Abstract
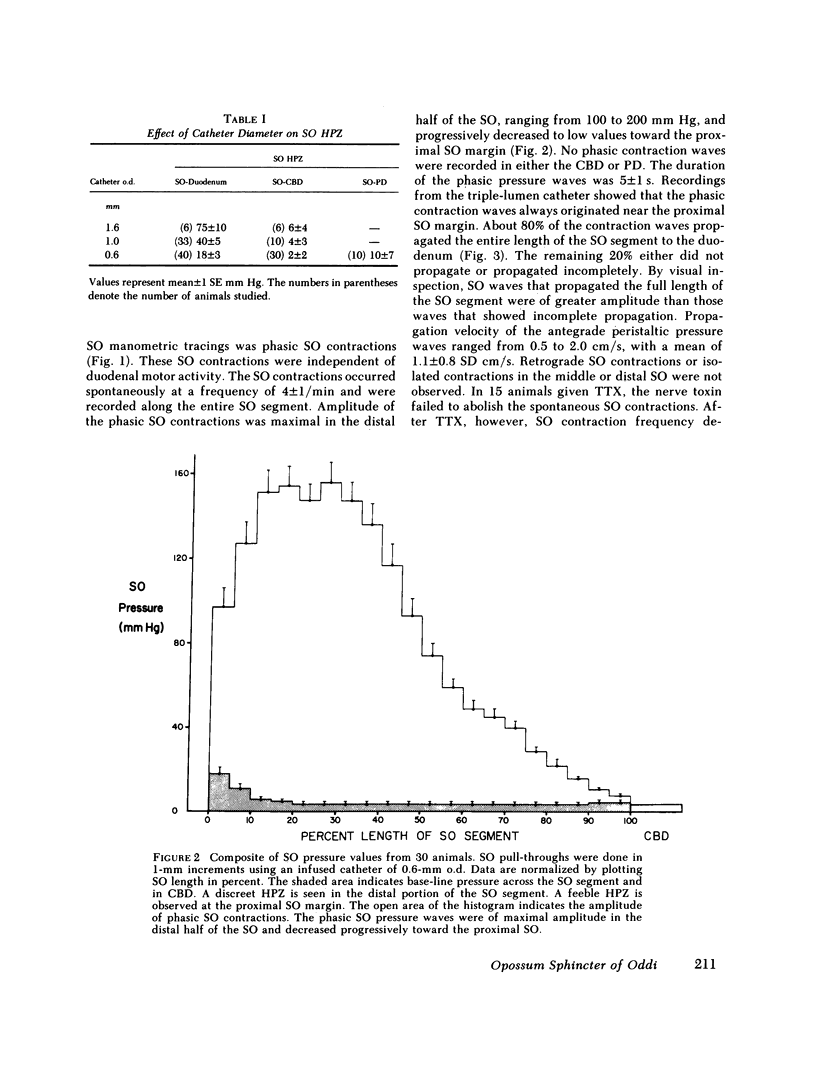
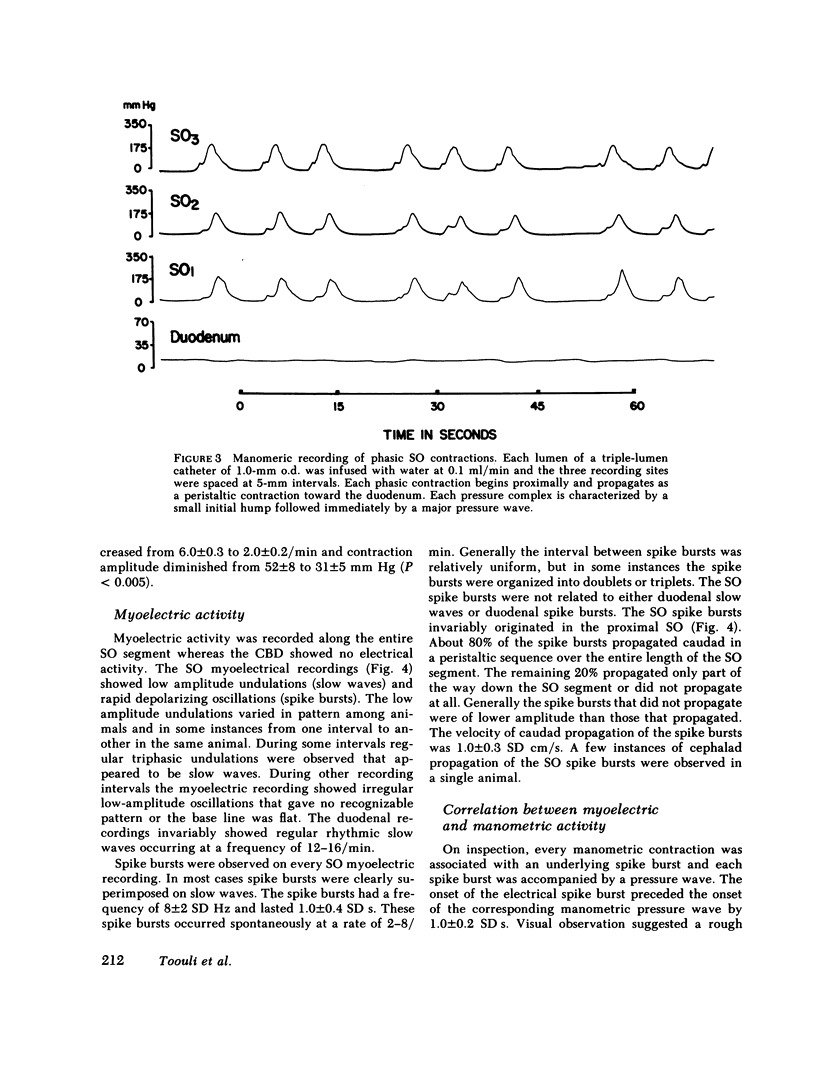
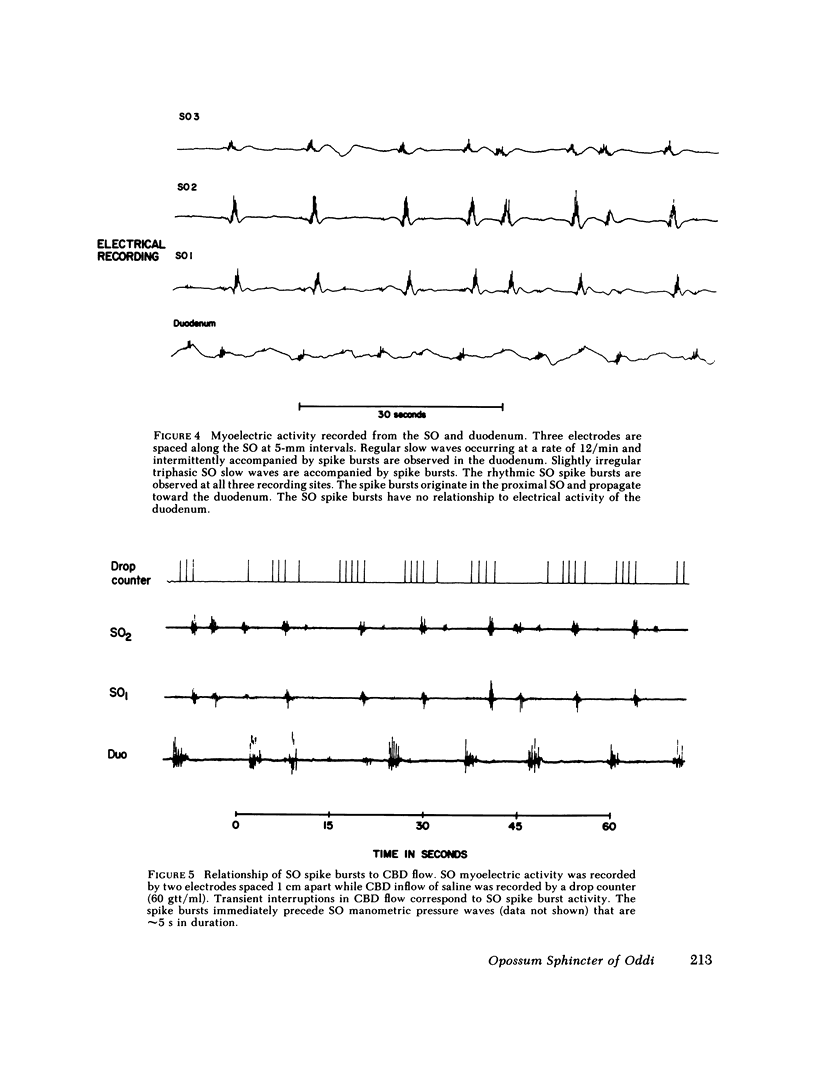
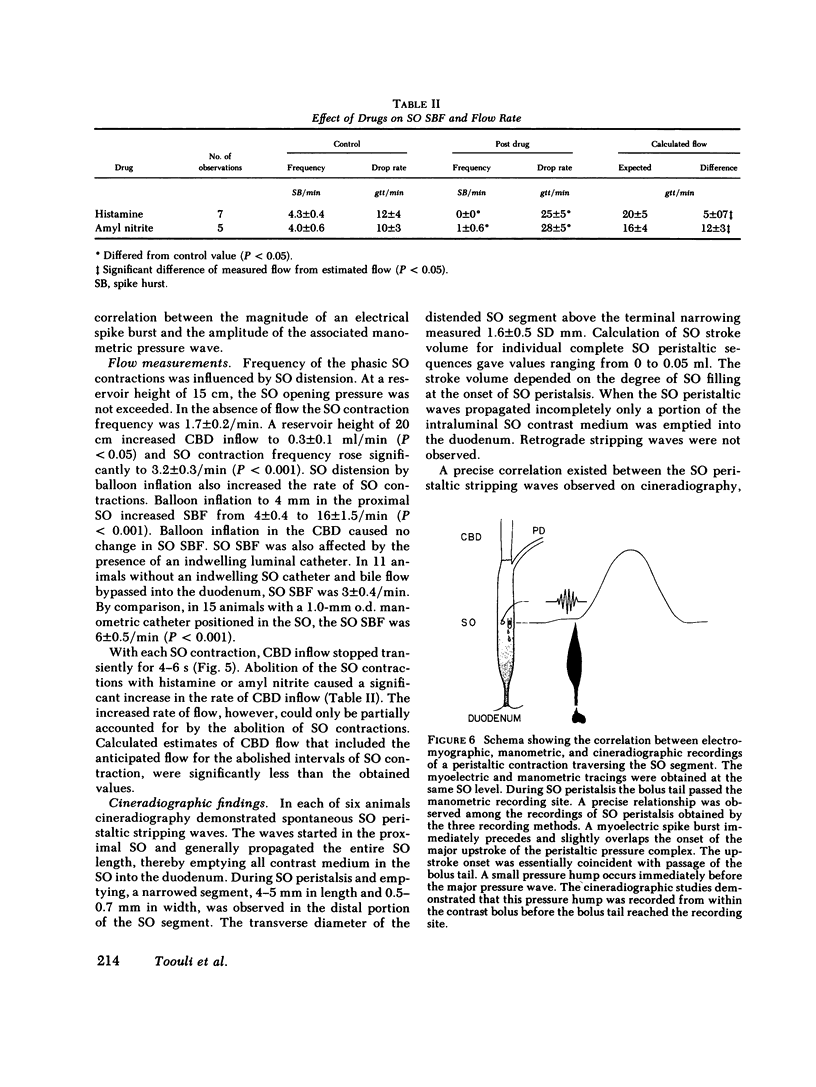
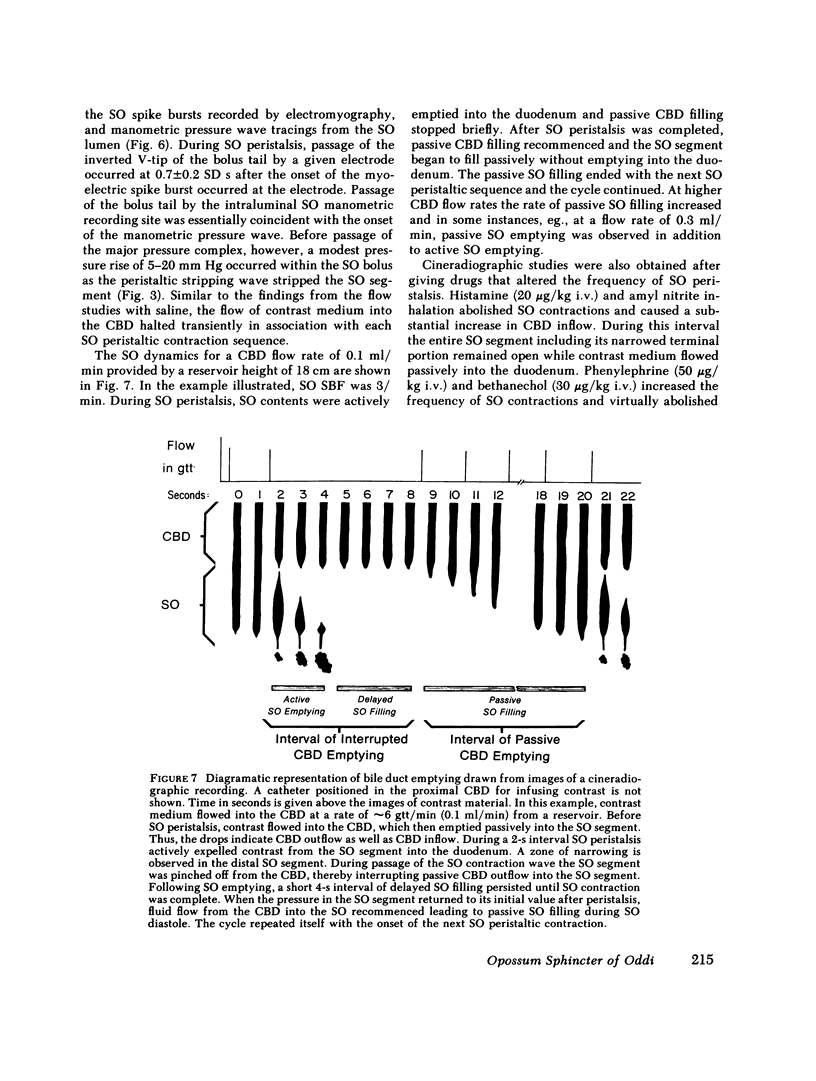
We studied the opossum sphincter of Oddi (SO) because in this species the SO is approximately 3 cm in length and its extraduodenal location permits recording of motor activity with negligible interference from duodenal motor activity. The SO segment of 120 animals was evaluated by one or more of the following: (a) intraluminal manometry; (b) electromyography; (c) common bile duct (CBD) flow monitored by a drop counter; (d) cineradiography of intraductal contrast medium; and (e) histologic examination. SO pull-throughs using an infused catheter of 0.6-mm o.d. invariably showed a high pressure zone (HPZ) of 18 +/- 3 SE mm Hg in the terminal 4-5 mm of the SO segment. This HPZ had a narrow lumen, 0.5-0.7 mm in diam, and prominent circular muscle. The HPZ in the terminal SO had both active and passive components. HPZ with minimal amplitude and a paucity of underlying smooth muscle were present inconstantly at the junction of the SO segment with the CBD and pancreatic duct, respectively. The dominant feature of the SO segment was rhythmic peristaltic contractions that originated in the proximal SO and propagated toward the duodenum. These contractions occurred spontaneously at a rate of 2-8/min, ranged up to 200 mm Hg in magnitude, had a duration of approximately 5 s and were not abolished by tetrodotoxin. Concurrent myoelectric and manometric recordings showed that each phasic contraction was immediately preceded by an electrical spike burst. Simultaneous recordings of cineradiography, CBD inflow of contrast medium, SO manometry, and SO electromyography indicated that rhythmic peristaltic contractions stripped contrast medium from the SO into the duodenum. During SO systole, CBD emptying was transiently interrupted, whereas SO filling occurred during the diastolic interval between SO peristaltic contractions. SO distention increased the frequency of SO peristalsis. We conclude that (a) the dominant feature of the opossum SO is rhythmic peristaltic contractions that originate in the proximal SO and propagate toward the duodenum; (b) these forceful SO peristaltic contractions are myogenic in origin and serve as a peristaltic pump that actively empties the SO segment; (c) CBD outflow occurs passively during SO diastole, but is interrupted transiently during each SO peristaltic contraction; and (d) a short HPZ with active as well as passive components exists in the distal SO segment and acts as a variable resistor to SO outflow.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndorfer R. C., Stef J. J., Dodds W. J., Linehan J. H., Hogan W. J. Improved infusion system for intraluminal esophageal manometry. Gastroenterology. 1977 Jul;73(1):23–27. [PubMed] [Google Scholar]
- BOYDEN E. A. The anatomy of the choledochoduodenal junction in man. Surg Gynecol Obstet. 1957 Jun;104(6):641–652. [PubMed] [Google Scholar]
- Becker J. M., Duff W. M., Moody F. G. Myoelectric control of gastrointestinal and biliary motility: a review. Surgery. 1981 Apr;89(4):466–477. [PubMed] [Google Scholar]
- Becker J. M., Moody F. G., Zinsmeister A. R. Effect of gastrointestinal hormones on the biliary sphincter of the opossum. Gastroenterology. 1982 Jun;82(6):1300–1307. [PubMed] [Google Scholar]
- Behar J., Biancani P. Effect of cholecystokinin and the octapeptide of cholecystokinin on the feline sphincter of Oddi and gallbladder. Mechanisms of action. J Clin Invest. 1980 Dec;66(6):1231–1239. doi: 10.1172/JCI109974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr-Locke D. L., Gregg J. A. Endoscopic manometry of pancreatic and biliary sphincter zones in man. Basal results in healthy volunteers. Dig Dis Sci. 1981 Jan;26(1):7–15. doi: 10.1007/BF01307970. [DOI] [PubMed] [Google Scholar]
- Csendes A., Kruse A., Funch-Jensen P., Oster M. J., Ornsholt J., Amdrup E. Pressure measurements in the biliary and pancreatic duct systems in controls and in patients with gallstones, previous cholecystectomy, or common bile duct stones. Gastroenterology. 1979 Dec;77(6):1203–1210. [PubMed] [Google Scholar]
- Dent J., Dodds W. J., Hogan W. J., Arndorfer R. C., Teeter B. C. Effect of cholecystokinin-octapeptide on opossum lower esophageal sphincter. Am J Physiol. 1980 Sep;239(3):G230–G235. doi: 10.1152/ajpgi.1980.239.3.G230. [DOI] [PubMed] [Google Scholar]
- Dodds W. J., Stewart E. T., Hodges D., Zboralske F. F. Movement of the feline esophagus associated with respiration and peristalsis. An evaluation using tantalum markers. J Clin Invest. 1973 Jan;52(1):1–13. doi: 10.1172/JCI107152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen J. E., Hogan W. J., Dodds W. J., Stewart E. T., Arndorfer R. C. Intraluminal pressure recording from the human sphincter of Oddi. Gastroenterology. 1980 Feb;78(2):317–324. [PubMed] [Google Scholar]
- Goyal R. K., Gidda J. S. Relation between electrical and mechanical activity in esophageal smooth muscle. Am J Physiol. 1981 Apr;240(4):G305–G311. doi: 10.1152/ajpgi.1981.240.4.G305. [DOI] [PubMed] [Google Scholar]
- Honda R., Toouli J., Dodds W. J., Sarna S., Hogan W. J., Itoh Z. Relationship of sphincter of Oddi spike bursts to gastrointestinal myoelectric activity in conscious opossums. J Clin Invest. 1982 Apr;69(4):770–778. doi: 10.1172/JCI110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIOKA T. Electromyographic study of the choledochoduodenal junction and duodenal wall muscle. Tohoku J Exp Med. 1959 Jun 25;70:73–84. doi: 10.1620/tjem.70.73. [DOI] [PubMed] [Google Scholar]
- LaMorte W. W., Schoetz D. J., Jr, Birkett D. H., Williams L. F., Jr The role of the gallbladder in the pathogenesis of cholesterol gallstones. Gastroenterology. 1979 Sep;77(3):580–592. [PubMed] [Google Scholar]
- Lin T. M. Actions of gastrointestinal hormones and related peptides on the motor function of the biliary tract. Gastroenterology. 1975 Oct;69(4):1006–1022. [PubMed] [Google Scholar]
- Ono K., Watanabe N., Suzuki K., Tsuchida H., Sugiyama Y., Abo M. Bile flow mechanism in man. Arch Surg. 1968 Jun;96(6):869–874. doi: 10.1001/archsurg.1968.01330240015004. [DOI] [PubMed] [Google Scholar]
- Persson C. G. Dual effects on the sphincter of Oddi and gallbladder induced by stimulation of the right great splanchnic nerve. Acta Physiol Scand. 1973 Mar;87(3):334–343. doi: 10.1111/j.1748-1716.1973.tb05397.x. [DOI] [PubMed] [Google Scholar]
- Scott G. W., Smallwood R. E., Rowlands S. Flow through the bile duct after cholecystectomy. Surg Gynecol Obstet. 1975 Jun;140(6):912–918. [PubMed] [Google Scholar]
- Shaw B. W., Jr, Becker J. M., Moody F. G. Histaminergic responses of biliary sphincter in opossum. Surg Forum. 1979;30:400–402. [PubMed] [Google Scholar]
- Strasberg S. M., Redinger R. N., Dorn B. C., Small D. M., Egdahl R. H. Effects of alteration of biliary pressure on bile composition--a method for study: primate biliary physiology. V. Gastroenterology. 1971 Sep;61(3):357–362. [PubMed] [Google Scholar]
- Toouli J., Dodds W. J., Honda R., Hogan W. J. Effect of histamine on motor function of opossum sphincter of Oddi. Am J Physiol. 1981 Aug;241(2):G122–G128. doi: 10.1152/ajpgi.1981.241.2.G122. [DOI] [PubMed] [Google Scholar]
- Toouli J., Geenen J. E., Hogan W. J., Dodds W. J., Arndorfer R. C. Sphincter of Oddi motor activity: a comparison between patients with common bile duct stones and controls. Gastroenterology. 1982 Jan;82(1):111–117. [PubMed] [Google Scholar]
- Watts J. M., Dunphy J. E. The role of the common bile duct in biliary dynamics. Surg Gynecol Obstet. 1966 Jun;122(6):1207–1218. [PubMed] [Google Scholar]
- White T. T., Waisman H., Hopton D., Kavlie H. Radiomanometry, flow rates, and cholangiography in the evaluation of common bile duct disease. A study of 220 cases. Am J Surg. 1972 Jan;123(1):73–79. doi: 10.1016/0002-9610(72)90314-5. [DOI] [PubMed] [Google Scholar]