Abstract
Many recent accounts of the frog peripheral auditory system have reproduced Wever’s (1973) schematic cross-section of the ear of a leopard frog. We sought to investigate to what extent this diagram is an accurate and representative depiction of the anuran inner ear, using three-dimensional reconstructions made from serial sections of Rana pipiens, Eleutherodactylus limbatus and Xenopus laevis. In Rana, three discrete contact membranes were found to separate the posterior otic (=endolymphatic) labyrinth from the periotic (=perilymphatic) system: those of the amphibian and basilar recesses and the contact membrane of the saccule. The amphibian ‘tegmentum vasculosum’ was distinguishable as a thickened epithelial lining within a posterior recess of the superior saccular chamber. These features were also identified in Eleutherodactylus, but in this tiny frog the relative proportions of the semicircular canals and saccule resemble those of ranid tadpoles. There appeared to be a complete fluid pathway between the right and left periotic labyrinths in this species, crossing the cranial cavity. Xenopus lacks a tegmentum vasculosum and a contact membrane of the saccule; the Xenopus ear is further distinguished by a lateral passage separating stapes from periotic cistern and a more direct connection between periotic cistern and basilar recess. The basilar and lagenar recesses are conjoined in this species. Wever’s diagram of the inner ear of Rana retains its value for diagrammatic purposes, but it is not anatomically accurate or representative of all frogs. Although Wever identified the contact membrane of the saccule, most recent studies of frog inner ear anatomy have overlooked both this and the amphibian tegmentum vasculosum. These structures deserve further attention.
Keywords: inner ear, frog, amphibian papilla, basilar papilla, tegmentum vasculosum, contact membrane
INTRODUCTION
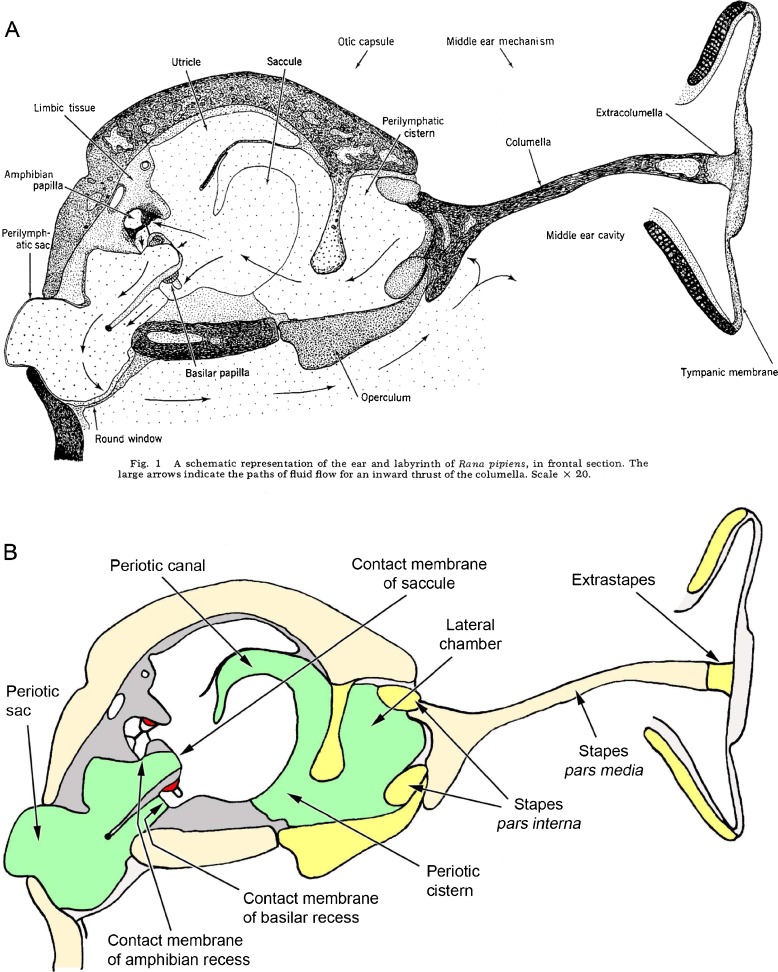
The inner ear structures of ranid frogs have been the subject of detailed anatomical accounts dating back over 150 years, many of which were written in German (see, e.g. Deiters 1862; Hasse 1868; Retzius 1881; Gaupp 1904). Among the best known of the English-language descriptions are those of Ernest Glen Wever. The first figure from Wever’s (1973) paper in Journal of Morphology (reproduced here as Fig. 1A) shows ‘A schematic representation of the ear and labyrinth of Rana pipiens, in frontal section’. An almost identical diagram appeared in two of Wever’s later publications including his 1985 book The Amphibian Ear, which remains the most comprehensive account of the subject. It has been reproduced, sometimes in a modified form, in review papers (e.g. Fay and Popper 1985; Lewis and Narins 1999; Simmons et al. 2007; Gridi-Papp and Narins 2010), a leading textbook on amphibian biology (Duellman and Trueb 1986) and several other articles. As such, it must be the world’s most widely consulted scientific illustration of an amphibian peripheral auditory system. However, as explained below, there is some uncertainty as to the species depicted, the orientation and the accuracy of this important diagram.
FIG. 1.
A Ernest Glen Wever’s schematic diagram of the peripheral auditory apparatus of a ranid frog. The original caption is included. From: Wever (1973) The ear and hearing in the frog, Rana pipiens. Journal of Morphology 141(4): 461-477. Copyright © 1973 Wiley-Liss, Inc. B A representation of the same diagram with the colour coding used elsewhere in this paper, to facilitate comparison between figures. Where Wever’s nomenclature differs from that used in the present paper, structures have been relabelled. Additionally, based on the interpretations of the present study, new labels have been introduced for structures that were not explicitly identified in Wever’s original diagram, including the contact membranes which separate otic and periotic labyrinths. Colour code: white = otic labyrinth (endolymph); green = periotic labyrinth (perilymph); red = sensory epithelium; dark grey = limbic tissue; cream = bone; yellow = cartilage.
The Species Examined
The taxonomy of the North American ranid frogs is currently in a state of flux (Dubois 2007; Hillis 2007; Pauly et al. 2009). Some authors place leopard frogs in the genus Lithobates, but following Hillis’ (2007) recommendation, we retain the genus Rana for North American ranids.
Wever (1973) stated that his illustration shows the ear of R. pipiens, but in his 1985 book, he distinguished between this species (the northern leopard frog) and the very similar ‘Rana utricularia sphenocephala’, now R. sphenocephala utricularia (the southern leopard frog). Wever (1985) did not make it clear which of these species is represented in the figure of interest to us here, which appears in slightly modified form as his Fig. 3-17. However, another illustration also reproduced from his 1973 paper (Fig. 3-79 in Wever 1985) is labelled as R. sphenocephala. Wever’s (1973) paper may therefore describe the ear of the southern leopard frog, but it is likely that the ears of these two species are practically identical. The articles that have reproduced Wever’s illustration have often implied that a generalised anuran morphology is represented.
The Orientation of Wever’s Illustration
Within his 1973 paper, Wever stated that he sectioned his specimens in ‘a horizontal plane (dorsal to ventral)… frontally (anterior to posterior) or laterally (right to medial, then continued from medial to left)’. This description is somewhat confusing, and perhaps for this reason, Wever later redefined the three planes. In Wever’s (1985) Fig. 3-34, a frontal plane is clearly shown dividing the head into dorsal and ventral components. Such a plane would pass through most of the teeth on both left and right maxillae and may be considered horizontal. A sagittal plane vertically divides the head into left and right components. A transverse plane is a vertical plane perpendicular to the frontal and sagittal planes, which divides the head into anterior and posterior components. These definitions agree with the standard veterinary anatomical nomenclature (Blood and Studdert 1999) and are those used in the present study. In our interpretation, ‘horizontal’ (Wever 1973) is actually frontal, ‘lateral’ (Wever 1973) is actually sagittal and ‘frontal’ (Wever 1973) is actually transverse.
The present study focuses on the first figure from Wever (1973), the caption of which reads ‘the ear and labyrinth… in frontal section’ (Fig. 1A). Was this in fact a transverse section, according to standard nomenclature?
The Accuracy of Wever’s Illustration
When preparing a previous article (van Dijk et al. 2011), two of the current authors had reason to question the accuracy of Wever’s 1973 diagram. Wever shows the extrastapes (=extracolumella) as a very short extension of the bony stapes shaft (stapes pars media), connecting it to the centre of the tympanic membrane (Fig. 1). It would be natural to assume that this apparatus must operate as a stiff piston, an inflection of the tympanic membrane driving the stapes directly into the inner ear. In reality, the extrastapes of ranid frogs is much longer than this and has an angled articulation with the pars media: the stapes/extrastapes system works as a flexible, first-order lever (Jørgensen and Kanneworff 1998; Mason and Narins 2002; Werner 2003).
Turning to the inner ear, Wever shows three pathways for ‘fluid flow’ to pass between the stapes on the right of his diagram to the periotic (=perilymphatic) sac on the left, one via the amphibian papilla, a second via the basilar papilla and a third between the two papillae, each being indicated by arrows in Fig. 1A. The endolymph within the otic (=endolymphatic) labyrinth in frogs is separated from the perilymph within the periotic labyrinth by so-called contact membranes (Fig. 1B), so fluid cannot actually flow between the two systems and the three arrows should instead be taken to indicate three pathways of acoustic energy flow. In their more recent account of energy flow pathways through the ear of the bullfrog (Rana catesbeiana), Purgue and Narins (2000a, b) considered the routes passing through the amphibian and basilar recesses but made no mention of Wever’s middle pathway. Purgue and Narins regarded the periotic canal as an alternative route for low-frequency energy flow which bypasses the otic system entirely, but this is not labelled in Wever’s diagram and there is scant reference to it in his written descriptions. Four potential pathways for sound energy flow between stapes and periotic sac have therefore been described in frogs, but are all four consistently present?
In this study, histological sections were made from the inner ears of three species of frogs, including leopard frogs (these were believed to be R. pipiens rather than R. sphenocephala, but this could not be confirmed beyond doubt by the suppliers). Photomicrographs and three-dimensional (3D) reconstructions were used to assess the accuracy of Wever’s accounts and other recent descriptions of ranid inner ear morphology.
While leopard frogs are in the family Ranidae, within the Ranoidea clade of the Neobatrachia, Eleutherodactylus limbatus (Eleutherodactylidae) is placed within the other major neobatrachian clade, the Hyloidea (Hoegg et al. 2004). Xenopus laevis, family Pipidae, is an aquatic ‘archaeobatrachian’, the ‘Archaeobatrachia’ being a paraphyletic assemblage of frogs which diverged before the Neobatrachia (Hoegg et al. 2004). In order to assess whether Wever’s diagram is representative of a more diverse range of frogs, the leopard frog ear was compared here with the ears of E. limbatus, one of the world’s smallest frogs, and with those of X. laevis.
MATERIALS AND METHODS
Twelve frogs from three different species were used in this study. Three leopard frogs believed to be R. pipiens (40–50 g body mass) were obtained from Charles D. Sullivan Co. Inc. (Nashville, TN, U.S.A.) via Exoterra Schaudi GmbH, Holzheim, Germany. They were housed at the University of Groningen laboratory animal facilities. The frogs were euthanized using the double pith procedure and then decapitated. The lower jaw was removed and the remaining part of the head was divided sagittally. Skin was removed and small holes were made in various places in the skull, away from the structures of interest, to improve fluid impregnation. The ears were fixed by immersion in a 10 % neutral buffered formalin solution (pH 7.4) for at least 24 h at 4 °C. The fresh corpse of a male R. pipiens, originating from Nasco (Fort Atkinson, WI, USA), was used for micro-computed tomography (micro-CT) scanning, as described below.
Two E. limbatus specimens (each around 0.2 g body mass) were captured at Las Terrazas, Artemisa province, Cuba. They were euthanized by double pithing and decapitation, the palatal skin was removed and their heads were preserved in 10 % formalin and sent to Groningen for further processing. The head of one specimen was halved prior to sectioning, while the other was sectioned whole.
Six X. laevis specimens (males 55–60 g, females 120–220 g, all gonadectomized body masses) were obtained as fresh corpses from a breeding colony in the Wellcome Trust/Cancer Research UK Gurdon Institute, Cambridge, UK. They had been euthanized via tricaine overdose followed by cooling, as part of another study. The otic capsules of one male and one female specimen were cut out and placed in 4 % buffered formaldehyde solution within two hours of euthanasia. They were then sent to the University of Groningen where they were processed as the Rana specimens. A micro-CT scan was made of the head of another male specimen at the University of Cambridge, and the head was then dissected under light microscopy. The remaining three specimens, two females and a male, were also dissected.
Animal care and euthanasia procedures conformed to local and national regulations and were approved by the appropriate institutional Animal Care and Use Committees.
Histological Procedures
After fixation the Rana and Xenopus specimens were rinsed in distilled water, refreshed several times. All subsequent steps were performed on a rolling bank to keep the specimens moving in the experimental solutions. Decalcification took place in a 10 % EDTA solution (Sigma, ED5SS, pH 7.34) at a temperature of 50 °C in a microwave oven (T/T MEGA microwave histo-processor, Milestone), in four sessions of twelve hours. After decalcification the specimens were rinsed again in distilled water and dehydrated in a graded, seven-step ethanol series (30, 50, 70, 90, 96, 100, 100 %) where each step took one hour and solutions were refreshed three times. If necessary, specimens were stored overnight in 70 % ethanol. Next, specimens were placed in a 100 % ethanol/hydroxypropyl methacrylate (HPMA) solution (50:50) for 4–8 h and then put in pure HPMA solution for 24–48 h. The specimens were then embedded in pure HPMA solution with addition of a plasticizer (around 25:1). The HPMA solution contained 45 ml HPMA, 5 ml ethylene glycol monobutyl ether, 0.5 g benzoyl peroxide, 1.25 ml glycerol and 0.25 ml ethylene glycol dimethacrylate. The plasticizer consisted of 1 ml n,n-dimethylaniline and 10 ml polyethylene glycol 400.
The Eleutherodactylus sections were prepared using a faster procedure owing to time constraints. The decalcification was performed in only two steps of 7 and 12 h, and the ethanol dehydration series was also slightly altered (30 % for 30 min, three times; 70 % for 30 min, three times; 90 % for 10 min, three times; 96 % for 10 min, three times and 100 % for 15 min, twice). The specimens were placed in the ethanol/HPMA solution for two sessions of 1 h, then overnight.
After polymerisation, transverse sections of 4 μm thickness were cut using a motorised microtome (HM350S, Microm, Heidelberg, Germany). In some cases, the otoconial mass of the saccule, if identified during the sectioning procedure, was removed from the embedded specimen using a fine needle so as to avoid damaging the microtome. A subset of sections was stained with toluidine blue 1 % (10 min) and contrast-stained with basic fuchsin (15–20 s).
3D Reconstruction from Serial Sections
Digital photographs of Rana and Xenopus sections were made with an Olympus Camedia C-5050 digital camera and stored as tiff files. Digital photographs of the smaller Eleutherodactylus specimens were made using a Leica DM RXA microscope fitted with a Colorview 1 MP camera (Soft Imaging System), working with AnalySiS software (Olympus). Individual files, in some cases reduced in size by cropping and/or conversion to greyscale, were then loaded into ImageJ 1.45 s (W. Rasband, 2011, National Institutes of Health) and autoregistered using the StackReg plug-in (Thévenaz 2011, Biomedical Imaging Group, Swiss Federal Institute of Technology, Lausanne; see Thévenaz et al. 1998). StackReg uses a recursive procedure based on rigid-body translation and rotation to align each consecutive section. WinSurf 4.0 (Moody and Lozanoff 1998) was then used to construct three-dimensional images, following visual identification of relevant structures. Where wall thickness was significant, the internal rather than external walls of the otic and periotic labyrinths were traced and modelled. The choice of interval between sections used to make the final reconstruction depended upon the size of the structure being reconstructed and the level of detail required. In the production of Fig. 12, MicroView 2.1.2 (GE Healthcare, 2006) was used to reorient the registered image stacks.
FIG. 12.
Reconstructions created from a stack of serial section images of the ear of Rana pipiens, digitally resectioned in a plane as close as possible to Wever’s (1973) illustration (Fig. 1). See text for details. The extrastapes, tympanic membrane, periotic sac and round window were not within the original sections and are consequently not shown in these reconstructions. A ‘Virtual section’; the faint, diagonal striations indicate the planes of the original section photomicrographs from which this was reconstructed. B Expanded, diagrammatic illustration of the same. Out of the plane of this particular section, the periotic canal is in communication with the periotic fluid which abuts the contact membranes of the amphibian and basilar recesses; these regions are all components of the periotic labyrinth and are hence shaded in green. The asterisk indicates a process of the stapes pars media which articulates with the otic capsule. C WinSurf reconstructions of the right inner ear of R. pipiens from (left) lateral, (middle) posterior and (right) dorsal views, showing the position of the ‘virtual section’ as a grey plane. The stapes and operculum are included in these reconstructions but the internal walls of the otic capsule are not. Both scale bars, 2 mm. Colour code: white = otic labyrinth (endolymph); green = periotic labyrinth (perilymph); dark grey = limbic tissue; lighter grey = looser periotic tissue; cream = bone; yellow = cartilage
One potential problem with 3D reconstruction from serial sections is systematic misalignment of the sections, resulting in a distorted (skewed or twisted) representation, and it can also be difficult to determine orientation. Eleutherodactylus was small enough that a whole head could be sectioned and reconstructions from right and left ears compared. In the case of Rana and Xenopus, the reconstructions from serial sections were compared with micro-CT reconstructions of the whole skull and the ear regions within it (see below). Although soft tissue could not be visualised in our CT scans, hard-tissue structures including otic capsule walls and stapes shaft provided sufficient landmarks for comparison with the serial section reconstructions.
Photomicrographs and reconstructions were laterally inverted where necessary, to facilitate comparison.
Micro-CT Reconstructions
Micro-CT images were obtained of the head of one male Xenopus specimen at the University of Cambridge. The posterior part of the head was skinned and tissues between the mandibles were removed. The head was then wrapped in cellophane to reduce the rate of drying, and the head was scanned using a Metris X-Tek HMX 160 micro-CT scanner operating at 50 kV and 50 μA with no prefilter. The stepping rotational angle was 0.5 degrees. The software used in the processing of the scan data included iXS Integrated X-ray System Control version 4.1.29 (X-Tek Systems Ltd., 2002), NGI CT Control version 1.5.4 (X-Tek Systems Ltd., 2005) and CT-Pro 2.0 (Metris, 2008). At UCLA, a micro-CT scan was made of the head of one male R. pipiens specimen, immersed in a buffered salt solution within a sample holder. A desktop micro-CT machine was used (MicroCT 40; Scanco Medical, Bassersdorf, Switzerland), operating at 55 kV and 145 μA with a 0.5-mm Al prefilter. The stepping rotational angle was 0.36 degrees. The image was processed using Scanco proprietary software. For both animals, the voxels in the scan images were of 30 μm side length.
VGStudio Max 2.0.1 (Volume Graphics GmbH, 2008), MicroView 2.1.2 and WinSurf 4.0 were used to construct 3D images from the CT data obtained. The CT reconstructions were used to verify that the reconstructions made from serial sections of Rana and Xenopus were not distorted and to determine their orientation relative to the skull.
RESULTS
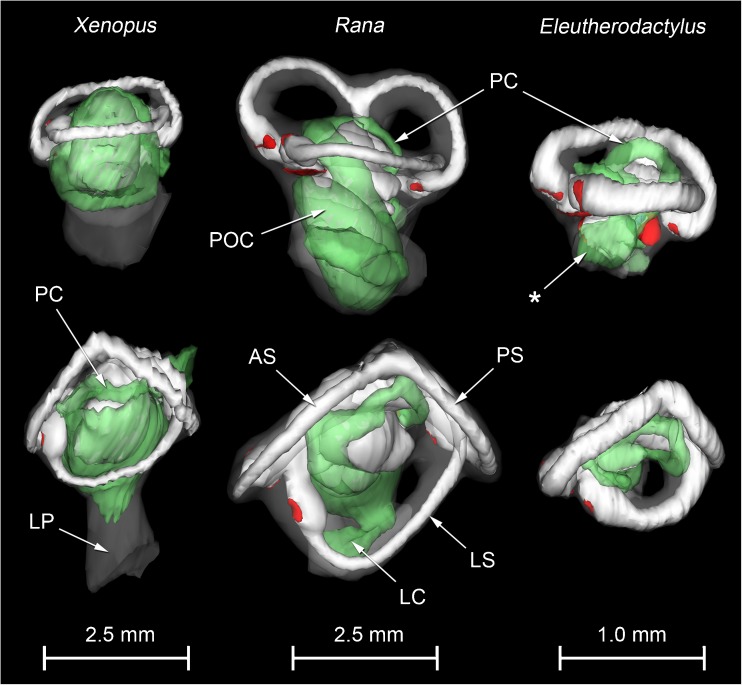
WinSurf reconstructions of the inner ears of the three anuran species are presented for comparison in Fig. 2. There was no evidence of systematic distortion of the reconstructions made from serial sections, as determined by comparison between different ears and/or comparison with micro-CT reconstructions. Histological artefacts inevitably affected the reconstructions, however, as described below.
FIG. 2.
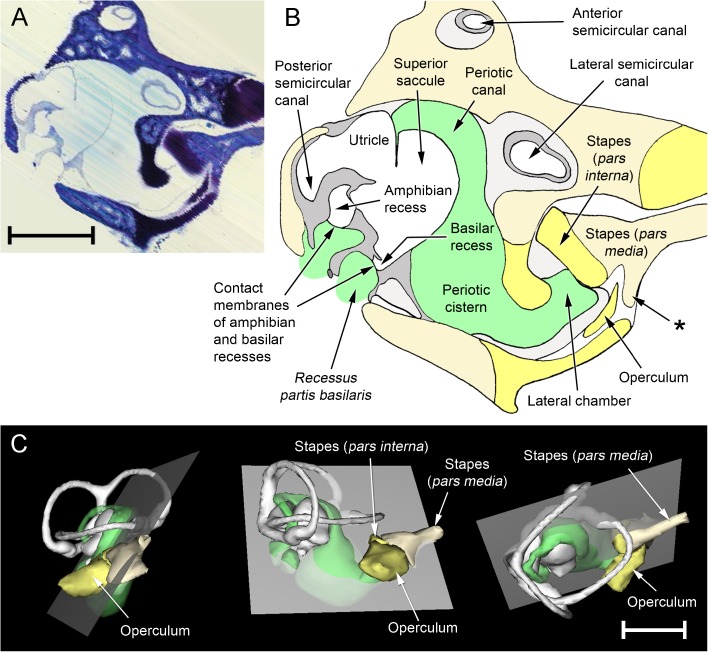
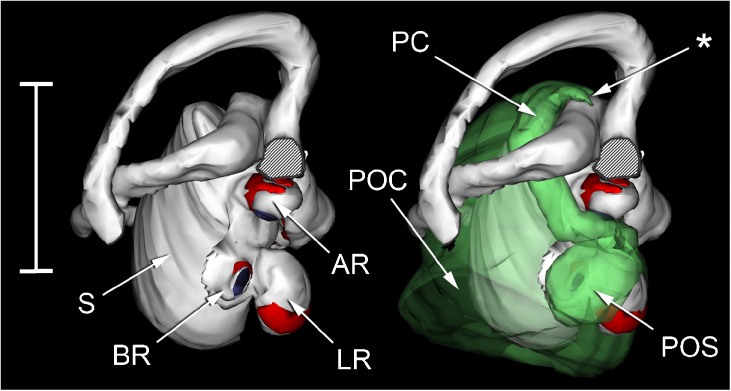
WinSurf reconstructions of the left inner ear structures of Xenopus laevis (left), male specimen, Rana pipiens (middle) and Eleutherodactylus limbatus (right). Lateral views are shown in the top row, dorsal views in the bottom row. Eleutherodactylus reconstructions are 2.5× enlarged relative to the others. In the Eleutherodactylus sections used for these reconstructions, the periotic cistern in the region marked with an asterisk, which lies lateral to the very small saccular cavity, had collapsed. Its approximate shape has been restored here by comparison with the contralateral ear and the extent of the space available for it within the otic capsule. Colour code: white = otic labyrinth (endolymph); green = periotic labyrinth (perilymph); red = sensory epithelium; semitranslucent grey = internal walls of the otic capsule. Key to this and subsequent figures: AR amphibian recess (endolymph), AS anterior semicircular canal, B brain, BR basilar recess (endolymph), CA contact membrane of amphibian recess, CB contact membrane of basilar recess, CC crus commune (confluence of anterior and posterior semicircular canals), CS contact membrane of saccule, IS inferior saccular chamber, LC lateral chamber, LP lateral passage, LR lagenar recess (endolymph), LS lateral semicircular canal, LT limbic tissue, O operculum, PC periotic canal, POC periotic cistern, POS periotic sac, PS posterior semicircular canal, PT periotic tissue, PU posterior utricular cavity, RPB recessus partis basilaris (perilymph), RW round window, S saccule, SPI stapes pars interna, SPM stapes pars media, SS superior saccular chamber, TV tegmentum vasculosum or the saccular diverticulum within which this epithelial lining is found, VIII branch of eighth cranial nerve.
Rana pipiens
Reconstructions of the inner ear of Rana are shown in Figs. 2 and 3, and photomicrographs of sections of particular interest are presented as Figs. 4 and 5.
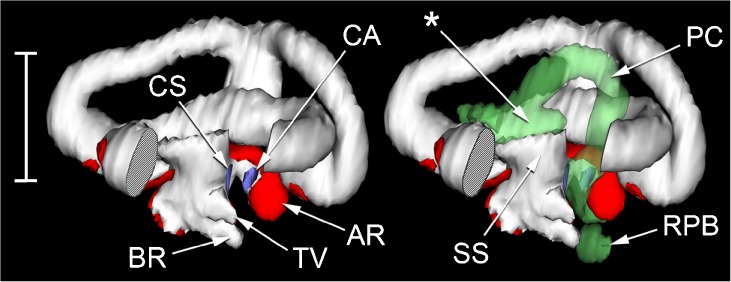
FIG. 3.
WinSurf reconstructions of left inner ear structures of Rana pipiens, seen from an approximately posterior view. The reconstruction on the left shows the otic labyrinth (white), sensory epithelia (red) and contact membranes separating endo- and perilymph (purple). Part of the posterior semicircular canal has been removed to reveal the diverticula of the superior saccule. The reconstruction on the right shows the same, with the periotic labyrinth added in (semitranslucent green). Scale bar, 2.5 mm. See Fig. 2 caption for full list of abbreviations.
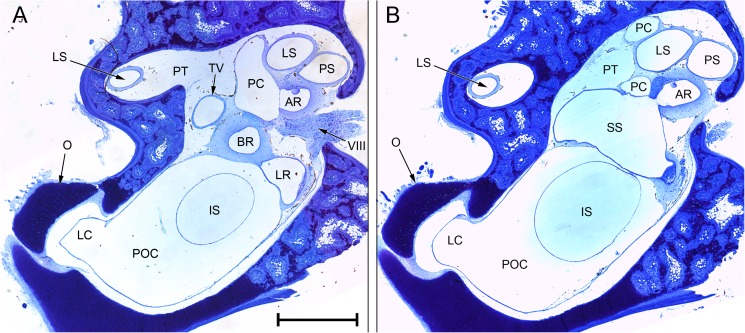
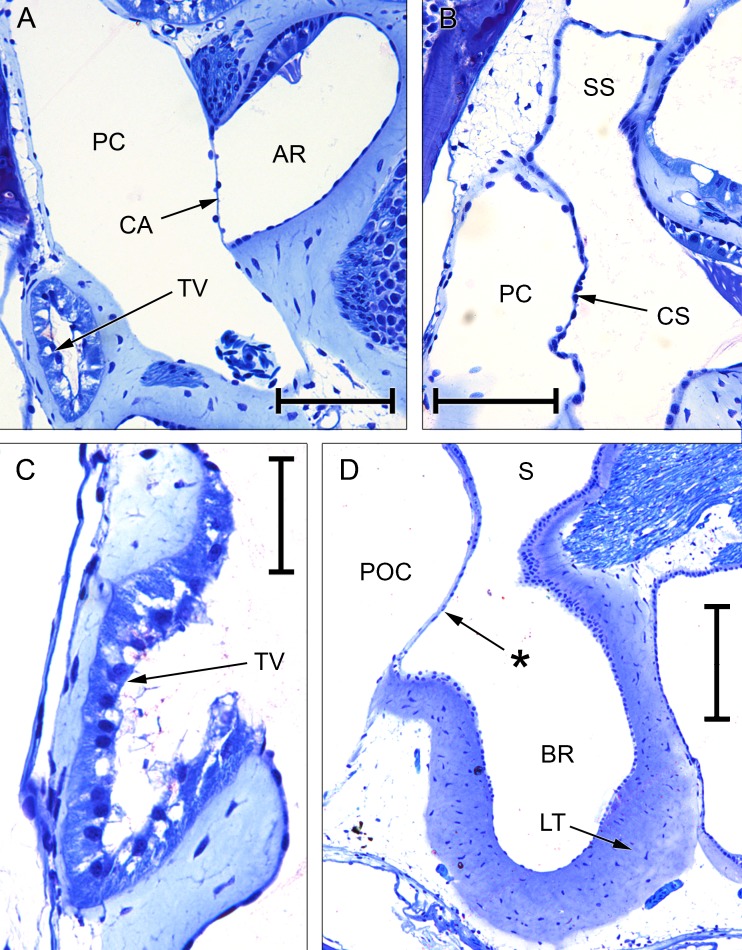
FIG. 4.
Composite photomicrographs of two approximately transverse sections through the posterior part of the inner ear of Rana pipiens. B A plane 240 μm anterior to (A). Relative to the centre of each photomicrograph, dorsal is upwards and slightly away from the viewer, lateral is to the left. Scale bar applies to both (A) and (B) and represents 1 mm. See Fig. 2 caption for full list of abbreviations.
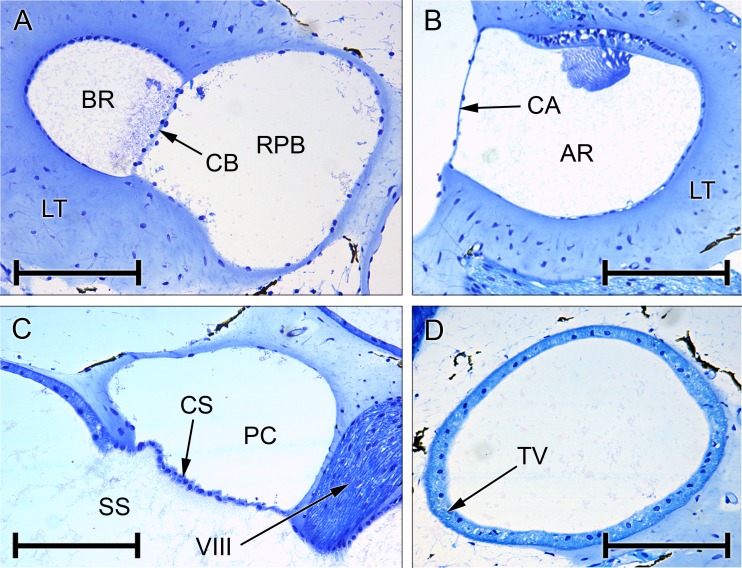
FIG. 5.
Photomicrographs of sections through the inner ear of Rana pipiens. A Basilar recess (BR; endolymph), recessus partis basilaris (RPB; perilymph) and contact membrane of basilar recess (CB) separating the two. B Amphibian recess (AR) and its contact membrane (CA), expanded from Fig. 4A. C Periotic canal (PC) and contact membrane of the saccule (CS), expanded from Fig. 4B. D Tegmentum vasculosum (TV), expanded from Fig. 4A. All scale bars, 200 μm. See Fig. 2 caption for full list of abbreviations.
Considering first the otic labyrinth, the saccule is partially divided by a central constriction into inferior and superior compartments (Fig. 3). The inferior saccule is an ovoid chamber, flattened rostromedially. The saccular macula (sensory epithelium) is at the centre of the flattened surface. The superior saccule has an expanded dorsal chamber and four relatively small, posterior diverticula:
The prominent amphibian recess (Figs. 3, 4A, B and 5B) extends medially from the dorsomedial part of the superior saccule before turning caudally. The sensory epithelium on its dorsal wall is known as the amphibian papilla, although this term is sometimes used to refer to the whole chamber and its contents.
The lagenar recess (Figs. 3 and 4A) extends medially from the caudoventral part of the superior saccule, below the amphibian recess. Its sensory epithelium covers its medial wall.
A third small diverticulum, the only one to lack a sensory end-organ, extends caudally from the dorsolateral part of the superior saccule (Fig. 3). The thick epithelium forming the internal lining of this diverticulum is known as the tegmentum vasculosum (Figs. 4A and 5D). This lining extends rostrally into the posterior part of the superior saccular chamber.
The basilar recess (Figs. 3, 4A and 5A) is located between the cavity of the tegmentum vasculosum and the lagenar recess. Its sensory epithelium (basilar papilla) lies on its medial wall.
Rostral to the amphibian recess, the superior saccule communicates via a constricted region, the utriculo-saccular foramen, with the elongated utricular chamber. The sensory epithelium of the utricle is on the ventral wall of the free, rostral portion of this chamber, which then divides to form the ampullae of the anterior and lateral semicircular canals (Fig. 2). From the caudal end of the utricular chamber arise the other end of the lateral semicircular canal and the crus commune, a short, vertical segment representing the convergence of the anterior and posterior semicircular canals (Fig. 3). The ampulla of the posterior semicircular canal is located just underneath the caudal-most part of the lateral semicircular canal; the two are not in contact.
We turn now to the periotic system, which may be divided (after Lombard 1977) into periotic tissue and the periotic labyrinth proper. Periotic tissue is the connective tissue found separating both otic and periotic labyrinths from the walls of the otic capsule. In places, it takes the form of a condensed and cartilage-like ‘limbic tissue’ (Wever 1973). Limbic tissue forms a thin layer around the membranes of the semicircular canals and utriculus, but it is much thicker around the amphibian and basilar recesses (Fig. 5A, B). The lagenar recess and part of the tegmentum vasculosum are also supported by limbic tissue. Elsewhere, the periotic tissue consists of little more than a diffuse collection of fibres within a fluid space. The semicircular canals, within their thin shells of limbic tissue, are separated from the otic capsule walls by such a fluid space, as is much of the superior saccule (Fig. 4A, B).
The other component of the periotic system, the periotic labyrinth, is a membranous sac of complex shape containing apparently acellular fluid. Its three main subdivisions are the periotic cistern, the periotic canal and the periotic sac. The capacious periotic cistern (Figs. 3 and 4A, B) almost completely surrounds the inferior saccule, extending around it on the medial side as far dorsally as the utricular chamber. A diverticulum of the lateral part of the periotic cistern extends through a narrow, oval-shaped foramen in the wall of the otic capsule and turns sharply rostrally to expand into a lateral chamber (Figs. 2, 3 and 4). The cartilaginous operculum lies immediately over the foramen (Fig. 4A, B), while the stapes footplate is rostral to this. The footplate comprises the expanded medial part of the bony pars media and, around its periphery, the U-shaped, cartilaginous pars interna. The operculum and stapes footplate interlock: a flange of the pars interna extends a short distance medial to the operculum, while the rostrolateral corner of the operculum fills the gap between the pars interna and a ventral process of the pars media which articulates with the otic capsule.
The periotic canal is a long, narrow tube which ascends dorsally from the lateral part of the periotic cistern and wraps closely around the anterior aspect of the superior saccule (Figs. 2 and 3). There is a thin, shared membrane between periotic and otic labyrinths throughout this course. The periotic canal then parts from the saccule near the crus commune, turns sharply caudolaterally and bends down around the lateral semicircular canal to meet the superior saccule again between the amphibian recess and the diverticulum of the tegmentum vasculosum. The oval region of apposition found here between otic and periotic labyrinths is the contact membrane of the saccule (Figs. 3 and 5C), identified in all three specimens of R. pipiens just lateral to the contact membrane of the amphibian recess. The contact membrane of the saccule has 33–76 % (n = 3 ears) of the area of the contact membrane of the amphibian recess, and it is more than twice as thick but still represents a relatively thin window between the otic and periotic labyrinths, in a region where much of the otic system is surrounded by a thick limbic tissue.
The periotic canal then turns ventromedially to form an elongated, curved contact membrane with the lateral wall of the amphibian recess (Fig. 5B). Leaving the otic capsule, the canal runs for a short distance parallel to the recessus partis basilaris, a blind-ending periotic diverticulum heading rostrally towards the basilar recess (Figs. 3 and 5A). There is a small contact membrane between the apposed tips of the recessus partis basilaris and the basilar recess (Fig. 5A), which is 6–14 % (n = 3 ears) of the area of the contact membrane of the amphibian recess. The sections of the three Rana specimens stopped at this point, so the relationship between the recessus partis basilaris and the rest of the periotic system could not be examined. From the literature (see, e.g. Lewis and Narins 1999), the recessus partis basilaris and the periotic canal are expected to communicate with each other via the periotic sac, a caudal expansion of the periotic canal which projects out of the otic capsule.
Eleutherodactylus limbatus
Reconstructions of the inner ear of Eleutherodactylus are shown in Figs. 2 and 6, and photomicrographs of sections of particular interest are presented as Figs. 7, 8 and 9.
FIG. 6.
WinSurf reconstructions of left inner ear structures of Eleutherodactylus limbatus, seen from an approximately lateral view. Part of the lateral semicircular canal has been removed to reveal the contact membrane of the amphibian recess (CA) and the contact membrane of the saccule (CS). The reconstruction on the left shows the otic labyrinth and associated structures only; the reconstruction on the right includes the dorsal and posterior parts (only) of the periotic labyrinth. The asterisk indicates the ventral diverticulum of the central part of the periotic canal, which is closely apposed to the superior saccular chamber (SS). Scale bar, 0.5 mm. Colour code: white = otic labyrinth (endolymph); semitranslucent green = periotic labyrinth (perilymph); red = sensory epithelium; purple = contact membrane separating endo- and perilymph. See Fig. 2 caption for full list of abbreviations.
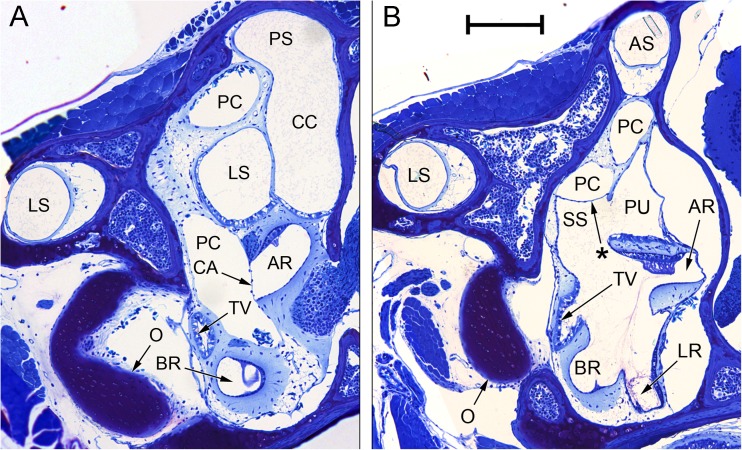
FIG. 7.
Composite photomicrographs of two sections through the inner ear of Eleutherodactylus limbatus. A An approximately transverse plane, through the posterior part of the inner ear. Relative to the centre of the photomicrograph, dorsal is upwards and slightly away from the viewer, lateral is to the left. B An oblique plane (between transverse and sagittal), taken from the ear on the contralateral side to (A) and laterally inverted to aid comparison. Relative to the centre of the photomicrograph, dorsal is upwards, lateral is to the left and away from the viewer. Note in particular the membrane marked with an asterisk, located between the ventral diverticulum of the central part of the periotic canal (PC) and the superior saccular chamber (SS). The membranous labyrinth has pulled away from the otic capsule wall on the right-hand side. Scale bar applies to both (A) and (B) and represents 200 μm. See Fig. 2 caption for full list of abbreviations.
FIG. 8.
Photomicrographs of sections through the inner ears of Eleutherodactylus limbatus and Xenopus laevis. A Tegmentum vasculosum (TV), amphibian recess (AR) and its contact membrane (CA) in E. limbatus; scale bar, 100 μm. B Periotic canal (PC) and the contact membrane of the saccule (CS) in E. limbatus; scale bar, 100 μm. C Tegmentum vasculosum of E. limbatus; scale bar, 50 μm. D Basilar recess (BR) of X. laevis (female specimen); scale bar, 200 μm. The asterisk indicates the additional ‘tympanal area’ identified by Paterson (1949, 1960), located just rostrolateral to the basilar recess (see text). See Fig. 2 caption for full list of abbreviations.
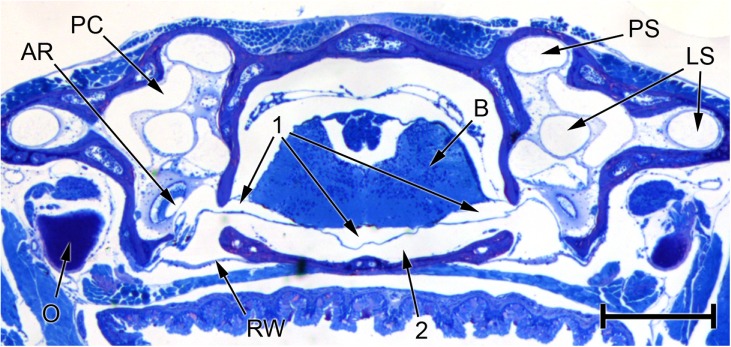
FIG. 9.
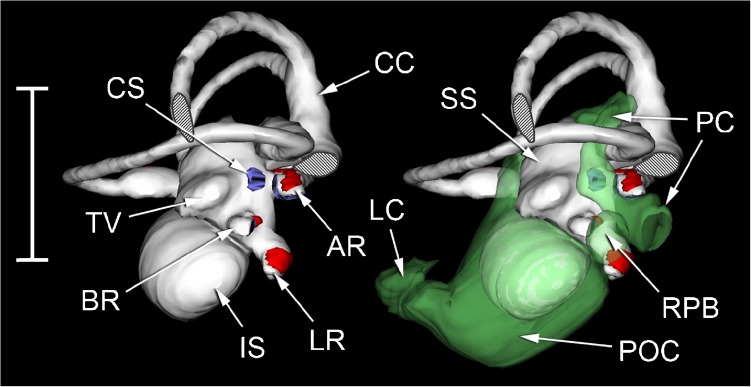
Photomicrograph of a transverse section through the head of Eleutherodactylus limbatus, at the level of the posterior half of the otic capsule. The three fluid compartments collectively denoted ‘1’ appear to be separate in this section, but inspection of other sections in the same series suggests that they are actually part of one continuous fluid system extending between the periotic labyrinths of each ear and passing underneath the brain (B). The left-hand arrow marked ‘1’ points to the left periotic sac, which can be seen emerging from the left otic capsule. Within the capsule, it is immediately adjacent to the amphibian recess (AR), from which it is separated by a thin contact membrane. Between fluid space 1 and the base of the skull is a second fluid space (2), which is also continuous across the head and extends between the two round windows (RW). Distortion resulting from shrinkage may have changed the relative sizes of fluid spaces 1 and 2. Scale bar, 0.5 mm. See Fig. 2 caption for full list of abbreviations.
There is no distinct lateral chamber in Eleutherodactylus, but the footplate and operculum lie at an angle to each other such that their inner surfaces form a bowl-like concavity. The stapes footplate is relatively small; as in Rana, it sends a prominent cartilaginous flange under the large operculum. The inferior saccule/periotic cistern region had evidently collapsed to a greater or lesser extent in all four ears examined because it had pulled away from surrounding structures, but its original shape could be determined as the region enclosed between otic capsule, stapes and operculum. The other parts of the inner ear escaped distortion in at least one specimen.
Given the size of the chamber in which it is contained, the inferior saccule must be relatively much smaller than that of Rana or Xenopus, while the semicircular canals are much wider relative to their length (Fig. 2). A narrow diverticulum lined with a tegmentum vasculosum extends from the saccular cavity just dorsolateral to the basilar recess (Figs. 6, 7A, B and 8A, C).
The periotic canal (Fig. 6) is relatively longer and more convoluted than in Rana. Because the superior saccule is little inflated in Eleutherodactylus, the canal, where it emerges from the periotic cistern, is initially not in such close contact with the saccular cavity. However, after turning caudally a diverticulum of the central part of the periotic canal extends downwards and comes into intimate apposition with the superior saccular cavity (Figs. 6 and 7B). As in Rana, the periotic canal then separates from the otic labyrinth and runs across the lateral semicircular canal en route to the amphibian recess. The contact membrane of the saccule (Figs. 6 and 8B) is located just rostral to the contact membrane of the amphibian recess (Figs. 6, 7A and 8A). The contact membrane of the saccule has 26–41 % (n = 3 ears) of the area of the contact membrane of the amphibian recess. The contact membrane of the basilar recess is around 5–15 % (n = 4 ears) of the area of the amphibian recess contact membrane.
The recessus partis basilaris of the periotic labyrinth (Fig. 6) originates from the periotic sac, which extends out of the otic capsule and into the brain case. In the whole-head sections which were made from one Eleutherodactylus specimen, the periotic sac appears to extend underneath the brain to meet and freely communicate with its contralateral counterpart (Fig. 9). A second fluid space just below this periotic space extends between the right and left round windows. The two fluid spaces are separated by a membrane which may be meningeal in origin. It was unclear whether this membrane had simply separated from the basicranial bones due to shrinkage, or whether it really does separate two fluid compartments in vivo. The membrane was everted into both the periotic sac and the recessus partis basilaris in all four ears examined, perhaps due to shrinkage of the periotic system.
Xenopus laevis
Reconstructions of the inner ear of Xenopus are shown in Figs. 2 and 10, and photomicrographs of sections of particular interest are presented as Figs. 8D and 11.
FIG. 10.
WinSurf reconstructions of left inner ear structures of Xenopus laevis (female specimen), seen from an approximately caudoventral view. Part of the posterior semicircular canal has been removed to reveal the diverticula of the saccule. The reconstruction on the left shows the otic labyrinth and associated structures only; the reconstruction on the right includes all but the most rostrolateral part of the periotic labyrinth too. The anterior semicircular canal had been obliterated in the slides used to make this reconstruction and the saccular cavity was distorted owing to the presence of a bubble. The presumed original shape of the saccule (S) has been restored here. The asterisk indicates a short, blind branch of the periotic canal (see text). Scale bar, 1.5 mm. Colour code: white = otic labyrinth (endolymph); semitranslucent green = periotic labyrinth (perilymph); red = sensory epithelium; purple = contact membrane separating endo- and perilymph. See Fig. 2 caption for full list of abbreviations.
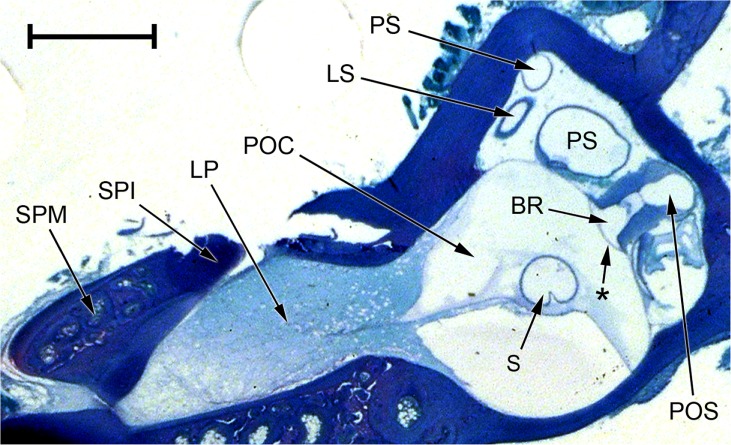
FIG. 11.
Photomicrograph of an oblique section through the inner ear of Xenopus laevis (male specimen). Note that the periotic cistern (POC), the contents of which have picked up only a small amount of stain, is separated from the stapes footplate (SPI and SPM) by the lateral passage (LP), which is filled with diffuse material staining pale blue. The contact membrane of the basilar recess is the thin membrane between the basilar recess (BR) and the periotic sac (POS); the membrane marked with an asterisk between the basilar recess and the POC is Paterson’s (1949, 1960) additional ‘tympanal area’ (see text). Relative to the centre of the picture, dorsal is upwards and away from the viewer, lateral is to the left and towards the viewer. Scale bar, 1 mm. See Fig. 2 caption for full list of abbreviations.
In the sectioned female Xenopus specimen, the anterior semicircular canal was damaged, there was a bubble in the saccular region and the sections did not include the lateral passage or stapes. In the male specimen, the utricular and lagenar cavities had collapsed, as judged from a comparison of shapes between the two specimens and the fact that these structures had pulled away from the otic capsule walls. The periotic cistern had pulled away from the otic capsule wall in both specimens. Despite these shrinkage artefacts, the essential features of the inner ear remained intact in at least one of the two specimens, permitting the following description.
The saccular chamber is relatively large and shifted dorsally compared with that of Rana (Fig. 2); it is not divided into superior and inferior compartments. The anterior and especially the lateral semicircular canals are elongated rostro-caudally; the posterior canal is shorter. The amphibian recess projects as a diverticulum from the caudomedial end of the saccule, and ventral to this extends a second diverticulum which divides into the basilar recess laterally and the prominent lagenar recess medially (Fig. 10). No special subcavity of the saccular chamber containing a tegmentum vasculosum could be found.
The periotic cistern completely enwraps the saccule (Fig. 10) and is interposed between this and the basilar recess, giving rise to a ‘tympanal area’ rostrolateral to the basilar recess (Figs. 8D and 11; see ‘DISCUSSION’). The relatively short, sickle-shaped periotic canal runs very close to the dorsal part of the periotic cistern, but the two remain separate (Fig. 2). Although the periotic canal is also close to the dorsal wall of the saccular cavity, the two are not in such close apposition as in Rana and Eleutherodactylus and there is no distinct contact membrane of the saccule. The periotic canal forms a small contact membrane with the amphibian recess before turning caudally and expanding into the periotic sac, which forms a second contact membrane directly with the basilar recess (Figs. 10 and 11). The periotic sac then extends out of the otic cavity.
No trace of an operculum was identified in Xenopus. The stapes footplate caps the end of a tubular passage projecting laterally from the otic capsule (Figs. 2 and 11). The contents of this passage had picked up a pale blue stain in the histological sections, suggesting that a precipitate had formed there. The periotic cistern was not similarly stained and was clearly separated from the stapes footplate by whatever was in this lateral passage. In gross dissection of frogs of both sexes, the lateral passage was found to be filled with a clear, colourless fluid. A very thin membrane was seen at the medial end of the passage, separating its contents from the periotic cistern. The lateral end was sealed by the tough membrane of the oval window.
Although the reconstructions made from the male and female Xenopus specimens were generally very similar, the female’s inner ear apparatus, particularly the saccular chamber, was more elongated rostro-caudally. The contact membrane of the amphibian recess was just over twice the area of the contact membrane of the basilar recess in the female, whereas in the male the contact membrane of the basilar recess was 1.5 times the area of that of the amphibian recess.
DISCUSSION
Wever’s Diagram
Wever’s (1973) schematic section through the ear of a leopard frog (Fig. 1A) has been widely reproduced in the literature. Although presented as a ‘frontal section’, Wever did not claim that his diagram was based on a single, histological section and he may have amalgamated several slides in its construction. In order to address this possibility, MicroView software was used to reorient a stack of registered Rana section photomicrographs and section it in a new plane, thus revealing a ‘virtual section’ through the inner ear. The orientation was chosen such that the ‘virtual section’ (Fig. 12A) was as close to Wever’s illustration as possible, the main criteria being that the section should show both amphibian and basilar recesses as well as the lateral chamber of the inner ear. Falling somewhere between frontal and transverse planes, its orientation is best described as oblique (Fig. 12C).
Assuming that our original section photomicrographs were well-aligned, which by comparison with CT scan data appeared to be the case, our ‘virtual section’ shows Wever’s schematic figure to be anatomically inaccurate in several respects. The orientation of the stapes footplate in our ‘virtual section’ differs substantially, revealing the process of the stapes pars media which articulates with the otic capsule (marked with an asterisk in Fig. 12B). It is easy to visualise the stapes footplate rocking about this process, as has been shown to be the case in ranid frogs (Jørgensen and Kanneworff 1998; Mason and Narins 2002; Werner 2003), rather than acting as a piston as Wever’s diagram might imply. Our ‘virtual section’ also passes through all three semicircular canals but only touches the periphery of the operculum. It does not include the contact membrane of the saccule.
Wever’s figure therefore appears not to represent a single, real section through the ear, but it is useful in diagrammatically illustrating the likely pathways for acoustic energy flow from the stapes to the amphibian and basilar papillae, and thence to the round window. Acoustic energy is also thought to be able to pass from periotic cistern to round window via the periotic canal, bypassing the otic labyrinth and auditory papillae entirely (Purgue and Narins 2000a, b). That portion of the periotic canal which ascends from the periotic cistern is visible in both our ‘virtual section’ (Fig. 12B) and Wever’s diagram (Fig. 1B), but Wever (1973, 1985) made surprisingly little mention of the canal in his otherwise detailed descriptions of frog inner ears.
Of the other schematic illustrations of the frog inner ear which exist in the literature, that of Frishkopf and Goldstein (1963) may be the best known. More obviously diagrammatic than Wever’s illustration, this older representation shows the periotic and semicircular canals, and it represents the extrastapes more accurately.
The Ears of Eleutherodactylus and Xenopus
The inner ear of E. limbatus was found generally to resemble that of Rana, but there were some pronounced differences in terms of the relative sizes and shapes of the various structures. These differences were not highlighted by Wever (1985), who examined three other Eleutherodactylus species. The very small saccule and the relatively short, wide semicircular canals (Fig. 2) closely resemble reconstructions of the inner ear in ‘stage 8’ Rana temporaria tadpoles (30 mm long, just before emergence of hindlimbs) made by Birkmann (1940). E. limbatus has direct development which omits a tadpole stage, but our frogs had been vocalising in life and were therefore believed to be reproductively mature. The inner ear of this species may therefore be paedomorphic.
The inner ear of X. laevis has been described, among others, by Paterson (1949, 1960), Wever (1985) and Bever et al. (2003). Our reconstructions of Xenopus ears largely agree with their descriptions. The saccular cavity contains a dense otoconial mass (very obvious in the CT scans) which, relative to the rest of the inner ear, is much larger and more dorsally positioned than its equivalent in Rana and Eleutherodactylus. The size and position of the saccular cavity in Xenopus gives its inner ear a striking morphological similarity to that of the fish Gobius niger, as illustrated by Retzius (1881). The possible functional convergence between these two aquatic species remains to be explored.
The association of the basilar papilla with the lagenar recess in urodeles, caecilians and amniotes has been said to be the ‘single most influential piece of evidence supporting a [basilar papilla] homology among all terrestrial vertebrates’, but the separate opening of the basilar recess into the saccule in frogs was seen as a complication to this theory (Smotherman and Narins 2004). We have found that the basilar and lagenar recesses are in fact conjoined in Xenopus (Fig. 10), suggesting that this represents the primitive condition for all lissamphibians and perhaps tetrapods in general.
At the caudal end of the basilar recess, periotic contact occurs via the periotic sac directly in Xenopus, rather than via a recessus partis basilaris. Rostrally, the recess is separated from the periotic cistern by a thin ‘tympanal area’, discussed later. These features have been previously described by Paterson (1949, 1960).
In Xenopus, the periotic cistern is separated from the stapes footplate by the fluid contained within a tubular extension of the otic capsule (Fig. 11). A shorter separation between stapes and cistern is shown in Wever’s (1985) diagrams of the ear of this frog, but Wever found a much longer, fluid-filled ‘lateral passage’ in the related species Pipa pipa. In both our histological slides of Xenopus and Wever’s slides of Pipa, it looked like a precipitate had formed within this lateral passage but not in the periotic labyrinth. Paterson (1960) found only a short lateral passage in her immature specimen of Pipa which she refers to as a ‘fossa fenestrae ovalis’, filled with ‘delicate connective tissue’; she did not describe anything similar in Xenopus. Perhaps the separation between footplate and periotic labyrinth increases in pipids as the skull grows, such that it is less obvious in younger specimens. The lateral chamber of Rana differs from the lateral passage of Xenopus because the ranid lateral chamber has a narrower connection with the main otic capsule, it bends sharply rostrally to reach the stapes footplate and it is filled with a diverticulum of the periotic cistern. The ranid lateral chamber is also in contact with the operculum, an element lacking in Xenopus.
The otic labyrinth in the female Xenopus was around 1.5 times the linear dimensions of that of the male. The saccular cavity was more elongated in the female, and there was a difference in the relative sizes of the contact membranes of the amphibian and basilar recesses, noted earlier. Further investigation of a larger number of specimens is needed in order to establish whether these inner ear differences represent sexual dimorphism, which has been observed in the middle ear of this species (Mason et al. 2009).
The Blind Branch of the Periotic Canal
The discrete ‘blind branch’ of the periotic canal which Purgue and Narins (2000b) found in the bullfrog R. catesbeiana could not be identified in the Rana or Eleutherodactylus specimens examined here. However, the membranous wall of the periotic canal as it curves around the superior saccule was particularly thin in R. pipiens, and in some slides, it appeared to be ruptured. An apparent short diverticulum found in the female Xenopus specimen only (Fig. 10) might also have been the result of periotic canal rupture. Purgue and Narins injected silicone into the periotic labyrinth of their frogs to make casts: it is possible that they experienced a similar problem.
The Contact Membranes
Harrison (1902) referred to three ‘tympanal areas’ in the frog inner ear where otic and periotic labyrinths are in particularly close apposition. One of these is the extensive, membranous division between periotic cistern and saccule, while the other two ‘tympanal areas’ are now more generally known as the ‘contact membranes’ of the amphibian and basilar recesses. To these three may be added the contact membrane of the saccule and an additional ‘tympanal area’ in Xenopus, discussed later.
Sound energy from stapedial vibrations is widely presumed to enter the otic labyrinth through the first ‘tympanal area’ between periotic cistern and saccule (the membrane surrounding the inferior saccular chamber, labelled IS in Fig. 4, forms part of this). The division between the periotic and otic systems remains very thin where the periotic canal wraps around the anterior wall of the superior saccule; a special, ventral diverticulum of the canal makes additional contact with the superior saccule in Eleutherodactylus (Figs. 6 and 7B).
In both Rana and Eleutherodactylus, the periotic canal separates from the saccule but returns to meet the otic labyrinth at three contact membranes within the otherwise thickened limbic tissue at the posterior end of the otic capsule. The contact membranes of the amphibian and basilar recesses represent pathways through which sound energy can travel from the otic labyrinth back into the periotic system, via the auditory epithelia of the amphibian and basilar papillae (Purgue and Narins 2000a, b). Although not considered by Purgue and Narins, Wever (1973) showed in his diagram a third contact membrane located between the two papillae, marked only with a tiny arrow (Fig. 1A). Wever wrote that ‘this thin area acts as a bypass and allows some fraction of the fluid motion to go directly into the perilymphatic duct [=periotic canal] without being detected’. This membrane between superior saccule and periotic canal was described in R. catesbeiana by Lewis (1976), who referred to it as the ‘contact membrane of the saccule’. Lewis and Narins (1999) state that it is found in ‘the more derived anurans’, referring this statement to Lewis (1984); Lewis and Narins may have meant Lewis’ 1976 publication.
The contact membrane of the saccule was identified in this study in both Rana (Figs. 3 and 5C) and Eleutherodactylus (Figs. 6 and 8B), located close to the contact membrane of the amphibian recess. Although it appears to be thicker than the other two contact membranes, it might indeed represent a second route by which sound energy could bypass the amphibian and basilar papillae, additional to the periotic canal route described by Purgue and Narins (2000a, b). The frequency-dependent impedance of such a bypass, and hence its functional significance, remains to be determined. The so-called round window within the metotic fissure, which may not be homologous with the round window of other tetrapods (Henson 1974), represents a point of pressure release for all these routes (Wever 1985).
As well as the usual contact membrane at the posterior end of the basilar recess, Xenopus has an additional ‘tympanal area’ between the rostrolateral wall of this recess and a posterior extension of the periotic cistern (Paterson 1949, 1960). Of the frogs studied here, only Xenopus has this ‘tympanal area’ (Figs. 8D and 11) because its basilar and lagenar recesses both arise from the same caudoventral diverticulum of the saccular cavity (Fig. 10; see earlier), and part of the periotic cistern has come to occupy the space between this diverticulum and the saccule proper. Elsewhere, the membranous walls of the basilar recess are enclosed within thick limbic tissue (Fig. 8D). In principle, acoustic energy might flow from periotic cistern through this ‘tympanal area’ directly into the basilar recess, exiting at the posterior end of the recess via the contact membrane formed here with the periotic sac. This short and direct pathway through the basilar recess was represented diagrammatically by Wever (1985).
The Tegmentum Vasculosum
‘Tegmentum vasculosum’, literally meaning ‘vascular covering’, is a term most often used to describe the well-vascularized, thickened wall of the cochlear duct in birds and crocodilians. Separating the scala media from the scala vestibuli, this archosaur tegmentum vasculosum is believed to combine the roles of the stria vascularis and Reissner’s membrane in mammals (Baird 1974; Lewis et al. 1985; Hossler et al. 2002). The same term has long been used in the German anatomical literature to describe the thickened layer of epithelial cells found in the superior saccule of certain frogs (e.g. Deiters 1862; Hasse 1868; Kuhn 1880; Retzius 1881; Gaupp 1904; Birkmann 1940; Hagmann and Giebel 1978). Retzius (1881) described a tegmentum vasculosum in Bufo, Hyla and Pelobates but found it to be very poorly developed in Alytes; it is not found in Ascaphus or Leiopelma (Wagner 1934) or in pipids including Xenopus (Paterson 1960; this study). Although lacking in some ‘archaeobatrachians’, the tegmentum vasculosum has apparently been identified in all neobatrachian ears in which it has been sought. It is not found in urodeles (Birkmann 1940).
Retzius (1881) produced several illustrations of the otic labyrinth of Rana esculenta, which were redrawn and modified by Gaupp (1904). Retzius and Gaupp both labelled the whole of the superior saccular wall as the tegmentum vasculosum, as did Birkmann (1940) in his reconstructions of the otic labyrinth of R. temporaria. Several illustrations from Gaupp and Birkmann were redrawn by Wever (1985) in The Amphibian Ear, but in each case the region originally labelled as tegmentum vasculosum was relabelled as part of the saccule. In the present study, the tegmentum vasculosum was readily identifiable in Rana and Eleutherodactylus as a thickened epithelium lining an otherwise unoccupied diverticulum of the saccular chamber. However, the extent of its vascularization could not be ascertained and, as Gaupp (1904) noted, its rostral borders are indistinct in Rana. A tegmentum vasculosum was not found in Xenopus.
Wever (1985, p. 78) made only one, brief mention of the anuran tegmentum vasculosum in his book, in which he commented on the mistake of ‘early anatomists’ in assigning to it a sensory function. Nevertheless, the unusual epithelium suggests a functional distinction from the rest of the superior saccular chamber. Hagmann and Giebel (1978), working on R. temporaria, confirmed that this region is richly vascularized and found high levels of metabolic enzymatic activity. This supports the contention that the tegmentum vasculosum in frogs is responsible for the secretion of endolymph, like the tegmentum of archosaurs and the stria vascularis of mammals. Subsequent studies of endolymph secretion in frogs, however, have focused on the dark cells located in the utricle and semicircular canal ampullae (Burnham and Stirling 1984; Bernard et al. 1986); the anuran tegmentum vasculosum has fallen into obscurity.
The Apparent Interaural Fluid Connection in Eleutherodactylus
Of the frogs examined, sections through the whole head were only available for one specimen of Eleutherodactylus. Although our interpretation may have been affected by shrinkage, these sections appeared to show the left and right periotic sacs converging to form a fluid space immediately beneath the brain (Fig. 9). Wever (1978) showed that vibrations applied to the operculum of one ear in a salamander can excite the contralateral ear: his proposed mechanism involved a similar intracranial pathway below the brain, but he believed that the two periotic sacs in his species communicated only indirectly, via the cerebrospinal fluid. Harrison (1902) challenged the notion of earlier authors that amphibians possess a connection between the periotic system and a ‘subdural space’.
Such a fluid system extending between the two ears via the cranial cavity might be functionally significant in (1) communicating vibrations from the cerebrospinal fluid to the inner ears, (2) acoustically coupling the two ears, which might affect sound localization or (3) providing increased possibilities for pressure release from the inner ear, affecting sensitivity. Further work is clearly needed to confirm the presence of an interaural fluid connection in Eleutherodactylus and other frogs, so as to assess whether this condition is widespread among anurans.
CONCLUSION
The anuran inner ear is a complex, 3D structure consisting of the intertwined canals of the periotic and otic labyrinths. Although his representation appears not to be anatomically accurate, Wever’s (1973) diagram of the leopard frog inner ear does an admirable job of clearly illustrating some of the possible routes of acoustic energy flow from stapes to round window. Its main shortcoming in this respect is that it does not label the periotic canal, which represents another potential route. Although some anatomical differences were identified, the inner ear of Eleutherodactylus is broadly similar to that of Rana, so Wever’s diagram is clearly representative of a wider range of neobatrachian frogs. The illustration is less useful in describing the ear of Xenopus; to what extent this reflects the ‘archaeobatrachian’ status of Xenopus or its aquatic habits remains to be determined.
The tegmentum vasculosum and the contact membrane of the saccule, found here in Rana and Eleutherodactylus but not Xenopus, have been neglected in the recent literature and deserve further attention from auditory physiologists. The intriguing possibility of a fluid pathway extending between right and left ears also demands investigation.
Acknowledgements
The authors wish to thank Emanuel Mora for his help and support with this project. Dave Simpson kindly provided the Xenopus specimens. The CT scan of Xenopus was made by Alan Heaver of the University of Cambridge Department of Engineering, with thanks going also to Norman Fleck for the use of his equipment. The authors are very grateful to Dolores Bozovic, Alan D. Grinnell, Tammy Hoang, Victoria Sandoval and Felix E. Schweizer for facilitating the Rana CT scan, which was made by Ting-Ling Chang at the UCLA School of Dentistry, Division of Advanced Prosthodontics. Stephan Kamrad helped with translations. The research of JMS and PvD was supported by the Heinsius Houbolt Foundation and is part of the research programme Healthy Ageing and Communication of the Department of Otorhinolaryngology at the University Medical Center Groningen. Finally, the authors wish to thank the reviewers and editors of the manuscript for their very helpful comments.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Contributor Information
Matthew J. Mason, Phone: +44 1223 333829, Email: mjm68@cam.ac.uk
Johannes M. Segenhout, Email: jmsegenhout@hotmail.com
Ariadna Cobo-Cuan, Email: cobo_cuan@fbio.uh.cu.
Patricia M. Quiñones, Email: yukiq@ucla.edu
Pim van Dijk, Email: p.van.dijk@umcg.nl.
References
- Baird IL. Anatomical features of the inner ear in submammalian vertebrates. In: Keidel WD, Neff WD, editors. Handbook of sensory physiology, volume V/1: auditory system. Berlin: Springer; 1974. pp. 159–212. [Google Scholar]
- Bernard C, Ferrary E, Sterkers O. Production of endolymph in the semicircular canal of the frog Rana esculenta. J Physiol. 1986;371:17–28. doi: 10.1113/jphysiol.1986.sp015959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever MM, Jean YY, Fekete DM. Three-dimensional morphology of inner ear development in Xenopus laevis. Dev Dyn. 2003;227:422–430. doi: 10.1002/dvdy.10316. [DOI] [PubMed] [Google Scholar]
- Birkmann K. Morphologisch-anatomische Untersuchungen zur Entwicklung des häutigen Labyrinthes der Amphibien. Zeitschrift für Anatomie und Entwicklungsgeschichte. 1940;110:443–488. doi: 10.1007/BF02118691. [DOI] [Google Scholar]
- Blood DC, Studdert VP. Saunders comprehensive veterinary dictionary. 2. Edinburgh: W.B. Saunders; 1999. [Google Scholar]
- Burnham JA, Stirling CE. Quantitative localization of Na-K pump site in frog inner ear dark cells. Hear Res. 1984;13:261–268. doi: 10.1016/0378-5955(84)90079-0. [DOI] [PubMed] [Google Scholar]
- Deiters O. Ueber das innere Gehörorgan der Amphibien. Archiv für Anatomie. Physiologie und Wissenschaftliche Medicin. 1862;1862:262–275. [Google Scholar]
- Dubois A. Naming taxa from cladograms: a cautionary tale. Mol Phylogenet Evol. 2007;42:317–330. doi: 10.1016/j.ympev.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Duellman WE, Trueb L. Biology of amphibians. Baltimore: The Johns Hopkins University Press; 1986. [Google Scholar]
- Fay RR, Popper AN. The octavolateralis system. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional vertebrate morphology. London: Belknap; 1985. pp. 291–316. [Google Scholar]
- Frishkopf LS, Goldstein MH. Responses to acoustic stimuli from single units in the eighth nerve of the bullfrog. J Acoust Soc Am. 1963;35:1219–1228. doi: 10.1121/1.1918676. [DOI] [Google Scholar]
- Gaupp E (1904) A. Ecker’s und R. Wiedersheim’s Anatomie des Frosches, part 3, 2nd edn. Druck und Verlag von Friedrich Vieweg und Sohn, Braunschweig
- Gridi-Papp M, Narins PM. Seismic detection and communication in amphibians. In: O’Connell-Rodwell CE, editor. The use of vibrations in communication: properties, mechanisms and function across Taxa. Kerala: Research Signpost; 2010. pp. 69–83. [Google Scholar]
- Hagmann B, Giebel W. Enzymhistochemische Untersuchungen am Innenohr des Frosches (Rana temporaria) Archives Oto-Rhino-Laryngol. 1978;220:89–103. doi: 10.1007/BF00456303. [DOI] [PubMed] [Google Scholar]
- Harrison HS. On the perilymphatic spaces of the amphibian ear. Int Monatsschrift für Anatomie Physiol. 1902;19:221–261. [Google Scholar]
- Hasse C. Das Gehörorgan der Frösche, Reprinted from Zeitschrift für wissenschaftliche Zoologie, Bd. 18. Leipzig: Verlag von Wilhelm Engelmann; 1868. [Google Scholar]
- Henson OW. Comparative anatomy of the middle ear. In: Keidel WD, Neff WD, editors. Handbook of sensory physiology, volume V/1: auditory system. Berlin: Springer; 1974. pp. 39–110. [Google Scholar]
- Hillis DM. Constraints in naming parts of the tree of life. Mol Phylogenet Evol. 2007;42:331–338. doi: 10.1016/j.ympev.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Hoegg S, Vences M, Brinkmann H, Meyer A. Phylogeny and comparative substitution rates of frogs inferred from sequences of three nuclear genes. Mol Biol Evol. 2004;21:1188–1200. doi: 10.1093/molbev/msh081. [DOI] [PubMed] [Google Scholar]
- Hossler FE, Olson KR, Musil G, McKamey MI. Ultrastructure and blood supply of the tegmentum vasculosum in the cochlea of the duckling. Hear Res. 2002;164:155–165. doi: 10.1016/S0378-5955(01)00427-0. [DOI] [PubMed] [Google Scholar]
- Jørgensen MB, Kanneworff M. Middle ear transmission in the grass frog, Rana temporaria. J Comp Physiol A. 1998;182:59–64. doi: 10.1007/s003590050158. [DOI] [PubMed] [Google Scholar]
- Kuhn Ueber das häutige Labyrinth der Amphibien. Arch Mikrosk Anat. 1880;17:479–550. doi: 10.1007/BF02952589. [DOI] [Google Scholar]
- Lewis ER. Surface morphology of the bullfrog amphibian papilla. Brain Behav Evol. 1976;13:196–215. doi: 10.1159/000123810. [DOI] [PubMed] [Google Scholar]
- Lewis ER (1984) On the frog amphibian papilla. Scanning Electron Microscopy 1984 (IV):1899–1913 [PubMed]
- Lewis ER, Narins PM. The acoustic periphery of amphibians: anatomy and physiology. In: Fay RR, Popper AN, editors. Comparative hearing: fish and amphibians. New York: Springer; 1999. pp. 101–154. [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The vertebrate inner ear. Boca Raton: CRC Press, Inc.; 1985. [Google Scholar]
- Lombard RE. Comparative morphology of the inner ear in salamanders (Caudata: Amphibia) Basel: S. Karger; 1977. [Google Scholar]
- Mason MJ, Narins PM. Vibrometric studies of the middle ear of the bullfrog Rana catesbeiana. I. The extrastapes. J Exp Biol. 2002;205:3153–3165. doi: 10.1242/jeb.205.20.3153. [DOI] [PubMed] [Google Scholar]
- Mason MJ, Wang M, Narins PM. Structure and function of the middle ear apparatus of the aquatic frog, Xenopus laevis. Proc Inst Acoustics. 2009;31:13–21. [PMC free article] [PubMed] [Google Scholar]
- Moody D, Lozanoff S (1998) SURFdriver: A practical computer program for generating three-dimensional models of anatomical structures using a PowerMac. Clin Anat 11:132
- Paterson NF (1949) The development of the inner ear of Xenopus laevis. Proc Zool Soc London 119:269–291
- Paterson NF. The inner ear of some members of the Pipidae (Amphibia) Proc Zool Soc London. 1960;134:509–546. doi: 10.1111/j.1469-7998.1960.tb05598.x. [DOI] [Google Scholar]
- Pauly GB, Hillis DM, Cannatella DC. Taxonomic freedom and the role of official lists of species names. Herpetologica. 2009;65:115–128. doi: 10.1655/08-031R1.1. [DOI] [Google Scholar]
- Purgue AP, Narins PM. A model for energy flow in the inner ear of the bullfrog (Rana catesbeiana) J Comp Physiol A. 2000;186:489–495. doi: 10.1007/s003590050447. [DOI] [PubMed] [Google Scholar]
- Purgue AP, Narins PM. Mechanics of the inner ear of the bullfrog (Rana catesbeiana): the contact membranes and the periotic canal. J Comp Physiol A. 2000;186:481–488. doi: 10.1007/s003590050446. [DOI] [PubMed] [Google Scholar]
- Retzius G. Das Gehörorgan der Wirbelthiere. morphologisch-Histologische Studien. I. Das Gehörorgan der Fische und Amphibien. Stockholm: Samson & Wallin; 1881. [Google Scholar]
- Simmons DD, Meenderink SWF, Vassilakis PN. Anatomy, physiology, and function of auditory end-organs in the frog inner ear. In: Narins PM, Feng AS, Fay RR, Popper AN, editors. Hearing and sound communication in amphibians. New York: Springer; 2007. pp. 184–220. [Google Scholar]
- Smotherman M, Narins P. Evolution of the amphibian ear. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer; 2004. pp. 164–199. [Google Scholar]
- Thévenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- van Dijk P, Mason MJ, Schoffelen RLM, Narins PM, Meenderink SWF. Mechanics of the frog ear. Hear Res. 2011;273:46–58. doi: 10.1016/j.heares.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DS. The structure of the inner ear in relation to the reduction of the middle ear in the Liopelmidae (Noble) Anat Anz. 1934;79:20–36. [Google Scholar]
- Werner YL. Mechanical leverage in the middle ear of the American bullfrog, Rana catesbeiana. Hear Res. 2003;175:54–65. doi: 10.1016/S0378-5955(02)00709-8. [DOI] [PubMed] [Google Scholar]
- Wever EG. The ear and hearing in the frog, Rana pipiens. J Morphol. 1973;141:461–477. doi: 10.1002/jmor.1051410406. [DOI] [PubMed] [Google Scholar]
- Wever EG. Sound transmission in the salamander ear. Proc Natl Acad Sci U S A. 1978;75:529–530. doi: 10.1073/pnas.75.1.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever EG. The amphibian ear. Princeton: Princeton University Press; 1985. [Google Scholar]