Abstract
In mammals, efferent projections to the cochlear receptor are constituted by olivocochlear (OC) fibers that originate in the superior olivary complex. Medial and lateral OC neurons make synapses with outer hair cells and with auditory nerve fibers, respectively. In addition to the OC system, there are also descending projections from the auditory cortex that are directed towards the thalamus, inferior colliculus, cochlear nucleus, and superior olivary complex. Olivocochlear function can be assessed by measuring a brainstem reflex mediated by auditory nerve fibers, cochlear nucleus neurons, and OC fibers. Although it is known that the OC reflex is activated by contralateral acoustic stimulation and produces a suppression of cochlear responses, the influence of cortical descending pathways in the OC reflex is largely unknown. Here, we used auditory cortex electrical microstimulation in chinchillas to study a possible cortical modulation of cochlear and auditory nerve responses to tones in the absence and presence of contralateral noise. We found that cortical microstimulation produces two different peripheral modulations: (i) changes in cochlear sensitivity evidenced by amplitude modulation of cochlear microphonics and auditory nerve compound action potentials and (ii) enhancement or suppression of the OC reflex strength as measured by auditory nerve responses, which depended on the intersubject variability of the OC reflex. Moreover, both corticofugal effects were not correlated, suggesting the presence of two functionally different efferent pathways. These results demonstrate that auditory cortex electrical microstimulation independently modulates the OC reflex strength and cochlear sensitivity.
Keywords: olivocochlear, efferent system, auditory cortex, top-down, corticofugal, electrical microstimulation
INTRODUCTION
Neural reflexes are fundamental in animals for homeostasis, survival, and reproduction. The basic circuit of a reflex is constituted by a sensory receptor, a central integrator, and an efferent pathway, and their presence is ubiquitous in the neural system (Clarac 2005). Some examples are the vestibule-ocular reflex which is fundamental to maintain visual fixation during head rotations (Straka 2010) or muscle stretch reflexes which are important for locomotion and posture control (Clarac 2008). In addition to this basic circuit, there are control mechanisms that suppress or enhance the reflex strength in different situations (Feldman and Orlovsky 1972). For instance, muscle stretch reflexes are enhanced by the arousal level (McIntyre et al. 2004), while spinal flexor reflexes are suppressed during anesthesia (Tang and Schroeder 1973).
The olivocochlear (OC) reflex is activated by sounds, and its physiological effect is to suppress auditory nerve and cochlear responses (Buño 1978; Liberman 1989). The neural circuit of this reflex is located in the brainstem and is constituted by auditory nerve fibers, postero-ventral cochlear nucleus neurons, and medial olivocochlear (MOC) fibers, which in turn make synapses bilaterally with cochlear receptor cells (de Venecia et al. 2005). The relevance of this reflex in hearing is still debated, and several functions have been attributed to it, including unmasking of auditory stimuli in background noise (Kawase and Liberman 1993) and protection to acoustic trauma (Maison and Liberman 2000). In addition, the arousal level of subjects is a factor that modulates the magnitude of the OC reflex, as it is known that compared to the awake state, different types of anesthesia reduce the strength of this reflex on cochlear responses (Guitton et al. 2004; Chambers et al. 2012).
In mammals, the olivocochlear system is part of an efferent neural network that comprises pathways from the auditory cortex to the cochlear receptor (Saldaña et al. 1996; Robles and Delano 2008; Schofield 2010; Malmierca and Ryugo 2011). Cortical descending projections originate from pyramidal neurons located in layers V and VI of the auditory cortex and are directed to the inferior colliculus (Bajo and Moore 2005), cochlear nucleus (Schofield and Coomes 2005), and even directly to medial olivocochlear neurons (Mulders and Robertson 2000).
The physiological influence of cortical descending projections in cochlear functioning has been demonstrated by auditory cortex microstimulation in bats and humans, as it modulates the amplitude of cochlear responses and of otoacoustic emissions (Xiao and Suga 2002a; Perrot et al. 2006). Moreover, auditory cortex deactivations or lesions in rodents and humans modulate the amplitude of thalamic, inferior colliculus, brainstem, and cochlear responses (Antunes and Malmierca 2011; Anderson and Malmierca 2013; Lamas et al. 2013; Khalfa et al. 2001; Leon et al. 2012). However, there is still no direct evaluation of how cortical descending pathways influence the olivocochlear reflex.
Here, we evaluated possible control mechanisms of auditory cortex descending projections on cochlear sensitivity and on the olivocochlear reflex circuit. The OC reflex strength was assessed by measuring cochlear and auditory nerve potentials with and without contralateral acoustic stimulation, before, during, and after auditory cortex electrical microstimulation (MS).
MATERIALS AND METHODS
Experimental Animals and Anesthetic Protocol
Fifteen adult chinchillas of either sex (Chinchilla laniger) weighing between 400 and 700 g were anesthetized (ketamine 30 mg/kg, xylazine 4 mg/kg, and atropine 0.04 mg/kg) and maintained under anesthesia with repeated half doses every 30–45 min, or when necessary, judging by the foot-withdrawal reflex. Rectal temperature was maintained at 36–37 °C by means of a heating pad. All procedures involving animals were made in accordance with NIH Guidelines for the Care and Use of Laboratory Animals, publication no. 86–23, revised 1996, and were approved by the Institutional Bioethics Committee (Comité de Bioética de Investigación en Animales, Facultad de Medicina, Universidad de Chile, permit number CBA #0262). At the end of each experiment, deeply anesthetized animals were humanely euthanized with an overdose of sodium thiopental (120 mg/kg).
Surgical Procedures
All surgical procedures were performed in a stereotaxic device that allowed free access to both external auditory meatus, tympanic membranes, and temporal bones with the head stabilized by means of a nose clamp and an occipital fixation device.
Auditory Cortex Surgery
A craniotomy was performed in the left temporal bone following anatomic descriptions and vascular landmarks given by Harrison et al. (1996) and Harel et al. (2000). The dura mater was incised and, in order to record and stimulate deep cortical layers (V and VI), a low impedance (<5 kΩ, 200 μm diameter) Nichrome® electrode was positioned and lowered 1,000–1,500 μm into the left auditory cortex using a 3D-microdrive arm (SM-11, Narishige®). The penetration site was covered with agar, and auditory cortex-evoked potential (ACEP) latencies (<25 ms) were measured to confirm that the electrode was positioned within auditory cortices (Delano et al. 2008).
Cochlear Surgery
The right cochlea was accessed by a dorsal aperture of the tympanic bulla, and another Nichrome® electrode (200 μm) was placed in the right round window niche.
Auditory Stimuli
Ipsilateral (right) tones (1–8 kHz, 30–90 dB sound pressure level (SPL)) and contralateral (left) broadband noise (55–60 dB SPL) were digitally generated by two synchronized National Instruments® PCI boards (6071-E) at 100,000 samples/s, attenuated by PA-5 programmable attenuators (Tucker Davis Technologies® system III), and delivered with ER-2 (Etymotic Research®) transducers through tubes sealed to the external auditory meatus. Tones were presented with alternating polarity at a rate of 4 Hz, with duration of 15–30 ms, and 5 ms rise/fall time. Noncontinuous broadband noise was presented at 4 Hz with duration of 170–200 ms. Sound pressure levels were calibrated in both ears of every chinchilla using an Etymotic® or Knowles® microphone. The signal was analyzed using a custom-made spectrum analyzer program written in C language (LabWindows®), assuring that the second and subsequent harmonic components in the signal were ≥40 dB below the level of the fundamental frequency at the highest output level.
Electrophysiological Recordings
ACEPs were always recorded from the left hemisphere and cochlear responses from the right round window. The electrical signal from the round window was amplified 10,000× and filtered between 300 and 10,000 and 300–20,000 Hz in response to low- and high-frequency tones, respectively, using a BMA-200 differential preamplifier (Cwe-inc®) and a second stage filter (Krohn-Hite®, model 3323). The cortical signal was amplified 10,000× and bandpass filtered (1–1,000 Hz) using a BMA-200 differential amplifier (Cwe-inc®) and in a second stage low-pass filtered at 200 Hz (Frequency devices® 901). Both signals were acquired and digitized at 40,000 samples/s with a National Instruments® board (6071-E). The ground and reference electrodes were inserted in midline nose and in the occipital region, respectively.
Auditory Cortex Electrical Microstimulation
Trains of four biphasic square electrical pulses (2–50 μA, 0.25 ms each, separated by 2.2 ms) were delivered at different rates (1–32 Hz) to the auditory cortex during 5 min, using an isolated pulse generator (2100, AM-Systems®). However, as electric pulses delivered at low rates did not produce changes in the amplitude of cochlear potentials, all data presented in this article were obtained with 32 Hz MS rate. This protocol was based on previous experiments of auditory cortex MS performed in the mustached bat (Xiao and Suga 2002a, b).
Experimental Protocols
Experiments were controlled with custom-made programs developed in C language (LabWindows®). We performed two types of experiments: in protocol 1 (n = 15), cochlear and auditory nerve responses were evaluated before and after but not during auditory cortex microstimulation, using current intensities from 2 to 50 μA. In order to evaluate the temporal course of descending effects on cochlear potentials, we designed a second protocol (n = 10), in which we evaluated changes produced in cochlear and auditory nerve potentials during simultaneous cortical MS. Considering that current intensities larger than 10 μA produced large MS artifacts, only currents from 2 to 8 μA were applied in this protocol. In addition, to avoid the superposition of the electrical artifacts produced by MS with cochlear responses, tones were presented with a time delay of 10 ms. The second protocol also included the evaluation of the effect of contralateral noise presented in the left ear with and without left auditory cortex MS. This protocol allowed us to evaluate the influence of descending projections from the left auditory cortex to the ipsilateral superior olivary complex and to the contralateral cochlea (Fig. 1).
FIG. 1.
Schematic illustration of experimental procedures. A Round window electrical responses; CAP (N1-P1 and N2-P2) and CM were recorded from the right round window before, during, and after left auditory cortex electrical microstimulation. A second protocol also included the evaluation of the effect of contralateral noise presented to the left ear. B Time courses of the experimental protocols, including a control period without microstimulation (i), a microstimulation period of 5 min (ii), and a recovery period after cortical MS (v). The experiments with contralateral noise included a condition in which noncontinuous broadband noise (without cortical microstimulation) was presented during 2 min (iii) and another condition in which the contralateral noise (iv) was preceded by auditory cortex microstimulation (ii) which began 90 s before the contralateral noise onset and was delivered during 5 min. Consequently, from 120 to 240 s, contralateral noise and auditory cortex microstimulation were presented simultaneously. C Temporal representation of tone bursts at a presentation rate of 4 Hz (i) without microstimulation and (ii) with trains of four biphasic electrical pulses presented at 32 Hz rate. In order to avoid a time coincidence between electrical microstimulation artifacts and cochlear responses, tones and electrical microstimulation onsets were synchronized with a 10-ms time offset.
Data Analysis
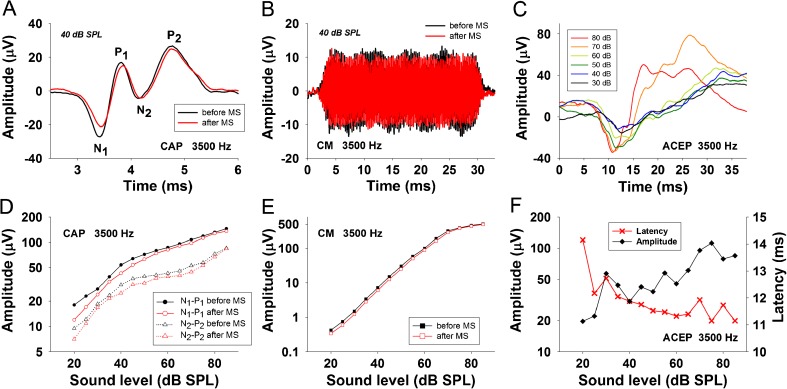
Data were analyzed using a custom-made C program (LabWindows®). Compound action potential waveforms from the auditory nerve (CAP) were obtained by averaging eight trials of alternating tone polarity. From the averaged CAP waveform, we obtained two different neural measures: (i) N1 to P1 and (ii) N2 to P2 (Fig. 2A). The N1 to P1 response is considered to be originated from distal auditory nerve, while the N2 to P2 response is proposed to be generated from the proximal auditory nerve and ipsilateral cochlear nucleus (Sellick et al. 2003; Brown and Patuzzi 2010). Both neural measures (N1-P1) and (N2-P2) were analyzed separately in this article. Fast Fourier transforms in 12.8 ms windows that excluded the CAP response were used to compute the power spectrum of cochlear microphonics (CM) from the same eight trials used to measure the CAP waveform. Standard deviation from N1-P1, N2-P2, and CM responses were obtained from these eight trials that were presented at 4 Hz rate, meaning that we have CAP and CM measures every 2 s of recordings. Mean ± standard errors are displayed in figure plots. The amplitudes and latencies of ACEP responses to auditory stimuli of different intensities and frequencies (1 to 8 kHz and 30–90 dB SPL) were calculated before cortical MS (Fig. 2C, F).
FIG. 2.
Examples of CAP (N1-P1 and N2-P2) and CM changes produced in the right cochlea with a 3,500-Hz tone after left auditory cortex microstimulation delivered at 32 Hz rate. A Averaged CAP waveforms showing an example of the measures used to compute N1-P1 and N2-P2 neural potentials before and after cortical microstimulation. B Averaged CM waveforms were obtained by inverting the polarity of odd trials. Notice an amplitude decrease of CM after cortical microstimulation. C Averaged ACEP waveforms obtained at different sound pressure levels. D CAP and E CM input–output level functions before and after auditory cortex MS, respectively. Cochlear potentials (N1-P1 (circles), N2-P2 (triangles), and CM (squares)) recorded before (black symbols) and after (red symbols) cortical microstimulation. F ACEP input–output amplitude (left axis) and latency (right axis) level functions obtained before cortical MS.
In protocol 1, the effects of cortical microstimulation were evaluated by comparing the amplitudes of CAP and CM obtained in the first 30 s with those obtained in the last 30 s of the experimental protocol (periods (i) and (v) in Fig. 1). In protocol 2, contralateral noise effects were assessed by the reduction (or increase) in N1-P1, N2-P2, and CM amplitudes compared in decibel to baseline amplitudes (dB = 20*LOG10[amplitude/reference amplitude]), obtained without contralateral noise and without cortical microstimulation. Average effects of contralateral noise were calculated in decibel from (i) the complete period of OC reflex activation (120 s) or (ii) dividing this period into three periods: the first, middle, and last 40 s of contralateral noise stimulation. The significance of the differences between data with normal distribution (evaluated by the Shapiro-Wilk test) in control and contralateral noise conditions was determined by paired t tests or with ANOVA in auditory cortex microstimulation experiments, while the Mann-Whitney or Kruskal-Wallis tests were used for nonnormal distributions. Tukey post hoc tests were used to evaluate significant differences between groups. Spearman correlation indexes were calculated to evaluate possible associations between contralateral noise and cortical MS effects on cochlear potentials (SigmaPlot®, v12.5).
RESULTS
Auditory cortex electrical microstimulation delivered at 32 Hz rate, but not at 1, 2, 4, or 8 Hz rate (not shown), produced a variety of changes in the amplitude of N1-P1, N2-P2, and CM responses to tones. In these experiments (n = 15), the amplitudes of N1-P1, N2-P2, and CM were evaluated before and after a 5-min period of microstimulation with current intensities between 2 and 50 μA. The average of maximum reductions obtained after MS that were at least 0.5 dB of change for N1-P1 and N2-P2 and 0.2 dB of change for CM responses was as follows: −1.6 ± 0.5 dB for CM (n = 6), −2.8 ± 0.9 dB for N1-P1 (n = 8), and −1.8 ± 1.1 dB for N2-P2 (n = 5), while average maximum increases obtained after cortical MS were 1.5 ± 1.1 dB for CM (n = 8), 1.8 ± 0.9 dB for N1-P1 (n = 3), and 1.5 ± 0.4 dB for N2-P2 (n = 5). The most common type of CAP change was N1-P1 amplitude reduction (Fig. 2A, D) found in eight cases, four of them accompanied with parallel CM reduction (Fig. 2B, E), and three of them with CM increase.
Figure 3 shows examples of the variety of descending effects of auditory cortex MS with 10 and 50 μA on cochlear responses to stimuli at different frequencies and sound pressure levels. The main effects of 10 μA cortical MS were CM reductions produced in the 1–4-kHz range of frequencies, at low to moderate sound pressure levels and almost no effect in N1-P1 amplitudes and mixed effects at 1 kHz in N2-P2 amplitudes, with reductions at low pressure levels accompanied by small increases at high pressure levels. The peripheral effects of 50 μA MS at the same cortical site (Fig. 3B) were similar in CM responses to those observed with 10 μA MS, CM reductions at 2 to 8 kHz, but accompanied by CM enhancements at 1 and 8 kHz and higher sound levels, while in the case of N2-P2 potentials, the efferent effect was inverted at 1 kHz, and a new amplitude reduction appeared at 2 kHz. These results suggest differential effects of cortical MS at different intensities on cochlear hair cell and neural responses.
FIG. 3.
Amplitude changes in cochlear potentials at different frequencies and sound levels induced after auditory cortex microstimulation at two current intensities. The upper and lower rows display amplitude changes (dB) produced with 10 and 50 μA, respectively. Green color represents no amplitude changes, while blue and violet represent significant decreases and yellow and red significant increases (frequency and intensity level paired t tests, p < 0.05). A Amplitude changes produced by 5 min of MS at 10 μA. A reduction of CM is seen between 1 and 4 kHz at low pressures, while N1-P1 presents tiny amplitude changes at 4 and 8 kHz. A strong N2-P2 reduction was observed at 1 kHz at 20 and 30 dB SPL, while focused increases were observed at 50 and 70 dB SPL at the same frequency. B Corticofugal effects obtained at 50 μA. Note that a CM reduction in the 2-kHz region, similar to the 10-μA changes, was observed, but it extended to the 4- and 8-kHz frequencies. The change pattern was also modified for the N1-P1, in which the reduction moved from 4 to 1 kHz. In the case of the N2-P2 response, there was a frequency shift of the diminishing effect to the 2-kHz region, while changes at 1 kHz were inverted, with an amplitude increase at 20 dB SPL and reductions at 50 and 70 dB SPL.
To evaluate the temporal course of corticofugal effects produced by auditory cortex MS, in the following experiments, we recorded and analyzed round window responses (at 4 Hz rate) throughout the period of cortical MS (delivered at 32 Hz rate). The aim of this type of experiments was to evaluate ongoing efferent effects produced during cortical MS in cochlear and auditory nerve responses. Only currents from 2 to 8 μA were used, as intensities higher than 10 μA produced large artifacts in the round window signal. Figure 4 shows an example of the temporal dynamics of N1-P1 and CM amplitude changes produced during cortical MS, evaluated at different stimulus frequencies (2, 4, and 8 kHz).
FIG. 4.
Auditory cortex electrical microstimulation modulates the amplitudes of N1-P1 and CM responses. Amplitudes of round window responses (at 4 Hz rate) were referenced to baseline amplitude (first 30 s) and displayed as decibel of change. Circles represent N1-P1 responses and red squares CM responses to tones. The red-shaded area represents the period of cortical microstimulation (4 μA at 32 Hz rate). A black dotted line represents baseline amplitude. A–C Changes for tones of 2, 4, and 8 kHz correspondingly, obtained from a single experiment. Notice that auditory cortex microstimulation produced significant decreases of CM responses to 2 and 8 kHz, while significant N1-P1 reductions were obtained only at 4 kHz stimuli.
A two-way ANOVA (frequency and MS as factors) for CM responses showed significant effects of frequency (F2, 84 = 142.329, p < 0.001) and MS (F1, 84 = 288.345, p < 0.001) and a significant interaction between frequency and MS (F2, 84 = 137.227, p < 0.001) in CM amplitudes. The Tukey post hoc test revealed significant effects of MS on CM responses at 2 and 8 kHz. On the other hand, a two-way ANOVA (frequency and MS as factors) for N1-P1 responses showed no effect for frequency (F2, 84 = 2.378, p = 0.099), a significant effect of MS (F1, 84 = 7.351, p < 0.008), and a significant interaction between frequency and MS (F2, 84 = 3.605, p = 0.031) in N1-P1 amplitudes. The Tukey post hoc test revealed a significant effect of MS only for N1-P1 responses obtained at 4 kHz stimuli.
The temporal course of the efferent effects was slow, in the order of tens of seconds, most of the times requiring between 1 and 2 min to obtain a significant buildup of the efferent effects. Fast effects (immediate changes with cortical MS) were not observed with this protocol.
Taken together, these results demonstrate that auditory cortex MS modulates cochlear and auditory nerve sensitivity probably by changing the activity of descending projections originated in layers V and VI of the auditory cortex. In addition, the differential effects observed at CM responses with different frequencies could be reflecting cortico-olivocochlear frequency specificity. However, whether these corticofugal effects were affecting the functioning of the olivocochlear reflex circuit remained uncertain. Next, we evaluated the effects of cortical MS on the strength of the olivocochlear reflex, assessed by the presentation of a broadband noise to the left ear, which is ipsilateral to the stimulated auditory cortex and contralateral to the recorded round window (Fig. 1A).
Contralateral noise produced amplitude increases, and reductions of the N1-P1 and N2-P2 responses, however, did not produce any changes in CM responses. Therefore, in the following results, only CAP data (N1-P1 and N2-P2) are shown. Figures 5, 6, 7, and 8 show characteristic examples from different single experiments that illustrate the variety of effects obtained. Figure 5A shows that the presentation of a contralateral noise without auditory cortex MS produced a small reduction effect at the onset of the contralateral acoustic stimulation in the N1-P1 responses, which was enlarged by auditory cortex MS (repeated measures ANOVA, F5, 60 = 122.08, p < 0.001, Tukey post hoc test). In contrast, there was no difference between the effect of the contralateral noise on N2-P2 responses obtained with and without cortical MS (Fig. 5B). Figure 6 shows an example in which the polarity of the contralateral noise effect on N1-P1 and N2-P2 responses was inverted with auditory cortex MS (repeated measures ANOVA, F5, 60 = 78.58, p < 0.001, Tukey post hoc test), while CM responses remained stable (not shown). Figure 7 shows an example in which a large effect of the contralateral noise on the amplitude of N1-P1 was reduced by cortical MS (Kruskal-Wallis, H5 = 255.284, p < 0.001).
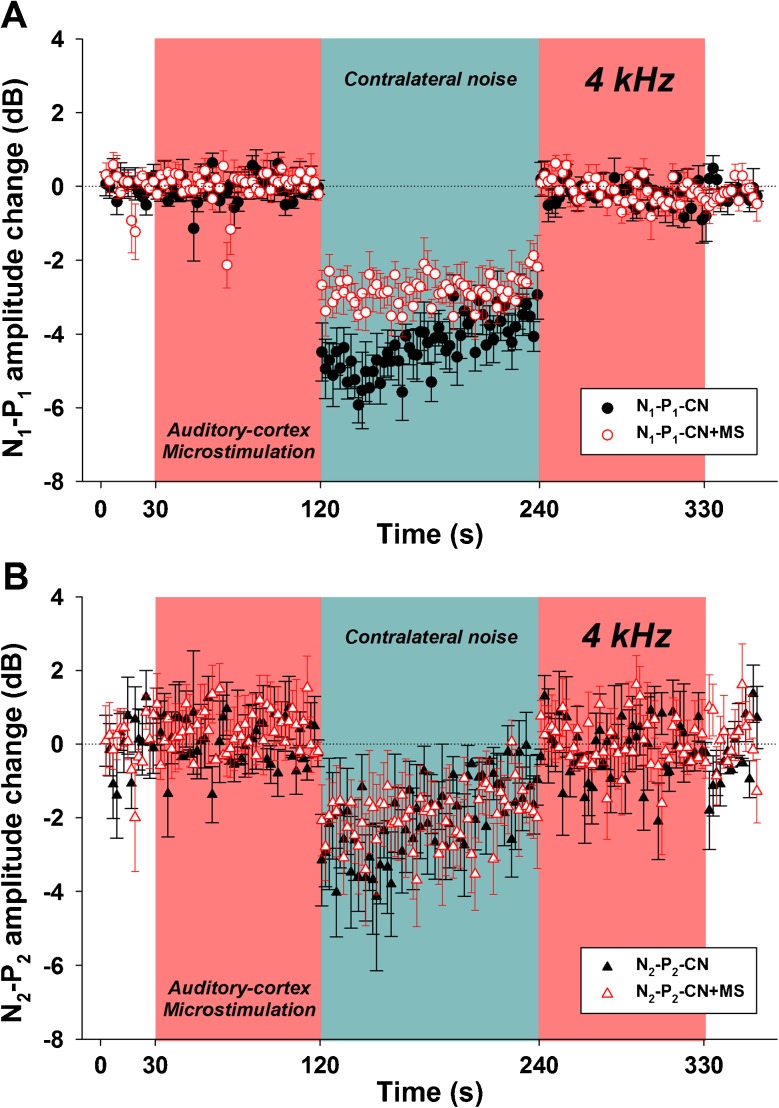
FIG. 5.
Auditory cortex electric microstimulation enhances the suppressive effect of contralateral noise on N1-P1 responses. The red-shaded area represents the period of cortical microstimulation (4 μA at 32 Hz rate), while the blue-shaded area represents the period in which the contralateral noise was presented. Black-filled symbols (circles and triangles) represent N1-P1 and N2-P2 potentials recorded without microstimulation, while white-filled, red border symbols (circles and triangles) represent N1-P1 and N2-P2 responses recorded with auditory cortex microstimulation. All amplitudes are referenced to the first 30 s, displayed as decibel of change. Notice that auditory cortex microstimulation A produced a significant increase of the suppressive effect of contralateral noise in N1-P1, while B in the case of the N2-P2 potential did not alter the suppressive effect of contralateral noise.
FIG. 6.
Auditory cortex microstimulation inverts the polarity of the contralateral noise effect on N1-P1 and N2-P2 responses. A, B Without cortical MS, there is an amplitude increase of N1-P1 and N2-P2 potentials with contralateral noise stimulation that was inverted to N1-P1 and N2-P2 suppressions when presenting contralateral noise preceded by cortical microstimulation.
FIG. 7.
Auditory cortex electric microstimulation reduces the suppressive effect of contralateral noise on N1-P1 responses. A Notice that auditory cortex microstimulation diminished the suppressive effect of contralateral noise on N1-P1 B but did not affect the suppressive effect of contralateral noise on N2-P2 responses.
FIG. 8.
The magnitude of corticofugal effects on CAP responses depends on the OC reflex strength without cortical MS. Left panels were obtained with 4-kHz stimuli, while right panels with 2 kHz. A Notice that auditory cortex microstimulation did not affect the suppressive effects of contralateral noise on N1-P1 and N2-P2 at 4 kHz. B But it increased the suppressive effect of contralateral noise on N1-P1 and N2-P2 at 2 kHz.
It is known that the OC reflex strength is variable in different subjects (Maison and Liberman 2000; Backus and Guinan 2007); therefore, next we explored whether the variability of the OC reflex strength on CAP responses was a factor that influences the level of modulation produced by the auditory cortex MS on the OC reflex strength on N1-P1 and N2-P2 responses. Figure 8 shows an example of a large CAP effect (N1-P1 and N2-P2) of contralateral noise without microstimulation at 4 kHz (Fig. 8A, C), which was not affected by cortical MS. In contrast, at 2 kHz, there was no CAP effect of contralateral noise without MS, but with cortical MS and simultaneous contralateral noise, an increasing suppressive effect appeared (Fig. 8B, D) (N1-P1: repeated measures ANOVA, F5, 60 = 23.61, p < 0.001, Tukey post hoc test; N2-P2: repeated measures ANOVA, F5, 60 = 31.22, p < 0.001, Tukey post hoc test).
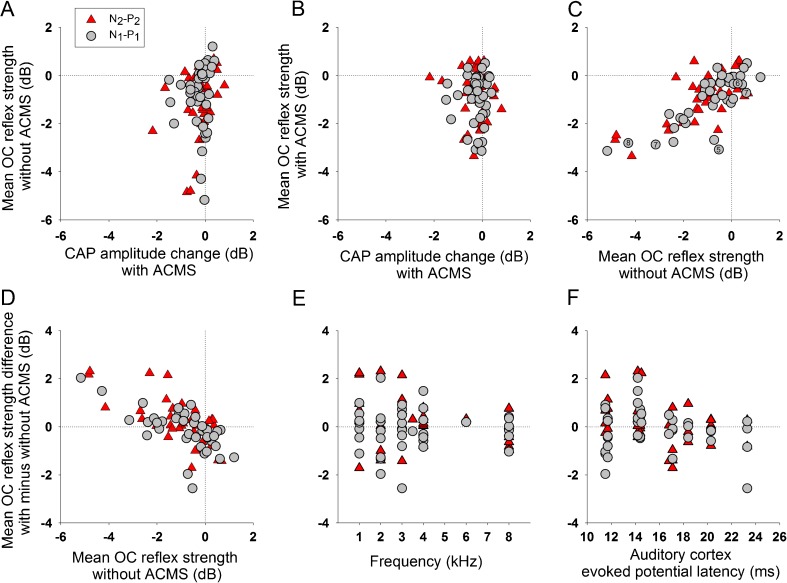
Taken together, data from Figures 5, 6, 7, and 8 suggest that cortical MS increases the suppressive strength of the OC reflex in those cases of positive or small reduction effects without MS (Figs. 5A, 6, and 8B, D), while large OC suppressive effects obtained without MS are diminished by cortical MS (Figs. 7A and 8A, C). This pattern of cortical MS effects on the strength of the OC reflex was confirmed by the analysis of the average effect of contralateral noise measured in all experiments, as displayed in Figure 9. Moreover, we found that both types of corticofugal effects were independent, as there was no correlation between them (Fig. 9A, B). On the other hand, there was a linear correlation between the strength of the OC reflex without and with cortical MS (Fig. 9C), while OC reflex strength without cortical MS correlated with the differential OC reflex effect obtained with MS (Fig. 9D). In addition, the effects of cortical MS on the OC reflex strength were mostly centered in auditory stimuli with frequencies between 1 and 4 kHz (Fig. 9E). The largest effects on N1-P1 were obtained with ACEP latencies <15 ms, as the median absolute value of these effects was 0.545 dB, while for those experiments with latencies >15 ms was 0.229 dB (Mann-Whitney test, U = 100, p = 0.009) (Fig. 9F). There was no significant difference in the ACEP latencies of N2-P2 effects.
FIG. 9.
Average effect of contralateral noise stimulation (CNS) calculated during the 120 s period of OC activation, with and without auditory cortex microstimulation (ACMS) in all experiments. In this graph, data was obtained at 70 dB SPL and from different frequencies (n = 40 recordings with and without ACMS). The ordinates show the following: In panel A, mean OC reflex strength without ACMS; in panels B and C, mean reflex strength with cortical MS; and in panels D−F, the difference between average CNS effects with and without ACMS. Gray circles and red triangles represent N1-P1 and N2-P2 amplitude changes (dB), respectively. A There was no correlation between the effect of ACMS on CAP amplitudes (X-axis) and the magnitude of OC reflex strength without cortical MS (Y-axis) B and the magnitude of OC reflex with auditory cortex MS (Y-axis). Notice that positive effects larger than 1 dB and reductions larger than −4 dB elicited by OC reflex without MS (Y-axis in panel A) disappeared when eliciting OC reflex with ACMS (Y-axis in panel B), thus centering the effects around −1 to −2 dB. C There was a linear correlation between OC reflex strength without and with cortical MS (N1-P1: r 2 40 = 0.5947, Spearman p < 0.0001; N2-P2: r 2 40 = 0.502, Spearman p < 0.0001). The small numbers inside gray circles indicate the specific examples given in Figures 5, 6, (at 2 kHz), 7 (at 4 kHz), and 8 for N1-P1. D There was a significant linear correlation between OC reflex strength without cortical MS (X-axis in panel D) and OC reflex strength difference with and without cortical MS (N1-P1: r 2 40 = 0.4402, Spearman p < 0.001; N2-P2: r 2 40 = 0.472, Spearman p < 0.001). Although the mean OC reflex strength without ACMS is one of the values used to calculate the difference effects displayed in the ordinate of panel D, a priori, this fact does not allow to predict the effect of ACMS on OC reflex strength. E The majority of the effects were obtained with frequencies between 1 and 4 kHz (evaluated from 1 to 8 kHz). F Relation between ACEP latencies (ms) and the effect of cortical MS on CNS effect. Note that most of the large effects were obtained with latencies <15 ms.
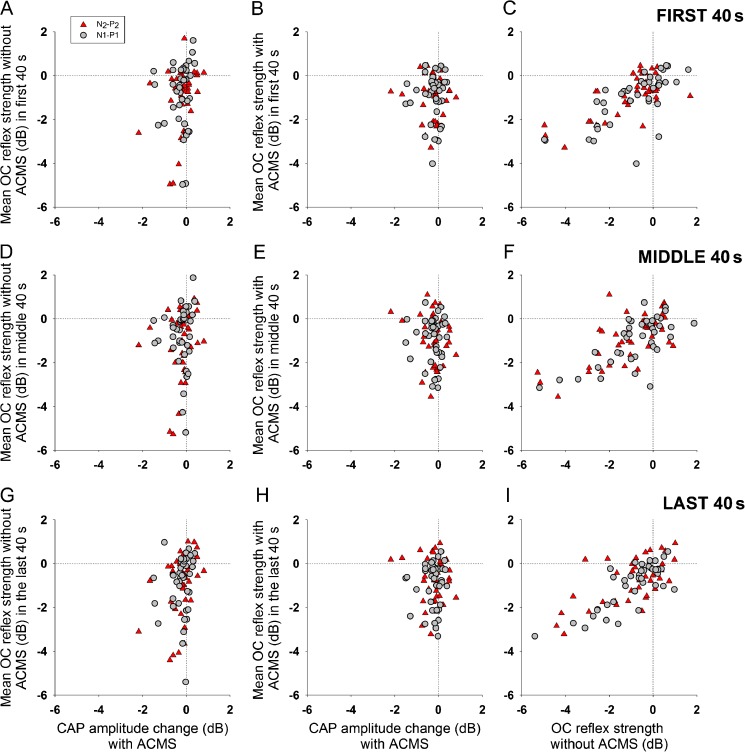
As the effects of contralateral noise on CAP responses presented a variety of temporal courses (Figs. 5, 6, 7, and 8), next, we separately analyzed the average effects obtained in the first, middle, and last 40 s of contralateral acoustic stimulation. Figures 10 and 11 show no systematic differences in contralateral noise effects on CAP responses between the three periods of stimulation and that these effects were similar to those obtained with average data for the whole period of contralateral stimulation displayed in Figure 9.
FIG. 10.
Average effect of contralateral noise stimulation evaluated at the first, middle, and last 40 s of the OC reflex with and without auditory cortex microstimulation. Similarly to the results displayed in Figure 9, in the first 40 s (A and B), middle 40 s (D and E), and in the last 40 s (G and H) of CNS, there was no correlation between the effect of ACMS on CAP amplitudes and the magnitude of OC reflex strength without cortical MS and the magnitude of OC reflex with auditory cortex MS. Linear correlations between OC reflex strength without and with cortical MS were also obtained in these three periods (C) (N1-P1: r 2 40 = 0.4906, Spearman p < 0.001; N2-P2: r 2 40 = 0.491, Spearman p < 0.001), (F) (N1-P1: r 2 40 = 0.4867, Spearman p < 0.001; N2-P2: r 2 40 = 0.4537, Spearman p < 0.001), and (I) (N1-P1: r 2 40 = 0.6345, Spearman p < 0.0001; N2-P2: r 2 40 = 0.4198, Spearman p < 0.001).
FIG. 11.
Significant correlations between OC reflex strength without cortical MS (X-axis in panels (A), (D), and (G)) and OC reflex strength difference with and without cortical MS in the first (N1-P1: r 2 40 = 0.4273, Spearman p < 0.001; N2-P2: r 2 40 = 0.5866, Spearman p < 0.001), middle (N1-P1: r 2 40 = 0.4402, Spearman p < 0.001; N2-P2: r 2 40 = 0.4894, Spearman p < 0.001), and last 40 s of CNS (N1-P1: r 2 40 = 0.4223, Spearman p < 0.001; N2-P2: r 2 40 = 0.5002, Spearman p < 0.001). Similarly, to results displayed in Figure 9, the majority of the effects in these three periods ((B), (E), and (H)) were obtained with frequencies between 1 and 4 kHz. In addition, most of the large effects in the first (C), middle (F), and last 40 s (I) of CNS were also obtained with latencies <15 ms.
DISCUSSION
Here, we demonstrated that auditory cortex electrical microstimulation produced two different types of corticofugal effects on cochlear and auditory nerve responses. The first type was an amplitude change of cochlear potentials, including CAP (N1-P1 and N2-P2) and CM responses, while the second type was a modulation of the strength of contralateral acoustic stimulation effects on CAP responses, without changes in CM amplitudes.
Previous studies of auditory cortex microstimulation found corticofugal effects at the most peripheral level of the auditory pathway, including frequency-specific amplitude changes of cochlear microphonics in bats (Xiao and Suga 2002a), amplitude reduction of otoacoustic emissions in humans (Perrot et al. 2006), and modulation of neural responses in the cochlear nucleus of mice (Luo et al. 2008). Some important differences of the present work with previous studies are (i) we recorded cochlear and auditory nerve responses simultaneously, (ii) we studied the temporal course of corticofugal effects in a millisecond scale, and (iii) we directly assessed the corticofugal effects of auditory cortex microstimulation on the strength of the olivocochlear reflex evoked by contralateral noise.
Corticofugal Effects on CAP and CM Responses
In addition to MOC neurons, the OC system is also constituted by lateral olivocochlear neurons (LOC) which make synapses with auditory nerve fibers. Consequently, a modulation of LOC activity would only affect CAP but not CM responses (Groff and Liberman 2003), while a MOC modulation can change CAP and CM amplitudes (Elgueda et al. 2011).
In a previous study of auditory cortex inactivation, we found a large diversity of corticofugal effects, suggesting the presence of an auditory cortex efferent basal tone that modulates the amplitudes of CAP and CM responses (Leon et al. 2012). Here, we found that auditory cortex electrical microstimulation also produced a variety of effects, including CAP amplitude changes (N1-P1 and N2-P2) that were in the range of ±3 dB, accompanied by CM changes in the range of ±1.6 dB. As the modulation of CM responses is only achieved through MOC fibers, these results suggest that cortical MS modified the basal tone of cortical neurons that constitute the direct or indirect (through inferior colliculus) descending pathways to the medial olivocochlear complex. However, a concomitant modulation of LOC neurons by cortical MS cannot be discarded (Fig. 12).
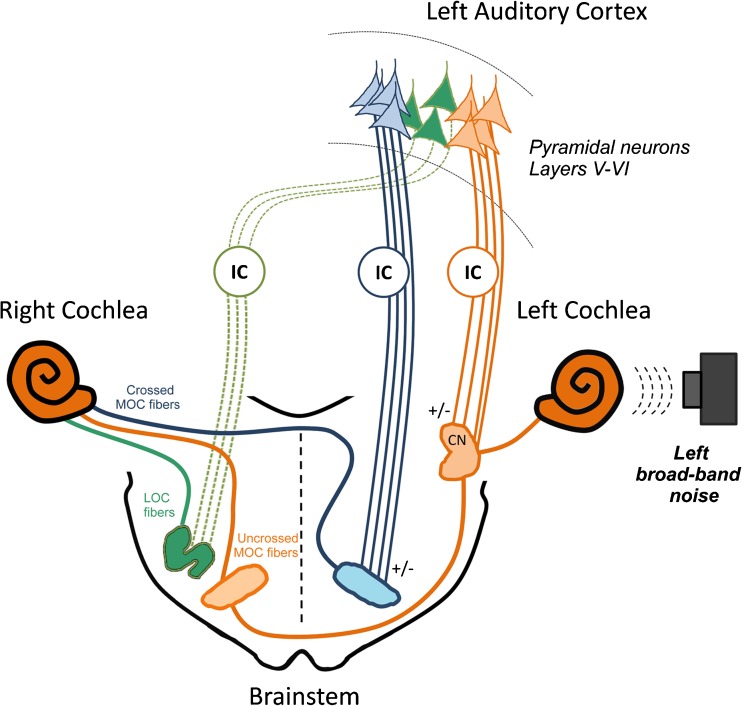
FIG. 12.
Proposed model for independent corticofugal modulation of cochlear sensitivity and olivocochlear reflex strength. Microstimulation was delivered to deep layers of the left auditory cortex. Descending projections from pyramidal neurons to the ipsilateral cochlear nucleus (Schofield and Coomes 2005) could be modulating the three-neuron olivocochlear reflex circuit (de Venecia et al. 2005), mediated by left auditory nerve fibers, left cochlear nucleus neurons, and right olivocochlear neurons that constitute the uncrossed olivocochlear bundle to the right cochlea (orange pathways). On the other hand, descending projections from pyramidal neurons to the ipsilateral superior olivary complex (Mulders and Robertson 2000) could be modulating left medial olivocochlear fibers that cross to the right cochlea and thus modulate cochlear sensitivity (blue pathways). Both descending pathways can be directly mediated from cortex to cochlear nucleus or superior olivary complex or indirectly through synapses in the left inferior colliculus. In addition, a third pathway from left auditory cortex to right inferior colliculus (Bajo and King 2013) and to right lateral olivocochlear neurons (Thompson and Thompson 1993) could be also modulating CAP responses (green pathways). CN cochlear nucleus, IC inferior colliculus, LOC lateral olivocochlear, MOC medial olivocochlear, OC olivocochlear neurons.
Frequency Dependence of Corticofugal Effects
Auditory cortex microstimulation produces sharp frequency-specific CM effects in bats (Xiao and Suga 2002a, b) and also tuned effects in ipsilateral and contralateral cochlear nucleus neurons in mice (Luo et al. 2008; Liu et al. 2010; Kong et al. 2014). Here, the majority of the descending effects were obtained at frequencies between 1 and 4 kHz, which is in agreement with our previous experiments of auditory cortex inactivation (Leon et al. 2012). Moreover, electrical stimulation of the medial olivocochlear bundle in chinchillas also produced the largest CAP effects at 2 kHz (Elgueda et al. 2011), a frequency that corresponds to a location at about the middle of the chinchilla cochlea, which receives most of the olivocochlear innervation (Eldredge et al. 1981; Azeredo et al. 1999). In relation with this frequency preference of the corticofugal effects, the present study has two limitations: (i) we evaluated a limited set of frequencies (1 to 8 kHz, in steps of 1.0 or 0.5 kHz), and (ii) we used ACEP responses obtained by averaging local field potentials, a less frequency-specific technique than unitary recordings (Gaucher et al. 2012). For these reasons, we cannot discard that cortico-olivocochlear effects were actually more frequency-specific than the results described in this work.
Temporal Course of Corticofugal Effects
The electrical activation of medial olivocochlear neurons can elicit two effects with different temporal course: a fast effect which has a time constant of tens of milliseconds and a slow effect with a time constant of tens of seconds (Sridhar et al. 1995). Here, as corticofugal effects of electrical microstimulation were evaluated within pulse trains (Fig. 1C), we were able to study the temporal course of the cortico-olivocochlear effects in a more precise time scale than previous cortico-olivocochlear studies (Xiao and Suga 2002a; Perrot et al. 2006), evaluating fast and slow effects. In all cases, corticofugal effects were obtained only in a slow time scale requiring tens of seconds to appear at the cochlear level, and for that reason, the second protocol (simultaneous MS and contralateral acoustic stimulation) was designed with a period of 90 s of cortical microstimulation before contralateral noise stimulation.
Corticofugal Effects of Auditory Cortex MS on the Strength of the OC Reflex
Several authors have described a large variability in the strength of the olivocochlear reflex, which has been attributed to different functions, like protection to acoustic trauma (Maison and Liberman 2000), or as a factor that prevents age-related hearing loss or presbycusis (Liberman et al. 2014). Moreover, Larsen and Liberman (2009) have suggested that slow fluctuations in the strength of the OC reflex can be attributed to central modulation of olivocochlear neurons. Here, we directly demonstrated that auditory cortex activity modulates the strength of the olivocochlear reflex in CAP (N1-P1 and N2-P2) but not in CM responses.
The effect of cortical MS on OC reflex strength depends on the magnitude of the OC reflex without cortical microstimulation. In the cases of CAP enhancements or weak reductions (<1 dB effect) with contralateral noise without MS, the average suppressive effect of the reflex is enhanced with MS (Figs. 5A and 6A, B), while in those cases of large OC suppressions without MS (>2 dB), the effect is reduced with MS (Fig. 7A). Moreover, these data suggest that auditory cortex MS overrides the suppressive effect of the OC reflex to an optimal average strength of about 0.97 dB for N1-P1 suppression and about 0.86 dB for N2-P2 suppression, values calculated using the average values of the OC reflex strength with auditory cortex MS.
The ACEP latency in chinchillas is an electrophysiological value used to delimit primary (<15 ms) and secondary (>15 ms) auditory cortex responses (Harel et al. 2000). Cortical modulations of the OC reflex strength in N1-P1 responses were larger in cortical sites with ACEP latencies <15 ms (Fig. 9F), suggesting that electric microstimulation of primary auditory cortex sites was more effective in modifying the OC reflex strength than that of secondary auditory cortex sites. This latency dependent difference on the strength of the OC reflex is in agreement with neuroanatomical studies showing that the primary auditory cortex is the main source of descending projections to the inferior colliculus and to the superior olivary complex (Bajo and King 2013; Doucet et al. 2002).
The anesthetic state of the animals could have reduced the magnitude of the cortical effect on the OC reflex strength and could also explain the lack of effect of contralateral noise on CM responses, as it is known that MOC activity is influenced by the arousal level (Guitton et al. 2004; Chambers et al. 2012). These questions should be addressed in future experiments in awake animals.
Two Independent Corticofugal Effects
Figure 12 proposes a working model to explain independent corticofugal effects on (i) cochlear sensitivity and (ii) olivocochlear reflex strength. In the experiments presenting modulation of cochlear sensitivity (affecting right CAP and CM responses), descending pathways from the left auditory cortex to the ipsilateral medial olivocochlear system are probably modulated, and thus crossed fibers from the left olivocochlear complex could mediate this cortico-olivocochlear effect. On the other hand, in experiments in which there was a modulation of the olivocochlear reflex strength on right CAP responses, descending pathways from left auditory cortex to the ipsilateral cochlear nucleus (Schofield and Coomes 2005) could be modulating the three neuron pathways of the uncrossed OC reflex. Therefore, we propose that the effect of the broadband noise presented in the left ear is probably modulated by descending pathways reaching the left cochlear nucleus and that the corticofugal modulation of the olivocochlear reflex strength found in this work could be mediated by uncrossed fibers from the right olivocochlear complex. An alternative model could also include the LOC circuit, as Darrow et al. (2006) have suggested that LOC neurons can reduce or increase the excitability of auditory nerve neurons. In our work, the modulation of right LOC activity by left auditory cortex microstimulation could explain why in some cases we found N1-P1 and CM parallel reductions, while in others these cochlear responses were dissociated.
Functional Relevance of Auditory Cortex Descending Pathways: Cognitive Processes and Tinnitus Perception
Some of the proposed functions of the cortico-olivocochlear network are modulation of cochlear sensitivity during selective attention to visual or auditory stimuli (Oatman 1971; Delano et al. 2007; Srinivasan et al. 2012; Wittekindt et al. 2014) and during awake and sleep stages (Velluti et al. 1989). Dissociated effects on cochlear potentials during selective attention to visual stimuli were obtained in chinchillas (Delano et al. 2007), that is CAP reductions concomitant to CM augmentations, while parallel changes of CAP and CM responses have been reported during sleep stages (Velluti et al. 1989). Based on the present results, we propose that the different effects obtained in these works can be mediated by two independent mechanisms, which are modulation of cochlear sensitivity and regulation of the olivocochlear reflex strength.
Cortical descending pathways could be involved in the mechanism for diminishing the perception of tinnitus. One of the most studied treatments to alleviate tinnitus is the electrical stimulation of auditory cortex using direct current stimulation (Fenoy et al. 2006; Fregni et al. 2006; Zhang et al. 2011) or indirect repetitive transcranial magnetic stimulation (De Ridder et al. 2005; Langguth and De Ridder 2013). Several mechanisms have been proposed to explain how the cortical stimulation can suppress tinnitus, including (i) modulation of neural hyperactivity related to tinnitus, (ii) modulation of cortical synchronization and coherence within brain centers, and (iii) modulation of peripheral gain (Zhang 2013). Here, we show that the activation of descending projections from the auditory cortex can modulate the OC reflex strength. This mechanism could be involved in tinnitus suppression and should be considered as an important factor to measure in future clinical trials.
In summary, we demonstrated that auditory cortex electrical microstimulation independently modulates cochlear sensitivity and the strength of the olivocochlear reflex probably through parallel efferent pathways from cortex to the brainstem and cochlear receptor.
Acknowledgments
This study was funded by Fondecyt 1120256 to PHD, Apoyo de viaje de la Vicerrectoría de Investigación de la Universidad de Chile to PHD, and Beca CONICYT to CDD and Fundación Puelma. We thank Fernando Vergara for his technical assistance.
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Constantino D. Dragicevic, Email: cdragicevic@gmail.com
Cristian Aedo, Email: caedosanchez@gmail.com.
Alex León, Email: alexsamir@gmail.com.
Macarena Bowen, Email: macabowen@gmail.com.
Natalia Jara, Email: njarao@gmail.com.
Gonzalo Terreros, Email: gonzalobenjamin.terreros@gmail.com.
Luis Robles, Email: lrobles@med.uchile.cl.
Paul H. Delano, Phone: 56 2 29786037, Email: pdelano@med.uchile.cl, Email: phdelano@gmail.com
References
- Anderson LA, Malmierca MS. The effect of auditory cortex deactivation on stimulus-specific adaptation in the inferior colliculus of the rat. Eur J Neurosci. 2013;37:52–62. doi: 10.1111/ejn.12018. [DOI] [PubMed] [Google Scholar]
- Antunes FM, Malmierca MS. Effect of auditory cortex deactivation on stimulus-specific adaptation in the medial geniculate body. J Neurosci. 2011;31:17306–16. doi: 10.1523/JNEUROSCI.1915-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeredo WJ, Kliment ML, Morley BJ, Relkin E, Slepecky NB, Sterns A, Warr WB, Weekly JM, Woods CI. Olivocochlear neurons in the chinchilla: a retrograde fluorescent labelling study. Hear Res. 1999;134:57–70. doi: 10.1016/S0378-5955(99)00069-6. [DOI] [PubMed] [Google Scholar]
- Backus BC, Guinan JJ., Jr Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions. J Assoc Res Otolaryngol. 2007;8:484–96. doi: 10.1007/s10162-007-0100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Moore DR. Descending projections from the auditory cortex to the inferior colliculus in the gerbil, Meriones unguiculatus. J Comp Neurol. 2005;486:101–16. doi: 10.1002/cne.20542. [DOI] [PubMed] [Google Scholar]
- Bajo VM, King AJ. Cortical modulation of auditory processing in the midbrain. Front Neural Circuits. 2013;6:114. doi: 10.3389/fncir.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DJ, Patuzzi RB. Evidence that the compound action potential (CAP) from the auditory nerve is a stationary potential generated across dura mater. Hear Res. 2010;267:12–26. doi: 10.1016/j.heares.2010.03.091. [DOI] [PubMed] [Google Scholar]
- Buño W., Jr Auditory nerve fiber activity influenced by contralateral ear sound stimulation. Exp Neurol. 1978;59:62–74. doi: 10.1016/0014-4886(78)90201-7. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Hancock KE, Maison SF, Liberman MC, Polley DB. Sound-evoked olivocochlear activation in unanesthetized mice. J Assoc Res Otolaryngol. 2012;13:209–17. doi: 10.1007/s10162-011-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarac, F (2005) The history of reflexes. Part 1: from Descartes to Pavlov. IBRO History of Neuroscience. [http://ibro.info/wp-content/uploads/2012/12/The-History-of-Reflexes-Part-1.pdf]
- Clarac F. Some historical reflections on the neural control of locomotion. Brain Res Rev. 2008;57:13–21. doi: 10.1016/j.brainresrev.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Cochlear efferent feedback balances interaural sensitivity. Nat Neurosci. 2006;9:1474–6. doi: 10.1038/nn1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano PH, Elgueda D, Hamame CM, Robles L. Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J Neurosci. 2007;27:4146–53. doi: 10.1523/JNEUROSCI.3702-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano PH, Pavez E, Robles L, Maldonado PE. Stimulus-dependent oscillations and evoked potentials in chinchilla auditory cortex. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:693–700. doi: 10.1007/s00359-008-0340-4. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Verstraeten E, Van der Kelen K, De Mulder G, Sunaert S, Verlooy J, Van de Heyning P, Moller A. Transcranial magnetic stimulation for tinnitus: influence of tinnitus duration on stimulation parameter choice and maximal tinnitus suppression. Otol Neurotol. 2005;26:616–9. doi: 10.1097/01.mao.0000178146.91139.3c. [DOI] [PubMed] [Google Scholar]
- de Venecia RK, Liberman MC, Guinan JJ, Jr, Brown MC. Medial olivocochlear reflex interneurons are located in the posteroventral cochlear nucleus: a kainic acid lesion study in guinea pigs. J Comp Neurol. 2005;487:345–60. doi: 10.1002/cne.20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet JR, Rose L, Ryugo DK. The cellular origin of corticofugal projections to the superior olivary complex in the rat. Brain Res. 2002;925:28–41. doi: 10.1016/S0006-8993(01)03248-6. [DOI] [PubMed] [Google Scholar]
- Eldredge DH, Miller JD, Bohne BA. A frequency-position map for the chinchilla cochlea. J Acoust Soc Am. 1981;69:1091–1095. doi: 10.1121/1.385688. [DOI] [PubMed] [Google Scholar]
- Elgueda D, Delano PH, Robles L. Effects of electrical stimulation of olivocochlear fibers in cochlear potentials in the chinchilla. J Assoc Res Otolaryngol. 2011;12:317–27. doi: 10.1007/s10162-011-0260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AG, Orlovsky GN. The influence of different descending systems on the tonic stretch reflex in the cat. Exp Neurol. 1972;37:481–94. doi: 10.1016/0014-4886(72)90091-X. [DOI] [PubMed] [Google Scholar]
- Fenoy AJ, Severson MA, Volkov IO, Brugge JF, Howard MA., 3rd Hearing suppression induced by electrical stimulation of human auditory cortex. Brain Res. 2006;1118:75–83. doi: 10.1016/j.brainres.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Marcondes R, Boggio PS, Marcolin MA, Rigonatti SP, Sanchez G, Nitsche MA, Pascual-Leone A. Transient tinnitus suppression induced by repetitive transcranial magnetic stimulation and transcranial direct current stimulation. Eur J Neurol. 2006;13:996–1001. doi: 10.1111/j.1468-1331.2006.01414.x. [DOI] [PubMed] [Google Scholar]
- Gaucher Q, Edeline JM, Gourévitch B. How different are the local field potentials and spiking activities? Insights from multi-electrodes arrays. J Physiol Paris. 2012;106:93–103. doi: 10.1016/j.jphysparis.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Groff JA, Liberman MC. Modulation of cochlear afferent response by the lateral olivocochlear system: activation via electrical stimulation of the inferior colliculus. J Neurophysiol. 2003;90:3178–200. doi: 10.1152/jn.00537.2003. [DOI] [PubMed] [Google Scholar]
- Guitton MJ, Avan P, Puel JL, Bonfils P. Medial olivocochlear efferent activity in awake guinea pigs. Neuroreport. 2004;15:1379–82. doi: 10.1097/01.wnr.0000131672.15566.64. [DOI] [PubMed] [Google Scholar]
- Harel N, Mori N, Sawada S, Mount RJ, Harrison RV. Three distinct auditory areas of cortex (AI, AII, and AAF) defined by optical imaging of intrinsic signals. Neuroimage. 2000;11:302–12. doi: 10.1006/nimg.1999.0537. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Kakigi A, Hirakawa H, Harel N, Mount RJ. Tonotopic mapping in auditory cortex of the chinchilla. Hear Res. 1996;100:157–63. doi: 10.1016/0378-5955(96)00120-7. [DOI] [PubMed] [Google Scholar]
- Kawase T, Liberman MC. Antimasking effects of the olivocochlear reflex. I. Enhancement of compound action potentials to masked tones. J Neurophysiol. 1993;70:2519–32. doi: 10.1152/jn.1993.70.6.2519. [DOI] [PubMed] [Google Scholar]
- Khalfa S, Bougeard R, Morand N, Veuillet E, Isnard J, Guenot M, Ryvlin P, Fischer C, Collet L. Evidence of peripheral auditory activity modulation by the auditory cortex in humans. Neuroscience. 2001;104:347–58. doi: 10.1016/S0306-4522(01)00072-0. [DOI] [PubMed] [Google Scholar]
- Kong L, Xiong C, Li L, Yan J (2014) Frequency-specific corticofugal modulation of the dorsal cochlear nucleus in mice. Front Syst Neurosci 1;8:125. [DOI] [PMC free article] [PubMed]
- Lamas V, Alvarado JC, Carro J, Merchán MA. Long-term evolution of brainstem electrical evoked responses to sound after restricted ablation of the auditory cortex. PLoS One. 2013;8(9):e73585. doi: 10.1371/journal.pone.0073585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B, De Ridder D. Tinnitus: therapeutic use of superficial brain stimulation. Handb Clin Neurol. 2013;116:441–67. doi: 10.1016/B978-0-444-53497-2.00036-X. [DOI] [PubMed] [Google Scholar]
- Larsen E, Liberman MC. Slow build-up of cochlear suppression during sustained contralateral noise: central modulation of olivocochlear efferents? Hear Res. 2009;256:1–10. doi: 10.1016/j.heares.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon A, Elgueda D, Silva MA, Hamamé CM, Delano PH. Auditory cortex basal activity modulates cochlear responses in chinchillas. PLoS One. 2012;7(4):e36203. doi: 10.1371/journal.pone.0036203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Rapid assessment of sound-evoked olivocochlear feedback: suppression of compound action potentials by contralateral sound. Hear Res. 1989;38:47–56. doi: 10.1016/0378-5955(89)90127-5. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Liberman LD, Maison SF. Efferent feedback slows cochlear aging. J Neurosci. 2014;34:4599–607. doi: 10.1523/JNEUROSCI.4923-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Wang Q, Kashani A, Yan J. Corticofugal modulation of initial sound processing in the brain. J Neurosci. 2008;28:11615–21. doi: 10.1523/JNEUROSCI.3972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yan Y, Wang Y, Yan J. Corticofugal modulation of initial neural processing of sound information from the ipsilateral ear in the mouse. PLoS One. 2010;5(11):e14038. doi: 10.1371/journal.pone.0014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci. 2000;20:4701–7. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca and Ryugo (2011) Descending connections of the auditory cortex to the midbrain and the brainstem. Chapter 9, The auditory cortex, Eds. Winer JA and Schreiner CE, Springer.
- McIntyre D, Ring C, Carroll D. Effects of arousal and natural baroreceptor activation on the human muscle stretch reflex. Psychophysiology. 2004;41:954–60. doi: 10.1111/j.1469-8986.2004.00235.x. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Evidence for direct cortical innervation of medial olivocochlear neurones in rats. Hear Res. 2000;144:65–72. doi: 10.1016/S0378-5955(00)00046-0. [DOI] [PubMed] [Google Scholar]
- Oatman LC. Role of visual attention on auditory evoked potentials in unanesthetized cats. Exp Neurol. 1971;32:341–356. doi: 10.1016/0014-4886(71)90003-3. [DOI] [PubMed] [Google Scholar]
- Perrot X, Ryvlin P, Isnard J, Guénot M, Catenoix H, Fischer C, Mauguière F, Collet L. Evidence for corticofugal modulation of peripheral auditory activity in humans. Cereb Cortex. 2006;16:941–8. doi: 10.1093/cercor/bhj035. [DOI] [PubMed] [Google Scholar]
- Robles L, Delano PH. Efferent system. The senses: a comprehensive reference. London: Academic Press; 2008. pp. 413–445. [Google Scholar]
- Saldaña E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol. 1996;371:15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Central descending auditory pathways. Auditory and vestibular efferents. New York: Springer; 2010. pp. 261–290. [Google Scholar]
- Schofield BR, Coomes DL. Auditory cortical projections to the cochlear nucleus in guinea pigs. Hear Res. 2005;199:89–102. doi: 10.1016/j.heares.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Sellick P, Patuzzi R, Robertson D. Primary afferent and cochlear nucleus contributions to extracellular potentials during tone-bursts. Hear Res. 2003;176:42–58. doi: 10.1016/S0378-5955(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sridhar TS, Liberman MC, Brown MC, Sewell WF. A novel cholinergic “slow effect” of efferent stimulation on cochlear potentials in the guinea pig. J Neurosci. 1995;15:3667–78. doi: 10.1523/JNEUROSCI.15-05-03667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Keil A, Stratis K, Woodruff Carr KL, Smith DW. Effects of cross-modal selective attention on the sensory periphery: cochlear sensitivity is altered by selective attention. Neuroscience. 2012;223:325–32. doi: 10.1016/j.neuroscience.2012.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka H. Ontogenetic rules and constraints of vestibulo-ocular reflex development. Curr Opin Neurobiol. 2010;20:689–95. doi: 10.1016/j.conb.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Tang AH, Schroeder LA. Spinal-cord depressant effects of ketamine and etoxadrol in the cat and the rat. Anesthesiology. 1973;39:37–43. doi: 10.1097/00000542-197307000-00007. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC. Relationship of descending inferior colliculus projections to olivocochlear neurons. J Comp Neurol. 1993;335:402–12. doi: 10.1002/cne.903350309. [DOI] [PubMed] [Google Scholar]
- Velluti R, Pedemonte M, Garcia-Austt E. Correlative changes of auditory nerve and microphonic potentials throughout sleep. Hear Res. 1989;39:203–208. doi: 10.1016/0378-5955(89)90091-9. [DOI] [PubMed] [Google Scholar]
- Wittekindt A, Kaiser J, Abel C. Attentional modulation of the inner ear: a combined otoacoustic emission and EEG study. J Neurosci. 2014;34:9995–10002. doi: 10.1523/JNEUROSCI.4861-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nat Neurosci. 2002;5:57–63. doi: 10.1038/nn786. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Reorganization of the cochleotopic map in the bat’s auditory system by inhibition. Proc Natl Acad Sci USA. 2002;99:15743–8. doi: 10.1073/pnas.242606699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang Y, Zhang X. Auditory cortex electrical stimulation suppresses tinnitus in rats. J Assoc Res Otolaryngol. 2011;12:185–201. doi: 10.1007/s10162-010-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Auditory cortex stimulation to suppress tinnitus: mechanisms and strategies. Hear Res. 2013;295:38–57. doi: 10.1016/j.heares.2012.05.007. [DOI] [PubMed] [Google Scholar]