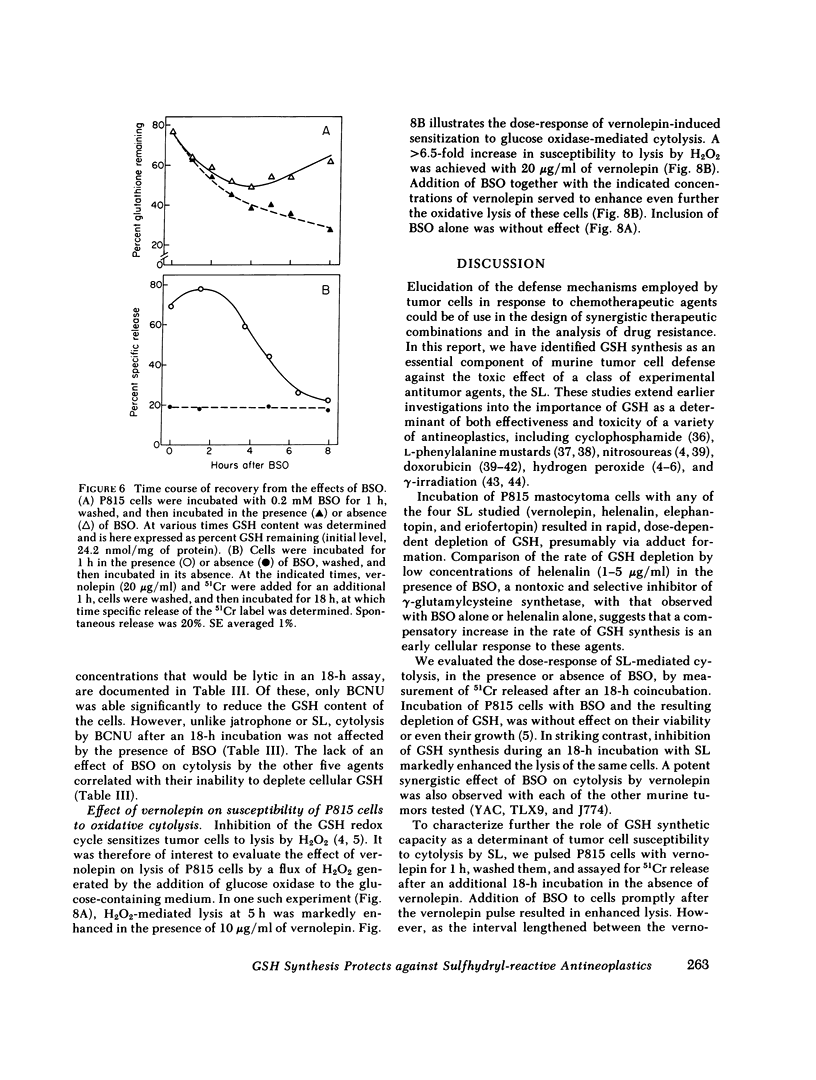
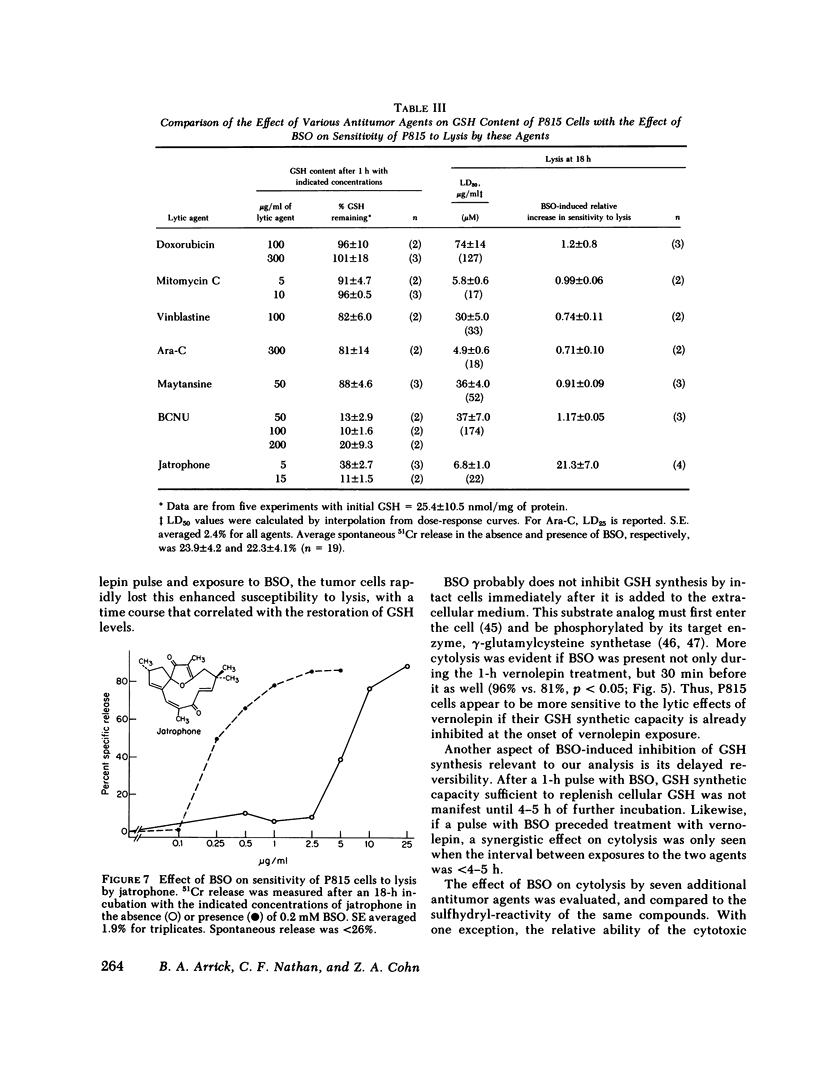
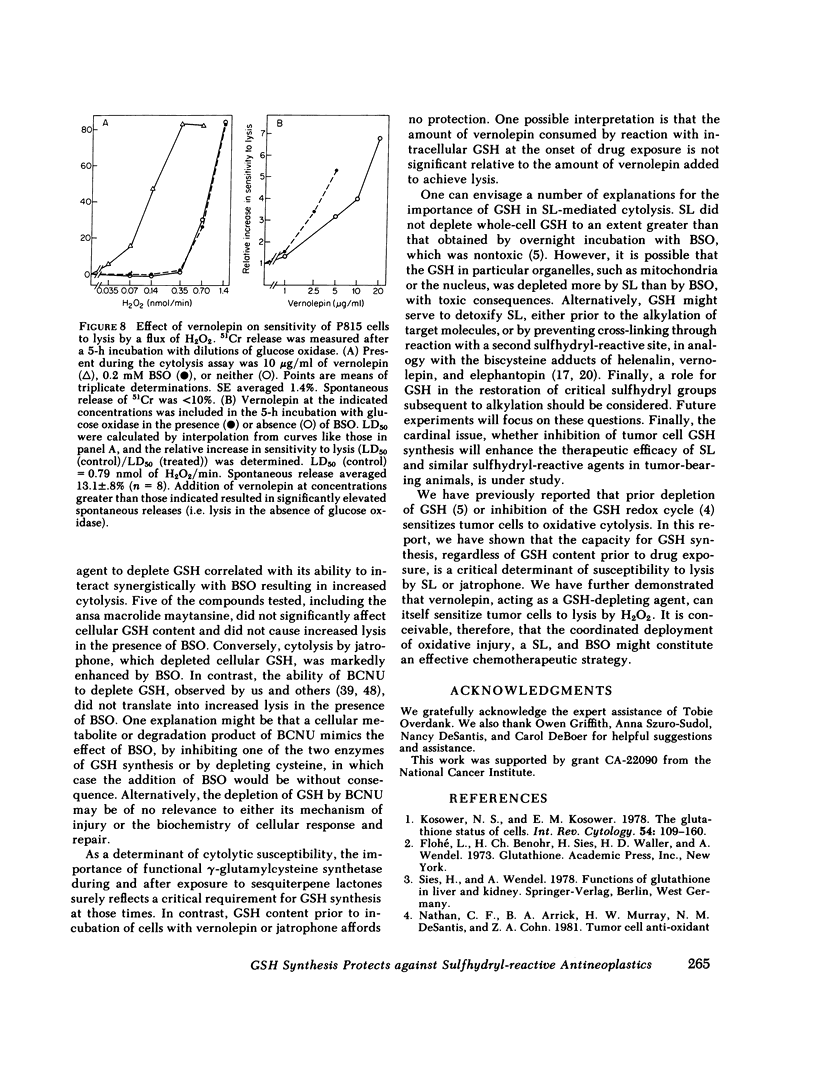
Abstract
GSH plays an important role in cellular defense against a wide variety of toxic electrophiles via the formation of thioether conjugates. We studied the role of GSH in murine tumor cell defense against a novel class of sulfhydryl-reactive antineoplastics, the sesquiterpene lactones (SL). Incubation of P815 mastocytoma cells with any of the four SL tested (vernolepin, helenalin, elephantopin, and eriofertopin) for 1 h resulted in 70-97% depletion of GSH. The importance of GSH resynthesis upon exposure of tumor cells to SL was evaluated with the use of buthionine sulfoximine (BSO), a selective, nontoxic inhibitor of gamma-glutamylcysteine synthetase. Inhibition of GSH synthesis with 0.2 mM BSO markedly enhanced SL-mediated cytolysis of four murine tumor cell lines. A 6- to 34-fold reduction in the amount of SL causing 50% lysis was obtained with BSO. Addition of BSO to P815cells either during or immediately after a 1-h pulse with 10 micrograms/ml of vernolepin increased cytolysis from less than 3% to 78-82%. However, a 1.5-h delay in the addition of BSO to such cells, which allowed for substantial resynthesis of GSH, reduced cytolysis to 30%. Recovery of GSH synthetic capacity after BSO treatment correlated with loss of the synergistic effect of BSO on lysis by vernolepin. BSO did not augment cytolysis by six other antineoplastics (doxorubicin, mitomycin C, vinblastine, cytosine arabinoside, maytansine, and 1,3-bis-[2-chloroethyl]-1-nitrosourea [BCNU]). Of these, only BCNU depleted cellular GSH. Lysis by jatrophone, another GSH-depleting antitumor agent, was increased 21-fold by BSO. Since prolonged incubation with BSO alone results in near-complete GSH depletion without loss of cell viability, SL-mediated cytolysis is probably not a result of GSH depletion. We have demonstrated, however, a critical role for GSH synthetic capacity as a determinant of tumor cell susceptibility to cytolysis by SL. GSH also plays an important role in cellular defense against oxidative injury. Vernolepin, acting as a GSH-depleting agent, markedly sensitized tumor cells to lysis by H2O2 (greater than 6.5-fold increase with 20 micrograms/ml of vernolepin). These findings suggest the possibility that the coordinated deployment of sulfhydryl-reactive antitumor agents, BSO, and oxidative injury might constitute an effective chemotherapeutic strategy.
Full text
PDF
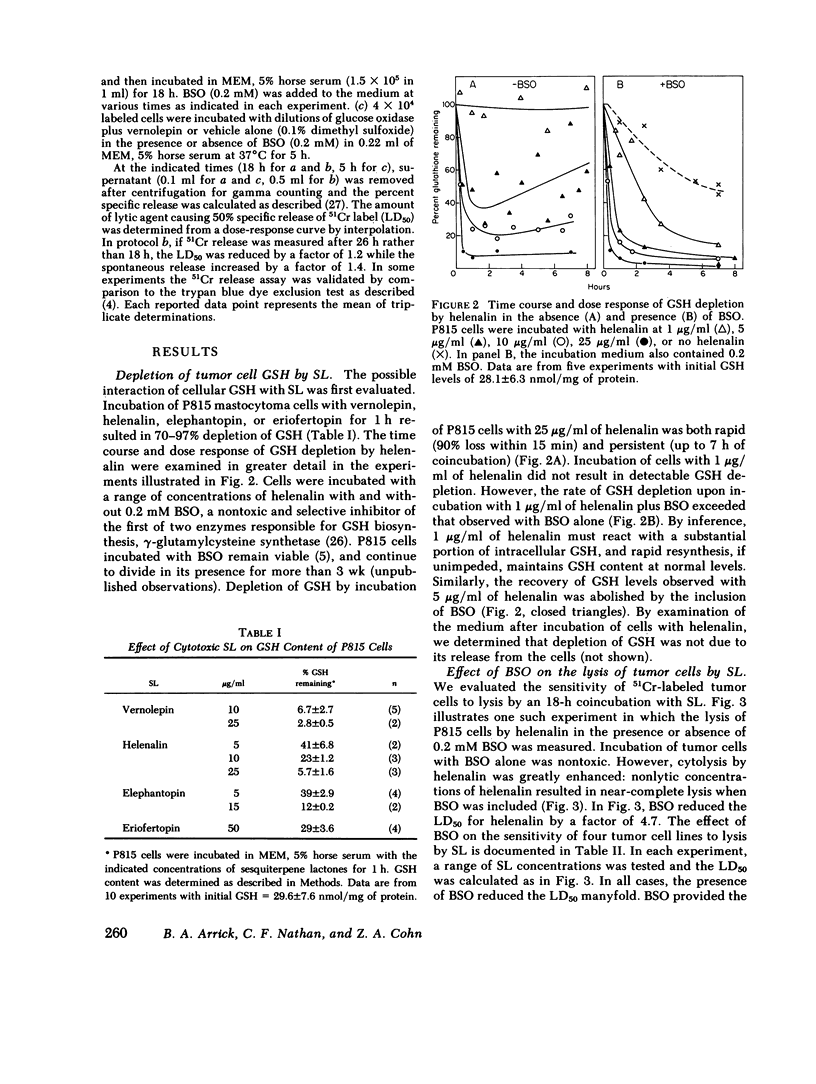
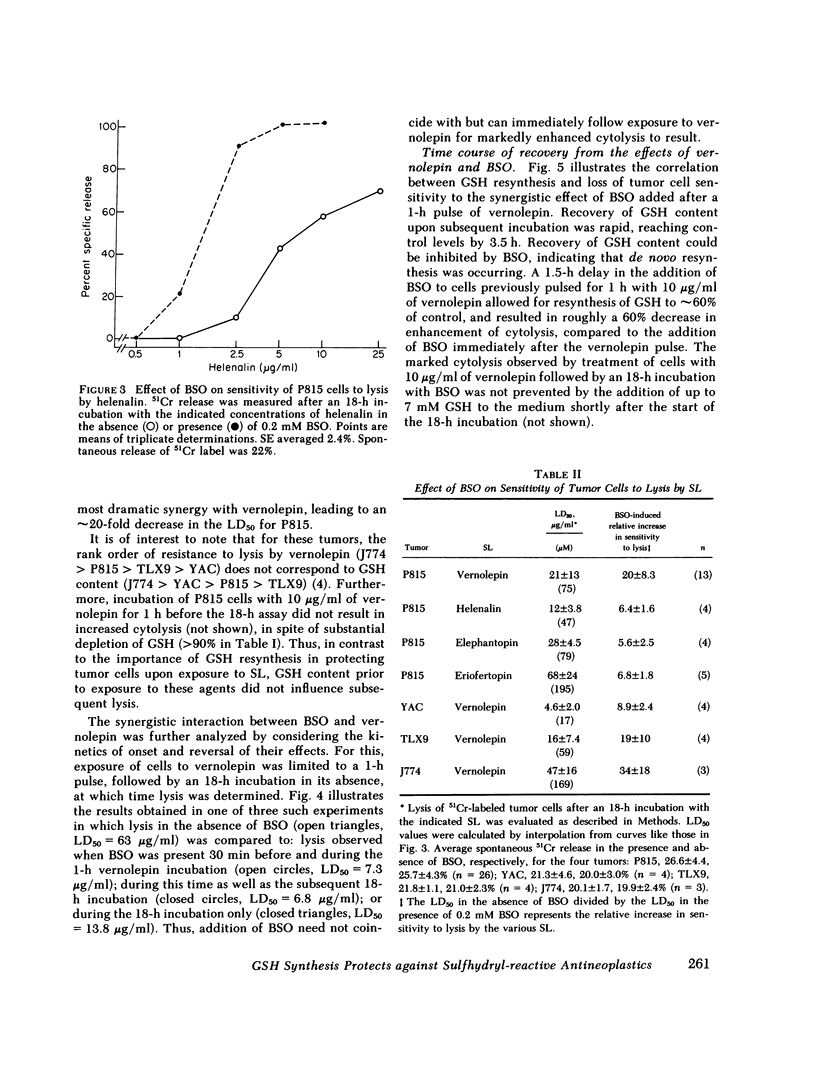
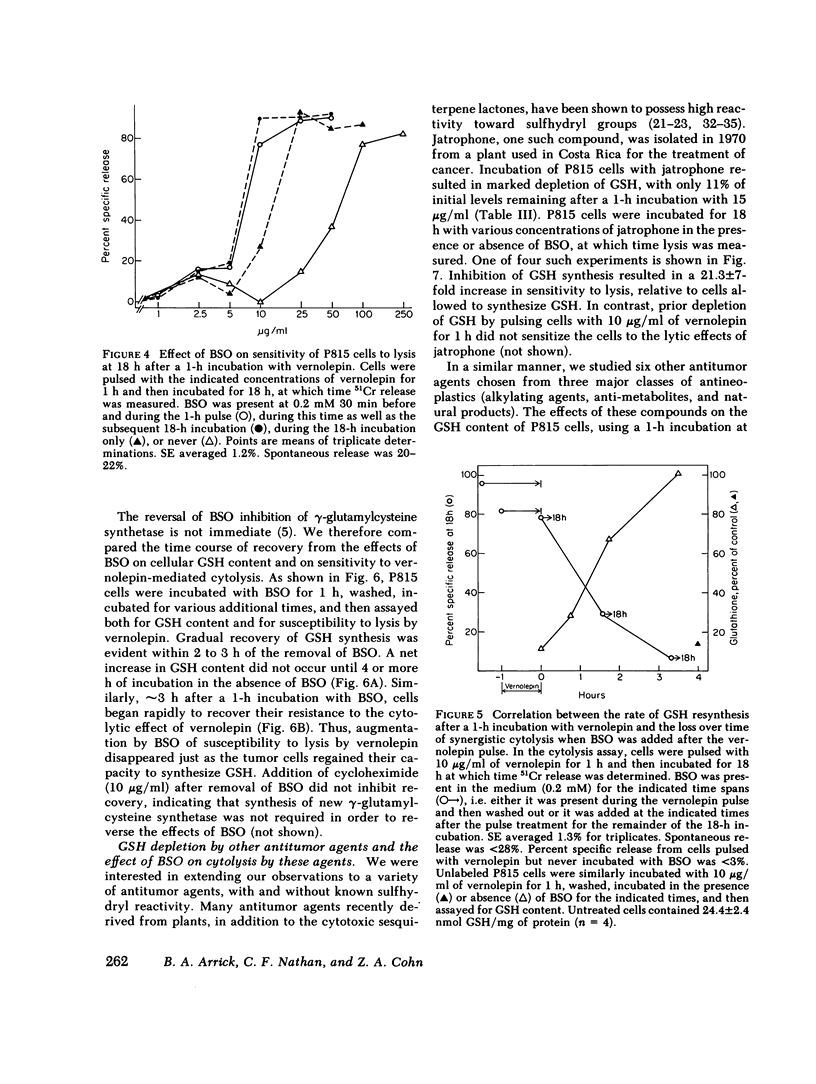


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Nathan C. F., Griffith O. W., Cohn Z. A. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem. 1982 Feb 10;257(3):1231–1237. [PubMed] [Google Scholar]
- Babson J. R., Abell N. S., Reed D. J. Protective role of the glutathione redox cycle against adriamycin-mediated toxicity in isolated hepatocytes. Biochem Pharmacol. 1981 Aug 15;30(16):2299–2304. doi: 10.1016/0006-2952(81)90102-7. [DOI] [PubMed] [Google Scholar]
- CALCUTT G., CONNORS T. A. TUMOUR SULPHYDRYL LEVELS AND SENSITIVITY TO THE NITROGEN MUSTARD MEROPHAN. Biochem Pharmacol. 1963 Aug;12:839–845. doi: 10.1016/0006-2952(63)90114-x. [DOI] [PubMed] [Google Scholar]
- Deschavanne P. J., Malaise E. P., Révész L. Radiation survival of glutathione-deficient human fibroblasts in culture. Br J Radiol. 1981 Apr;54(640):361–362. doi: 10.1259/0007-1285-54-640-361. [DOI] [PubMed] [Google Scholar]
- Dethmers J. K., Meister A. Glutathione export by human lymphoid cells: depletion of glutathione by inhibition of its synthesis decreases export and increases sensitivity to irradiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7492–7496. doi: 10.1073/pnas.78.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshow J. H., Locker G. Y., Baldinger J., Myers C. E. The effect of doxorubicin on hepatic and cardiac glutathione. Res Commun Chem Pathol Pharmacol. 1979 Nov;26(2):285–295. [PubMed] [Google Scholar]
- Doroshow J. H., Locker G. Y., Myers C. E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980 Jan;65(1):128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douros J., Suffness M. New natural products of interest under development at the National Cancer Institute. Cancer Chemother Pharmacol. 1978;1(2):91–100. doi: 10.1007/BF00254042. [DOI] [PubMed] [Google Scholar]
- Dupuis G., Brisson J. Toxic effect of alantolactone and dihydroalantolactone in in vitro cultures of leukocytes. Chem Biol Interact. 1976 Nov;15(3):205–217. doi: 10.1016/0009-2797(76)90147-2. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Depletion of glutathione by inhibition of biosynthesis. Methods Enzymol. 1981;77:59–63. doi: 10.1016/s0076-6879(81)77011-3. [DOI] [PubMed] [Google Scholar]
- Griffith O. W. Glutathione turnover in human erythrocytes. Inhibition by buthionine sulfoximine and incorporation of glycine by exchange. J Biol Chem. 1981 May 25;256(10):4900–4904. [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Gurtoo H. L., Hipkens J. H., Sharma S. D. Role of glutathione in the metabolism-dependent toxicity and chemotherapy of cyclophosphamide. Cancer Res. 1981 Sep;41(9 Pt 1):3584–3591. [PubMed] [Google Scholar]
- Hall I. H., Lee K. H., Eigebaly S. A. Antitumor agents XXVII: Effects of helenalin on anaerobic and aerobic metabolism of Ehrlich ascites cells. J Pharm Sci. 1978 Apr;67(4):552–554. doi: 10.1002/jps.2600670430. [DOI] [PubMed] [Google Scholar]
- Hanson R. L., Lardy H. A., Kupchan S. M. Inhibition of phosphofructokinase by quinone methide and alpha-methylene lactone tumor inhibitors. Science. 1970 Apr 17;168(3929):378–380. doi: 10.1126/science.168.3929.378. [DOI] [PubMed] [Google Scholar]
- Hladoń B., Drozdz B., Grabarczyk H., Bobkiewicz T., Olszewski J. Sesquiterpene lactones. Part XIII. Cytotoxic activity of eupatolide and eupatoriopicrin on human and animal malignant cells in tissue culture in vitro. Pol J Pharmacol Pharm. 1975 Jul-Aug;27(4):429–438. [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- Kupchan S. M., Eakin M. A., Thomas A. M. Tumor inhibitors. 69. Structure-cytotoxicity relationships among the sesquiterpene lactones. J Med Chem. 1971 Dec;14(12):1147–1152. doi: 10.1021/jm00294a001. [DOI] [PubMed] [Google Scholar]
- Kupchan S. M., Fessler D. C., Eakin M. A., Giacobbe T. J. Reactions of alpha methylene lactone tumor inhibitors with model biological nucelophiles. Science. 1970 Apr 17;168(3929):376–378. doi: 10.1126/science.168.3929.376. [DOI] [PubMed] [Google Scholar]
- Kupchan S. M., Lacadie J. A. Dehydroailanthinone, a new antileukemic quassinoid from Pierreodendron kerstingii. J Org Chem. 1975 Mar 7;40(5):654–656. doi: 10.1021/jo00893a024. [DOI] [PubMed] [Google Scholar]
- Kupchan S. M. Novel natural products with antitumor activity. Fed Proc. 1974 Nov;33(11):2288–2295. [PubMed] [Google Scholar]
- Kupchan S. M. Novel plant-derived tumor inhibitors and their mechanisms of action. Cancer Treat Rep. 1976 Aug;60(8):1115–1126. [PubMed] [Google Scholar]
- Kupchan S. M. Recent advances in the chemistry of tumor inhibitors of plant origin. Trans N Y Acad Sci. 1970 Jan;32(1):85–106. doi: 10.1111/j.2164-0947.1970.tb02046.x. [DOI] [PubMed] [Google Scholar]
- Kupchan S. M., Schubert R. M. Selective alkylation: a biomimetic reaction of the antileukemic triptolides? Science. 1974 Aug 30;185(4153):791–793. doi: 10.1126/science.185.4153.791. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. H., Hall I. H., Mar E. C., Starnes C. O., ElGebaly S. A., Waddell T. G., HADGRAFT R. I., Ruffner C. G., Weidner I. Sesquiterpene antitumor agents: inhibitors of cellular metabolism. Science. 1977 Apr 29;196(4289):533–536. doi: 10.1126/science.191909. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Huang E. S., Piantadosi C., Pagano J. S., Geissman T. A. Cytotoxicity of sesquiterpene lactones. Cancer Res. 1971 Nov;31(11):1649–1654. [PubMed] [Google Scholar]
- Lillehaug J. R., Kleppe K., Sigel C. W., Kupchan S. M. Reaction of biological thiols with the tumor inhibitor jatrophone. Inhibition of RNA polymerase. Biochim Biophys Acta. 1973 Nov 15;327(1):92–100. doi: 10.1016/0005-2744(73)90106-x. [DOI] [PubMed] [Google Scholar]
- McConnell W. R., Kari P., Hill D. L. Reduction of glutathione levels in livers of mice treated with N,N'-bis (2-chloroethyl)-N-nitrosourea. Cancer Chemother Pharmacol. 1979;2(3):221–223. doi: 10.1007/BF00258299. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Cohn Z. A. Antitumor effects of hydrogen peroxide in vivo. J Exp Med. 1981 Nov 1;154(5):1539–1553. doi: 10.1084/jem.154.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson R. D., MacDonald J. S., vanBoxtel C. J., Boerth R. C., Harbison R. D., Slonim A. E., Freeman R. W., Oates J. A. Regulatory role of glutathione and soluble sulfhydryl groups in the toxicity of adriamycin. J Pharmacol Exp Ther. 1980 Nov;215(2):450–454. [PubMed] [Google Scholar]
- Picman A. K., Rodriguez E., Towers G. H. Formation of adducts of parthenin and related sesquiterpene lactones with cysteine and glutathione. Chem Biol Interact. 1979;28(1):83–89. doi: 10.1016/0009-2797(79)90116-9. [DOI] [PubMed] [Google Scholar]
- Roos D., Weening R. S., Voetman A. A., van Schaik M. L., Bot A. A., Meerhof L. J., Loos J. A. Protection of phagocytic leukocytes by endogenous glutathione: studies in a family with glutathione reductase deficiency. Blood. 1979 May;53(5):851–866. [PubMed] [Google Scholar]
- Rosowsky A., Papathanasopoulos N., Lazarus H., Foley G. E., Modest E. J. Cysteine scavengers. 2. Synthetic alpha-methylenebutyrolactones as potential tumor inhibitors. J Med Chem. 1974 Jul;17(7):672–676. doi: 10.1021/jm00253a003. [DOI] [PubMed] [Google Scholar]
- Smith C. H., Larner J., Thomas A. M., Kupchan S. M. Inactivation of glycogen synthase by the tumor inhibitor vernolepin. Biochim Biophys Acta. 1972 Jul 13;276(1):94–104. doi: 10.1016/0005-2744(72)90011-3. [DOI] [PubMed] [Google Scholar]
- Suzukake K., Petro B. J., Vistica D. T. Reduction in glutathione content of L-PAM resistant L1210 Cells confers drug sensitivity. Biochem Pharmacol. 1982 Jan 1;31(1):121–124. doi: 10.1016/0006-2952(82)90249-0. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Woynarowski J. M., Konopa J. Inhibition of DNA biosynthesis in HeLa cells by cytotoxic and antitumor sesquiterpene lactones. Mol Pharmacol. 1981 Jan;19(1):97–102. [PubMed] [Google Scholar]



