Abstract
Aim
To agree a draft pressure ulcer risk factor Minimum Data Set to underpin the development of a new evidenced-based Risk Assessment Framework.
Background
A recent systematic review identified the need for a pressure ulcer risk factor Minimum Data Set and development and validation of an evidenced-based pressure ulcer Risk Assessment Framework. This was undertaken through the Pressure UlceR Programme Of reSEarch (RP-PG-0407-10056), funded by the National Institute for Health Research and incorporates five phases. This article reports phase two, a consensus study.
Design
Consensus study.
Method
A modified nominal group technique based on the Research and Development/University of California at Los Angeles appropriateness method. This incorporated an expert group, review of the evidence and the views of a Patient and Public Involvement service user group. Data were collected December 2010–December 2011.
Findings
The risk factors and assessment items of the Minimum Data Set (including immobility, pressure ulcer and skin status, perfusion, diabetes, skin moisture, sensory perception and nutrition) were agreed. In addition, a draft Risk Assessment Framework incorporating all Minimum Data Set items was developed, comprising a two stage assessment process (screening and detailed full assessment) and decision pathways.
Conclusion
The draft Risk Assessment Framework will undergo further design and pre-testing with clinical nurses to assess and improve its usability. It will then be evaluated in clinical practice to assess its validity and reliability. The Minimum Data Set could be used in future for large scale risk factor studies informing refinement of the Risk Assessment Framework.
Keywords: consensus study, nursing, pressure ulcer, risk factors, tissue viability
Why is this research or review needed?
There are limitations associated with development methodologies and content validity for risk assessment scales and a lack of agreement of the risk factors required to adequately identify risk.
A recent systematic review highlighted the need to agree a pressure ulcer risk factor Minimum Data Set to facilitate meta-analysis and underpin risk assessment.
What are the key findings?
Consensus methods facilitated agreement of a pressure ulcer risk factor Minimum Data Set incorporating nine risk factors and associated assessment items.
The development of a draft pressure ulcer Risk Assessment Framework incorporating the Minimum Data Set in preparation for pre-testing and clinical evaluation.
How should the findings be used to influence policy/practice/research/education?
The Minimum Data Set could be used by healthcare professionals to record key pressure ulcer risk factors, facilitating clinical risk assessment, case mix adjustment, multivariable analyses and future meta-analysis.
The draft pressure ulcer Risk Assessment Framework is being further evaluated to assess its reliability and validity in preparation for eventual long-term implementation in clinical practice.
Introduction
Pressure Ulcers (PUs) are associated with ill health and poor mobility and have a detrimental effect on patients’ quality of life (Gorecki et al. 2009, 2012). They are defined as ‘localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear’ and are numerically classified according to the severity of the ulcer and the tissue layers involved (National Pressure Ulcer Advisory Panel/European Pressure Ulcer Advisory Panel (NPUAP/EPUAP 2009). PUs remain a substantial problem (Schoonhoven et al. 2007, Vowden & Vowden 2009, Pieper 2012) and present a financial burden to healthcare organisations worldwide (Severens et al. 2002, Bennett et al. 2004, Schuurman et al. 2009, Berlowitz et al. 2011, Dealey et al. 2012).
Background
In clinical practice PU risk assessment is considered key to prevention (AHCPR 1992, NICE 2003, NPUAP/EPUAP 2009) and despite limited evidence of clinical effectiveness risk assessment tools/scales are routinely used. These incorporate the assessment of PU risk factors and usually use a scoring system to allocate the patients level of risk, e.g. ‘high risk, moderate risk, at risk’.
There are several limitations associated with development methodologies, content validity and evaluation of PU risk assessment scales. ‘Gold standard’ methods include multivariable modelling (either from single studies or meta-analysis from a number of studies) to identify items for a risk tool, with subsequent model testing on a ‘new’ prospective target population (Steyerberg 2010). The majority of risk assessment tools have been developed using non-systematic reviews of the epidemiological literature and expert opinion (Cullum et al. 1995, Nixon & McGough 2001) with postdevelopment evaluation of reliability and validity (Beeckman et al. 2012).
Where ‘gold standard’ methods have been used in tool development methodological limitations are apparent including the use of single rather than multiple centre populations and inadequate sample sizes for both model derivation and testing (Suriadi et al. 2008, Slowikowski & Funk 2010, Page et al. 2011). The development and predictive validity testing of PU risk assessment scales is further complicated by:
the absence of a reference standard for PU ‘risk’ with ‘PU presence’ being commonly used as an alternative despite their differences (Kottner & Balzer 2010).
the instigation of preventative interventions being a key element of standard clinical practice which will impact tool performance in the study population (Deeks 1996, Defloor & Grypdonck 2004).
There are also practical problems associated with the use of existing risk assessment scales. While many were designed for use on patients without PUs to identify those at ‘risk’, they are in practice often used for all patients (i.e. those with and without PUs) and they do not differentiate between these two groups. Furthermore, in clinical practice risk assessment and skin assessment are viewed as two separate processes. This is a limitation for two reasons. Firstly, nurses may disregard the presence of an existing PU in clinical assessment and fail to initiate appropriate secondary prevention and treatment interventions, leading to the progression of a severe PU (Pinkney et al. 2014). Secondly, our risk factor systematic review indicated that alterations to intact skin and the presence of a category 1 PU are key predictors of subsequent ≥category 2 PUs (Coleman et al. 2013). Another issue is that a full detailed risk assessment is undertaken on all patients even those who are clearly not at risk. This unnecessarily diverts nursing time away from other priorities. There is a need, therefore to streamline the assessment process to incorporate a screening stage that would allow those who are obviously ‘not at risk’ to be quickly identified, preventing the need for a more detailed full assessment.
We therefore embarked on a work package to develop and validate a robust risk assessment tool to facilitate the assessment and stratification of PU risk in adult patient populations. This was undertaken as part of the PU Programme Of reSEarch (PURPOSE: RP-PG-0407-10056), funded by the National Institute for Health Research (NIHR) and comprised five distinct phases:
a systematic review of the existing evidence to identify risk factors associated with increased probability of PU development (Coleman et al. 2013)
a consensus study to agree a draft risk factor Minimum Data Set (MDS) to underpin the development of a Risk Assessment Framework (RAF)
the proposal of a new PU conceptual framework building on the phase 2 consensus study (Coleman et al. 2014)
the design and pre-testing of the draft RAF with clinical nurses to assess and improve usability
the clinical evaluation of the RAF to assess reliability, validity, data completeness and clinical usability
Phase one the systematic review, provided the foundation for the work (Coleman et al. 2013). The review comprised 54 eligible studies (34,449 patients) and identified a large number of potential risk factors (15 domains, 46 sub-domains including over 250 named variables), lack of comparable data fields for measurement of the same constructs and key risk factors not being routinely recorded in all studies (Coleman et al. 2013). Due to these limitations, meta-analysis was not possible and a narrative synthesis was undertaken.
The narrative synthesis of the systematic review found that the most consistently emerging risk factor domains were immobility, skin/PU status and perfusion (including diabetes). Other important but less consistently emerging risk factor domains included nutrition, moisture, age, haematological measures, general health status, sensory perception and mental status (Coleman et al. 2013). A small number of studies suggest a relationship between body temperature and immunity and PU development and these factors require further confirmatory research. The evidence regarding race and gender was equivocal (Coleman et al. 2013). While immobility assessment is included in existing PU risk assessment tools, the inclusion of skin/PU status and perfusion (including diabetes) is not universal.
The systematic review highlighted the need to re-consider which risk factors should be considered in PU risk assessment, how these should be assessed and the overall assessment process. In addition, a key recommendation of the review was the development of a risk factor MDS, to encourage the use of consistent risk factors across PU studies facilitating large scale multivariable analysis, meta-analysis and case mix adjustment (Berlowitz et al. 2001). It was also proposed that to enable the routine recording of the MDS in practice, the MDS would be incorporated into the RAF. This paper reports phase two of the work, the consensus study.
The study
Aim
To develop a draft PU risk factor Minimum Data Set (MDS) and Risk Assessment Framework (RAF) for pre-testing and clinical evaluation. The objectives were:
To agree a list of patient characteristics to form an MDS suitable for routine collection of key risk factors in adult patient populations.
-
To develop a RAF incorporating the MDS with:
a simple screening stage to quickly identify not at risk patients
a detailed full assessment stage for patients who are at potential/actual risk or have an existing PU
Decision pathways, i.e. not currently at risk, primary prevention (at risk) or secondary prevention and treatment pathway (with PU).
Design
A consensus study involving a modified nominal group technique based on the RAND/UCLA (Research and Development/University of California at Los Angeles) appropriateness method (Fitch et al. 2001) was used. This incorporated face-to-face interaction of an expert group and pre- and postmeeting questionnaire completion. In addition, face-to-face interaction of a Patient and Public Involvement (PPI) service user group (PU Research Service User Network: PURSUN) to consider the acceptability of proposed risk assessment elements was undertaken. The face-to-face element of the methodology was considered necessary due to the complexity of the subject area.
Sample/participants
The expert group comprised internationally recognized clinical/academic leaders identified via their publication record in PU or relevant research. The group was purposively sampled to include the perspectives of nurses (academic and clinical nurse specialists), doctors (diabetologist, vascular surgeon, elderly care medicine and public health), bioengineers, epidemiologist and individuals with organisational development and clinical decision-making expertise. A multi-specialty group was developed to take account of a wide range of opinions (Hutchings & Raine 2006). Seventeen members were recruited to allow for attrition, as 12 was considered the optimum number in relation to preventing co-ordination problems while maximizing reliability (Murphy et al. 1998).
The service user group, involved members of PURSUN UK (web address: http://www.pursun.org.uk/), which was set up to improve the quality of PPI in PU research. Seven members were involved in the study and included people with direct experience of a PU, people with experience of living with PU risk and carers.
Data collection
Data collection was undertaken December 2010–December 2011. The consensus process incorporated an initial expert group meeting and an initial PURSUN meeting, followed by two consensus cycles. The first consensus cycle focussed on agreeing the risk factors to be included in the MDS and RAF, while the second consensus cycle focussed on agreeing the assessment items. Each cycle comprised an expert group face-to-face meeting and pre- and postmeeting consensus questionnaire completion (Figure1). A PURSUN meeting was also undertaken at the end of cycle 1 (Figure1).
Figure 1.
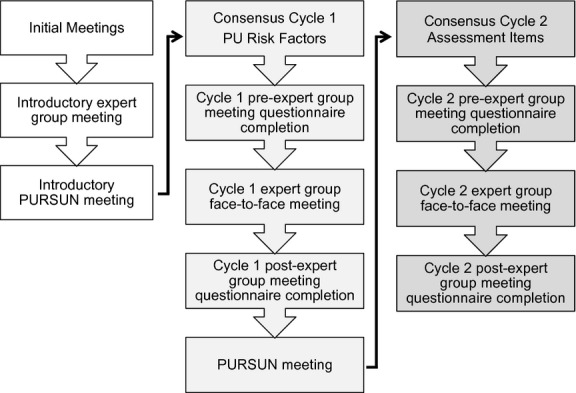
Overview consensus cycle.
Reviewing the PU risk factor evidence was an important element of the study and was integrated throughout all cycles of the consensus process. The systematic review, through its identification of risk factor domains and sub-domains provided the foundation for considering which risk factor variables were important for identifying PU risk. Other wider scientific evidence was also drawn from the expertise of the group. The relevance of the evidence to clinical practice and the practicalities of PU risk assessment were also considered.
Questionnaires were completed by all expert group members privately before and after cycle 1 and 2 meetings (Figure1). This allowed individuals to change their ratings in light of discussions and/or where necessary for questionnaire items to be clarified and amended. In each questionnaire participants were asked to rate their level of support for statements (relating to the inclusion of risk factors/assessment items to the MDS and RAF) on a 9-point Likert scale where 1 indicated strong disagreement and 9 indicated strong agreement (Figure2). Each statement was preceded by summaries of the PU systematic review evidence, expert group discussions, PURSUN group discussions (as applicable) and follow-up/explanatory notes (as applicable). Electronic links to the full systematic review evidence tables and the full summary of the preceding expert group discussions were also available in the questionnaires. Questionnaires were administered and completed via a commercial online survey platform.
Figure 2.
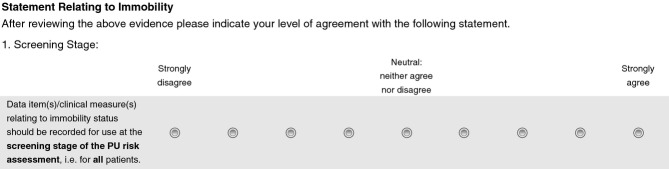
Example questionnaire items from the cycle 1 questionnaire.
All expert group meetings were led by trained facilitators and were audio-taped. Unlike a traditional RAND/UCLA method where the first face-to-face meeting occurs following questionnaire completion, an initial face-to-face meeting was undertaken to review the PU evidence and consider the views of the group. This informed the development of the cycle 1 risk factor questionnaire (Raine et al. 2005). At cycle 1 and 2 expert group meetings (Figure1), the pre-meeting collective questionnaire responses were anonymously fed back to the group. Members were also provided with a reminder report of their individual questionnaire responses and a copy of the summary of the previous expert group meeting discussions. The questionnaire results highlighted areas of agreement and areas of uncertainty and disagreement. This provided a focus for the group discussions to ascertain whether there was genuine uncertainty or disagreement, or if there was ambiguity in the wording of the questionnaire.
As PU risk assessment practice is part of routine care there was a need to explore the acceptability of proposed risk assessment elements with patients and carers. This was undertaken through facilitated PURSUN meetings. At the initial PURSUN meeting (Figure1) participants were introduced to the aims of the study, the purpose of the meetings and discussed potential assessment components of the MDS and RAF. Views were fed back to the expert group by the Patient and Public Involvement Officer (cycle 1). At the second PURSUN meeting (cycle 1, Figure1) members were asked to consider the risk factors that the expert group had agreed should be included in the MDS and RAF, potential assessment items and the acceptability of collecting this information on a routine basis. Views were fed back to the expert group via the cycle 2 pre-meeting questionnaire (which included a summary PURSUN discussions) prompting discussion at the expert group meeting.
Ethical considerations
The study was reviewed and approved by a University Research Ethics Committee. Informed consent was gained from expert group members prior to participation and they remained free to withdraw from the study without giving reasons.
Data analysis
The researcher (SC) listened to the audio-tapes and read the associated transcripts in total to ensure completeness. The data were coded with categories based on the PU risk factor systematic review, in keeping with a directed content analysis approach (Hsieh & Shannon 2005). As new themes were identified from the expert group discussions, further codes were added. A summary report of each meeting was generated by the researcher (SC). The report was reviewed by the facilitators and members of a working group (sub-group of expert group) to ensure it reflected group discussions.
Careful notes were taken throughout the PURSUN meetings and a summary of discussions was written by the researcher (SC). The summary was circulated to the facilitator and group participants to ensure it reflected the discussions of the meeting.
Questionnaire statements were summarized using the median group response as a measure of central tendency. In keeping with the RAND/UCLA Appropriateness methods and other studies (Scott & Black 1991, Fitch et al. 2001, Shiffman et al. 2003, Kroger et al. 2007) Likert scale group median responses for each statement were categorised into 3 tertiles. For this study the categories were 1–3 disagree, 4–6 uncertain, 7–9 agree. Within-group agreement was measured using the RAND Disagreement index (Fitch et al. 2001), which considers the dispersion of individual scores and identifies areas of disagreement (where panellists rate at both ends of the Likert Scale). This involves calculating the interpercentile range (IPR: 0·3–0·7) and the IPR adjusted for symmetry (IPRAS) to detect disagreement (if the IPR is larger than the IPRAS there is disagreement) (Fitch et al. 2001). By calculating the ratio of these an index of >1 indicates disagreement.
Using the group median response and the disagreement index for each statement (about risk factors/assessment items) the following principles were applied following postmeeting questionnaire completion (Figure1):
Group medians of 1–3 without disagreement would be excluded
Group medians of 7–9 without disagreement would be included
Where the disagreement index was >1 or where the median was 4–6 they would be excluded but are potential areas for further research.
Validity and reliability
It has been recognized that it is difficult to determine the validity of consensus judgements (i.e. whether ‘good judgements’ are made) at the time the judgements are made (Black et al. 1999). It is, therefore, important that the consensus process is as rigorous as possible (Raine et al. 2005). This study applied principles of good practice in the planning and delivery of the consensus process incorporating the involvement of a mixed-speciality expert group (Hutchings & Raine 2006) and the views of service users (PURSUN). Other key principles included careful preparation and consideration of relevant evidence throughout the consensus process, questionnaire content informed by expert group discussions (and reviewed by a working group to ensure content validity), private completion of questionnaires by expert group members, facilitated face-to-face meetings and the inclusion of a measure of dispersion and central tendency in the reporting (Black et al. 1999). While the reliability of expert group judgements were not assessed in this study, future work is being planned to check the representativeness of the expert group views with the wider community (Raine et al. 2005).
Results
The expert group comprised of 17 international experts in the PU field, comprising nine female and eight male participants. There was 100% completion of all questionnaires and 86% attendance at the face-to-face meetings (17 of 17 attended the first meeting, 13 of 17 attended the second meeting and 14 of 17 attended the third meeting). The results concerning the risk factors (cycle 1) and assessment items (cycle 2) of the MDS and RAF are detailed below.
Cycle 1: risk factors
The expert group agreed that three risk factors should be incorporated into the screening stage of the MDS and RAF for the assessment of all patients and comprised immobility, existing and previous PU. Table1 indicates the questionnaire responses before and after the expert group meetings. In the pre-meeting questionnaire responses there was support for inclusion of three risk factors and exclusion of 13 risk factors, with uncertainty for 10 risk factors (three with disagreement). Following the consensus meeting and discussion of the areas of uncertainty and disagreement the post meeting questionnaire responses indicated agreement for inclusion of three risk factors and exclusion of 21 risk factors (Table1).
Table 1.
Risk factors for screening stage of MDS and RAF
| Pre-meeting questionnaire responses |
Postmeeting questionnaire responses |
||||
|---|---|---|---|---|---|
| Group median | Disagreement index | Group median | Disagreement index | ||
| Immobility status | 9·00 | 0·00 | 9·00 | 0·00 | |
| Existing pressure status | 9·00 | 0·13 | 9·00 | 0·00 | |
| Previous PU status | 7·00 | 0·29 | 8·00 | 0·29 | |
| General skin status | 5·00 | 1·87* | 3·00 | 0·74 | |
| Sensory perception | 4·00 | 0·68 | 3·00 | 0·72 | |
| Acute illness | 5·00 | 0·59 | 3·00 | 0·54 | |
| Infection | 5·00 | 0·98 | 2·00 | 0·33 | |
| Body temperature | 5·00 | 0·97 | 2·00 | 0·29 | |
| Nutrition | 5·00 | 0·55 | 2·00 | 0·75 | |
| Friction and shear | 2·00 | 0·16 | 2·00 | 0·29 | |
| Chronic wounds | 3·00 | 0·65 | 2·00 | 0·29 | |
| Diabetes | 4·00 | 0·55 | 2·00 | 0·37 | |
| Summary measure of general health status. | 2·00 | 0·20 | 2·00 | 0·13 | |
| Perfusion | – | – | 2·00 | 0·75 | |
| Albumin | 3·00 | 0·48 | 2·00 | 0·29 | |
| Skin moisture | 4·00 | 1·61* | 2·00 | 0·29 | |
| Dual incontinence | 5·00 | 1·70* | 2·00 | 0·33 | |
| Medication | 3·00 | 0·33 | 1·00 | 0·02 | |
| Mental status | 2·00 | 0·65 | 1·00 | 0·13 | |
| Age | 4·00 | 0·67 | 1·00 | 0·16 | |
| Race | 2·00 | 0·49 | 1·00 | 0·02 | |
| Gender | 1·00 | 0·29 | 1·00 | 0·02 | |
| Haemoglobin | 2·00 | 0·37 | 1·00 | 0·16 | |
| Pitting oedema | 3·00 | 0·67 | 1·00 | 0·13 | |
| BP | 3·00 | 0·67 | – | – | |
| Smoking | 2·00 | 0·37 | – | – | |
| Cardiovascular disease | 3·00 | 0·67 | – | – | |
Dark grey: group median 1–3 (inclusion not supported).
Mid grey: group median 4–6 (uncertain).
Light grey: group median 7–9 (inclusion supported).
Disagreement.
The expert group agreed that eleven risk factors, namely immobility, existing and previous PU, general skin status, perfusion, skin moisture, dual incontinence, diabetes, sensory perception, nutrition and albumin should be incorporated into the detailed full assessment stage of the MDS and RAF. This would be for patients, who were considered to be at potential/actual risk or have an existing PU from the screening stage. Table2 indicates the questionnaire responses before and after the expert group meetings. In the pre-meeting questionnaire responses there was support for inclusion of 12 risk factors and exclusion of two risk factors, with uncertainty for 12 risk factors (two with disagreement). Following the consensus meeting and discussion of the areas of uncertainty and disagreement the postmeeting questionnaire responses indicated agreement for inclusion of 11 risk factors, exclusion of 4 risk factors and uncertainty for nine risk factors (1 with disagreement) (Table2). After reviewing the evidence the postmeeting questionnaire was revised and Blood Pressure (BP), smoking and cardiovascular disease were combined into a general category of ‘perfusion’. A summary of the key discussion points relating to the uncertain risk factors is detailed in Table3.
Table 2.
Risk factors for the detailed full assessment stage of MDS and RAF
| Pre-meeting questionnaire responses |
Postmeeting questionnaire responses |
||||
|---|---|---|---|---|---|
| Group median | Disagreement index | Group median | Disagreement index | ||
| Immobility status | 9·00 | 0·16 | 9·00 | 0·00 | |
| Existing PU status | 9·00 | 0·13 | 9·00 | 0·16 | |
| Previous PU status | 7·00 | 0·40 | 8·00 | 0·16 | |
| General skin status | 8·00 | 0·23 | 8·00 | 0·29 | |
| Skin moisture | 8·00 | 0·29 | 8·00 | 0·33 | |
| Diabetes | 8·00 | 0·29 | 8·00 | 0·33 | |
| Nutrition | 7·00 | 0·67 | 8·00 | 0·16 | |
| Perfusion | – | – | 8·00 | 0·40 | |
| Albumin | 7·00 | 0·20 | 7·00 | 0·45 | |
| Sensory perception | 8·00 | 0·29 | 7·00 | 0·29 | |
| Dual incontinence | 8·00 | 0·19 | 7·00 | 0·33 | |
| Friction and shear | 5·00 | 1·10* | 6·00 | 0·52 | |
| Chronic wounds | 6·00 | 0·42 | 6·00 | 0·37 | |
| Medication | 5·00 | 0·41 | 5·00 | 0·08 | |
| Acute illness | 7·00 | 0·07 | 5·00 | 0·59 | |
| Infection | 5·00 | 1·10* | 5·00 | 0·41 | |
| Body temperature | 7·00 | 0·52 | 5·00 | 0·88 | |
| Pitting oedema | 6·00 | 0·30 | 5·00 | 1·04* | |
| Age | 5·00 | 0·49 | 5·00 | 0·50 | |
| Summary measure of general health status | 4·00 | 0·62 | 4·00 | 0·65 | |
| Haemoglobin | 5·00 | 0·32 | 3·00 | 0·72 | |
| Mental status | 5·00 | 0·72 | 2·00 | 0·75 | |
| Race | 2·00 | 0·49 | 1·00 | 0·13 | |
| Gender | 2·00 | 0·29 | 1·00 | 0·02 | |
| BP | 5·00 | 0·52 | – | – | |
| Smoking | 5·00 | 0·59 | – | – | |
| Cardiovascular disease | 6·00 | 0·42 | – | – | |
Dark grey: group median 1–3 (inclusion not supported).
Mid grey: group median 4–6 (uncertain).
Light grey: group median 7–9 (inclusion supported).
Disagreement.
Table 3.
Uncertain risk factors
| Uncertain risk factors | Key discussion points |
|---|---|
| Friction and shear | • Important concept in relation to biomechanics and tissue loading • Debate about whether a patient characteristic • Difficult to measure in practice • Different definition of terms (e.g. nurses and bioengineers) • Interlinked with immobility • Should to be minimized in care |
| Acute illness Infection Body temperature (elements of general health status) | • Felt to be important clinically • Links between the 3 elements recognized • Impact on mobility, perfusion and moisture acknowledged |
| Chronic wound | • Did not emerge as a strong risk factor in the systematic review • Link to other factors including nutritional depletion, moisture(exudate), oedema, diabetes and general skin condition recognized • Would be captured by other key risk factors e.g. general ‘skin status’, nutrition, moisture and diabetes |
| Pitting oedema | • Relatively unexplored area in the literature • Leads to changes in the mechanical properties of the tissues • May result in reduced mobility due to heavy oedematous legs • Some felt that oedema should be considered under the skin status umbrella |
| Medication | • Acknowledged that the systematic review evidence associated with medication was weak. • Links between specific medications and risk factors were made, e.g. the effects of sedation, epidurals and analgesia on sensation and movement and steroids on skin condition (tissue paper skin) • Use of vasoconstrictors in specialist areas important • Complicated by dose-dependent effects • Difficult to measure |
| Age | • Some felt that age formed an important element of assessment • Others felt it was a proxy for other measures e.g. skin condition and immobility |
Using the decision rules highlighted in the methods section, the MDS and RAF comprised only those risk factors where there was agreement (group median 7-9 without disagreement). The progression of risk factors through the consensus study are detailed in Figure3 (also see Tables2 and 3). This shows that of the original 15 risk factor domains and 46 sub-domains of the systematic review (Coleman et al. 2013), 26 risk factors were considered to potentially warrant inclusion in the MDS and RAF and progressed to consensus cycle 1.
Figure 3.

Risk factor progression.
The risk factors for inclusion were mainly agreed in the cycle 1 postmeeting questionnaire but there were some refinements of the risk factors in the cycle 2 pre-meeting questionnaire. The expert group recognized that albumin emerged strongly in the systematic review and that it was important in relation to potential changes in oncotic pressure and the development of oedema. Some also thought it was linked to nutritional status. The expert group agreed that albumin should be included at the second stage of the assessment (Table2). However, at a subsequent PURSUN meeting, concern was raised about the need to undertake an additional blood test for assessment of albumin. This concern was fed back to the expert group in the cycle 2 pre-meeting questionnaire. Members were asked whether there was a clinical indication for undertaking an additional blood test to measure albumin to establish level of PU risk. It was concluded that this was unnecessary and it would not be included in the MDS and RAF. The expert group also concluded that skin moisture and dual incontinence could be combined into one measure.
Cycle 2: assessment items for risk factors
There was good support (group median 7–9 without disagreement) for all statements in the cycle 2 questionnaire concerning the assessment items of MDS and RAF. However, following group discussion at the cycle 2 meeting, it was said that some changes were necessary to specific items. As the group were content with the majority of the PU risk factor MDS items highlighted in the cycle 2 pre-meeting questionnaire, the postmeeting questionnaire focussed on items that required adjustment. The agreed assessment items for the screening and detailed full assessment stage are shown in Table4. In addition, the expert group agreed that the RAF would facilitate the identification of a risk profile for each patient, rather than condense the risk from different aspects into a single score. This would support care planning with interventions selected in response to specific risk factors.
Table 4.
MDS (to be incorporated in RAF)
| Screening Stage |
| Mobility: |
| a. Does the patient walk without help? |
| b. Does the patient change position? |
| PU status: |
| a. Current PU (≥1 category) |
| b. Reported history of PU |
| Detailed Full Assessment stage |
| Immobility items to incorporate the frequency of independent movement, e.g.: |
| a. Doesn't move |
| b. Moves occasionally |
| c. Moves frequently |
| Immobility items to incorporate the magnitude of independent movement, e.g.: |
| a. Doesn't move |
| b. Slight position changes |
| c. Major position changes |
| Immobility items to incorporate general, clinically relevant descriptions of movement, e.g.: |
| a. Bedfast |
| b. Chairfast |
| c. Walks with assistance |
| Sensory perception: |
| a. Does the patient feel and respond appropriately to discomfort from pressure |
| PU (existing and previous PU): |
| a. Category of PU (where possible for previous PU) |
| b. Site of PU |
| c. Presence of scar tissue (for previous PU) |
| General skin status: |
| a. Confirmation of vulnerable skin, e.g. dryness, paper thin and redness |
| b. Pressure area skin site |
| Perfusion: |
| a. Conditions affecting central circulation, e.g. shock, heart failure and hypotension |
| b. Conditions affecting peripheral circulation, e.g. peripheral vascular/arterial disease. |
| Diabetes: |
| a. Presence of diabetes |
| Moisture: |
| a. Presence of moisture due to perspiration, urine, faeces or exudate. |
| Frequency: |
| b. Frequent (1 or 2 times a day) |
| c. Constant |
| Nutrition: |
| a. Unplanned weight loss |
| b. Poor nutritional intake |
| c. Low BMI |
| d. High BMI |
Draft RAF
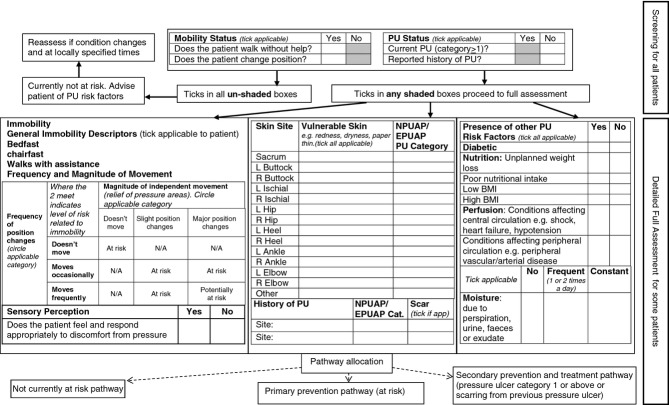
Using the results from cycle 1 and 2 of the study an initial draft of the RAF (Figure4) was made incorporating the screening and detailed full assessment stage and decision pathways of the assessment, i.e. not currently at risk, primary prevention (at risk) or secondary prevention and treatment pathway (existing PU or scarring from a previous PU). This will undergo further graphic design in preparation for pre-testing.
Figure 4.
Draft RAF with underpinning MDS.
Discussion
Using structured consensus methods, the risk factors and assessment items for a draft MDS and RAF were agreed. The consensus methods were particularly useful in allowing us to identify the risk factors for inclusion in the RAF and MDS. While they were also useful in identifying the key principles of the assessment items, the method was inappropriate for considering the specific wording of items. Of note was the agreement that the risk factors and assessment items should be the same for the MDS and the RAF, i.e. no additional risk factor information to supplement the MDS was considered necessary for a RAF for assessment in clinical practice. The draft RAF differs from other risk assessment tools in two main ways. First, the incorporation of a screening stage allows those who are obviously ‘not at risk’ to be quickly identified preventing the need for a more detailed full assessment, which will save time in clinical practice. Second, the integration of the skin assessment items enables the identification of vulnerable skin or an existing PU (and/or scarring from a previous PU) to be a key consideration in the risk assessment and subsequent care planning. Where a PU is identified, the patient will be allocated to the secondary prevention and treatment pathway, which has the potential to facilitate escalation of interventions to prevent deterioration and promote healing. Further research is required to confirm this.
The use of the systematic review evidence (Coleman et al. 2013) provided the foundation for the evidence base of the consensus study. The expert group also considered wider scientific evidence, clinical and practical implications and the views of PURSUN when deciding which risk factors should be included at the screening stage and the detailed full assessment stage of the MDS and RAF. This enabled the expert group to agree the key risk factors to summarize patient risk, i.e. those that were considered to increase the probability of PU development. However, it was recognized that other excluded risk factors may still have a role in the PU causal pathway via their relationship with the primary risk factors and may be important at an individual patient level, e.g. the use of inotropes have an impact on perfusion. PU causal pathways were considered more closely in a follow-up piece of work which proposes a new conceptual framework (Coleman et al. 2014).
The risk factors included in the MDS and RAF included those with strong epidemiological evidence (immobility, existing PU, general skin status, perfusion (including diabetes), and those with less consistent epidemiological evidence which were felt to be important in clinical practice (moisture, nutrition, sensory perception). Previous PU was included on the basis of clinical and service user opinion and theoretical bioengineering evidence, rather than by the epidemiological evidence. Conversely, albumin, which has strong epidemiological evidence was initially agreed for inclusion in the MDS and RAF by the expert group, but was subsequently excluded due to concerns raised by PURSUN. In these examples, where the group diverged from the epidemiological evidence the reasons were in keeping with some of those previously reported including clinical experience and patient preference (Raine et al. 2004).
There was strong commitment from the expert group to be involved throughout the study, though there were a few occasions where participants were unable to attend the face-to-face meetings (13/17 attended the second meeting and 14/17 attended the third meeting). On these occasions, special arrangements were made to ensure they were properly updated and could continue to participate in the process. One to one telephone meetings were organized between the researcher and these individuals after the expert group meeting. The participant was sent the same information considered at the expert group meeting and the researcher presented the same power point presentations and summarized the discussions of the expert group meeting. The participant then completed the online questionnaire (all completed the pre-meeting questionnaires at the same time as the rest of the group). Every effort was made to ensure that these participants remained engaged in the consensus process but there remains the possibility that these participants might have made different questionnaire responses had they been subject to the actual expert group discussions.
The integration of the PURSUN perspective throughout the study proved invaluable and to our knowledge is the first study to use such an approach. While others using consensus methods have incorporated patient/carer representation to their expert groups (Rycroft-Malone 2001, Jackson et al. 2009) we decided to use an alternative approach when developing the study methodology. This was due to concern that the complexity of the epidemiological and wider scientific evidence, and the complex nature of facilitating a mixed group of patients and professionals could have impeded the patients and carers input into the process. Difficulties in involving patients and carers in the development of technical and clinical guidelines have been raised previously (Rolls & Elliott 2008). For this study, there seemed to be more value in devoting whole meetings to patient/carer insights, with particular emphasis on the acceptability of elements of assessment. This allowed us to consider the views of a larger number of service users. We were conscious of the need to integrate PURSUN members' perspectives into the consensus process. This was achieved by feedback at the expert group meetings or inclusion of their comments into questionnaires, so that the group could consider the patient/carer perspective alongside other evidence.
Limitations
While the study involved an expert group with considerable experience a weakness of the methodology relates to reliability and whether the results of this study are representative of the views of other experts in the field. This could prove especially important for uncertain areas such as friction and shear (excluded) where the expert group identified a close relationship with immobility and difficulties in measuring this risk factor in clinical practice. Raine, Sanderson and Black proposed a new approach in developing clinical guidelines which includes checking the representativeness of the group's ratings with a large similarly composed group (Raine et al. 2005). With this in mind, further work is currently being planned to consider the risk factors that should be considered in the MDS and RAF with a larger group. This will also allow new evidence to be brought forward and integrated into the work.
While the consensus study provided us with a draft MDS and RAF further development is underway. This incorporates further liaison with the expert group and PURSUN, and the subsequent phases of the work package (conceptual framework proposal, design, pre-testing and clinical evaluation of the RAF). Of particular note is that the design and pre-test will address issues of usability, clarification of areas of confusion and guidance for decision-making of assessment outcomes. It will also facilitate the development of a User Manual to accompany the RAF where assessment components and operational definitions can be fully explained. This was considered important by the expert group who recognized areas of practice where operational definitions are vague, for example in the assessment of general skin status. The pre-test will also prepare the RAF for clinical evaluation where further assessment of reliability and validity can be undertaken. In the longer-term future large scale statistical modelling will be undertaken to refine the RAF.
Conclusion
Using a modified nominal group technique based on the RAND/UCLA appropriateness method, incorporating an expert group, review of the PU evidence and the views of a PPI service user group (PURSUN) we have agreed risk factors, assessment items and have drafted the MDS and RAF. The RAF comprises two stages of assessment, the screening stage for all patients and the detailed full assessment stage for patients at potential/actual risk or with an existing PU. The RAF allows patients to be allocated to a not currently at risk, primary prevention (at risk) or secondary prevention and treatment pathway (existing PU or scarring from a previous PU).
The continuing development of the RAF is being to be taken forward by the NIHR funded programme of research (PURPOSE: RP-PG-0407-10056). This involves further design and pre-testing of the RAF with clinical nurses, and evaluation in clinical practice. It is hoped that this will give an up-to-date, valid and reliable tool for use with adult populations in clinical practice. Further testing will be needed to assess if this translates to better care and reduced PU incidence or severity. The MDS will encourage the use of consistent risk factors across PU studies facilitating meta-analysis and case mix adjustment. In addition, the MDS will allow further statistical modelling to be undertaken to refine the RAF.
Acknowledgments
The authors thank the Pressure Ulcer Research Service User Network (PURSUN).
Funding
This publication presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (RP-PG-0407-10056). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest
No conflict of interest has been declared by the author(s).
Author contributions
All authors have agreed on the final version and meet at least one of the following criteria [recommended by the ICMJE (http://www.icmje.org/ethical_1author.html)]:
substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
drafting the article or revising it critically for important intellectual content.
References
- AHCPR. Pressure Ulcers in Adults: Prediction and Prevention: Quick Reference Guide for Clinicians. Rockville, MD: Services, U. D. o. H. a. h; 1992. [Google Scholar]
- Beeckman D, Mathei C, Van Lancker A, Van Houdt S, Vanwalleghem G, Gryson L, Heyman H, Thyse C, Toppets A, Stordeur S. Van Heede Den K. 2012. & Good Clinical Practice: A National Guideline for the Prevention of Pressure Ulcers KCE Report 193C.
- Bennett G, Dealey C. Posnett J. The cost of pressure ulcers in the UK. Age and Ageing. 2004;33(3):230–235. doi: 10.1093/ageing/afh086. &. [DOI] [PubMed] [Google Scholar]
- Berlowitz DR, Brandeis GH, Morris JN, Ash AS, Anderson JJ, Kader B. Moskowitz MA. Deriving a risk-adjustment model for pressure ulcer development using the Minimum Data Set. Journal of the American Geriatrics Society. 2001;49(7):866–871. doi: 10.1046/j.1532-5415.2001.49175.x. &. [DOI] [PubMed] [Google Scholar]
- Berlowitz D, VanDeusen Lukas C, Parker V, Niederhauser A, Silver J, Logan C. Ayello E. Preventing Pressure Ulcers in hospitals: A Toolkit for Improving Quality of Care. Agency of Healthcare Research and Quality, Rockville, MD; 2011. &. [Google Scholar]
- Black N, Murphy M, Lamping D, McKee M, Sanderson C, Askham J. Marteau T. Consensus development methods: a review of best practice in creating clinical guidelines. Journal of Health Services & Research Policy. 1999;4(4):236–248. doi: 10.1177/135581969900400410. &. [DOI] [PubMed] [Google Scholar]
- Coleman S, Gorecki C, Nelson EA, Close SJ, Defloor T, Halfens R, Farrin A, Brown J, Schoonhoven L. Nixon J. Patient risk factors for pressure ulcer development: systematic review. International Journal of Nursing Studies. 2013;50:974–1003. doi: 10.1016/j.ijnurstu.2012.11.019. &. [DOI] [PubMed] [Google Scholar]
- Coleman S, Nixon J, Keen J, Wilson L, McGinnis E, Dealey C, Stubbs N, Farrin A, Dowding D, Schols JMGA, Cuddigan J, Berlowitz D, Jude E, Vowden P, Schoonhoven L, Bader DL, Gefen A, Oomens CWJ. Nelson EA. A new pressure ulcer conceptual framework. Journal of Advanced Nursing. 2014 doi: 10.1111/jan.12405. &. doi: 10.1111/jan.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullum N, Deeks J, Fletcher A, Mouneimne H, Sheldon T, Song F. Long A. The Prevention and Treatment of Pressure Sores: how useful are the measures for scoring people's risk of developing a pressure sore? Effective Health Care Bulletin. 1995;2(1):1–18. &. [Google Scholar]
- Dealey C, Posnett J. Walker A. The cost of pressure ulcers in the United Kingdom. Journal of Wound Care. 2012;21(6):261–266. doi: 10.12968/jowc.2012.21.6.261. &. [DOI] [PubMed] [Google Scholar]
- Deeks JJ. Pressure sore prevention: using and evaluating risk assessment tools. British Journal of Nursing. 1996;5(5):313–314. doi: 10.12968/bjon.1996.5.5.313. 316–320. [DOI] [PubMed] [Google Scholar]
- Defloor T. Grypdonck MF. Validation of pressure ulcer risk assessment scales: a critique. Journal of Advanced Nursing. 2004;48(6):613–621. doi: 10.1111/j.1365-2648.2004.03250.x. &. [DOI] [PubMed] [Google Scholar]
- Fitch K, Bernstein SJ, Aguilar MS, Burnand B, LaCalle JR, Lazaro P, Loo MVH, McDonnell J, Vader J. Khan JP. The RAND/UCLA Appropriateness Method User's Manual. Santa Monica, CA: RAND Corporation; 2001. &. [Google Scholar]
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, Defloor T, Nixon J European Quality of Life Pressure Ulcer Project g. Impact of pressure ulcers on quality of life in older patients: a systematic review. Journal of the American Geriatrics Society. 2009;57(7):1175–1183. doi: 10.1111/j.1532-5415.2009.02307.x. &. [DOI] [PubMed] [Google Scholar]
- Gorecki C, Nixon J, Madill A, Firth J. Brown JM. What influences the impact of pressure ulcers on health-related quality of life? A qualitative patient-focused exploration of contributory factors. Journal of Tissue Viability. 2012;21(1):3–12. doi: 10.1016/j.jtv.2011.11.001. &. [DOI] [PubMed] [Google Scholar]
- Hsieh HF. Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. &. [DOI] [PubMed] [Google Scholar]
- Hutchings A. Raine R. A systematic review of factors affecting the judgments produced by formal consensus development methods in health care. Journal of Health Services & Research Policy. 2006;11(3):172–179. doi: 10.1258/135581906777641659. &. [DOI] [PubMed] [Google Scholar]
- Jackson A, Hettinga DM, Mead J. Mercer C. Using consensus methods in developing clinical guidelines for exercise in managing persistent low back pain. Physiotherapy. 2009;95(4):302–311. doi: 10.1016/j.physio.2009.08.001. &. [DOI] [PubMed] [Google Scholar]
- Kottner J. Balzer K. Do pressure ulcer risk assessment scales improve clinical practice? Journal of Multidisciplinary Healthcare. 2010;3:103–111. doi: 10.2147/jmdh.s9286. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger E, Tourigny A, Morin D, Cote L, Kergoat MJ, Lebel P, Robichaud L, Imbeault S, Proulx S. Benounissa Z. Selecting process quality indicators for the integrated care of vulnerable older adults affected by cognitive impairment or dementia. BMC Health Services Research. 2007;7:195. doi: 10.1186/1472-6963-7-195. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J. Marteau T. Consensus development methods and their use in clinical guideline development. Health Technology Assessment. 1998;2(3):i–iv. &, 1–88. [PubMed] [Google Scholar]
- NICE. CG7 Pressure Relieving Devices. London: National Institute of Clinical Excellence; 2003. [Google Scholar]
- Nixon J. McGough A. Principles of patient assessment: screening for pressure ulcers and potential risk. In: Morison M, editor; The Prevention and Treatment of Pressure Ulcers. Edinburgh: Mosby; 2001. pp. 55–74. [Google Scholar]
- NPUAP/EPUAP. Prevention and Treatment of Pressure Ulcers:Clinical Practice Guideline. Washington, DC: National Pressure Ulcer Advisory Panel; 2009. [Google Scholar]
- Page KN, Barker AL. Kamar J. Development and validation of a pressure ulcer risk assessment tool for acute hospital patients. Wound Repair and Regeneration. 2011;19(1):31–37. doi: 10.1111/j.1524-475X.2010.00647.x. &. [DOI] [PubMed] [Google Scholar]
- Pieper BE. Pressure Ulcers: Prevalence, Incidence and Implications for the Future. Washinton, DC: NPUAP; 2012. [Google Scholar]
- Pinkney L, Nixon J, Wilson L, Coleman S, McGinnis E, Stubbs N, Dealey C, Nelson A, Patterson M. Keen J. Why do patients develop severe pressure ulcers? A retrospective case study. BMJ Open. 2014;4(1) doi: 10.1136/bmjopen-2013-004303. & ). Retrieved from http://bmjopen.bmj.com/content/4/1/e004303.full.pdf on 20 January 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine R, Sanderson C, Hutchings A, Carter S, Larkin K, Black N, Raine R, Sanderson C, Hutchings A, Carter S, Larkin K. Black N. An experimental study of determinants of group judgments in clinical guideline development.[see comment] Lancet. 2004;364(9432):429–437. doi: 10.1016/S0140-6736(04)16766-4. &. [DOI] [PubMed] [Google Scholar]
- Raine R, Sanderson C. Black N. Developing clinical guidelines: a challenge to current methods. BMJ. 2005;331(7517):631–633. doi: 10.1136/bmj.331.7517.631. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls KD. Elliott D. Using consensus methods to develop clinical practice guidelines for intensive care: the intensive care collaborative project.[see comment] Australian Critical Care. 2008;21(4):200–215. doi: 10.1016/j.aucc.2008.08.003. &. [DOI] [PubMed] [Google Scholar]
- Rycroft-Malone J. Formal consensus: the development of a national clinical guideline. Quality in Health Care. 2001;10(4):238–244. doi: 10.1136/qhc.0100238... [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonhoven L, Bousema MT. Buskens E. The prevalence and incidence of pressure ulcers in hospitalised patients in The Netherlands: a prospective inception cohort study. International Journal of Nursing Studies. 2007;44(6):927–935. doi: 10.1016/j.ijnurstu.2006.02.011. &. [DOI] [PubMed] [Google Scholar]
- Schuurman J-P, Schoonhoven L, Defloor T, van Engelshoven I, van Ramshorst B. Buskens E. Economic evaluation of pressure ulcer care: a cost minimization analysis of preventive strategies. Nursing Economics. 2009;27(6):390–400. &. [PubMed] [Google Scholar]
- Scott EA. Black N. Appropriateness of cholecystectomy in the United Kingdom–a consensus panel approach. Gut. 1991;32(9):1066–1070. doi: 10.1136/gut.32.9.1066. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severens JL, Habraken JM, Duivenvoorden S. Frederiks CMA. The cost of illness of pressure ulcers in The Netherlands. Advances in Skin & Wound Care. 2002;15(2):72–77. doi: 10.1097/00129334-200203000-00008. &. [DOI] [PubMed] [Google Scholar]
- Shiffman RN, Shekelle P, Overhage JM, Slutsky J, Grimshaw J. Deshpande AM. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Annals of Internal Medicine. 2003;139(6):493–498. doi: 10.7326/0003-4819-139-6-200309160-00013. &. [DOI] [PubMed] [Google Scholar]
- Slowikowski GC. Funk M. Factors associated with pressure ulcers in patients in a surgical intensive care unit. Journal of Wound, Ostomy, and Continence Nursing. 2010;37(6):619–626. doi: 10.1097/WON.0b013e3181f90a34. &. [DOI] [PubMed] [Google Scholar]
- Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation and Updating. New York: Springer; 2010. [Google Scholar]
- Suriadi, Sanada H, Sugama J, Thigpen B. Subuh M. Development of a new risk assessment scale for predicting pressure ulcers in an intensive care unit. Nursing in Critical Care. 2008;13(1):34–43. doi: 10.1111/j.1478-5153.2007.00250.x. &. [DOI] [PubMed] [Google Scholar]
- Vowden KR. Vowden P. The prevalence, management, equipment provision and outcome for patients with pressure ulceration identified in a wound care survey within one English health care district. Journal of Tissue Viability. 2009;18(1):20–26. doi: 10.1016/j.jtv.2008.11.001. &. [DOI] [PubMed] [Google Scholar]