Abstract
Purpose
As our society becomes increasingly sedentary, compliance with exercise regimens that require numerous high-energy activities each week become less likely. Alternatively, given an osteogenic exercise intervention that required minimal effort, it is reasonable to presume that participation would be enhanced. Insertion of brief rest-intervals between each cycle of mechanical loading holds potential to achieve this result as substantial osteoblast function is activated by many fewer loading repetitions within each loading bout. Here, we examined the complementary hypothesis that the number of bouts/wk of rest-inserted loading could be reduced from 3/wk without loss of osteogenic efficacy.
Methods
We conducted a series of 3 wk in vivo experiments that non-invasively exposed the right tibiae of mice to either cyclic (1 Hz) or rest-inserted loading interventions and quantified osteoblast function via dynamic histomorphometry.
Results
While reducing loading bouts from 3/wk (i.e., 9 total bouts) to 1/wk (3 total bouts) effectively mitigated the osteogenic benefit of cyclic loading, the same reduction did not significantly reduce periosteal bone formation parameters induced by rest-inserted loading. The osteogenic response was robust to the timing of the rest-inserted loading bouts (3 bouts in the first week vs 1 bout/wk for three weeks). However, elimination of any single bout of the three 1/wk bouts mitigated the osteogenic response to rest-inserted loading. Finally, periosteal osteoblast function assessed after the 3 wk intervention was not sensitive to the timing or number of rest-inserted loading bouts.
Conclusions
We conclude that rest-inserted loading holds potential to retain the osteogenic benefits of mechanical loading with significantly reduced frequency of bouts of activity while also enabling greater flexibility in the timing of the activity.
Keywords: Bone, Exercise, Mechanical Loading, Osteoblast
Introduction
Exercise is an epigenetic stimulus for enhanced bone mass. The potential of this anabolic intervention has been illustrated in a variety of pre-clinical animal models and in cross-sectional studies of single limb sport activities (7,10,14,29). However, the results of longitudinal human exercise interventions have been equivocal (16,23,25). Critically, the enhanced periosteal osteoblast activity so clearly induced by mechanical loading in pre-clinical studies (and that most effectively augments bone’s structural integrity; (5)) has been modest in human studies (as assessed by periosteal expansion; (15,17)).
There are many possible explanations for the inability of exercise to enhance periosteal adaptation in the human skeleton, including the normal challenges associated with translating pre-clinical observations into viable clinical interventions (13). One particularly challenging phenotypic aspect of bone’s response to loading is that only stimuli perceived as sufficiently different from normal are capable of eliciting an adaptive response. To achieve this objective, the bone cell syncytium possesses great sensitivity to distinguish between those epigenetic stimuli requiring a biological response from the great majority of stimuli that do not. In the specific context of exogenous mechanical loading interventions, a threshold number of loading cycles (itself dependent upon factors such as magnitude, rate, and frequency) are required to initiate a sustained osteoblastic response eventually resulting in bone accretion (27,30,38). In terms of human exercise interventions, this threshold phenomenon means that the mild magnitude activities that are most readily complied with by the broadest populations must be performed for many more load cycles (or at higher frequencies) to elicit an anabolic response (1). Extending these observations, one might surmise that the human skeleton ignores the vast majority of skeletal loading induced by modest exercise (8).
The existence of this threshold behavior has led us to explore strategies that might enable mild mechanical stimuli to be perceived as potently anabolic. In an initial study, we observed that inserting a 10 s zero load rest interval between each loading cycle transformed a cyclic loading intervention that did not induce bone formation into a highly osteogenic regimen (35). We and other groups have since demonstrated that rest-inserted loading significantly elevates bone formation compared with cyclic loading interventions of equal cycle numbers, magnitudes, and frequencies (11,18,33,36). These studies support the thesis that brief zero-load rest-intervals significantly augment the osteogenic potential of otherwise moderately or non-stimulatory interventions and can be used to reduce the threshold of cycle numbers or strain magnitude required to achieve an anabolic response (33).
One clear limitation in developing osteogenic exercise regimens is the need to repeatedly and vigorously load the skeleton across days and weeks. This limitation has been little explored, despite ultimately being a critical aspect of any exercise regimen (i.e., how often must one exercise to achieve the desired result). Given the ability of rest-inserted loading to greatly reduce the thresholds required to achieve an osteogenic response (either the number of loading cycles or strain magnitude within each loading bout), we hypothesized that the number of bouts/wk of rest-inserted loading could be reduced from 3/wk without loss of osteogenic efficacy. We tested this hypothesis via a series of 3 wk long in vivo exogenous loading regimens that varied in the number and timing of loading bouts.
Methods
We pursued four complementary in vivo experiments in which young adult female C57BL/6 mice (16 wk) were subjected to external mechanical loading of the right tibia. Briefly, with mice under inhaled anesthesia (2% isoflurane), the right tibia was placed in cantilever bending by gripping the tibia proximal to the tuberosity and by applying controlled loads to the lateral surface of the distal tibia (9). Using previous strain gaging and FEA based calibrations (33), an applied load magnitude of 0.56 N was anticipated to induce 2350 με peak longitudinal normal strain at the tibia mid-shaft. Based upon pilot studies, we anticipated that this magnitude of stimulus would induce rapid saturation of the osteoblastic response to loading. This strain magnitude is similar to that observed on the human tibia mid-shaft during vigorous activity (24), is greater than tibia normal strains during mouse locomotion (~1600 με) but less than that experienced when mice jump off a 20 cm ledge (~ 2600 με; (19)). To quantify animal specific normal strains, the right tibia mid-shaft of each mouse was imaged (vivaCT40, Scanco, Inc) prior to the study and animal specific peak normal strains were quantified via application of beam theory to individualized mid-shaft morphology (33). Across all experiments, each bout consisted of 50 loading cycles in which peak loads were reached in 100 ms, held for 700 ms, and unloading reached in 100 ms and dwell time prior to initiation of the subsequent loading cycle was 100 ms (i.e., 1 s per load cycle). For rest-inserted loading, each load cycle was separated by a 10 s no load rest interval. Normal cage activity was permitted between loading sessions and food and water provided ad libitum with body weights assessed on loading days and the day of sacrifice. All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Experiment 1
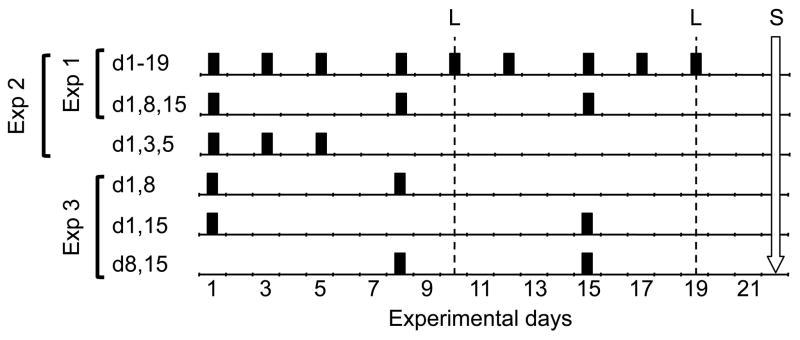
The first experiment separately assessed whether cyclic loading or rest-inserted loading interventions could be reduced from 3/wk to 1/wk without loss of osteogenic efficacy. The right tibiae of separate groups of mice were exposed to equal magnitude mechanical loading applied as either a cyclic (1 Hz) or a 10 s rest-inserted waveform (i.e., 10 s zero-load rest between each load cycle). The interventions consisted of 9 bouts applied three days per week for three weeks (day 1, 3, 5, 8, 10, 12, 15, 17, 19; d1-19) or 3 bouts applied once per week over the same 3 wk period (i.e., day 1, 8, 15; d1,8,15; Fig 1). All groups contained n=8, with the exception of the 9 bout rest-inserted loading group (n=10). All mice in Experiment 1 received calcein labels on d 10 and 19, with sacrifice on d 22. Bone formation induced at the tibia mid-shaft was compared to that of the contralateral tibia to assess whether a given intervention was osteogenic and across regimens to determine whether a reduced number of bouts compromised the osteogenic response to loading.
Figure 1. Loading intervention schematic for Experiments 1 (d1-19; d1,8,15), 2 (d1,3,5; d1,8,15; d1-19,) and 3 (d1,8; d1,15; d8,15).
Loading bouts for each group are noted by a black bar with calcein labels noted by dashed lines (L). For Experiment 4, d1-19, d1,8,15 and d1,3,5 loading interventions were implemented, but calcein labels occurred on days 19 and 28, with sacrifice on d 31.
Experiment 2
We next assessed whether the osteogenic benefit induced by 3 bouts of rest-inserted loading was robust to the timing of the bouts within the 3 wk intervention. While numerous perturbations could be used to explore this thesis, we contrasted the osteoblastic response to 3 bouts of rest-inserted loading applied over the 1st week of the 3-wk protocol (i.e., loading on d 1, 3, 5; d1,3,5; n=7) with that achieved by 3 bouts of 1/wk rest-inserted loading (d 1, 8, 15; d1,8,15; n=8) or 9 bouts of rest-inserted loading (d1, 3, 5, 8, 10, 12, 15, 17, 19; d1-19; n=10). This design provided a stringent assessment of the impact of timing of loading bouts, as the final loading event for the d1,3,5 group occurred the furthest in time from the final calcein label on d19. Mice received calcein labels on d 10 and 19, with sacrifice on d 22. In order to reduce animal use, we used data for the latter two groups generated in Experiment 1.
Experiment 3
In this experiment, we used a point mutation paradigm to assess whether removing any single bout of the once weekly rest-inserted interventions (d1,8,15) mitigated the osteogenic potential of the intervention. We therefore exposed mice to two bouts of rest-inserted loading with the following temporal perturbations: 1) d 1 and d 8 (d1,8; n=8), d 1 and d 15 (d1,15; n=8), or 3) d 8 and d 15 (d8,15; n=7). As in the previous experiments, each loading bout consisted of 50 cycles of equal magnitude rest-inserted loading (10 s rest) with the osteogenic response of the interventions contrasted with contralateral tibiae and across interventions. Additionally, all data for 2 bouts of rest-inserted loading were pooled and then compared with the pooled data for 3 bouts of rest-inserted loading (from Experiment 2) and the data for 9 bouts of rest-inserted loading (from Experiment 1). All mice received calcein labels on d 10 and 19, with sacrifice on d 22.
Experiment 4
In the final experiment, we used a separate cohort of mice to determine whether the equally osteogenic interventions of Experiment 2 resulted in osteoblast activation that differentially extended past the cessation of loading on d19. The experiment groups and the timing of loading interventions were identical to those of Experiment 2: 1) 3 bouts of loading during the first week (d1,3,5; n=7), 2) 3 bouts of loading occurring once per week for three weeks (d1,8,15; n=7), or 3) 9 bouts of loading occurring 3/wk for 3 weeks (d1-19; n=7). Mice freely ambulated following their respective last loading events and then received calcein labels on d 19 and d 28 and were sacrificed on d 31.
Dynamic Histomorphometry and Statistics
Upon sacrifice (d 22 for Experiments 1, 2 and 3; d 31 for Experiment 4), the right (experimentally loaded) and left tibiae (contralateral) were dissected of soft tissue, and tibia mid-shaft cross-sections (300 μm) were obtained using a diamond wheel saw. Sections were hand ground to 90 μm, cover-slipped and imaged using an epiflourescent microscope (Nikon). Upon imaging, composite images were constructed, anatomically oriented and analyzed using customized Image J software. As in previous studies (33), we determined the standard dynamic histomorphometry parameters including: surface referent mineralizing surface (MS/BS; %), mineral apposition rate (MAR; mm/d) and bone formation rate (BFR/BS; mm3/mm2/d) at the endocortical and periosteal surfaces. Preliminary analysis of endocortical data indicated that, consistent with the minimal endocortical response induced previously in our loading model, only one intervention significantly altered endocortical bone formation (rest-inserted, 9 bouts) and these data were therefore not subjected to further analysis. Group size varied due to: 1) Experiment 1, 2 and 3 were conducted via a series of unbalanced smaller groups that always contained mice exposed to 9 bouts of rest-inserted loading as a sentinel (resulting in n=10 for that group), 2) one mouse each was excluded from the d1,3,5 rest group (Experiment 2) and the d8,15 rest group (Experiment 3) due to hyperactive osteoblast function on the contralateral control tibia suggesting systemic distress (> 3 S.D.), and 3) Experiment 4 was powered at n=7 based on preliminary pilot data. Statistical analyses of the periosteal osteoblastic response were performed via factorial ANOVA (2×3 design, except Experiment 1 with separate 2×2 for cyclic and rest-inserted loading), with p < 0.05 considered to be statistically significant for all comparisons. Main effects for each comparison were loading (i.e., experimental vs contralateral tibiae) and protocol (i.e., varied bout timing). Prior to each factorial, data were assessed for homogeneity of variance using Levine’s test. For those data sets that did not meet the assumption of homogeneity (Exp 2: p.BFR, Exp 3: p.BFR, Exp 4: p.MAR, p.MS, p.BFR), data were square root transformed prior to statistical analysis. Post-hoc analyses (Tukey’s, p=0.05) were performed for those comparisons demonstrating significant interactions between loading and protocol. All results are reported as mean ± S.E.
Results
Tibia mid-shaft peak normal strains induced by the external loading protocols did not differ with or across experiments (Table 1). Across all groups, peak normal strains averaged 2440 ± 25 με. Across experiments, body weight alterations were not significant over time, nor were interactions between protocol and time on body weight observed.
Table 1.
Mean (± S.E.) peak induced tibia mid-shaft normal strains for each experimental group. No significant differences were noted within or across experiments.
| Experiment | Group | Mean | S.E. | |
|---|---|---|---|---|
|
| ||||
| 1 | Cyclic (d1-19) | 2370 με | 85 με | |
| Cyclic (d1,8,15) | 2345 με | 40 με | ||
| Rest (d1-19) | 2410 με | 65 με | ||
| Rest (d1,8,15) | 2290 με | 50 με | ||
|
| ||||
| 2 | Rest (d1,3,5) | 2440 με | 80 με | |
| Rest (d1,8,15) | 2290 με | 50 με | (same as Exp 1) | |
| Rest (d1-19) | 2410 με | 65 με | (same as Exp 1) | |
|
| ||||
| 3 | Rest (d1,8) | 2470 με | 60 με | |
| Rest (d1,15) | 2490 με | 80 με | ||
| Rest (d8,15) | 2500 με | 50 με | ||
|
| ||||
| 4 | Rest (d1,3,5) | 2525 με | 100 με | |
| Rest (d1,8,15) | 2520 με | 60 με | ||
| Rest (d1-19) | 2500 με | 50 με | ||
|
| ||||
| Across Exp | 2440 με | 25 με | ||
Experiment 1
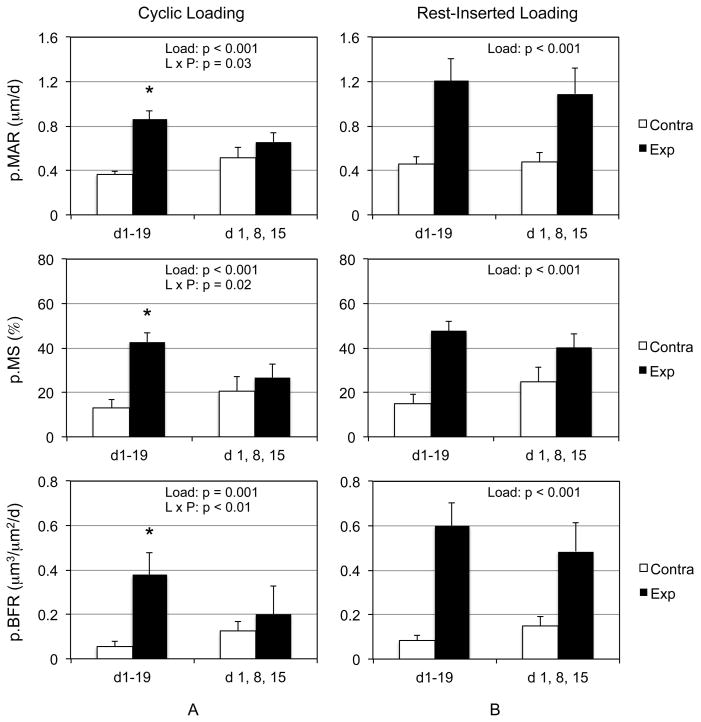
We first assessed whether cyclic loading could be reduced from 3/wk to 1/wk interventions without loss of the respective osteogenic benefit of each protocol. Cyclic loading induced a significant main effect for p.MS, p.MAR, and p.BFR compared to contralateral tibiae (Fig 2a). While the main effect of protocol was not significant for any parameter, there was a significant interaction between loading and protocol. Follow up tests indicated that 3/wk of cyclic loading significantly enhanced p.MS, p.MAR, and p.BFR compared to contralateral tibiae, whereas the 1/wk intervention did not.
Figure 2. Rest-inserted loading, but not cyclic loading, can be reduced from 3/wk to 1/wk loading interventions without loss of osteogenic efficacy.
The combination of significant loading main effect and loading and protocol interaction indicated that 3/wk cyclic loading, but not 1/wk cyclic loading significantly elevated p.MAR, p.MS, and p.BFR in loaded tibiae compared to contralateral tibiae (A). In contrast, rest-inserted loading resulted in significantly elevated p.MAR, p.MS, and p.BFR in experimentally loaded tibiae, but reducing the protocol from 3/wk to 1/wk did not influence this response (B). *, p<0.05 vs contralateral tibiae.
In a parallel experiment, rest-inserted loading induced a significant main effect for p.MS, p.MAR, p.BFR, that was independent of loading protocol (Fig 2b). That is, neither protocol main effect nor interactions between loading and protocol were significant. As a result reducing rest-inserted loading from 3 bouts/wk to 1 bout/wk did not diminished osteogenic efficacy.
Experiment 2
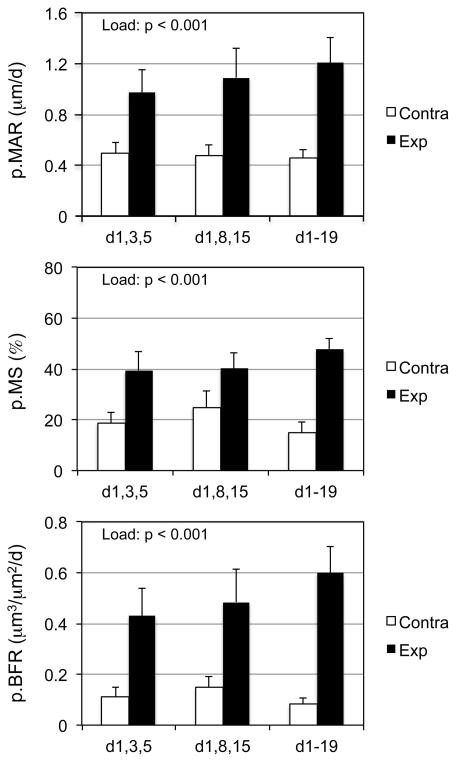
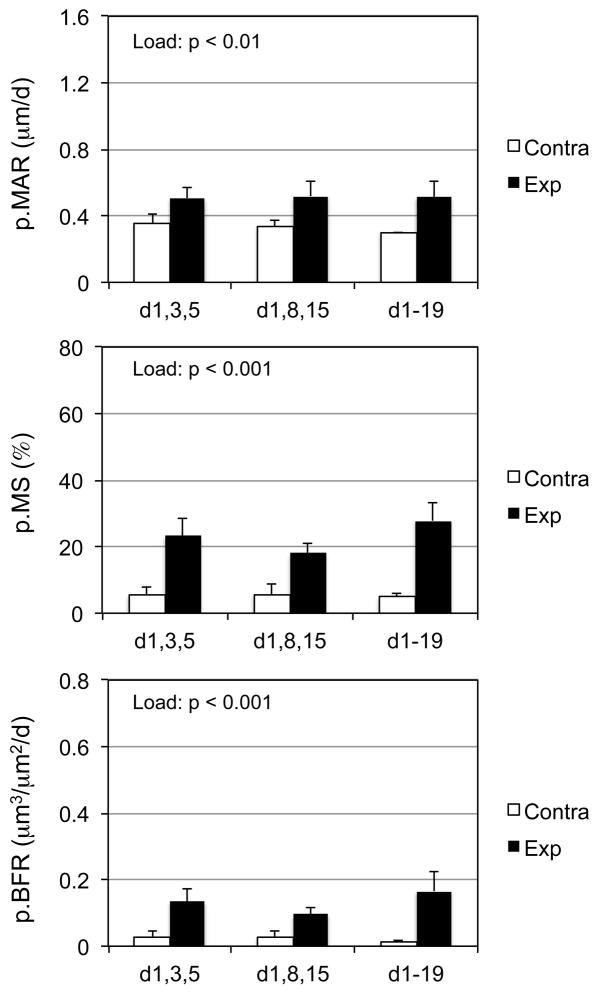
Here we sought to determine whether the osteogenic response to three total bouts of rest-inserted loading was robust to the timing of when the loading events were implemented (i.e. 3x in week one or 1x per week for 3 weeks). A significant loading main effect was observed for p.MAR, p.MS, and p.BFR (Fig 3). The absence of a protocol main effect or interaction between loading and protocol indicated that osteogenic effect of rest-inserted loading was not altered by the studied permutations in bout timing.
Figure 3. The osteogenic response to rest-inserted loading was robust to the timing of 3 loading bouts during a 3 wk intervention.
A significant loading main effect upon p.MAR, p.MS, and p.BFR was observed when 3 bouts of rest-inserted loading in the first week (d,1,3,5), or 1/wk (d,1,18,15) and 3/wk (d1-19; 9 total bouts) interventions were contrasted. The absence of protocol main effect or load and protocol interactions indicated that the compression all three rest-inserted loading bouts into the first week did not alter the induced osteogenic response.
Experiment 3
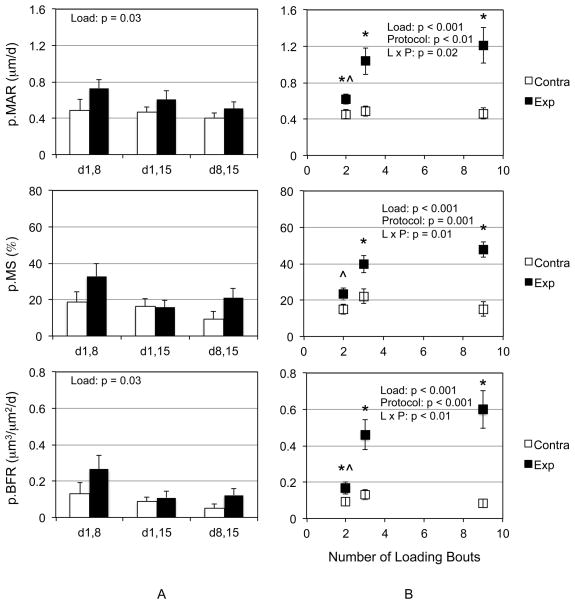
We then used a point mutation strategy to determine whether each of the 1/wk rest-inserted loading bouts were required to induce an osteogenic response to mechanical loading during a 3 wk intervention. Both p.MAR and p.BFR demonstrated main effects due to loading, but protocol main effects or interaction between loading and protocol were not observed (Fig 4a). To further clarify protocol efficacy, we then pooled all of the 2 loading bout groups, pooled all the three loading bout groups and contrasted those data with those generated by 3/wk loading. We observed significant loading and protocol main effects and loading and protocol interactions for p.MAR, p.MS, and p.BFR (Fig 4b). Post hoc analysis indicated that 2, 3 and 9 bouts of loading significantly enhanced p.MAR and p.BFR compared to contralateral tibiae, while 3 and 9 bouts of loading were required to significantly enhance p.MS. Finally, while 3 and 9 bouts of loading generated greater p.MAR, p.MS, and p.BFR compared to 2 bouts of loading, 3 and 9 bouts of loading did not induce differential responses in any parameter.
Figure 4. Removing any single 1/wk rest-inserted loading bouts results in a modest osteogenic response to mechanical loading.
Two bouts of rest-inserted loading (d1,8, d1,15, d8,15) were sufficient to demonstrate significant load main effects for p.MAR and p.BFR, but not p.MS (A). There was no significant effect of protocol nor were there any treatment interactions. A comparison of pooled 2, and 3 bouts of loading data compared to 9 bouts of loading demonstrated significant loading and protocol main effects and interaction for p.MAR, p.MS, and p.BFR. Post hoc analysis indicated that 2, 3 and 9 bouts of loading significantly enhanced p.MAR and p.BFR compared to contralateral tibiae, while 3 and 9 bouts of loading were required to significantly enhance p.MS compared to contralateral tibia (*, p<0.05). While 2 bouts of loading generated significantly less p.MAR, p.MS, and p.BFR compared to 3 and 9 bouts of loading (^, p<0.05), no differences were observed between 3 and 9 bouts of loading.
Experiment 4
In the final experiment, we explored whether osteoblast activity induced during a 3 wk intervention extended past the cessation of exogenous loading and, if so, whether altering the timing of loading bouts during the 3 wk intervention influenced this response. Loading related main effects were significant for p.MAR, p.MS, and p.BFR (Fig 5). However, neither protocol main effects nor loading and protocol interactions were observed.
Figure 5. The osteoblastic response to rest-inserted loading extended past the cessation of loading but was not altered by reducing the number of loading bouts from 9 to 3 nor the timing of the 3 loading bouts.
A significant load main effect was observed for p.MAR, p.MS, and p.BFR for each of the three protocols. The absence of protocol main effect or load and protocol interactions indicated that the timing of the loading bouts did not alter the duration of the induced osteoblastic response.
Discussion
We assessed the potential of rest-inserted loading to enable reduction in the number of loading bouts required to elevate periosteal osteoblast function during a 3 wk loading intervention. We observed that rest-inserted loading retained osteogenic efficacy when the frequency of loading bouts was reduced from 3/wk to 1/wk. The anabolic response to rest-inserted loading was robust to varying the timing of the bouts, but was mitigated if fewer than 3 loading bouts were implemented during the 3 wk period. Finally, we found that reducing the number of loading bouts from 9 to 3 (either compressed within the first week or once weekly) did not impair periosteal bone formation induced by rest-inserted loading in the period following the 3 wk loading intervention.
These experiments were not designed to contrast the osteogenic effectiveness of rest-inserted and cyclic loading. However, data from our group and others have clearly demonstrated that rest-inserted loading induces a significantly enhanced osteogenic response compared to equivalent magnitude and cyclic number interventions or a similar magnitude osteogenic response with lesser magnitude and/or cyclic number interventions (11,32,33,36). The results of Experiment 1 add to the potential applications of rest-inserted loading.
It is clear that the magnitude of the exogenous loading protocol (whether cyclic or rest-inserted) affects the number of bouts required to reach a saturated response. The magnitude of the loading protocols implemented in these experiments was at the upper end of induced peak normal strains previously implemented with our cantilever bending device (32). While naïve control mice were not included in the study, the absence of altered contralateral osteoblast function across protocols suggests that the induced response was confined to the externally loaded tibiae. In contrast to other mouse bone loading models (such as tibial compression; (39)) the induced strain distribution in this model is characterized by equivalent peak normal strains spanning a 2.5 mm segment about the mid-shaft. The loading induced periosteal response within this region is constant and lamellar (33,35). Due to the mechanical advantage of the device, only small cantilever loads were required to induce a repeatable osteogenic response (5 to 10% of loads used in other models; 0.56 N). The mean induced normal strain magnitude of this study was within the range of current in vivo models using non-high frequency loading (1400 to 3500 με), was greater than normal strains during locomotion in the murine bone (~1600 με), but was similar to those mid-shaft strains induced in murine and human long bones during vigorous activities (19,24). While it is likely to prove challenging to implement activities of this magnitude in the elderly, rest-inserted loading has also demonstrated osteogenic efficacy at strain levels associated with modest activity (33).
Our series of experiments did not explore all possible permutations of the timing and number of loading bouts that might occur during a 3 wk intervention, but instead attempted to design experiments that would identify osteogenic rest intervals while minimizing animal use. For example, the point mutation approach of Experiment 3 and analysis of consolidated data suggested that while 2 bouts of rest-inserted loading can induce biosynthetic activity, the response is significantly less osteogenic than that induced by 3- bouts of loading. However, while it is possible that 2 bouts of loading might retain osteogenic potential if more strategically timed relative to the dynamic labeling period, in vivo exploration of each possible permutation is experimentally prohibitive. Therefore, we envision exploiting our data sets to assess the entire space of loading bout timing (and other variables such as magnitude and cycle number per bout) via in silico approaches such as agent based modeling (34). Such a protocol optimization strategy could utilize dynamic histomorphometry as an initial outcome measure, then transition to longer-term studies in which enhanced bone morphology will become the primary outcome to be optimized. Even if the optimized protocols vary with the duration of intervention and/or outcome measure, this computational strategy would enable rapid exploration of osteogenic regimens over a vast solution space that would be otherwise unapproachable via traditional experimental paradigms.
One challenge in interpreting this series of experiments arises with our use of a fixed interval of osteoblast function quantification (i.e., d 10 and 19) in the first three experiments. This experimental constant resulted in loading bouts occurring at varied intervals respective to the time during which osteoblast activity was assessed. However, comparison of varied timing of labeling and loading bouts is common across in vivo mechanical loading studies (26,33,39). Further, Experiment 4, in which loading did not occur during osteoblast activity assessment, was designed, in part, to mitigate this potential limitation.
Despite the lack of clarity regarding how mechanical loading of bone is transduced into bone cell function, the potential of rest-inserted loading as a means to augment bone mass runs counter to exercise interventions reported in the literature, which have primarily exploited the long held premise that activities generating the greatest loads and loading rates will precipitate the greatest anabolic response. As such, interventions such as vigorous jumping have been assessed for their potential to enhance bone mass in children (21). These high impact activities are clearly more stimulatory for osteoblast function than casual activities such as walking (which has no apparent anabolic effect in humans; (3)). However, vigorous activities also have the narrowest potential for implementation in the elderly. Our findings with rest-inserted loading suggest that it may be possible to identify exercise interventions that are osteogenic, do not require impact loading, and are effective without daily repetition.
In support of this view, we observed that p.BFR was not significantly enhanced when rest-inserted loading was increased from 3 to 9 bouts, but also could not be reduced to 2 bouts within a 3 wk intervention without attenuating osteogenic efficacy. Such a response is consistent with a saturated system (i.e., one in which further exposure to the stimulus confers little additional benefit), although addition protocols with greater number of loading bouts per week (e.g., 5 or 7) may prove useful to better define the extent of induced saturation. However, previous studies have observed that bone formation as an adaptive response to mechanical loading ultimately diminishes to near baseline levels despite the persistence of exogenous loading (31). As well, we observed a lack of differential osteoblastic response between d 19 and d 28 for osteogenic regimens that ceased loading interventions on either d 5 or d 19 consistent with the thesis that once exogenous loading is ‘coded’ into activation of periosteal osteoblasts, the cellular response is largely predetermined with additional loading events conferring minimal additional benefit.
The phenomenon of response saturation, or desensitization, has been observed in a variety of cells and contexts and is typical of homeostatic systems (4,6,20,40). Often, this goal is achieved via end-point or negative feedback mechanisms (i.e., finite Ca2+ stores or synthesis of autoregulatory proteins such as RCAN1; (12,37)). With respect to bone, saturation responses have been observed at a cellular level (22), within individual loading bouts (i.e., when load cycles are increased, the response of bone rapidly plateaus; (30)), and across loading bouts (e.g., a similar benefit is obtained even when the bouts per week or the weeks of loading are reduced (28,31)).
Interestingly, we have previously found that insertion of no load rest intervals between each load cycle serves to delay the saturation of periosteal osteoblast activity observed when cycle numbers are increased within each loading bout (33). In contrast to this seconds/minutes time scale effect, our current data suggest that over a longer time period (i.e., days/weeks), the beneficial response to rest-inserted loading rapidly saturates (i.e., increasing from 3 to 9 bouts within 3 wk conferred negligible additional benefit). Together, these data suggest that the mechanisms underlying saturation of periosteal osteoblast activity varies across time scales. As a result, we believe that effective strategies to optimize exercise regimens must capitalize on these time dependent phenomena.
Acutely (i.e., within and immediately after a bout of loading), rest-intervals appear to alter/augment of the earliest aspects of mechanotransduction. In this context, in vitro fluid flow systems have revealed that no flow rest-intervals enhance Ca2+ spiking and lead to differential gene expression in MC3T3-E1 osteoblasts compared to continuous flow conditions (2,41). Over a broader time frame (e.g., across loading bouts over days), our data are consistent with the current literature demonstrating that repeated activation of signaling pathways leads to post-transcriptional synthesis of autoregulatory feedback proteins that inhibit ability to subsequently activate the same pathways. If such feedback mechanisms were present during subsequent mechanotransduction events, the saturation of osteoblast function (and ultimately, decline to baseline levels) would not be unexpected. In this context, our preliminary in vitro studies have suggested the emergence of autoregulatory components downstream of MAPK and NFAT pathway activation in bone cells subjected to repeated bouts of fluid flow (41). In a simplified sense, if saturation to an exercise intervention is achieved via negative feedback, not only would loading implemented after that threshold is reached have limited benefit, it may actually have a negative influence (e.g., should upregulation of negative feedback persist through future loading events). We therefore believe that understanding autoregulatory mechanisms (which may also be critical to bone cell homeostasis) will be essential to overcoming the ‘premature’ saturation of bone’s response to repeated bouts of loading and to thereby optimize mechanical loading to augment bone morphology.
In conclusion, we found that rest-inserted loading has potential for substantially minimizing the number of loading bouts required to enhance periosteal osteoblast function and that there was flexibility in the timing of the bout intervals without diminishment of de novo bone formation. These pre-clinical data suggest that it may be possible to design rest-inserted exercise interventions in humans that require substantially reduced time and effort compared with repetitive cyclic exercise while maintaining, or potentially augmenting, the osteogenic benefit derived from the intervention.
Acknowledgments
This work was supported, in part, by the NIH (AR56652: TSG; AR56235: SS), the Sigvard T. Hansen, Jr. Endowed Chair (TSG), the Zimmer Fracture Biology Professorship (SDB), and startup up funding from the UW Department of Orthopaedics and Sports Medicine (RYK). The authors would also like to specifically acknowledge the collaborative contributions of Leah E. Worton, Ph.D. the technical assistance of Dewayne Threet and Chris Jerome, B.Vet.Med., Ph.D. for development of the Image J histomorphometry software.
Footnotes
Conflict of Interest: The authors have no conflict of interest to disclose and note that the results of this study do not constitute endorsement by the ACSM.
Funding Disclosure: This work was funded, in part, by NIH (NIAMS), University of Washington Endowments and Department of Orthopaedics and Sports Medicine start up funding.
References
- 1.Bailey CA, Brooke-Wavell K. Optimum frequency of exercise for bone health: randomised controlled trial of a high-impact unilateral intervention. Bone. 2010;46(4):1043–9. doi: 10.1016/j.bone.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Batra NN, Li YJ, Yellowley CE, et al. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech. 2005;38(9):1909–17. doi: 10.1016/j.jbiomech.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Bolam KA, van Uffelen JG, Taaffe DR. The effect of physical exercise on bone density in middle-aged and older men: A systematic review. Osteoporos Int. 2013;24(11):2749–62. doi: 10.1007/s00198-013-2346-1. [DOI] [PubMed] [Google Scholar]
- 4.Cannon WB. Organization for physiological homeostasis. Physiological Reviews. 1929;9:399–431. [Google Scholar]
- 5.Currey JD. The mechanical adaptations of bones. Princeton, NJ: Princeton University Press; 1984. [Google Scholar]
- 6.Dallos P. Response characteristics of mammalian cochlear hair cells. J Neurosci. 1985;5(6):1591–608. doi: 10.1523/JNEUROSCI.05-06-01591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ducher G, Bass SL, Saxon L, Daly RM. Effects of repetitive loading on the growth-induced changes in bone mass and cortical bone geometry: a 12-month study in pre/peri- and postmenarcheal tennis players. J Bone Miner Res. 2011;26(6):1321–9. doi: 10.1002/jbmr.323. [DOI] [PubMed] [Google Scholar]
- 8.Forwood MR, Burr DB. Physical activity and bone mass: exercises in futility? Bone Miner. 1993;21(2):89–112. doi: 10.1016/s0169-6009(08)80012-8. [DOI] [PubMed] [Google Scholar]
- 9.Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res. 2002;17(3):493–501. doi: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haapasalo H, Sievanen H, Kannus P, Heinonen A, Oja P, Vuori I. Dimensions and estimated mechanical characteristics of the humerus after long-term tennis loading. J Bone Miner Res. 1996;11(6):864–72. doi: 10.1002/jbmr.5650110619. [DOI] [PubMed] [Google Scholar]
- 11.Holguin N, Brodt MD, Sanchez ME, Kotiya AA, Silva MJ. Adaptation of tibial structure and strength to axial compression depends on loading history in both C57BL/6 and BALB/c mice. Calcif Tissue Int. 2013;93(3):211–21. doi: 10.1007/s00223-013-9744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung CT, Allen FD, Pollack SR, Brighton CT. Intracellular Ca2+ stores and extracellular Ca2+ are required in the real-time Ca2+ response of bone cells experiencing fluid flow. J Biomech. 1996;29(11):1411–7. doi: 10.1016/0021-9290(96)84536-2. [DOI] [PubMed] [Google Scholar]
- 13.Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. Jama. 2005;294(2):218–28. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- 14.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40(6):1333–9. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Karinkanta S, Heinonen A, Sievanen H, et al. A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int. 2007;18(4):453–62. doi: 10.1007/s00198-006-0256-1. [DOI] [PubMed] [Google Scholar]
- 16.Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in men: a meta-analysis of randomized controlled trials. Bone. 2013;53(1):103–11. doi: 10.1016/j.bone.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 17.Kukuljan S, Nowson CA, Sanders KM, et al. Independent and combined effects of calcium-vitamin D3 and exercise on bone structure and strength in older men: an 18-month factorial design randomized controlled trial. J Clin Endocrinol Metab. 2011;96(4):955–63. doi: 10.1210/jc.2010-2284. [DOI] [PubMed] [Google Scholar]
- 18.LaMothe JM, Zernicke RF. Rest insertion combined with high-frequency loading enhances osteogenesis. J Appl Physiol. 2004;96(5):1788–93. doi: 10.1152/japplphysiol.01145.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lee KC, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31(3):407–12. doi: 10.1016/s8756-3282(02)00842-6. [DOI] [PubMed] [Google Scholar]
- 20.Ligeti E, Csepanyi-Komi R, Hunyady L. Physiological mechanisms of signal termination in biological systems. Acta Physiol (Oxf) 2012;204(4):469–78. doi: 10.1111/j.1748-1716.2012.02414.x. [DOI] [PubMed] [Google Scholar]
- 21.Macdonald HM, Kontulainen SA, Khan KM, McKay HA. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? J Bone Miner Res. 2007;22(3):434–46. doi: 10.1359/jbmr.061205. [DOI] [PubMed] [Google Scholar]
- 22.Mantila Roosa SM, Liu Y, Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res. 2011;26(1):100–12. doi: 10.1002/jbmr.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2009;43(12):898–908. doi: 10.1136/bjsm.2008.052704. [DOI] [PubMed] [Google Scholar]
- 24.Milgrom C, Finestone A, Levi Y, et al. Do high impact exercises produce higher tibial strains than running? Br J Sports Med. 2000;34(3):195–9. doi: 10.1136/bjsm.34.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikander R, Sievanen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8(47) doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niziolek PJ, Warman ML, Robling AG. Mechanotransduction in bone tissue: The A214V and G171V mutations in Lrp5 enhance load-induced osteogenesis in a surface-selective manner. Bone. 2012;51(3):459–65. doi: 10.1016/j.bone.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998;16(4):482–9. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- 28.Raab-Cullen DM, Akhter MP, Kimmel DB, Recker RR. Bone response to alternate-day mechanical loading of the rat tibia. J Bone Miner Res. 1994;9(2):203–11. doi: 10.1002/jbmr.5650090209. [DOI] [PubMed] [Google Scholar]
- 29.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17(8):1545–54. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 30.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66(3):397–402. [PubMed] [Google Scholar]
- 31.Saxon LK, Robling AG, Alam I, Turner CH. Mechanosensitivity of the rat skeleton decreases after a long period of loading, but is improved with time off. Bone. 2005;36(3):454–64. doi: 10.1016/j.bone.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS. Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone. 2003;33(6):946–55. doi: 10.1016/j.bone.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan S, Ausk BJ, Poliachik SL, Warner SE, Richardson TS, Gross TS. Rest-inserted loading rapidly amplifies the response of bone to small increases in strain and load cycles. J Appl Physiol. 2007;102(5):1945–52. doi: 10.1152/japplphysiol.00507.2006. [DOI] [PubMed] [Google Scholar]
- 34.Srinivasan S, Ausk BJ, Prasad J, et al. Rescuing loading induced bone formation at senescence. PLoS Comput Biol. 2010;6(9) doi: 10.1371/journal.pcbi.1000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17(9):1613–20. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umemura Y, Sogo N, Honda A. Effects of intervals between jumps or bouts on osteogenic response to loading. J Appl Physiol. 2002;93(4):1345–8. doi: 10.1152/japplphysiol.00358.2002. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, De Keulenaer GW, Weinberg EO, et al. Direct biomechanical induction of endogenous calcineurin inhibitor Down Syndrome Critical Region-1 in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2002;283(2):H533–9. doi: 10.1152/ajpheart.00002.2002. [DOI] [PubMed] [Google Scholar]
- 38.Warden SJ, Turner CH. Mechanotransduction in the cortical bone is most efficient at loading frequencies of 5–10 Hz. Bone. 2004;34(2):261–70. doi: 10.1016/j.bone.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Weatherholt AM, Fuchs RK, Warden SJ. Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone. 2013;52(1):372–9. doi: 10.1016/j.bone.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams JT, Ingram SL, Henderson G, et al. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65(1):223–54. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Worton LE, Ausk BJ, Downey LM, et al. Systems-based identification of temporal processing pathways during bone cell mechanotransduction. PLoS One. 2013;8(9):e74205. doi: 10.1371/journal.pone.0074205. [DOI] [PMC free article] [PubMed] [Google Scholar]