Abstract
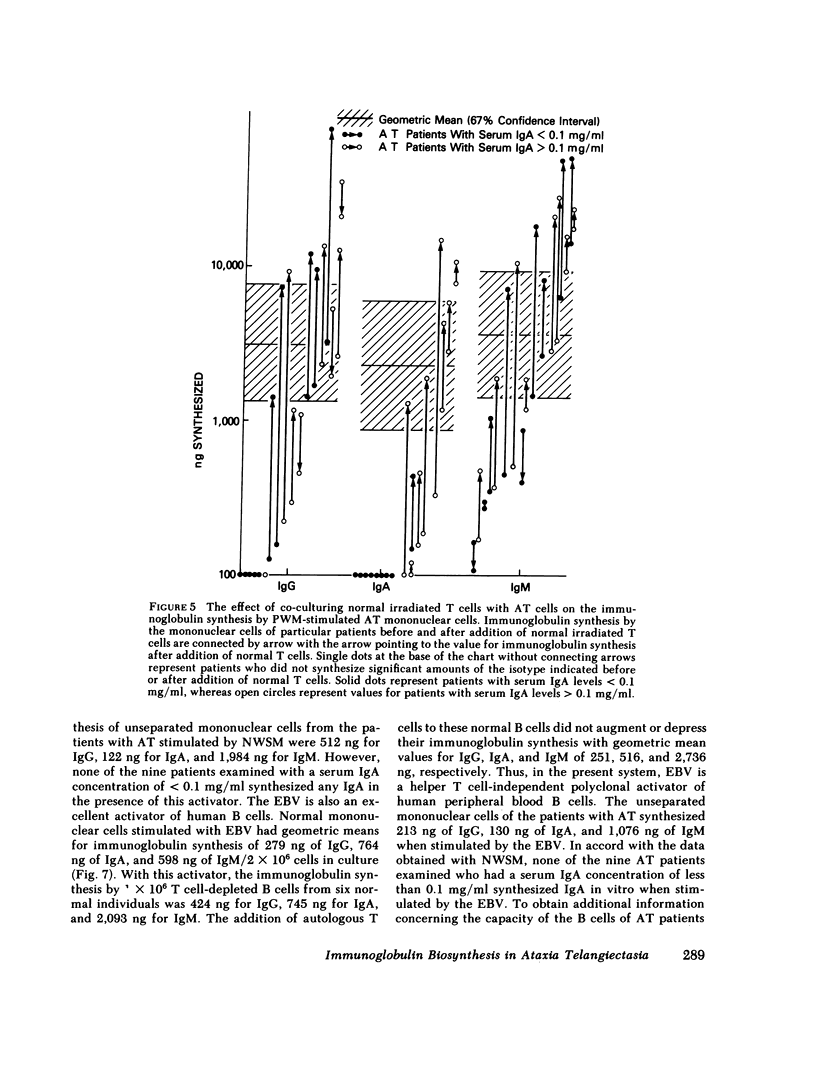
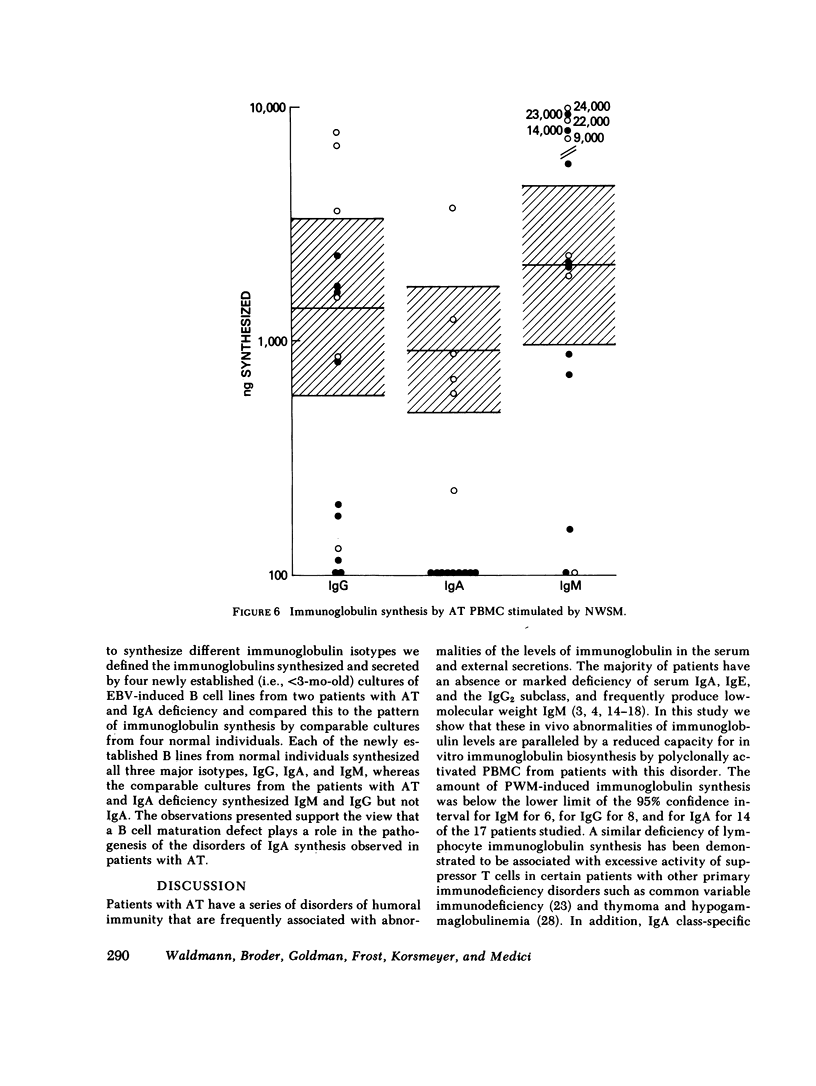
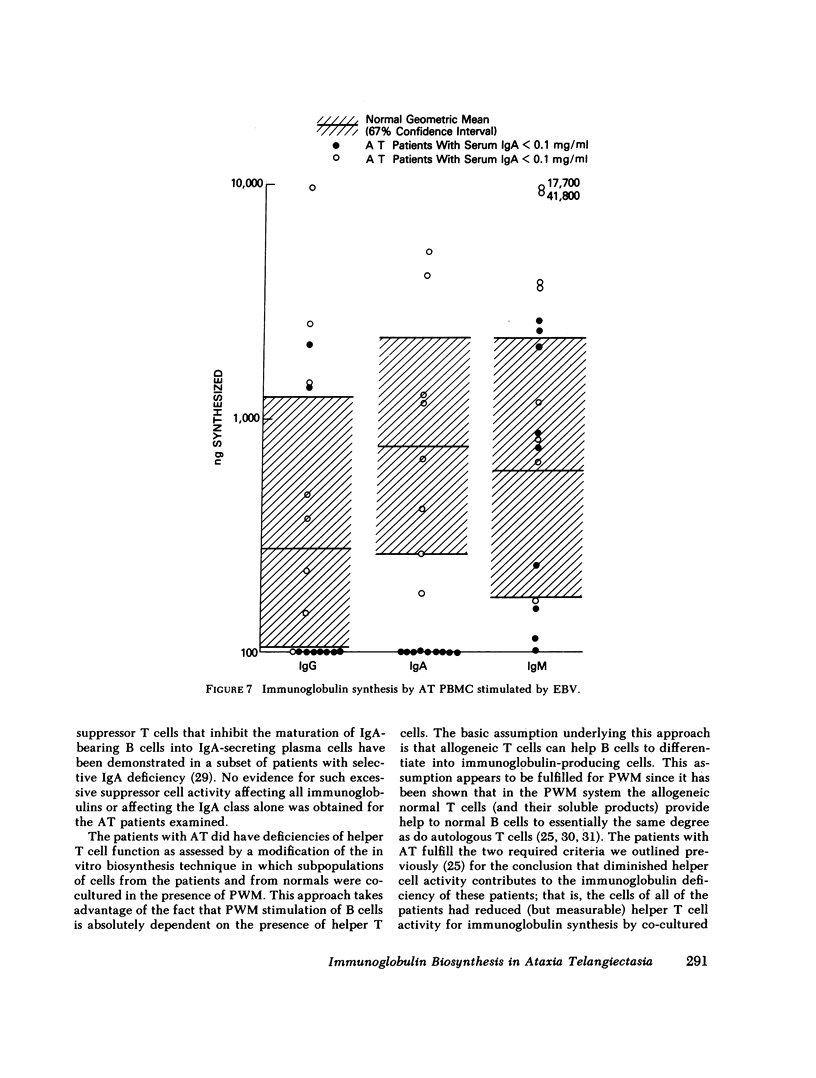
The pathogenesis of the immunoglobulin deficiency of 20 patients with ataxia telangiectasia was studied using an in vitro immunoglobulin biosynthesis system. 10 patients had no detectable IgA in their serum as assessed by radial diffusion in agar and 3 had a reduced serum IgA concentration. The peripheral blood mononuclear cells of 17 of the patients and 17 normal controls were cultured with pokeweed mitogen for 12 d and the immunoglobulin in the supernatants measured. The immunoglobulin synthesis was below the lower limit of the normal 95% confidence interval for IgM in 5 patients, for IgG in 8, and for IgA in 14. The mononuclear cells from 9 of the 10 patients with a serum IgA concentration less than 0.1 mg/ml failed to synthesize IgA in vitro. None of the patients manifested excessive suppressor cell activity. All patients had reduced but measurable helper T cell activity for immunoglobulin synthesis by co-cultured normal pokeweed mitogen-stimulated B cells (geometric mean 22% of normal). Furthermore, the addition of normal irradiated T cells to patient peripheral blood mononuclear cells led to an augmentation of IgM synthesis in 15 of 17 and to increased IgG synthesis in 9 of the 17 patients studied, including 9 of the 12 patients who had synthesized IgG before the addition of the irradiated T cells. In addition, IgA synthesis was increased in all eight patients examined that had serum IgA concentrations greater than 0.1 mg/ml. These studies suggest that a helper T cell defect contributes to the diminished immunoglobulin synthesis. However, a helper T cell defect does not appear to be the sole cause since there was no IgA synthesis by the peripheral blood mononuclear cells of 9 of the 10 patients with a profoundly reduced serum IgA even when co-cultured with normal T cells. Furthermore, the cells of the nine patients with profoundly reduced IgA levels examined also failed to produce IgA when stimulated with the relatively helper T cell-independent polyclonal activators, Nocardia water soluble mitogen or Epstein-Barr virus. Taken together these data support the view that the reduced immunoglobulin synthesis of these patients is due to defects of both B cells and helper T cells. Such a broad defect in lymphocyte maturation taken in conjunction with our demonstration of persistent alpha fetoprotein production by ataxia telangiectasia patients provides support for the proposal that these patients exhibit a generalized defect in tissue differentiation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUERBACH R. Morphogenetic interactions in the development of the mouse thymus gland. Dev Biol. 1960 Jun;2:271–284. doi: 10.1016/0012-1606(60)90009-9. [DOI] [PubMed] [Google Scholar]
- Ammann A. J., Cain W. A., Ishizaka K., Hong R., Good R. A. Immunoglobulin E deficiency in ataxia-telangiectasia. N Engl J Med. 1969 Aug 28;281(9):469–472. doi: 10.1056/NEJM196908282810904. [DOI] [PubMed] [Google Scholar]
- BODER E., SEDGWICK R. P. Ataxia-telangiectasia; a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics. 1958 Apr;21(4):526–554. [PubMed] [Google Scholar]
- Bona C., Damais C., Dimitriu A., Chedid L., Ciorbaru R., Adam A., Petit J. F., Lederer E., Rosselet J. P. Mitogenic effect of a water-soluble extract of Nocardia opaca: a comparative study with some bacterial adjuvants on spleen and peripheral lymphocytes of four mammalian species. J Immunol. 1974 Jun;112(6):2028–2035. [PubMed] [Google Scholar]
- Bridges B. A. Some DNA-repair-deficient human syndromes and their implications for human health. Proc R Soc Lond B Biol Sci. 1981 Jul 14;212(1188):263–278. doi: 10.1098/rspb.1981.0038. [DOI] [PubMed] [Google Scholar]
- Broder S., Edelson R. L., Lutzner M. A., Nelson D. L., MacDermott R. P., Durm M. E., Goldman C. K., Meade B. D., Waldmann T. A. The Sézary syndrome: a malignant proliferation of helper T cells. J Clin Invest. 1976 Dec;58(6):1297–1306. doi: 10.1172/JCI108585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Cohen M. M., Shaham M., Dagan J., Shmueli E., Kohn G. Cytogenetic investigations in families with ataxia-telangiectasia. Cytogenet Cell Genet. 1975;15(5):338–356. doi: 10.1159/000130530. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Shander M., Martinis J., Cicurel L., D'Ancona G. G., Dolby T. W., Koprowski H. Chromosomal location of the genes for human immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3416–3419. doi: 10.1073/pnas.76.7.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Epstein W. L., Fudenberg H. H., Reed W. B., Boder E., Sedgwick R. P. Immunologic studies in ataxia-telangiectasia. I. Delayed hypersensitivity and serum immune globulin levels in probands and first-degree relatives. Int Arch Allergy Appl Immunol. 1966;30(1):15–29. [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- Hecht F., McCaw B. K., Koler R. D. Ataxia-telangiectasia--clonal growth of translocation lymphocytes. N Engl J Med. 1973 Aug 9;289(6):286–291. doi: 10.1056/NEJM197308092890603. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kuritani T., Kishimoto T., Yamamura Y. In vitro immune response of human peripheral lymphocytes. I. The mechanism(s) involved in T cell helper functions in the pokeweed mitogen-induced differentiation and proliferation of B cells. J Immunol. 1977 Oct;119(4):1235–1241. [PubMed] [Google Scholar]
- Kataoka T., Kawakami T., Takahashi N., Honjo T. Rearrangement of immunoglobulin gamma 1-chain gene and mechanism for heavy-chain class switch. Proc Natl Acad Sci U S A. 1980 Feb;77(2):919–923. doi: 10.1073/pnas.77.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- McCaw B. K., Hecht F., Harnden D. G., Teplitz R. L. Somatic rearrangement of chromosome 14 in human lymphocytes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2071–2075. doi: 10.1073/pnas.72.6.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin D. E., Strober W., Waldmann T. A. Ataxia-telangiectasia. Medicine (Baltimore) 1972 Jul;51(4):281–314. doi: 10.1097/00005792-197207000-00002. [DOI] [PubMed] [Google Scholar]
- Misiti J., Waldmann T. A. In vitro generation of antigen-specific hemolytic plaque-forming cells from human peripheral blood mononuclear cells. J Exp Med. 1981 Oct 1;154(4):1069–1084. doi: 10.1084/jem.154.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxelius V. A., Berkel A. I., Hanson L. A. IgG2 deficiency in ataxia-telangiectasia. N Engl J Med. 1982 Mar 4;306(9):515–517. doi: 10.1056/NEJM198203043060905. [DOI] [PubMed] [Google Scholar]
- Oxford J. M., Harnden D. G., Parrington J. M., Delhanty J. D. Specific chromosome aberrations in ataxia telangiectasia. J Med Genet. 1975 Sep;12(3):251–262. doi: 10.1136/jmg.12.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON R. D., KELLY W. D., GOOD R. A. ATAXIA-TELANGIECTASIA. ITS ASSOCIATION WITH A DEFECTIVE THYMUS, IMMUNOLOGICAL-DEFICIENCY DISEASE, AND MALIGNANCY. Lancet. 1964 May 30;1(7344):1189–1193. doi: 10.1016/s0140-6736(64)91209-7. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Smith B. P., Lohman P. H., Anderson A. K., Fishman L. Defective excision repair of gamma-ray-damaged DNA in human (ataxia telangiectasia) fibroblasts. Nature. 1976 Apr 1;260(5550):444–447. doi: 10.1038/260444a0. [DOI] [PubMed] [Google Scholar]
- Peterson R. D., Cooper M. D., Good R. A. Lymphoid tissue abnormalities associated with ataxia-telangiectasia. Am J Med. 1966 Sep;41(3):342–359. doi: 10.1016/0002-9343(66)90080-5. [DOI] [PubMed] [Google Scholar]
- Polmar S. H., Waldmann T. A., Balestra S. T., Jost M. C., Terry W. D. Immunoglobulin E in immunologic deficiency diseases. I. Relation of IgE and IgA to respiratory tract disease in isolated IgE deficiency, IgA deficiency, and ataxia telangiectasia. J Clin Invest. 1972 Feb;51(2):326–330. doi: 10.1172/JCI106817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Tomasi T. B. A Low Molecular Weight Immunoglobulin Antigenically Related to 19 S IgM. J Clin Invest. 1967 Aug;46(8):1329–1337. doi: 10.1172/JCI105625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W., Wochner R. D., Barlow M. H., McFarlin D. E., Waldmann T. A. Immunoglobulin metabolism in ataxia telangiectasia. J Clin Invest. 1968 Aug;47(8):1905–1915. doi: 10.1172/JCI105881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T., Sawada T., Tozawa M., Kidowaki T., Kusunoki T., Yamaguchi N. Plasma levels of carcinoembryonic antigen in patients with ataxia-telangiectasia. J Pediatr. 1978 Mar;92(3):436–439. doi: 10.1016/s0022-3476(78)80440-5. [DOI] [PubMed] [Google Scholar]
- THIEFFRY S., ARTHUIS M., AICARDI J., LYON G. [Ataxiatelangiectasis. (7 personal cases)]. Rev Neurol (Paris) 1961 Nov;105:390–405. [PubMed] [Google Scholar]
- Taylor A. M., Harnden D. G., Arlett C. F., Harcourt S. A., Lehmann A. R., Stevens S., Bridges B. A. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975 Dec 4;258(5534):427–429. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Metcalfe J. A., Oxford J. M., Harnden D. G. Is chromatid-type damage in ataxia telangiectasia after irradiation at G0 a consequence of defective repair? Nature. 1976 Apr 1;260(5550):441–443. doi: 10.1038/260441a0. [DOI] [PubMed] [Google Scholar]
- Trompeter R. S., Layward L., Hayward A. R. Primary and secondary abnormalities of T cell subpopulations. Clin Exp Immunol. 1978 Dec;34(3):388–392. [PMC free article] [PubMed] [Google Scholar]
- Vasileiskii S. S., Akishina G. E., Kudashkina V. M. Mesto beta-fetoproteinov sredi drugikh fetal'nykh belkov. Ontogenez. 1975;6(2):183–186. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S., Durm M., Blackman M., Krakauer R., Meade B. Suppressor T cells in the pathogenesis of hypogammaglobulinemia associated with a thymoma. Trans Assoc Am Physicians. 1975;88:120–134. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S., Krakauer R., Durm M., Meade B., Goldman C. Defect in IgA secretion and in IgA specific suppressor cells in patients with selective IgA deficiency. Trans Assoc Am Physicians. 1976;89:215–224. [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Polyclonal B-cell activators in the study of the regulation of immunoglobulin synthesis in the human system. Adv Immunol. 1982;32:1–63. doi: 10.1016/s0065-2776(08)60720-8. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., McIntire K. R. Serum-alpha-fetoprotein levels in patients with ataxia-telangiectasia. Lancet. 1972 Nov 25;2(7787):1112–1115. doi: 10.1016/s0140-6736(72)92717-1. [DOI] [PubMed] [Google Scholar]
- Webb T., Harnden D. G., Harding M. The chromosome analysis and susceptibility to transformation by Simian Virus 40 of fibroblasts from ataxia-telangiectasia. Cancer Res. 1977 Apr;37(4):997–1002. [PubMed] [Google Scholar]



