Abstract
This research presents a multiple enzyme-doped thread-based microfluidic system for blood urea nitrogen (BUN) and glucose detection in human whole blood. A novel enzyme-doped thread coated with a thin polyvinylchloride (PVC) membrane is produced for on-site electrochemical detection of urea and glucose in whole blood. Multiple enzymes can be directly applied to the thread without delicate pretreatment or a surface modification process prior to sealing the thread with PVC membrane. Results indicate that the developed device exhibits a good linear dynamic range for detecting urea and glucose in concentrations from 0.1 mM–10.0 mM (R2 = 0.9850) and 0.1 mM–13.0 mM (R2 = 0.9668), which is suitable for adoption in detecting the concentrations of blood urea nitrogen (BUN, 1.78–7.12 mM) and glucose (3.89–6.11 mM) in serum. The detection result also shows that the developed thread-based microfluidic system can successfully separate and detect the ions, BUN, and glucose in blood. The calculated concentrations of BUN and glucose ante cibum (glucose before meal) in the whole blood sample are 3.98 mM and 4.94 mM, respectively. The developed thread-based microfluidic system provides a simple yet high performance for clinical diagnostics.
I. INTRODUCTION
The applications of Bio-MEMS grew exponentially in various fields including medical detection, food detection, agriculture, and other industrials in the past decades. Various microchips have been developed and have been demonstrated for their capabilities in high performance analytical applications.1 The idea of the miniaturized total analysis system was explored in the early 1990s,2 which brought several advantages including reduced sample and reagent consumption, faster analysis, higher throughput, and lower operation cost. Over the developed bio-chip devices, capillary electrophoresis (CE) chip is one of the most promising schemes since it is capable of separating and detecting the target bio-samples in the microfluidic system. In general, UV absorption or laser induced fluorescence technique is the most common scheme for the detector of a capillary electrophoresis system. However, fluorescence labeling or delicate optical detectors are required to build the detection system. Alternatively, electrochemical detection is a high sensitivity approach which also offers many desirable features such as low-cost for building portable/disposable systems, tunable detection potential for improving the selectivity, and full electronic signal for easily integrating with electronic circuits.3,4 In addition, the signal response is insensitive to the sensing area of the electrodes but only dependent on the sample volume and the diffusion path such that the sensitivity of electrochemical sensor is remained upon miniaturization.5,6 Amperometry is the most common scheme in electrochemical detection, which has been successfully integrated with electronic circuit in Lab-chip systems.7,8 Recently, several researchers have developed the so-called Lab-Chip systems for in-column enzymatic reactions and detections of multiple samples.9 Microfluidic devices integrated with the ion-selective membrane or modified nano-electrodes for high-performance analyzing the concentration of urea and glucose (GLU) in human serum were also reported.9,10 However, the fabrication for the sensing electrodes was comparatively delicate. Moreover, most biochip devices aimed to perform one biocatalytic reaction and detection in one test. Therefore, it is beneficial to develop a microfluidic device which is capable of performing simultaneous multi-reactions and detections for medical diagnosis applications.
The chip-based CE system is an important technique for separating such biological samples as ions, protein, and DNA.11 In addition, these microchip devices are usually produced in materials such as glass, silicon, polymer, and even silk yarn3,12–15 using various microfabrication technologies. Glass-based microfluidic chips have several advantages, including excellent mechanical and chemical properties and optical transparency. Though a rapid fabrication process has been developed to produce a microfluidic system in a low-cost soda-lime glass,16 the fabrication processes for glass-based microfluidic devices is usually time consuming and delicate.17–19 Some other CE-chips have also been used for simultaneously measuring hydrogen peroxide, ascorbic acid, and uric acid in glass-based microfluidic devices under the electrochemical detection scheme.20,21 Recently, an even lower cost microfluidic system comprising hydrophilic paper bounded by a hydrophobic polymer.22–24 Paper-based microfluidics have emerged as a multiple point-of-cares platform which might transcend the capabilities of existing assays in resource-limited settings.25 The developed microfluidic device suggested a way for developing simple, inexpensive, and portable diagnostic assays for detecting disease and monitoring health. Martinez et al.26 developed three-dimensional microfluidic paper-based analytical devices (3D μPADs) which used SU-8 to pattern the microfluidic channels on the paper substrate for biological assay tests. Hsu et al. also reported a paper-based microfluidic device for the detection of autoimmune antibodies in body fluid utilizing enzyme-linked immunosorbent assays (ELISA).27 Wet-chemical etching of paper for producing paper-based microfluidic devices have also been reported.28 Paper was successfully patterned for low-cost food detection. Alternatively, silk yarns with different properties were also produced as the fabric chip for immunoassay application. Strips of this fabric were then used to perform a proof-of-concept immunoassay with sample flow taking place by capillary action and detection being performed by a visual readout.29,30 Paper-based microfluidic systems and the recent developed cotton-based diagnostic devices have a number of advantages during practical applications, including high speed and low cost production processes, compatibility with commercial printing technology, and no requirement for an external driving force.15,31–33 However, the devices still have some practical issues including that it is delicate to define the hydrophilic/hydrophobic regions and the material is relatively fragile and is easy to damage.34 Therefore, the use of the polyester thread as the microfluidic channel has shown a great potential for rapid and low-cost detection of bio-samples.35,36 Biomolecules and ions could be transported and electrochemically detected on a thread such that time-consuming fabrication processes for producing sealed microfluidic channel can be excluded.
Monitoring the blood urea nitrogen (BUN) and blood glucose is important for patients with diabetic or kidney diseases. Microfluidic systems integrated with a solid-state sensor incorporated with enzyme-carrying alginate micro-beads have been reported for on-site analyze of urea, glucose, and creatinine in the human serum.28,37 Nevertheless, the operation procedures for detecting the biosamples using these devices were comparatively delicate since the chemical structure of urea and glucose are stable. Therefore, these biomolecules are difficult to detect with typical electrochemical procedures without the assist of enzymatic reaction. Nevertheless, urea is easily to be converted into ammonium hydroxide with in the presence of urease. Ammonium ion is a cation which has a reduction potential of around −0.2 V to −0.3 V and can be easily detected using a negative potential state. The ammonium ion is electrochemical active and can be detected using typical electrochemical electrodes. This enzymatic reaction is usually used in electrochemical detections for in situ analyzing of the urea concentration.38 Alternatively, glucose can be converted into hydrogen peroxide (H2O2) with the presence of glucose oxidase (GOD). However, the H2O2 is an oxidation regent and has a standard reduction potential of around 1.776 V. Therefore, it is difficult to simultaneously detect these two products with a single set of electrochemical detecting electrodes. It is essential to apply the secondary enzyme of horseradish peroxidase (HRP) to further react with hydrogen peroxide to form the reduction products at a low negative voltage.39 The HRP/GOD sensor with mediator (catechol) is a convenient model system for comparison of mono- and bi-enzymatic sensors.40,41 With this approach, the reaction products of glucose can be electrochemically detected at a low potential near the ammonium ion. Therefore, it is essential to use at least three consecutive enzymatic reactions to electrochemical detect BUN and glucose on a single set of electrochemical electrode.
This study develops a thread-based microfluidic with novel polyvinylchloride (PVC) coated membrane system for rapid and low-cost electrophoresis separation and electrochemical detection of BUN and glucose in whole human blood. Various enzymes of urease, glucose oxidase, and catechol are immobilized on a thread for on-site enzymatic reactions prior to the PVC coating process. The PVC coating on the enzyme coated thread will reduce the evaporation of buffer solution and also reduced the Joule heating effect. The enzyme and the mediator (catechol) can be simply applied on the thread without delicate sample pretreatment or surface modification process. The reaction products of these two biomolecules can be simultaneously detected with the same applied potential of −0.28 V. Whole blood sample is capable of lysing, eluting, electrophoresis separation, and electrochemical detection on the developed thread-based microfluidic system. The system developed in the present study is a simple and cost effective and fast diagnostic tool for detecting urea and glucose in whole blood.
II. MATERIALS AND METHODS
A. Working principle
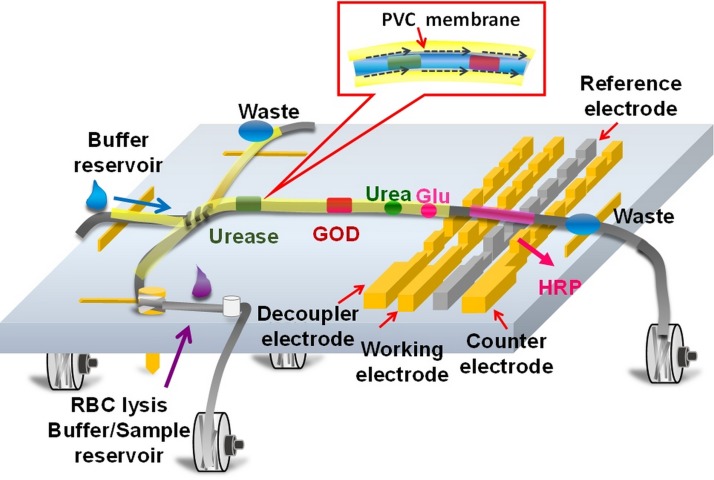
This research presents a multiple enzyme-doped thread-based microfluidic system for blood urea nitrogen and glucose detection in human whole blood. The developed thread-based CE-EC system is capable for on-site enzymatic reactions and detection of urea and glucose on a single thread. Figure 1 presents the schematic illustration of the working principle of the developed CE-EC micro-device. Since enzymes can be attached on the polyester thread simply by direct applying a drop of enzyme solution on the thread, multiple enzymes can be applied on the thread for various sample detections. The passing bio-samples on the thread will be digested with the immobilized enzymes, and the products of the enzymatic reactions are then electrochemically detected downstream. A simple coating technique for sealing the polyester thread with a thin PVC membrane is developed to prevent from the evaporation of the running buffer during electrophoresis separation such that the performance of the thread-based microfluidic system can be enhanced. Prior to the application of PVC solution, the enzyme-doped thread is immersed in buffer solution to fully soak the buffer solution on the thread to prevent form the infusion of PVC solution into the fiber bundle of the thread. Due to the immiscible property between the PVC solution and the water-based running buffer, a thin PVC layer on the thread can be formed after evaporating the organic solvent in the PVC solution. The formed PVC membrane not only prevents the buffer solution from rapid evaporation during CE test but also confines the fiber bundle of the polyester thread. Therefore, the applied electric field can be increased for sample separation, which would enhance the quality for the electrochemical signals of the samples.
FIG. 1.
Schematic of the enzyme-doped thread coated with PVC membrane for high-performance CE-EC detection.
B. Fabrication
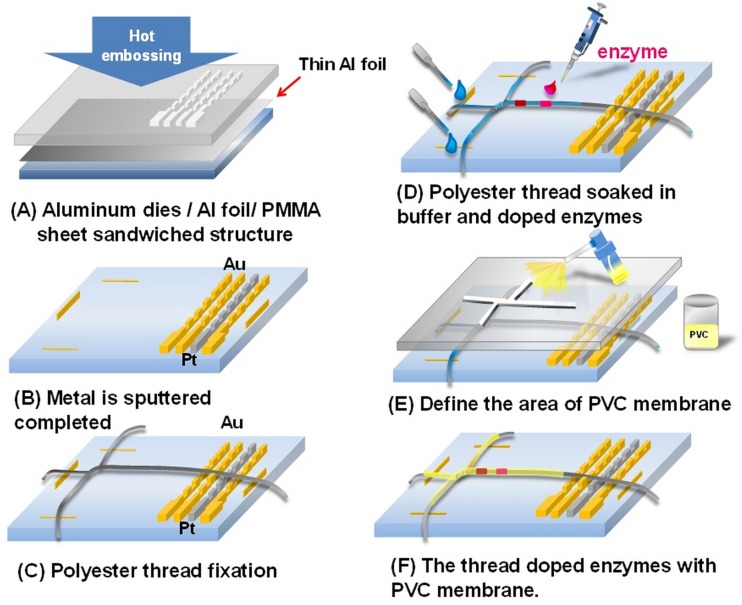
Figure 2 shows the simplified fabrication process for producing the proposed thread-based microfluidic system. The details for the fabrication process can be found in the previous report.42 A concave master mold was first fabricated on a 2-mm thick aluminum plate using a micro CNC machine. The convex electrode structures were then produced on a PMMA substrate using the hot embossing process (Fig. 2(a)). The working and counter electrodes for electrochemical detection were then produced by patterning sputtered Cr/Au layers on the embossed PMMA substrate, and the reference electrode was patterned with sputtered Cr/Pt layers (Fig. 2(b)). Polyester threads of around 200-μm in diameter (50 Denier, 72 filaments, Acelon Fiber Corp., Taiwan) were fixed on the PMMA substrate as the liquid routes and the enzymatic reaction sites (Fig. 2(c)). Specific enzymes including urease, GOD, and HRP were then directly applied on different sites of the thread with a 2-μl pipette (Fig. 2(d)). A thin PVC solution was sprayed on the desired region with the assistance of a plastic mask to seal the thread for capillary electrophoresis separation (Fig. 2(e)). A thin PVC membrane was then on the thread after evaporating the solvent of the applied PVC solution. Multiple enzyme-doped polyester thread coated with PVC membrane was finally produced for simultaneously EC detection of urea and glucose. It is noted that the PVC membrane was prepared by dissolving 200 mg (Fig. 2(f)) of the mixed powder composed of 33 wt. % of polyvinyl chloride (PVC, Fluka, Switzerland) and 66 wt. % of DBS (dibutyl sebacate, Fluka, Switzerland) with 2.5 ml of THF (Tetrahydrofuran, Alfa Aesar, Germany).
FIG. 2.
Schematic for the simplified fabrication process for the thread-based microfluidic device.
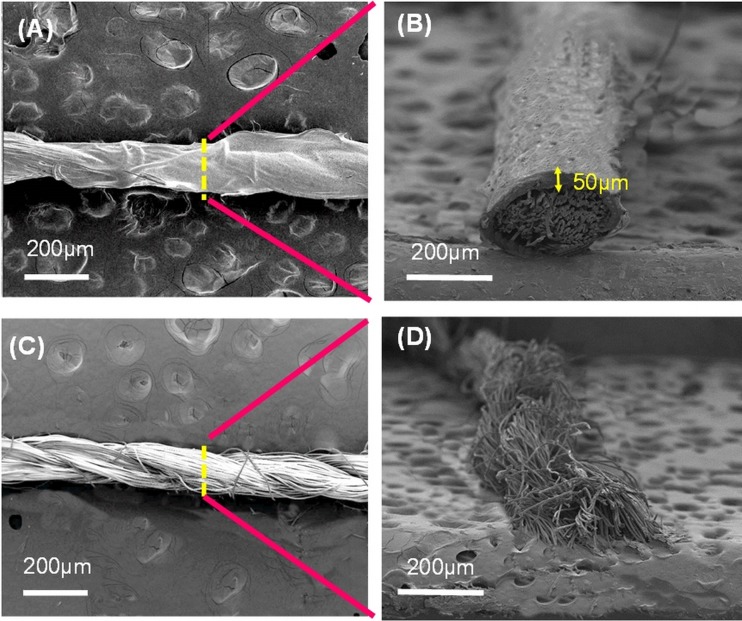
Since PVC is a hydrophobic material and may reduce the electroosmotic mobility of the thread-based microfluidic system, it is important to form a PVC shell on the thread without blocking the liquid flowing path inside the thread bundle. Figure 3 presents the SEM images of the threads with and without PVC coating. It is clear that a PVC layer was successfully formed on the thread surface (Fig. 3(a)). From the cross-section view, the fiber bundle was well constrained by the PVC membrane of around 50 μm in thickness (Fig. 3(b)). Moreover, no smear for the coated PVC coating layer such that the reduction for the electroosmotic mobility could be eliminated. Alternatively, a rougher surface can be observed for the thread without PVC coating (Fig. 3(c)). The fiber bundle was also loose (Fig. 3(d)) such that the CE separation performance was hindered in compare with the thread with PVC coating.
FIG. 3.
SEM images showing the polyester threads with (a) and (b) and without PVC coating (c) and (d). Note that (a) and (c) are eagle view and (b) and (d) are cross section view.
C. Experimental setup and apparatus
Figure 4 presents the experimental setup for on-site enzymatic reaction and capillary electrophoresis and electrochemical (CEEC) detection for the urea and glucose biomolecules using the developed thread-based microfluidic system. A high voltage power supply (MP-3500-250P, Major Science, Taiwan) was used for sample injection and separation. The separated biomolecules were then electrochemically detected with a commercial electrochemical analyzer (Model CHI611C, CH instruments, USA). A home-built circuit and four DC motors were used to control the tension of the polyester threads and automatically replace the polyester threads. In addition, to avoid the sample cross-contamination, replacement of the thread must be important to make the entire process more automated runner replacement. The DC motor module was powered by a 12 V DC power supply (DP-3630S, HILA, Taiwan). The running buffer for capillary electrophoresis was 1.0 mM MES (2-(N-morpholino) ethanesulfonic acid, Sigma-Aldrich, USA) prepared with Mini-Q deionized (DI) water. The standard bio-samples and enzymes are all reagent grade including catechol (CA, Sigma-Aldrich, USA), urease (Type III, Jack Beam, Germany), glucose oxidase (Type VII, Jack Beam, Germany), horseradish peroxidase (Type I, Jack Beam, Germany), Urea (Carbamide, 99%, Panreac Química, Spain), and glucose (Dextrose Anhydrous Powder, J. T. Baker, USA) and the red blood cell (RBC) lysing buffer (R7757, Sigma-Aldrich, USA).
FIG. 4.
Experimental setup for the developed thread-based CE-EC detection system.
III. RESULTS AND DISCUSSION
A. Electroosmotic flow (EOF) characteristic evaluations
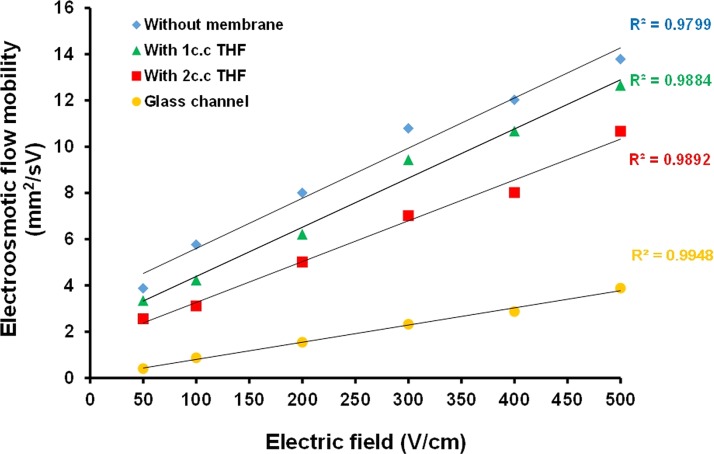
EOF mobility is a critical concern for evaluating the capability of an electrokinetically driving system such as the capillary electrophoresis. The CE system with a higher EOF mobility is able to provide a faster sample transportation rate. In this regards, this study compared the electroosmotic mobility for the conventional glass-based microfluidic channel and the polyester thread coated with the PVC membranes of different thicknesses. The PVC solutions were diluted with different amounts of THF to decrease the viscosity prior to the coating procedure. Figure 5 presents the measured EOF mobility of the threads with and without PVC coating and the conventional glass channel. Results showed that the EOF mobility of the thread-based microfluidic system was about four times higher than that of conventional glass-based microfluidic system. It is noted that the thread with PVC coating exhibited lower EOF mobility in compare with the thread without PVC coating due to the hydrophobic property of the PVC surface. A thinner PVC solution the lower the EOF mobility since the PVC solution might be soaked into the polyester thread due to capillary force. The dense PVC coating layer on the enzyme-doped thread greatly reduced the evaporation of the running buffer caused by Joule heating effect during CE separation. The eliminated buffer evaporation also reduced the heat accumulation on the thread such that the PVC coated thread could be operated at a higher separation electric field of 500 V/cm. Moreover, the PVC layer also formed a closed channel to prevent from the outside contamination.
FIG. 5.
Measured EOF mobility of the threads with and without PVC coating and conventional glass channel.
B. Enzyme concentration evaluation
It is important to convert all the low EC activity samples into EC detectable products by the adopted enzymatic reactions on the thread. The optimal concentration for each enzyme should be determined prior to the CEEC test. Figure 6(a) shows the measured current responses for detecting 10 mM urea using the thread modified with different concentrations of urease. The optimal enzyme concentration for modifying the thread was determined under the criterion of the minimum enzyme concentration for reaching a saturated current response. Results indicated that the optimized concentration for urease was 20 mg/ml. Figure 6(b) represents the measured current responses for measuring 10 mM glucose samples using a thread modified with different concentrations of glucose oxidase. Results indicated that the optimized concentration for GOD were 10 mg/ml. Note that the secondary enzyme of HRP was with the concentration of 5 mg/ml and 0.5 mM mediator (catechol).
FIG. 6.
Measured current responses for detecting urea and glucose with the threads coated with different concentrations of urease and GOD.
Since there were two enzymatic reaction products should be simultaneously detected using the EC electrodes, it was essential to determine the optimal detection potential of the biosamples prior to the potential state test. The redox potentials for each reaction product should be evaluated. Figure 7(a) illustrates the cyclic voltammogram for measuring the sample composed of 5 mM urea and 20 mg/ml urease. It is clear that there was an electrochemical peak around −0.3 V, corresponding to reduction peak of the ammonium ion. Figure 7(b) presents the CV response for measuring 5 mM glucose with the presence of 10 mg/ml GOD and 5 mg/ml HRP and 0.5 mM mediator. The CV result indicated that there was a significant redox peak around −0.2 to −0.3 V. On the other hand, the inset of Fig. 7(b) shows the CV response for detecting 5 mM GLU mixed the 10 mg/ml GOD without adding the HRP and mediator. In general, the product of GLU and GOD enzymatic reaction is hydrogen peroxide, which has a theoretical oxidation potential of +0.68 V. The CV response indicated that there was an oxidation potential around +0.6 to +0.8 V, corresponding to the oxidation of H2O2. This high EC potential made it impossible to simultaneously detect ammonium ion and hydrogen peroxide using potential state. Therefore, the second enzyme of HRP and the mediator of catechol were used to further react with hydrogen peroxide.43 These mediators have the common characteristics of small molecular weight, lower redox potential and reversibility. With this approach, a significant redox peak around −0.2 to −0.3 V was obtained such that these two enzymatic reaction products could be simultaneously detected using potential state. Although the set potential of −0.3 V might have great current response for detecting these two biosamples, it is also risky to activate the unknown species in serum and caused a higher noise level or signal interference. In this regards, the voltage level for the potential state measurement was set at −0.28 V. The method developed in the present study provides rapid and quantitative analyses for simultaneous detecting urea and glucose in blood.
FIG. 7.
Cyclic voltammogram for measuring (a) 5 mM of urea with the presence of 20 mg/ml urease. (b) 5 mM GLU with the presence of 10 mg/ml GOD and 5 mg/ml HRP and 0.5 mM mediator. Inset: without the HRP and mediator.
C. Electrophoresis of enzyme doped thread coated with PVC membrane
As described above, the PVC coating could eliminate the evaporation of the running buffer during electrophoresis. Figure 8 shows the electropherograms of measuring a mixed sample composed of 1 mM urea and 1 mM glucose using the threads without (Fig. 8(a)) and with (Fig. 8(b)) PVC coating. The applied potential for this potential state measurement was set at −0.28 V for detecting the reduction signals of the reaction products. The applied voltages for sample injection and separation were 300 V/cm for the thread without PVC coating since higher operation voltage might burn the thread due to Joule heat. Alternatively, the PVC coated thread could sustain a higher operation electric field up to 500 V/cm, resulting a faster separation and higher signal response for detecting the same sample. In addition, due to the well constrain, the coated thread appeared a lower and stable background noise during electrochemical detection in compare with the uncoated thread.
FIG. 8.
Electropherograms for on-site digesting and EC detecting 1 mM of glucose and urea using (a) thread without coating and (b) thread coated PVC membrane.
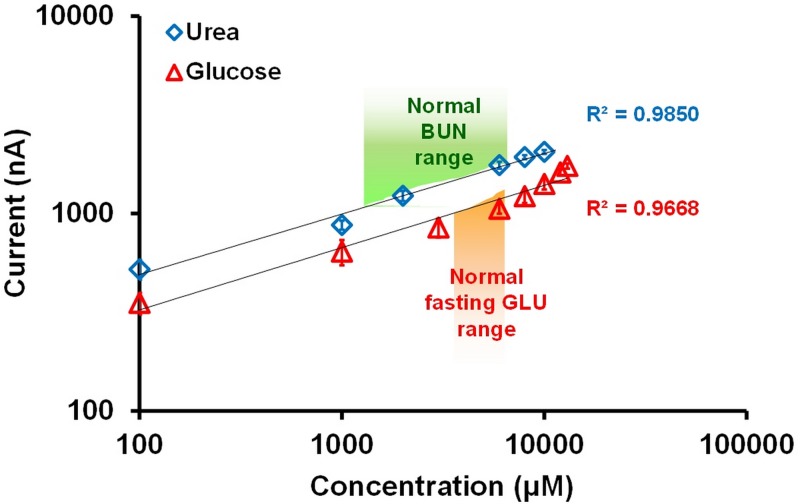
In general, the normal concentrations for BUN and blood GLU in serum are 1.78–7.12 mM and 3.89–6.11 mM, respectively. In order to evaluate the sensing performance of the system, mixed samples composed of urea and glucose with the concentrations of 0.1 mM–10.0 mM (R2 = 0.9850) and 0.1 mM–13.0 mM (R2 = 0.9668), respectively, were measured using the developed thread-based microfluidic system. Note that the enzyme concentrations for urease and GOD applied on the thread were 20 mg/ml and 10 mg/ml, respectively. As described above, 5 mg/ml of HRP and 0.5 mM catechol (mediator) were applied on the region above the EC electrodes for further reducing the detection potential of hydrogen peroxide. Figure 9 presents the measured current responses for EC detecting different concentrations of urea and glucose using the thread-based microfluidic system. The normal BUN and GLU concentrations in blood are indicated in Fig. 9. Results show that the developed system exhibited nice sensing performance for simultaneously detecting these two biomolecules. The reaction products of urea and glucose were successfully electrochemically detected with the developed system.
FIG. 9.
Measured current responses for detecting various concentrations of urea and glucose solutions.
D. Detection of human whole blood
Coagulation is one of the most important issues for whole blood detection using the microfluidic device. Coagulation greatly increases the viscosity of the blood such that it is difficult to flow in microchannel. In addition, the volume ratio for blood cell in whole blood is around 45%. The blood cells may block the thread-based microfluidic system. Although the structure of the thread bundle can be used as a filter to extract the blood cells, coagulation is still problematic for whole blood detection using the developed thread-based microfluidic system. In this regards, the 2-μl of RBC lysing buffer was applied on the filter zone of the thread-based microfluidic system. Blood cells could be lysed and the cell fragments could be simultaneously filtered without entering the CE-CE threads. Figure 10 presents the photo images showing the polyester thread at the filter zone after detecting whole blood without adding RBC lysing buffer (Fig. 10(a)) and added with 2-μl RBC lysing buffer (Fig. 10(b)). Significant blood coagulation was observed, and the thread-based liquid route was blocked if no lysing buffer was applied. On contrast, the thread with lysing buffer showed clear without blocking the thread.
FIG. 10.
Photo images showing the polyester thread at the filter zone after detecting whole blood (a) without adding RBC lysing buffer and (b) added with 2-μl RBC lysing buffer.
Figure 11 presents the electropherogram of two successive injection runs for detecting human whole blood with the developed thread-based microfluidic device. An injection potential of 300 V/cm was applied for 60 s to inject the sample into the thread, a separation potential of 500 V/cm was then applied for 80 s. Significant peaks corresponding to the reaction product of GLU and BUN as well as other ions in serum were successfully detected. The current response for the peaks corresponding to the ammonium ion and GLU reaction product were 2.02 and 1.41 μA, and the calculated BUN and GLU concentrations were 3.98 mM and 4.94 mM, respectively. The cations in the blood serum were also detected due to the conductivity change of the sample plugs at the EC electrode such that the EC currents were induced. The detecting results confirmed the feasibility of the developed system. The thread-based microfluidic system provides a simple yet high performance method for detecting BUN and GLU in whole blood.
FIG. 11.
Electropherogram for detecting human whole blood with the developed thread-based microfluidic device. The calculated BUN and GLU-AC in concentration for 3.87 mM and 4.94 mM. (Buffer: 0.1 mM MES, injection: 300 v/cm for 60 s, separation: 500 v/cm for 80 s, applied EC detection potential: −0.28 V).
IV. CONCLUSIONS
This study developed a novel technique to form a PVC coated thread doped with various enzymes of urease, glucose oxidase, and horseradish peroxidase for on-site bio-sample separation, bio-catalytic reaction, and electrochemical detection. Enzyme-modified polyester thread coated with a thin layer of PVC was used as the liquid route and the reactor for converting urea and glucose into ammonium ions and hydrogen peroxide. Multiple enzymes were directly applied onto the thread for enzymatic reactions without delicate pretreatment or a surface modification process. A thin PVC layer was coated on the enzyme-doped thread to further protect enzyme from contamination and to prevent the rapid evaporation of the running buffer due to the Joule heating effect. With this approach, a sealed microfluidic channel embedded with various enzymes could be easily produced. Results showed that the threads coated with PVC layer still exhibited high EOF mobility in comparison with that of the conventional glass microfluidic channel. Results also showed that the developed thread-based microfluidic system exhibit wide linear range for detecting GLU and BUN. For detecting GLU and BUN in whole blood, the blood cells were simultaneously lysed and filtered using the thread doped with RBC lysing buffer such that blood coagulation was prevented. The whole blood test result also indicated that the enzymatic reaction products of BLU and BUN could be successfully separated and detected with the EC electrodes downstream. The novel device developed in the present study provides a low-cost for rapid detection of bio-samples in whole blood.
References
- 1.Minerick A. R. and Ugaz V. M., Biomicrofluidics 1(2), 21504 (2007). 10.1063/1.2726342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manz A., Graber N., and Widmer H. á., Sens. Actuators, B 1(1), 244–248 (1990). 10.1016/0925-4005(90)80209-I [DOI] [Google Scholar]
- 3.Harrison D. J., Manz A., Fan Z., Luedi H., and Widmer H. M., Anal. Chem. 64(17), 1926–1932 (1992). 10.1021/ac00041a030 [DOI] [Google Scholar]
- 4.Jacobson S. C., Hergenroder R., Koutny L. B., and Ramsey J. M., Anal. Chem. 66(7), 1114–1118 (1994). 10.1021/ac00079a029 [DOI] [Google Scholar]
- 5.Graß B., Neyer A., Jöhnck M., Siepe D., Eisenbeiß F., Weber G., and Hergenröder R., Sens. Actuators, B 72(3), 249–258 (2001). 10.1016/S0925-4005(00)00643-2 [DOI] [Google Scholar]
- 6.Laugere F., Guijt R. M., Bastemeijer J., van der Steen G., Berthold A., Baltussen E., Sarro P., van Dedem G. W., Vellekoop M., and Bossche A., Anal. Chem. 75(2), 306–312 (2003). 10.1021/ac0157371 [DOI] [PubMed] [Google Scholar]
- 7.da Silva R. P., Lima A. W. O., and Serrano S. H., Anal. Chim. Acta 612(1), 89–98 (2008). 10.1016/j.aca.2008.02.017 [DOI] [PubMed] [Google Scholar]
- 8.Castaño-Álvarez M., Fernández-Abedul M. T., and Costa-García A., Electrophoresis 26(16), 3160–3168 (2005). 10.1002/elps.200500148 [DOI] [PubMed] [Google Scholar]
- 9.Zhu L., Yang R., Zhai J., and Tian C., Biosens. Bioelectron. 23(4), 528–535 (2007). 10.1016/j.bios.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Chiang T. Y. and Lin C. H., RSC Adv. 4(1), 379–385 (2014). 10.1039/C3RA45809A [DOI] [Google Scholar]
- 11.Meulemans A. and Delsenne F., J. Chromatogr. B: Biomed. Sci. Appl. 660(2), 401–404 (1994). 10.1016/0378-4347(94)00310-6 [DOI] [PubMed] [Google Scholar]
- 12.Chen C.-M., Chang G.-L., and Lin C.-H., J. Chromatogr. A 1194(2), 231–236 (2008). 10.1016/j.chroma.2008.04.056 [DOI] [PubMed] [Google Scholar]
- 13.Horng R.-H., Han P., Chen H.-Y., Lin K.-W., Tsai T.-M., and Zen J.-M., J. Micromech. Microeng. 15(1), 6 (2005). 10.1088/0960-1317/15/1/002 [DOI] [Google Scholar]
- 14.Hsieh B.-C., Hsiao H.-Y., Cheng T.-J., and Chen R. L., Anal. Chim. Acta 623(2), 157–162 (2008). 10.1016/j.aca.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 15.Li X., Ballerini D. R., and Shen W., Biomicrofluidics 6(1), 011301 (2012). 10.1063/1.3687398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin C.-H., Lee G.-B., Lin Y.-H., and Chang G.-L., J. Micromech. Microeng. 11(6), 726 (2001). 10.1088/0960-1317/11/6/316 [DOI] [Google Scholar]
- 17.Jia Z.-J., Fang Q., and Fang Z.-L., Anal. Chem. 76(18), 5597–5602 (2004). 10.1021/ac0494477 [DOI] [PubMed] [Google Scholar]
- 18.Lin C.-H., Tsai C.-H., and Fu L.-M., J. Micromech. Microeng. 15(5), 935 (2005). 10.1088/0960-1317/15/5/006 [DOI] [Google Scholar]
- 19.Zeng Y., Chen H., Pang D.-W., Wang Z.-L., and Cheng J.-K., Anal. Chem. 74(10), 2441–2445 (2002). 10.1021/ac0110247 [DOI] [PubMed] [Google Scholar]
- 20.Wang J., Chatrathi M. P., Tian B., and Polsky R., Anal. Chem. 72(11), 2514–2518 (2000). 10.1021/ac991489l [DOI] [PubMed] [Google Scholar]
- 21.Ruecha N., Siangproh W., and Chailapakul O., Talanta 84(5), 1323–1328 (2011). 10.1016/j.talanta.2011.02.040 [DOI] [PubMed] [Google Scholar]
- 22.Ballerini D. R., Li X., and Shen W., Microfluid. Nanofluid. 13(5), 769–787 (2012). 10.1007/s10404-012-0999-2 [DOI] [Google Scholar]
- 23.Wang P. P., Ge L., Yan M., Song X. R., Ge S. G., and Yu J. H., Biosens. Bioelectron. 32(1), 238–243 (2012). 10.1016/j.bios.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 24.Kao P. K. and Hsu C. C., Anal. Chem. 86(17), 8757–8762 (2014). 10.1021/ac501945q [DOI] [PubMed] [Google Scholar]
- 25.Coltro W. K. T., Cheng C. M., Carrilho E., and de Jesus D. P., Electrophoresis 35(16), 2309–2324 (2014). 10.1002/elps.201400006 [DOI] [PubMed] [Google Scholar]
- 26.Martinez A. W., Phillips S. T., Nie Z., Cheng C.-M., Carrilho E., Wiley B. J., and Whitesides G. M., Lab Chip 10(19), 2499–2504 (2010). 10.1039/c0lc00021c [DOI] [PubMed] [Google Scholar]
- 27.Hsu C. K., Huang H. Y., Chen W. R., Nishie W., Ujiie H., Natsuga K., Fan S. T., Wang H. K., Lee J. Y. Y., Tsai W. L., Shimizu H., and Cheng C. M., Anal. Chem. 86(9), 4605–4610 (2014). 10.1021/ac500835k [DOI] [PubMed] [Google Scholar]
- 28.Cai L. F., Xu C. X., Lin S. H., Luo J. T., Wu M. D., and Yang F., Biomicrofluidics 8(5), 056504 (2014). 10.1063/1.4898096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballerini D. R., Li X., and Shen W., Biomicrofluidics 5, 014105 (2011). 10.1063/1.3567094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhandari P., Narahari T., and Dendukuri D., Lab Chip 11(15), 2493–2499 (2011). 10.1039/c1lc20373h [DOI] [PubMed] [Google Scholar]
- 31.Dungchai W., Chailapakul O., and Henry C. S., Analyst 136(1), 77–82 (2011). 10.1039/C0AN00406E [DOI] [PubMed] [Google Scholar]
- 32.Li X., Tian J., Garnier G., and Shen W., Colloids Surf., B 76(2), 564–570 (2010). 10.1016/j.colsurfb.2009.12.023 [DOI] [PubMed] [Google Scholar]
- 33.Lin S. C., Hsu M. Y., Kuan C. M., Wang H. K., Chang C. L., Tseng F. G., and Cheng C. M., Sci. Rep.-UK 4, 6976 (2014). 10.1038/srep06976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu J., Wang S. Q., Wang L., Li F., Pingguan-Murphy B., Lu T. J., and Xu F., Biosens. Bioelectron. 54, 585–597 (2014). 10.1016/j.bios.2013.10.075 [DOI] [PubMed] [Google Scholar]
- 35.Cubaud T. and Mason T. G., New J. Phys. 11, 075029 (2009). 10.1088/1367-2630/11/7/075029 [DOI] [Google Scholar]
- 36.Yang Y. A., Kuo W. C., and Lin C. H., in Proceedings of the IEEE 27th International Conference on Micro Electro Mechanical Systems (MEMS) (2014), pp. 959–962. [Google Scholar]
- 37.Lin Y.-H., Wang S.-H., Wu M.-H., Pan T.-M., Lai C.-S., Luo J.-D., and Chiou C.-C., Biosens. Bioelectron. 43, 328–335 (2013). 10.1016/j.bios.2012.12.053 [DOI] [PubMed] [Google Scholar]
- 38.Wang J., Chatrathi M. P., and Tian B., Anal. Chem. 73(6), 1296–1300 (2001). 10.1021/ac001205t [DOI] [PubMed] [Google Scholar]
- 39.Ferri T., Maida S., Poscia A., and Santucci R., Electroanalysis 13(14), 1198–1202 (2001). [DOI] [Google Scholar]
- 40.Garguilo M. G., Huynh N., Proctor A., and Michael A. C., Anal. Chem. 65(5), 523–528 (1993). 10.1021/ac00053a007 [DOI] [PubMed] [Google Scholar]
- 41.Wang G., Xu J.-J., Chen H.-Y., and Lu Z.-H., Biosens. Bioelectron. 18(4), 335–343 (2003). 10.1016/S0956-5663(02)00152-5 [DOI] [PubMed] [Google Scholar]
- 42.Wei Y.-C., Fu L.-M., and Lin C.-H., Microfluid. Nanofluid. 14(3–4), 583–590 (2013). 10.1007/s10404-012-1076-6 [DOI] [Google Scholar]
- 43.Saleh Ahammad A., J. Biosens. Bioelectron. S9, 001 (2013). 10.4172/2155-6210.S9-001 [DOI] [Google Scholar]