Abstract
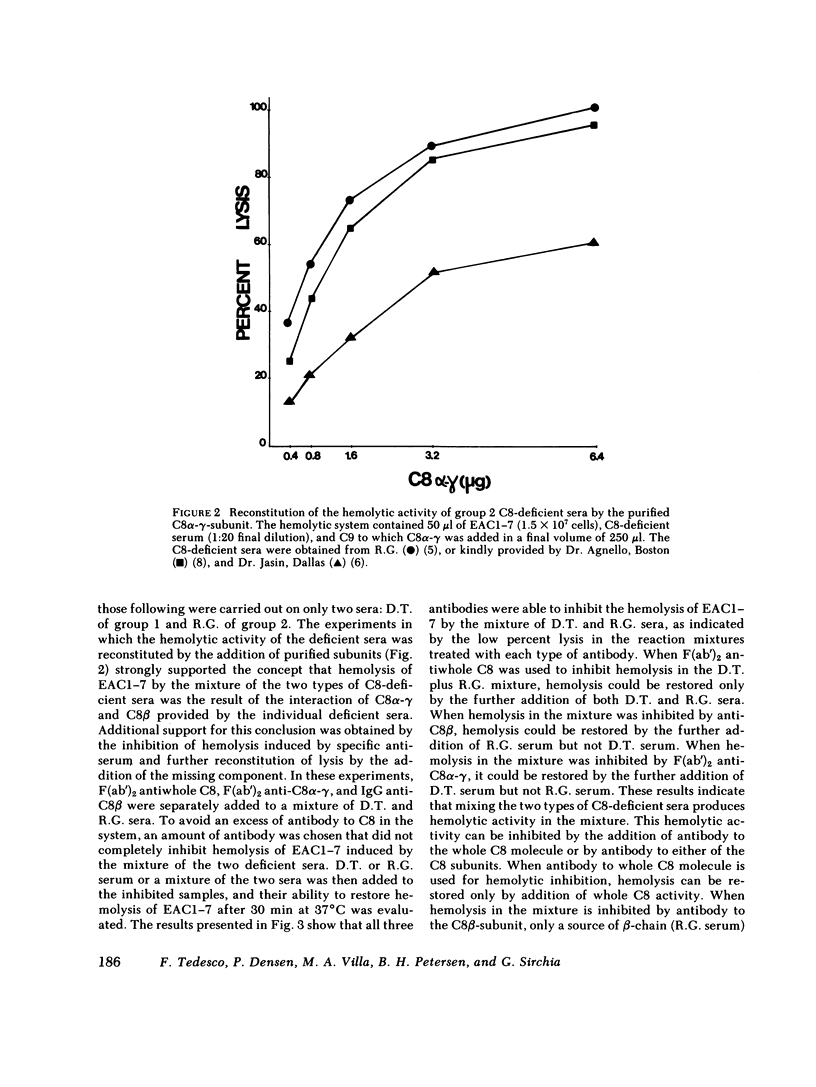
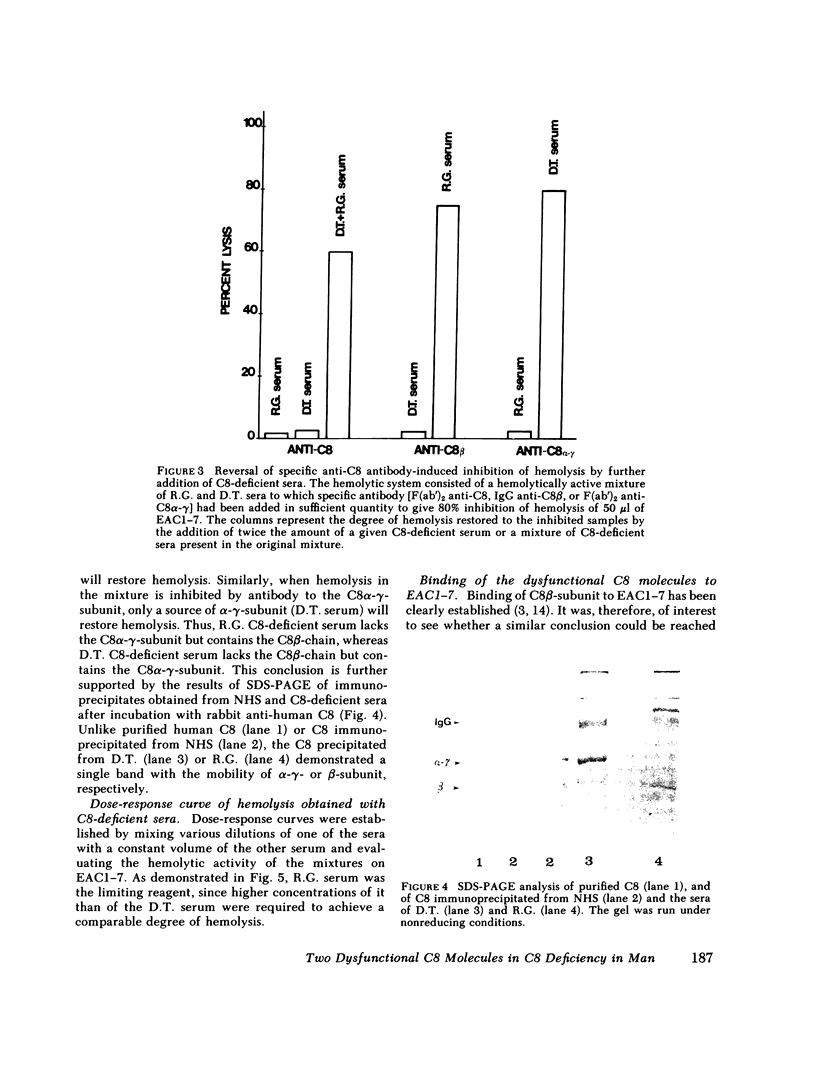
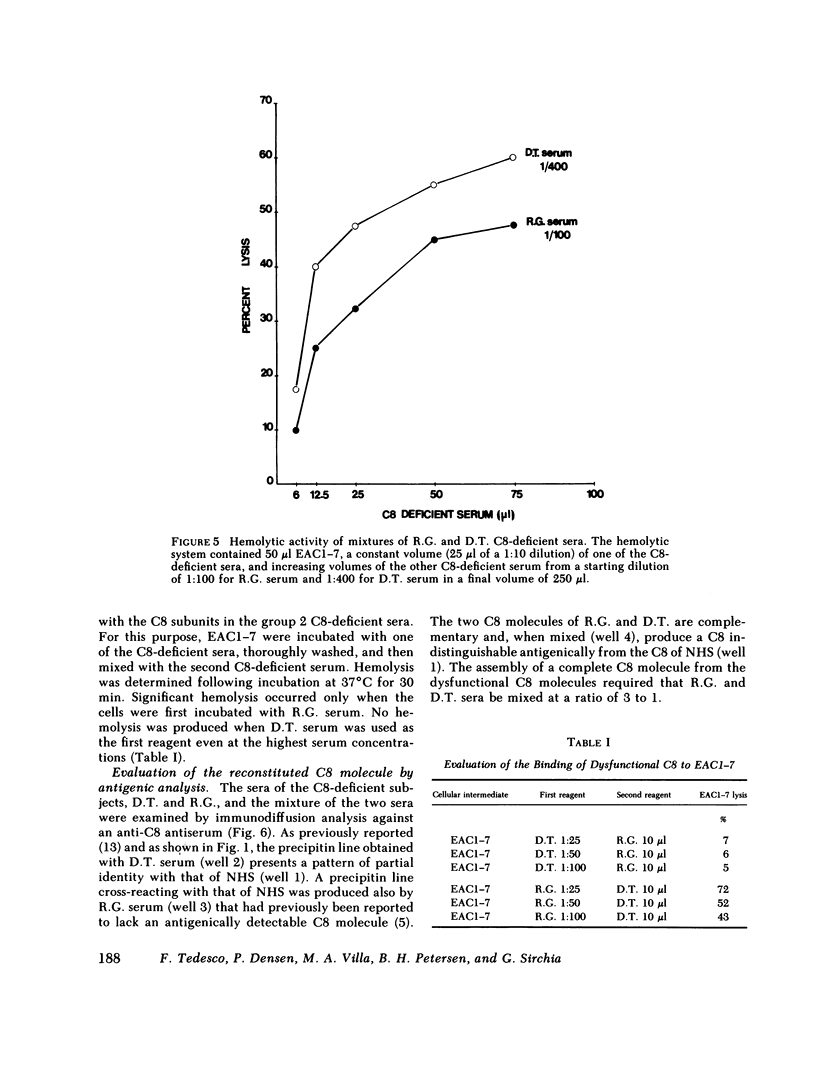
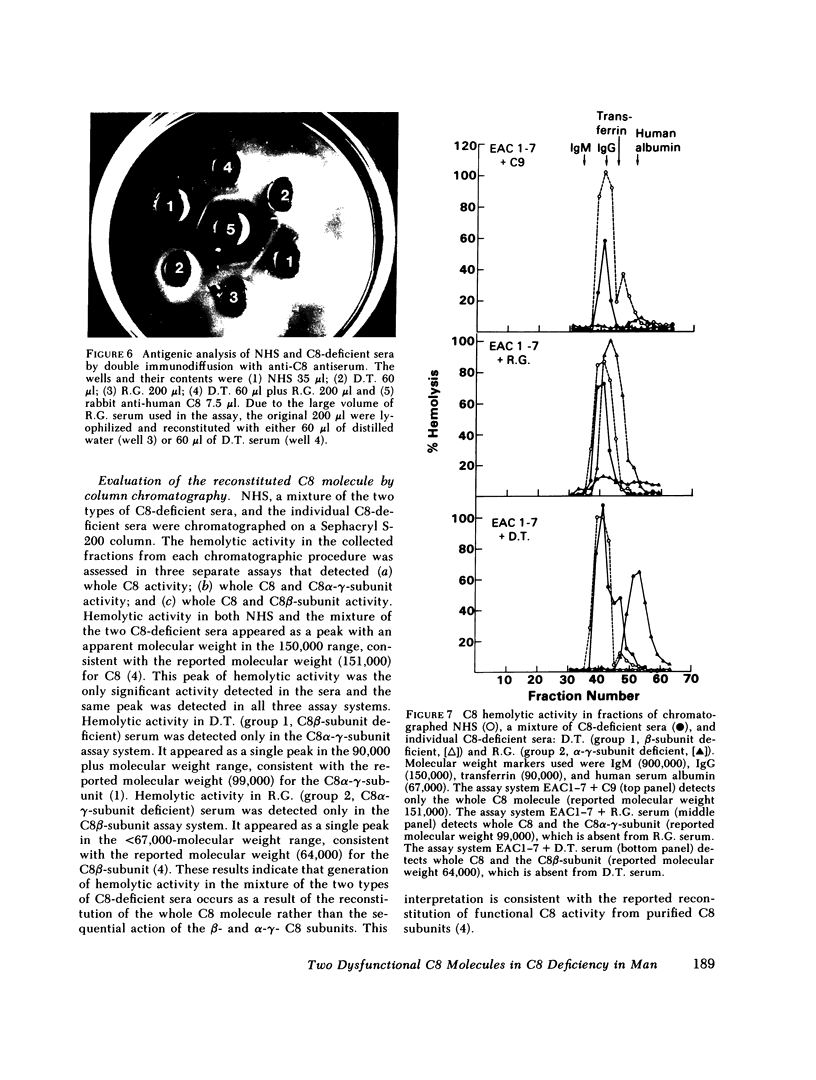
Restoration of hemolytic activity was examined in sera from seven unrelated eighth component of complement (C8)-deficient subjects. The sera fell into two groups, depending on whether hemolytic activity was restored by the addition of the beta-chain (group 1) or the alpha-gamma-subunit (group 2) purified from normal human C8. Antigenic analysis of these sera by double-immunodiffusion using anti-human C8 confirmed previous findings of a dysfunctional C8 in the four sera of group 1 and established the presence of a different dysfunctional C8 in one of the sera of group 2 when tested at a high concentration. Further characterization of the dysfunctional C8 molecules in the two sera by sodium dodecyl sulfate-polyacrylamide gel electrophoresis demonstrated that group 1 sera were missing the beta-subunit and group 2 sera were missing the alpha-gamma-subunit of the C8 molecule. Sera from either of these two groups alone did not produce hemolysis in hemolytic plates containing sheep erythrocytes coated with antibody and complement components up to C7 (EAC1-7) and C9. When sera from the two groups were added to adjacent wells in the hemolytic plates, a zone of hemolysis developed between the wells. The contribution of C8 alpha-gamma from the sera of group 1 and of C8 beta from those of group 2 to the lysis of EAC1-7 in the presence of C9 was confirmed by the inhibitory effect of specific antibodies against the two C8 subunits. In experiments in which hemolytic activity was reconstituted by mixing sera from group 1 with sera from group 2, the serum source of C8 beta (group 2) was the limiting reagent. The dysfunctional C8 molecule in this serum was able to bind to EAC1-7. Chromatographic analysis demonstrated that the generation of hemolytic activity in the mixture of the two sera resulted from the reconstitution of the C8 molecule rather than the sequential action of the two C8 subunits.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Tranum-Jensen J., Klump O. The terminal membrane C5b-9 complex of human complement. Evidence for the existence of multiple protease-resistant polypeptides that form the trans-membrane complement channel. J Immunol. 1980 May;124(5):2451–2457. [PubMed] [Google Scholar]
- Giraldo G., Degos L., Beth E., Sasportes M., Marcelli A., Gharbi R., Day N. K. C8 deficiency in a family with xeroderma pigmentosum. Lack of linkage to the HLA region. Clin Immunol Immunopathol. 1977 Nov;8(3):377–384. doi: 10.1016/0090-1229(77)90002-2. [DOI] [PubMed] [Google Scholar]
- Jasin H. E. Absence of the eighth component of complement in association with systemic lupus erythematosus-like disease. J Clin Invest. 1977 Sep;60(3):709–715. doi: 10.1172/JCI108823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klob W. P., Müller-Eberhard H. J. The membrane attack mechanism of complement: the three polypeptide chain structure of the eigth component (C8). J Exp Med. 1976 May 1;143(5):1131–1139. doi: 10.1084/jem.143.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J. The purification of specific antibody as F(ab')2 by the pepsin digestion of antigen-antibody precipitates, and its application to immunoglobulin and complement antigens. Immunochemistry. 1971 Jan;8(1):81–88. doi: 10.1016/0019-2791(71)90423-x. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Thompson R. A. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970 Apr 1;131(4):643–657. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matthews N., Stark J. M., Harper P. S., Doran J., Jones D. M. Recurrent meningococcal infections associated with a functional deficiency of the C8 component of human complement. Clin Exp Immunol. 1980 Jan;39(1):53–59. [PMC free article] [PubMed] [Google Scholar]
- Monahan J. B., Sodetz J. M. Binding of the eighth component of human complement to the soluble cytolytic complex is mediated by its beta subunit. J Biol Chem. 1980 Nov 25;255(22):10579–10582. [PubMed] [Google Scholar]
- Monahan J. B., Sodetz J. M. Role of the beta subunit in interaction of the eighth component of human complement with the membrane-bound cytolytic complex. J Biol Chem. 1981 Apr 10;256(7):3258–3262. [PubMed] [Google Scholar]
- Petersen B. H., Graham J. A., Brooks G. F. Human deficiency of the eighth component of complement. The requirement of C8 for serum Neisseria gonorrhoeae bactericidal activity. J Clin Invest. 1976 Feb;57(2):283–290. doi: 10.1172/JCI108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckel E. W., York R. G., Monahan J. B., Sodetz J. M. The eighth component of human complement. Purification and physicochemical characterization of its unusual subunit structure. J Biol Chem. 1980 Dec 25;255(24):11997–12005. [PubMed] [Google Scholar]
- Tedesco F., Bardare M., Giovanetti A. M., Sirchia G. A familial dysfunction of the eight component of complement (C8). Clin Immunol Immunopathol. 1980 Jun;16(2):180–191. doi: 10.1016/0090-1229(80)90202-0. [DOI] [PubMed] [Google Scholar]
- Thompson R. A., Lachmann P. J. Reactive lysis: the complement-mediated lysis of unsensitized cells. I. The characterization of the indicator factor and its identification as C7. J Exp Med. 1970 Apr 1;131(4):629–641. doi: 10.1084/jem.131.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J., Esser A. F., Spira T. J., Müller-Eberhard H. J. Occurrence of an incomplete C8 molecule in homozygous C8 deficiency in man. J Exp Med. 1981 Nov 1;154(5):1599–1607. doi: 10.1084/jem.154.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]