Abstract
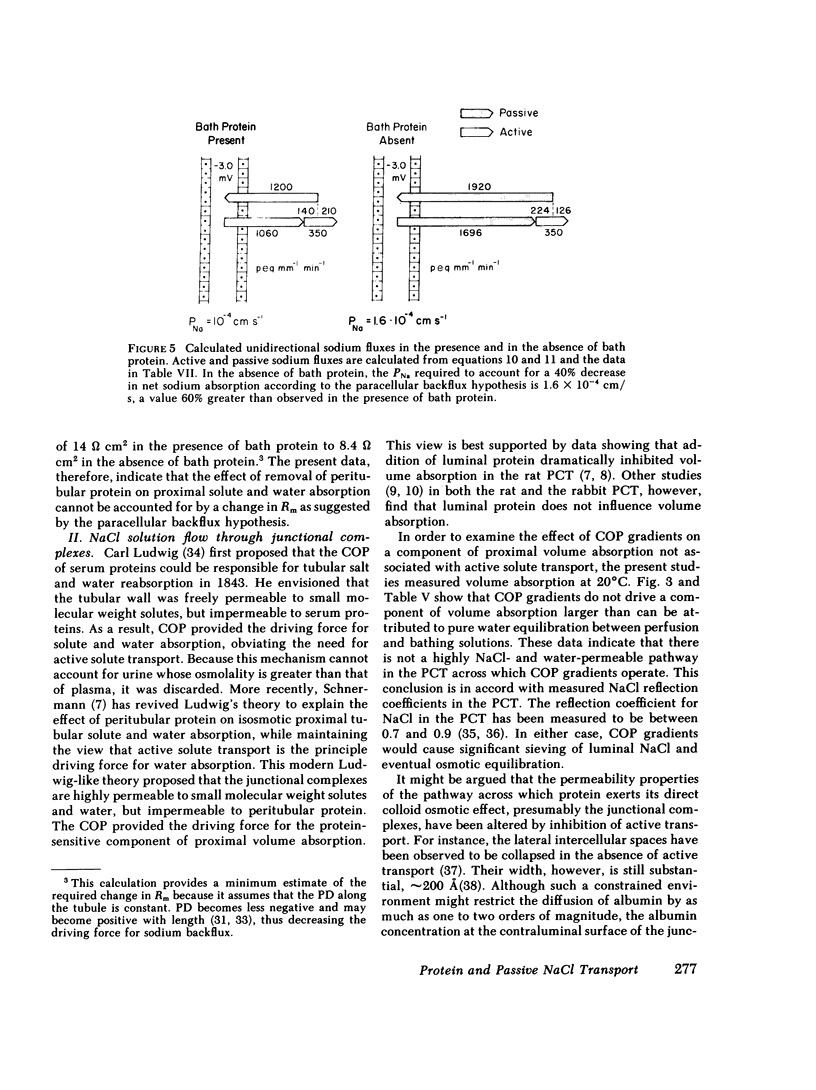
The effect of peritubular protein removal on passive NaCl transport was examined in the isolated rabbit proximal convoluted tubule (PCT). Three modes of passive NaCl transport were tested: (a) paracellular backflux of NaCl, (b) convective flow of NaCl through junctional complexes, and (c) anion gradient-dependent NaCl transport. The effect of peritubular protein removal on the paracellular permeability to NaCl was examined using transepithelial specific resistance. Eight PCT were perfused with ultrafiltrate (UF) and bathed in either serum or UF. Transepithelial specific resistance averaged 14.5 +/- 1.9 in the presence and 13.7 +/- 1.7 omega cm2 in the absence of peritubular protein. The effect of peritubular protein removal on the convective flow of a NaCl solution across functional complexes was examined in the absence of active transport by using colloid osmotic pressure (COP) gradients. 12 PCT were perfused with simple salt solutions in Donnan equilibrium with and without protein at 20 degrees C. A COP gradient of 60.1 and -60.1 mmHg drove only 0.06 and -0.23 nl/min, respectively. These values are approximately 10% of the value predicted for an effect of peritubular protein on NaCl solution flow (1.98 nl/min) and are approximately equal to the value predicted for pure water equilibration for the small osmotic pressure difference between solutions in Donnan equilibrium (0.17-0.18 nl/min). The effect of peritubular protein removal on the passive absorption of NaCl driven by anion concentration gradients was examined in seven PCT perfused with a high chloride solution simulating late proximal tubular fluid and bathed in either serum or UF at 20 degrees C. Volume absorption averaged 0.34 +/- 0.20 in the presence and 0.39 +/- 0.20 nl/mm min in the absence of peritubular protein. In conclusion, peritubular protein removal did not significantly affect any of the three distinct modes of passive NaCl transport tested. The lack of effect of peritubular protein removal on passive paracellular NaCl transport suggests that protein modulates an active transcellular NaCl transport process.
Full text
PDF

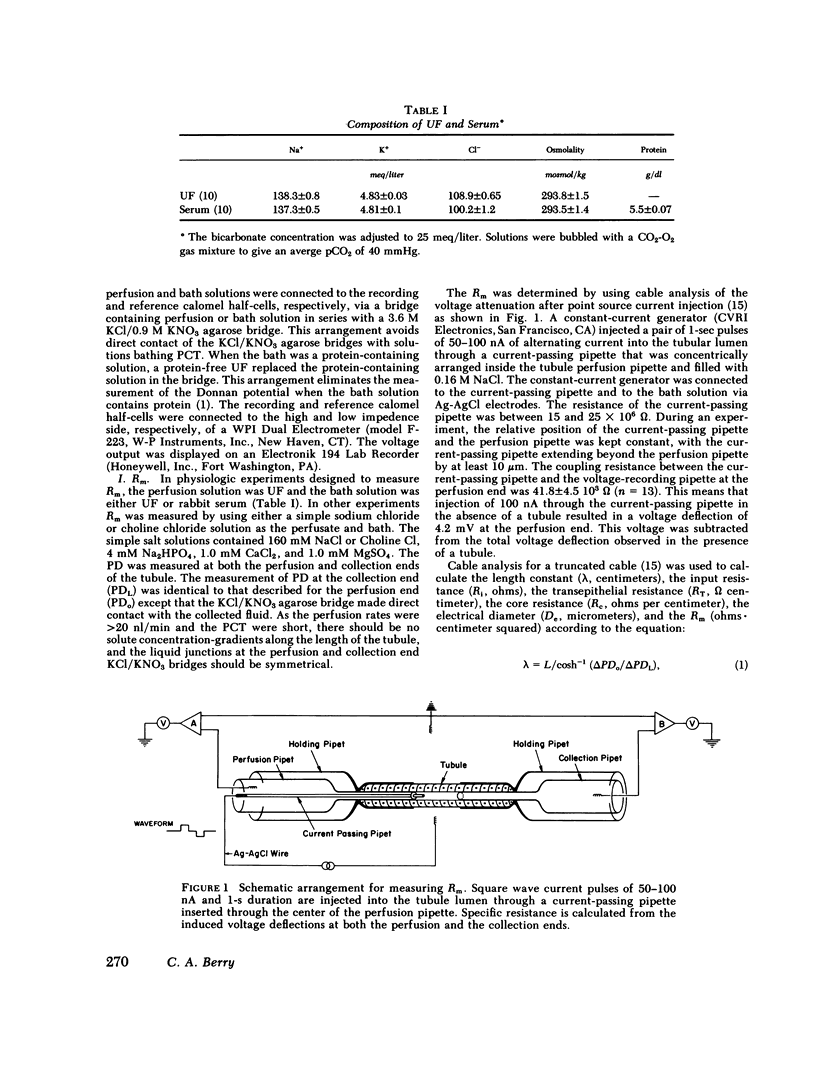


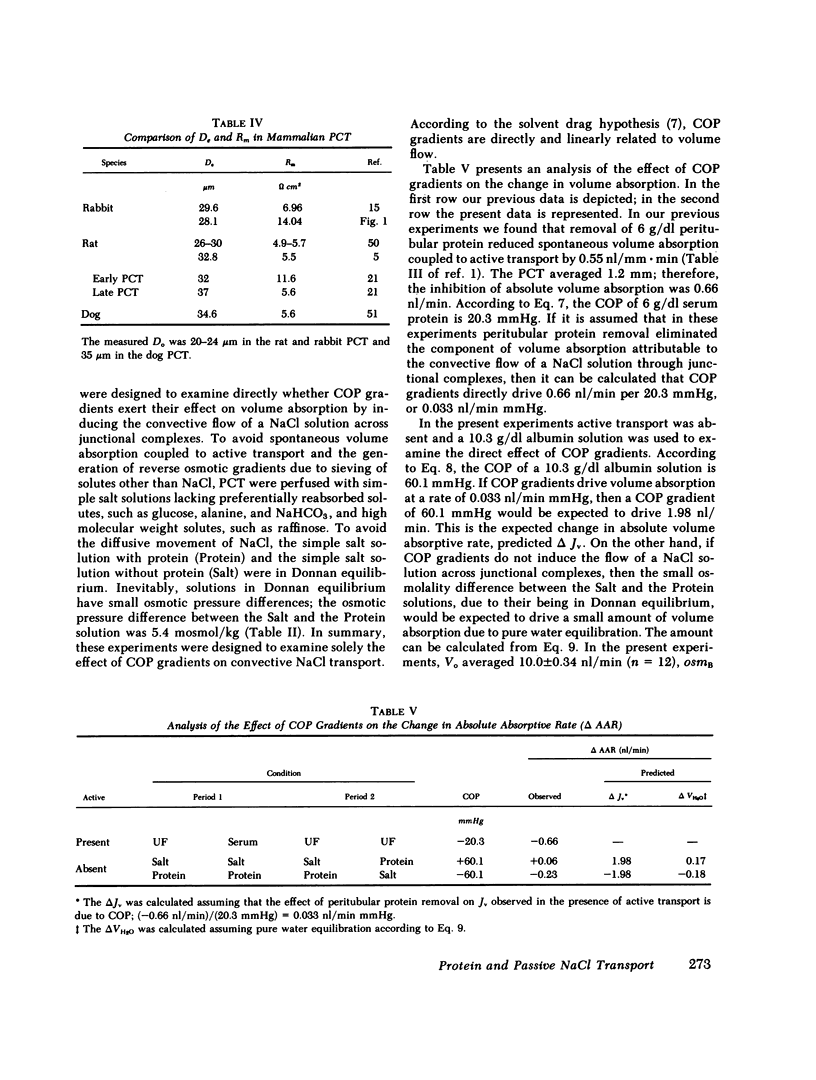






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agerup B. Influence of peritubular hydrostatic and oncotic pressures on fluid reabsorption in proximal tubules of the rat kidney. Acta Physiol Scand. 1975 Feb;93(2):184–194. doi: 10.1111/j.1748-1716.1975.tb05808.x. [DOI] [PubMed] [Google Scholar]
- Barratt L. J., Rector F. C., Jr, Kokko J. P., Seldin D. W. Factors governing the transepithelial potential difference across the proximal tubule of the rat kidney. J Clin Invest. 1974 Feb;53(2):454–464. doi: 10.1172/JCI107579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzel C. J., Parsa B., Hare D. K. Osmotic flow across proximal tubule of Necturus: correlation of physiologic and anatomic studies. Am J Physiol. 1969 Aug;217(2):570–580. doi: 10.1152/ajplegacy.1969.217.2.570. [DOI] [PubMed] [Google Scholar]
- Berry C. A., Cogan M. G. Influence of peritubular protein on solute absorption in the rabbit proximal tubule. A specific effect on NaCl transport. J Clin Invest. 1981 Aug;68(2):506–516. doi: 10.1172/JCI110282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. A. Electrical effects of acidification in the rabbit proximal convoluted tubule. Am J Physiol. 1981 May;240(5):F459–F470. doi: 10.1152/ajprenal.1981.240.5.F459. [DOI] [PubMed] [Google Scholar]
- Berry C. A., Rector F. C., Jr Relative sodium-to-chloride permeability in the proximal convoluted tubule. Am J Physiol. 1978 Dec;235(6):F592–F604. doi: 10.1152/ajprenal.1978.235.6.F592. [DOI] [PubMed] [Google Scholar]
- Berry C. A., Warnock D. G., Rector F. C., Jr Ion selectivity and proximal salt reabsorption. Am J Physiol. 1978 Sep;235(3):F234–F245. doi: 10.1152/ajprenal.1978.235.3.F234. [DOI] [PubMed] [Google Scholar]
- Biagi B., Kubota T., Sohtell M., Giebisch G. Intracellular potentials in rabbit proximal tubules perfused in vitro. Am J Physiol. 1981 Mar;240(3):F200–F210. doi: 10.1152/ajprenal.1981.240.3.F200. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Tucker B. J. Determinants of peritubular capillary fluid uptake in hydropenia and saline and plasma expansion. Am J Physiol. 1975 Jun;228(6):1927–1935. doi: 10.1152/ajplegacy.1975.228.6.1927. [DOI] [PubMed] [Google Scholar]
- Boulpaep E. L. Permeability changes of the proximal tubule of Necturus during saline loading. Am J Physiol. 1972 Mar;222(3):517–531. doi: 10.1152/ajplegacy.1972.222.3.517. [DOI] [PubMed] [Google Scholar]
- Boulpaep E. L., Seely J. F. Electrophysiology of proximal and distal tubules in the autoperfused dog kidney. Am J Physiol. 1971 Oct;221(4):1084–1096. doi: 10.1152/ajplegacy.1971.221.4.1084. [DOI] [PubMed] [Google Scholar]
- Brazy P. C., Balaban R. S., Gullans S. R., Mandel L. J., Dennis V. W. Inhibition of Renal Metabolism. Relative effects of arsenate on sodium, phosphate, and glucose transport by the rabbit proximal tubule. J Clin Invest. 1980 Dec;66(6):1211–1221. doi: 10.1172/JCI109972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Patlak C., Green N., Villey D. Organic solutes in fluid absorption by renal proximal convoluted tubules. Am J Physiol. 1976 Aug;231(2):627–637. doi: 10.1152/ajplegacy.1976.231.2.627. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Maddox D. A., Lucci M. S., Rector F. C., Jr Control of proximal bicarbonate reabsorption in normal and acidotic rats. J Clin Invest. 1979 Nov;64(5):1168–1180. doi: 10.1172/JCI109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Gessner K. Active transport potentials, membrane diffusion potentials and streaming potentials across rat kidney proximal tubule. Pflugers Arch. 1974;351(1):85–98. doi: 10.1007/BF00603513. [DOI] [PubMed] [Google Scholar]
- González E., Carpi-Medina P., Whittembury G. Cell osmotic water permeability of isolated rabbit proximal straight tubules. Am J Physiol. 1982 Apr;242(4):F321–F330. doi: 10.1152/ajprenal.1982.242.4.F321. [DOI] [PubMed] [Google Scholar]
- Grandchamp A., Boulpaep E. L. Pressure control of sodium reabsorption and intercellular backflux across proximal kidney tubule. J Clin Invest. 1974 Jul;54(1):69–82. doi: 10.1172/JCI107751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Qualizza P. B., Welling L. W. Influence of serum proteins on net fluid reabsorption of isolated proximal tubules. Kidney Int. 1972 Aug;2(2):66–75. doi: 10.1038/ki.1972.73. [DOI] [PubMed] [Google Scholar]
- Green R., Windhager E. E., Giebisch G. Protein oncotic pressure effects on proximal tubular fluid movement in the rat. Am J Physiol. 1974 Feb;226(2):265–276. doi: 10.1152/ajplegacy.1974.226.2.265. [DOI] [PubMed] [Google Scholar]
- Hegel U., Frömter E., Wick T. Der elektrische Wandwiderstand des proximalen Konvolutes der Rattenniere. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;294(4):274–290. [PubMed] [Google Scholar]
- Ichikawa I., Hoyer J. R., Seiler M. W., Brenner B. M. Mechanism of glomerulotubular balance in the setting of heterogeneous glomerular injury. Preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J Clin Invest. 1982 Jan;69(1):185–198. doi: 10.1172/JCI110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Effect of peritubular protein concentration on reabsorption of sodium and water in isolated perfused proxmal tubules. J Clin Invest. 1972 Feb;51(2):314–325. doi: 10.1172/JCI106816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Transtubular oncotic pressure gradients and net fluid transport in isolated proximal tubules. Kidney Int. 1974 Sep;6(3):138–145. doi: 10.1038/ki.1974.92. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R. Characteristics of volume reabsorption in rabbit superficial and juxtamedullary proximal convoluted tubules. J Clin Invest. 1979 Mar;63(3):410–418. doi: 10.1172/JCI109317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P., Burg M. B., Orloff J. Characteristics of NaCl and water transport in the renal proximal tubule. J Clin Invest. 1971 Jan;50(1):69–76. doi: 10.1172/JCI106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy J. E., Windhager E. E. Peritubular control of proximal tubular fluid reabsorption in the rat kidney. Am J Physiol. 1968 May;214(5):943–954. doi: 10.1152/ajplegacy.1968.214.5.943. [DOI] [PubMed] [Google Scholar]
- Lutz M. D., Cardinal J., Burg M. B. Electrical resistance of renal proximal tubule perfused in vitro. Am J Physiol. 1973 Sep;225(3):729–734. doi: 10.1152/ajplegacy.1973.225.3.729. [DOI] [PubMed] [Google Scholar]
- Ott C. E. Effect of saline expansion on peritubule capillary pressures and reabsorption. Am J Physiol. 1981 Feb;240(2):F106–F110. doi: 10.1152/ajprenal.1981.240.2.F106. [DOI] [PubMed] [Google Scholar]
- Persson A. E., Schnermann J., Agerup B., Eriksson N. E. The hydraulic conductivity of the rat proximal tubular wall determined with colloidal solutions. Pflugers Arch. 1975 Oct 16;360(1):25–44. doi: 10.1007/BF00584324. [DOI] [PubMed] [Google Scholar]
- Quinn M. D., Marsh D. J. Peritubular capillary control of proximal tubule reabsorption in the rat. Am J Physiol. 1979 May;236(5):F478–F487. doi: 10.1152/ajprenal.1979.236.5.F478. [DOI] [PubMed] [Google Scholar]
- Sackin H., Boulpaep E. L. Isolated perfused salamander proximal tubule: methods, electrophysiology, and transport. Am J Physiol. 1981 Jul;241(1):F39–F52. doi: 10.1152/ajprenal.1981.241.1.F39. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Patlak C. S., Andreoli T. E. Fluid absorption and active and passive ion flows in the rabbit superficial pars recta. Am J Physiol. 1977 Aug;233(2):F154–F167. doi: 10.1152/ajprenal.1977.233.2.F154. [DOI] [PubMed] [Google Scholar]
- Seely J. F. Effects of peritubular oncotic pressure on rat proximal tubule electrical resistance. Kidney Int. 1973 Jul;4(1):28–35. doi: 10.1038/ki.1973.77. [DOI] [PubMed] [Google Scholar]
- Seely J. F. Variation in electrical resistance along length of rat proximal convoluted tubule. Am J Physiol. 1973 Jul;225(1):48–57. doi: 10.1152/ajplegacy.1973.225.1.48. [DOI] [PubMed] [Google Scholar]
- Stoner L. C., Roch-Ramel F. The effects of pressure on the water permeability of the descending limb of Henle's loops of rabbits. Pflugers Arch. 1979 Oct;382(1):7–15. doi: 10.1007/BF00585898. [DOI] [PubMed] [Google Scholar]
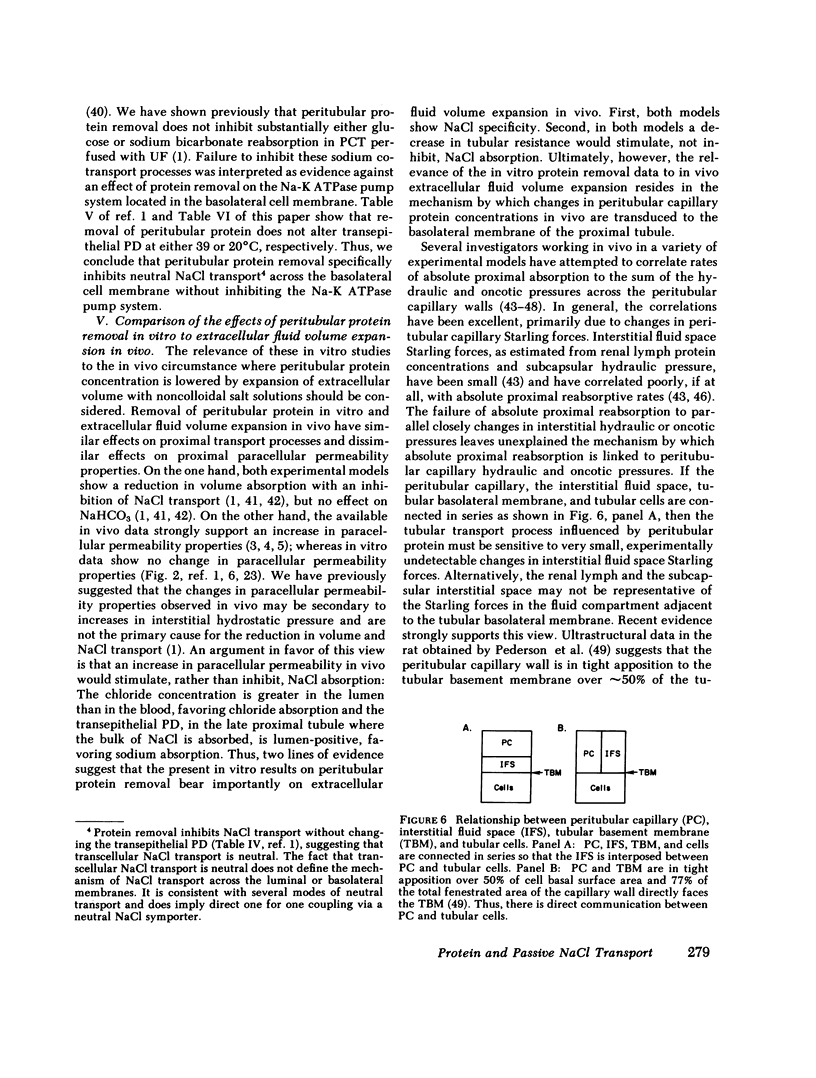
- Tucker B. J., Blantz R. C. Determinants of proximal tubular reabsorption as mechanisms of glomerulotubular balance. Am J Physiol. 1978 Aug;235(2):F142–F150. doi: 10.1152/ajprenal.1978.235.2.F142. [DOI] [PubMed] [Google Scholar]
- Weinman E. J., Kashgarian M., Hayslett J. P. Role of peritubular protein concentration in sodium reabsorption. Am J Physiol. 1971 Nov;221(5):1521–1528. doi: 10.1152/ajplegacy.1971.221.5.1521. [DOI] [PubMed] [Google Scholar]
- Welling D. J., Welling L. W., Hill J. J. Phenomenological model relating cell shape to water reabsorption in proximal nephron. Am J Physiol. 1978 Apr;234(4):F308–F317. doi: 10.1152/ajprenal.1978.234.4.F308. [DOI] [PubMed] [Google Scholar]
- Windhager E. E., Lewy J. E., Spitzer A. Intrarenal control of proximal tubular reabsorption of sodium and water. Nephron. 1969;6(3):247–259. doi: 10.1159/000179732. [DOI] [PubMed] [Google Scholar]