 Suppl 11:178–181. doi: 10.1111/j.1600-051X.2010.01674.x. [DOI] [PubMed] [Google Scholar]
- 10.Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3:9–16. doi: 10.1034/j.1600-0501.1992.030102.x. [DOI] [PubMed] [Google Scholar]
- 11.Marinello CP, Berglundh T, Ericsson I, Klinge B, Glantz PO, Lindhe J. Resolution of ligature-induced peri-implantitis lesions in the dog. J Clin Periodontol. 1995;22:475–479. doi: 10.1111/j.1600-051x.1995.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 12.Ericsson I, Nilner K, Klinge B, Glantz PO. Radiographical and histological characteristics of submerged and nonsubmerged titanium implants. An experimental study in the Labrador dog. Clin Oral Implants Res. 1996;7:20–26. doi: 10.1034/j.1600-0501.1996.070103.x. [DOI] [PubMed] [Google Scholar]
- 13.Persson LG, Lekholm U, Leonhardt A, Dahlen G, Lindhe J. Bacterial colonization on internal surfaces of Branemark system implant components. Clin Oral Implants Res. 1996;7:90–95. doi: 10.1034/j.1600-0501.1996.070201.x. [DOI] [PubMed] [Google Scholar]
- 14.Gotfredsen K, Berglundh T, Lindhe J. Bone reactions at implants subjected to experimental peri-implantitis and static load. A study in the dog. J Clin Periodontol. 2002;29:144–151. doi: 10.1034/j.1600-051x.2002.290209.x. [DOI] [PubMed] [Google Scholar]
- 15.Guncu GN, Tozum TF, Guncu MB, et al. Myeloperoxidase as a measure of polymorphonuclear leukocyte response in inflammatory status around immediately and delayed loaded dental implants: a randomized controlled clinical trial. Clin Implant Dent Relat Res. 2008;10:30–39. doi: 10.1111/j.1708-8208.2007.00058.x. [DOI] [PubMed] [Google Scholar]
- 16.Arikan F, Buduneli N, Kutukculer N. Osteoprotegerin levels in peri-implant crevicular fluid. Clin Oral Implants Res. 2008;19:283–288. doi: 10.1111/j.1600-0501.2007.01463.x. [DOI] [PubMed] [Google Scholar]
- 17.Candel-Marti ME, Flichy-Fernandez AJ, Alegre-Domingo T, Ata-Ali J, Penarrocha-Diago MA. Interleukins IL-6, IL-8, IL-10, IL-12 and periimplant disease. An update. Med Oral Patol Oral Cir Bucal. 2011;16:e518–521. doi: 10.4317/medoral.16.e518. [DOI] [PubMed] [Google Scholar]
- 18.Konttinen YT, Lappalainen R, Laine P, Kitti U, Santavirta S, Teronen O. Immunohistochemical evaluation of inflammatory mediators in failing implants. Int J Periodontics Restorative Dent. 2006;26:135–141. [PubMed] [Google Scholar]
- 19.Becker ST, Foge M, Beck-Broichsitter BE, et al. Induction of periimplantitis in dental implants. J Craniofac Surg. 2013;24:e15–18. doi: 10.1097/SCS.0b013e318266fb2d. [DOI] [PubMed] [Google Scholar]
- 20.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Journal of pharmacology & pharmacotherapeutics. 2010;1:94–99. doi: 10.4103/0976-500X.72351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang B, Cheong S, Chaichanasakul T, et al. Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J Bone Miner Res. 2013;28:1631–1640. doi: 10.1002/jbmr.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira RC, Stadmeyer LE, Smith DL, Rydziel S, Canalis E. CCAAT/Enhancer-binding protein homologous protein (CHOP) decreases bone formation and causes osteopenia. Bone. 2007;40:619–626. doi: 10.1016/j.bone.2006.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivares-Navarrete R, Raines AL, Hyzy SL, et al. Osteoblast maturation and new bone formation in response to titanium implant surface features are reduced with age. J Bone Miner Res. 2012;27:1773–1783. doi: 10.1002/jbmr.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung RE, Pjetursson BE, Glauser R, Zembic A, Zwahlen M, Lang NP. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin Oral Implants Res. 2008;19:119–130. doi: 10.1111/j.1600-0501.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 25.Berglundh T, Gotfredsen K, Zitzmann NU, Lang NP, Lindhe J. Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness: an experimental study in dogs. Clin Oral Implants Res. 2007;18:655–661. doi: 10.1111/j.1600-0501.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- 26.Bult CJ, Eppig JT, Blake JA, Kadin JA, Richardson JE. The mouse genome database: genotypes, phenotypes, and models of human disease. Nucleic Acids Res. 2013;41:D885–891. doi: 10.1093/nar/gks1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maddatu TP, Grubb SC, Bult CJ, Bogue MA. Mouse Phenome Database (MPD) Nucleic Acids Res. 2012;40:D887–894. doi: 10.1093/nar/gkr1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CM, Finger JH, Hayamizu TF, et al. The mouse Gene Expression Database (GXD): 2014 update. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhr A, Popa-Wagner A, Schmoll H, Schwahn C, Kocher T. Observations on experimental marginal periodontitis in rats. J Periodontal Res. 2004;39:101–106. doi: 10.1111/j.1600-0765.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 30.de Souza JA, Nogueira AV, de Souza PP, Cirelli JA, Garlet GP, Rossa C., Jr Expression of suppressor of cytokine signaling 1 and 3 in ligature-induced periodontitis in rats. Arch Oral Biol. 2011;56:1120–1128. doi: 10.1016/j.archoralbio.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Aljateeli M, Fu JH, Wang HL. Managing peri-implant bone loss: current understanding. Clin Implant Dent Relat Res. 2012;14 Suppl 1:e109–118. doi: 10.1111/j.1708-8208.2011.00387.x. [DOI] [PubMed] [Google Scholar]


