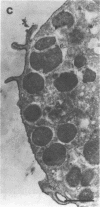
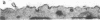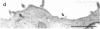Abstract
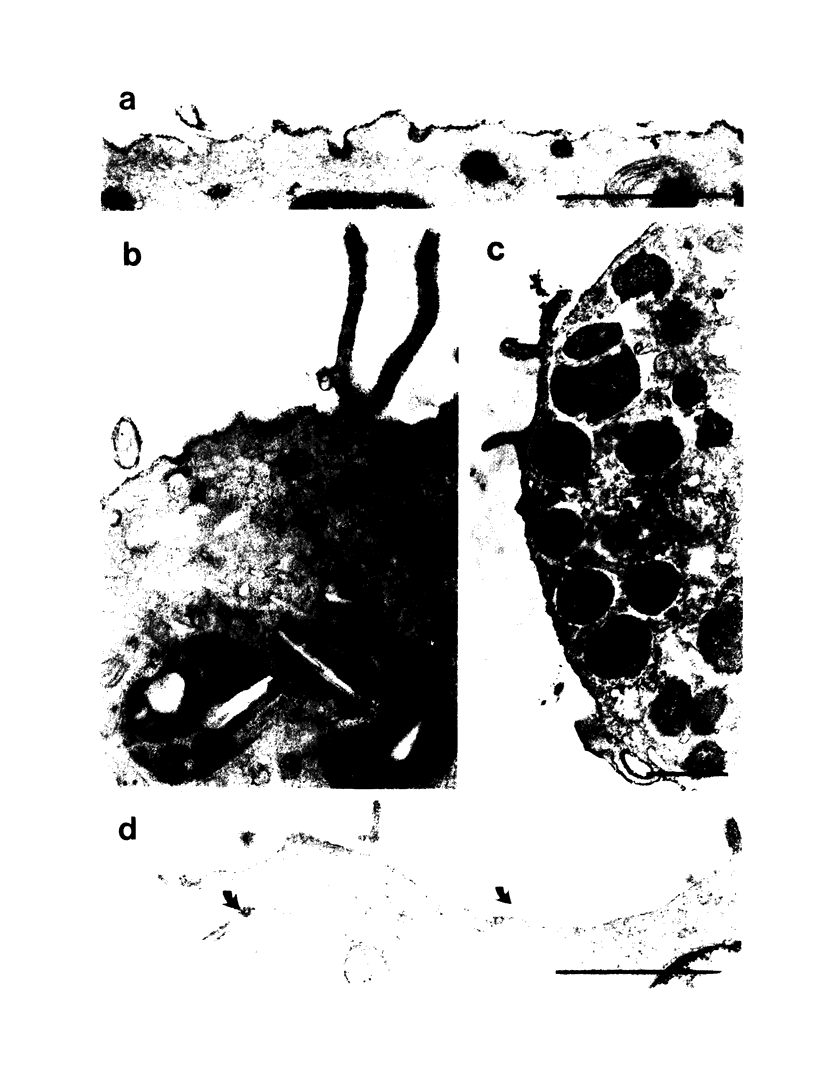
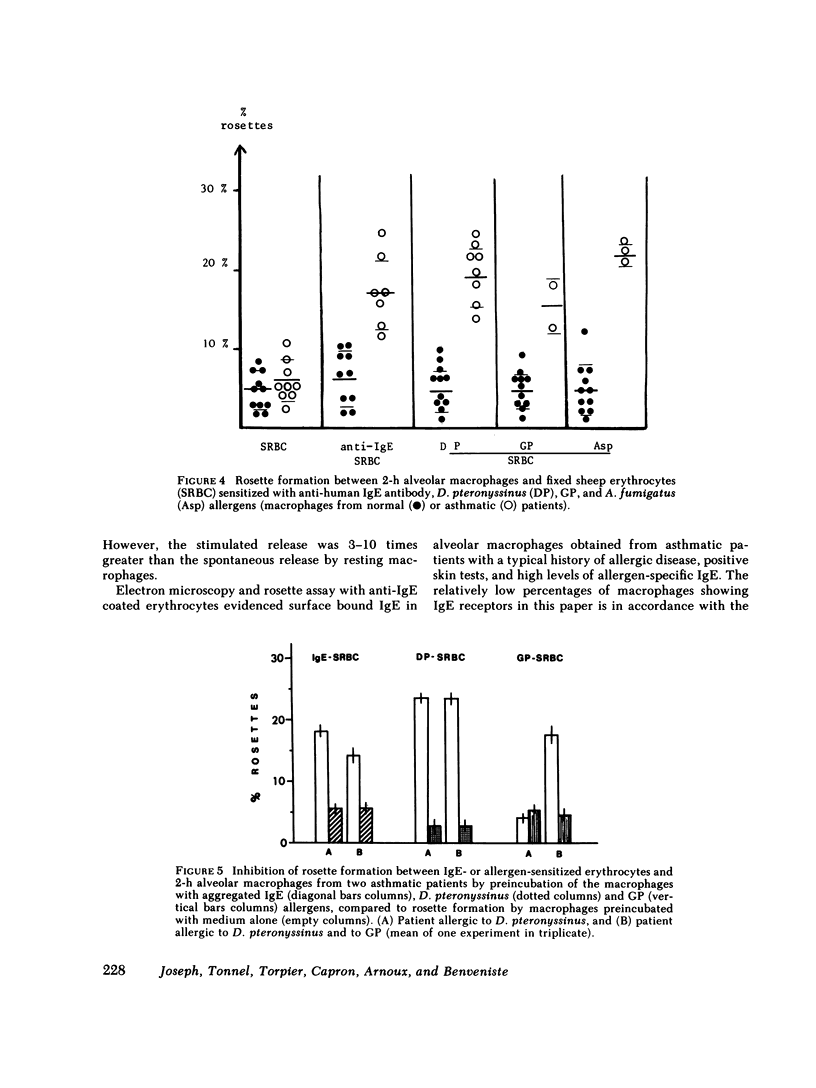
Alveolar macrophages from nonatopic donors were passively sensitized with allergen-specific IgE antibody from the serum of asthmatic patients. A selective release of 4-8% of the lysosomal beta-glucuronidase of these cells occurred within 30 min of contact with the related allergen or with anti-human IgE antibody, in the absence of any mast or basophil cells. The cell reactivity was dependent on the interaction of macrophages with IgE, as shown by the disappearance of the allergen-induced enzyme release after heating or IgE-immune adsorption of the sensitizing serum, but not after IgG-adsorption. Alveolar macrophages from asthmatic patients behaved similarly to passively sensitized normal macrophages. Contact with the related allergen or with anti-IgE antibody induced the same percentage of enzyme release, demonstrating that these cells possess allergen-specific IgE bound on their surface. 18% of them formed rosettes with anti-IgE-coated sheep erythrocytes, and 15-22% with allergen-coated erythrocytes, but lost this property after preincubation with the specific allergen. The presence of IgE-specific receptors on the macrophage surface was demonstrated both at the ultrastructural level with immunoperoxidase labeling, and at low magnification by the formation of 15-18% rosettes with human IgE-coated erythrocytes. The formation of such rosettes was inhibited after incubation of alveolar phagocytes with aggregated myeloma IgE. On the basis of these observations, the participation of the alveolar macrophages in IgE-mediated pulmonary hypersensitivity must be considered. Its precise involvement requires, however, further investigations.
Full text
PDF



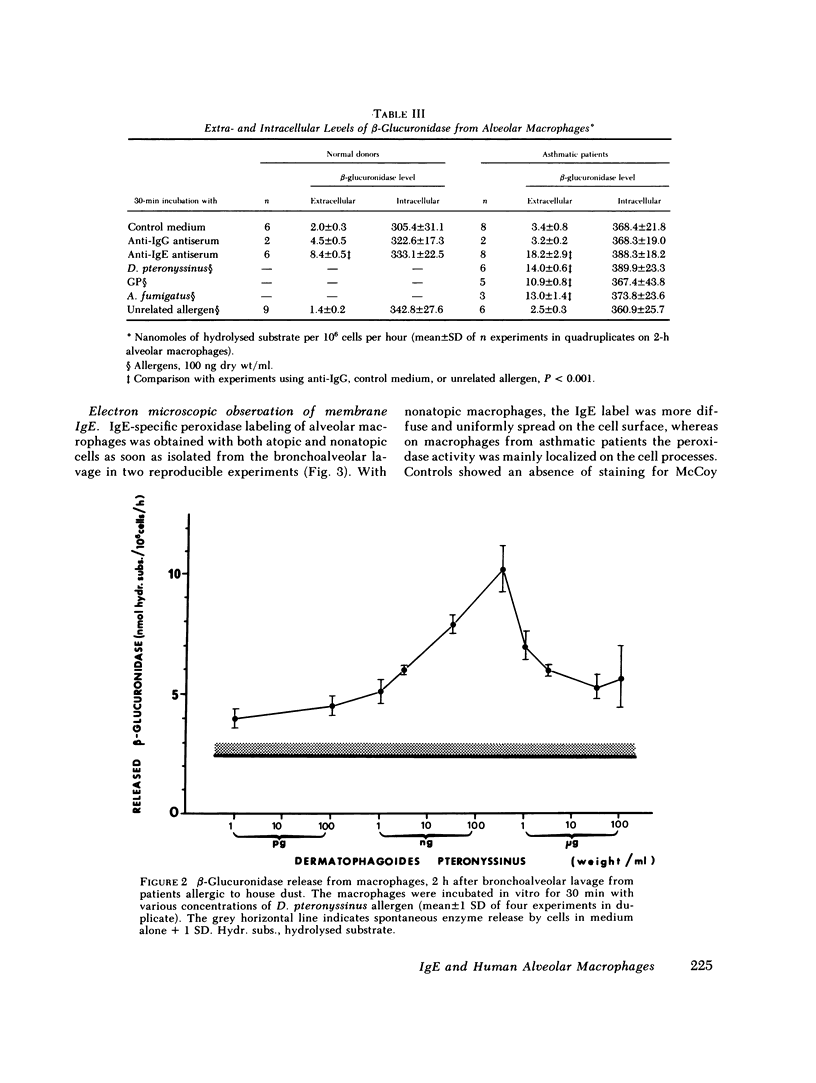



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman N. R., Beebe J. R., Schidlovsky G. Lysosomal enzyme release from guinea pig alveolar cells: biochemical and morphologic observations. Cytobios. 1977;20(77):7–20. [PubMed] [Google Scholar]
- Arnoux B., Duval D., Benveniste J. Release of platelet-activating factor (PAF-acether) from alveolar macrophages by the calcium ionophore A23187 and phagocytosis. Eur J Clin Invest. 1980 Dec;10(6):437–441. doi: 10.1111/j.1365-2362.1980.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Boltz-Nitulescu G., Plummer J. M., Spiegelberg H. L. Fc receptors for IgE on mouse macrophages and macrophage-like cell lines. J Immunol. 1982 May;128(5):2265–2268. [PubMed] [Google Scholar]
- Boltz-Nitulescu G., Spiegelberg H. L. Receptors specific for IgE on rat alveolar and peritoneal macrophages. Cell Immunol. 1981 Mar 15;59(1):106–114. doi: 10.1016/0008-8749(81)90438-x. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Bazin H. Specific IgE antibodies in immune adherence of normal macrophages to Schistosoma mansoni schistosomules. Nature. 1975 Feb 6;253(5491):474–475. doi: 10.1038/253474a0. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Joseph M., Rousseaux R., Capron M., Bazin H. Interaction between IgE complexes and macrophages in the rat: a new mechanism of macrophage activation. Eur J Immunol. 1977 May;7(5):315–322. doi: 10.1002/eji.1830070515. [DOI] [PubMed] [Google Scholar]
- Czarnetzki B. M., Behrendt H. Studies on the in vitro development of rat peritoneal mast cells. Immunobiology. 1981;159(3):256–268. doi: 10.1016/S0171-2985(81)80084-8. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaint J. P., Capron A., Joseph M., Bazin H. Cytophilic binding of IgE to the macrophage. II. Immunologic release of lysosomal enzyme from macrophages by IgE and anti-IgE in the rat: a new mechanism of macrophage activation. Cell Immunol. 1979 Aug;46(1):24–34. doi: 10.1016/0008-8749(79)90242-9. [DOI] [PubMed] [Google Scholar]
- Dessaint J. P., Torpier G., Capron M., Bazin H., Capron A. Cytophilic binding of IgE to the macrophage. I. Binding characteristics of IgE on the surface of macrophages in the rat. Cell Immunol. 1979 Aug;46(1):12–23. doi: 10.1016/0008-8749(79)90241-7. [DOI] [PubMed] [Google Scholar]
- Dessaint J. P., Waksman B. H., Metzger H., Capron A. Cytophilic binding of IgE to the macrophage. III. Involvement of cyclic GMP and calcium in macrophage activation by dimeric or aggregated rat myeloma IgE. Cell Immunol. 1980 May;51(2):280–292. doi: 10.1016/0008-8749(80)90260-9. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Haque A., Joseph M., Ouaissi M. A., Capron M., Capron A. IgE antibody-mediated cytotoxicity of rat macrophages against microfilaria of Dipetalonema citeae in vitro. Clin Exp Immunol. 1980 Jun;40(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. L., Kelman J. A., Crystal R. G. Activation of alveolar macrophage collagenase by a neutral protease secreted by the same cell. Nature. 1976 Dec 23;264(5588):772–774. doi: 10.1038/264772a0. [DOI] [PubMed] [Google Scholar]
- Joseph M., Capron A., Butterworth A. E., Sturrock R. F., Houba V. Cytotoxicity of human and baboon mononuclear phagocytes against schistosomula in vitro: induction by immune complexes containing IgE and Schistosoma mansoni antigens. Clin Exp Immunol. 1978 Jul;33(1):48–56. [PMC free article] [PubMed] [Google Scholar]
- Joseph M., Dessaint J. P., Capron A. Characteristics of macrophage cytotoxicity induced by IgE immune complexes. Cell Immunol. 1977 Dec;34(2):247–258. doi: 10.1016/0008-8749(77)90247-7. [DOI] [PubMed] [Google Scholar]
- Joseph M., Tonnel A. B., Capron A., Voisin C. Enzyme release and superoxide anion production by human alveolar macrophages stimulated with immunoglobulin E. Clin Exp Immunol. 1980 May;40(2):416–422. [PMC free article] [PubMed] [Google Scholar]
- Lynch S. M., Austen K. F., Wasserman S. I. Release of arylsulfatase A but not B from rat mast cells by noncytolytic secretory stimuli. J Immunol. 1978 Oct;121(4):1394–1399. [PubMed] [Google Scholar]
- Melewicz F. M., Kline L. E., Cohen A. B., Spiegelberg H. L. Characterization of Fc receptors for IgE on human alveolar macrophages. Clin Exp Immunol. 1982 Aug;49(2):364–370. [PMC free article] [PubMed] [Google Scholar]
- Melewicz F. M., Spiegelberg H. L. Fc receptors for IgE on a subpopulation of human peripheral blood monocytes. J Immunol. 1980 Sep;125(3):1026–1031. [PubMed] [Google Scholar]
- Melewicz F. M., Zeiger R. S., Mellon M. H., O'Connor R. D., Spiegelberg H. L. Increased peripheral blood monocytes with Fc receptors for IgE in patients with severe allergic disorders. J Immunol. 1981 Apr;126(4):1592–1595. [PubMed] [Google Scholar]
- Pestel J., Joseph M., Dessaint J. P., Capron A. Macrophage triggering by aggregated immunoglobulins. I. Delayed effect of IgG aggregates or immune complexes. J Immunol. 1981 May;126(5):1887–1891. [PubMed] [Google Scholar]
- Rodriguez R. J., White R. R., Senior R. M., Levine E. A. Elastase release from human alveolar macrophages: comparison between smokers and nonsmokers. Science. 1977 Oct 21;198(4314):313–314. doi: 10.1126/science.910131. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Segal D. M., Taurog J. D., Metzger H. Dimeric immunoglobulin E serves as a unit signal for mast cell degranulation. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois P. Release of mediators from free bronchoalveolar cells: effect of ionophore A23187 and antigen challenge. J Immunopharmacol. 1980;2(4):543–551. doi: 10.3109/08923978009026411. [DOI] [PubMed] [Google Scholar]
- Sirois P., Rola-Pleszczynski M., Bégin R. Phospholipase A activity and prostaglandin release from alveolar macrophages exposed to asbestos. Prostaglandins Med. 1980 Jul;5(1):31–37. doi: 10.1016/0161-4630(80)90088-9. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Melewicz F. M. Fc receptors specific for IgE on subpopulations of human lymphocytes and monocytes. Clin Immunol Immunopathol. 1980 Mar;15(3):424–433. doi: 10.1016/0090-1229(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Valone F. H., Franklin M., Sun F. F., Goetzl E. J. Alveolar macrophage lipoxygenase products of arachidonic acid: isolation and recognition as the predominant constituents of the neutrophil chemotactic activity elaborated by alveolar macrophages. Cell Immunol. 1980 Sep 1;54(2):390–401. doi: 10.1016/0008-8749(80)90219-1. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Chignard M., Benveniste J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur J Pharmacol. 1980 Jul 25;65(2-3):185–192. doi: 10.1016/0014-2999(80)90391-x. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Prancan A. V., Chignard M., Benveniste J. Platelet-lung in vivo interactions: an artifact of a multi-purpose model? Haemostasis. 1979;8(3-5):171–182. doi: 10.1159/000214309. [DOI] [PubMed] [Google Scholar]