Abstract
The POU motif, conserved among a family of eukaryotic transcription factors, contains two DNA-binding domains: an N-terminal POU-specific domain (POUS) and a C-terminal homeodomain (POUHD). Surprisingly, POUS is similar in structure to the helix-turn-helix domains of bacteriophage repressor and Cro proteins. Such similarity predicts a common mechanism of DNA recognition. To test this prediction, we have studied the DNA-binding properties of the human Oct-2 POU domain by combined application of chemical synthesis and site-directed mutagenesis. The POUS footprint of DNA contacts, identified by use of modified bases, is analogous to those of bacteriophage repressor-operator complexes. Moreover, a loss-of-contact substitution in the putative POUS recognition alpha-helix leads to relaxed specificity at one position in the DNA target site. The implied side chain-base contact is identical to that of bacteriophage repressor and Cro proteins. These results establish a functional analogy between the POUS and prokaryotic helix-turn-helix elements and suggest that their DNA specificities may be governed by a shared set of rules.
Full text
PDF
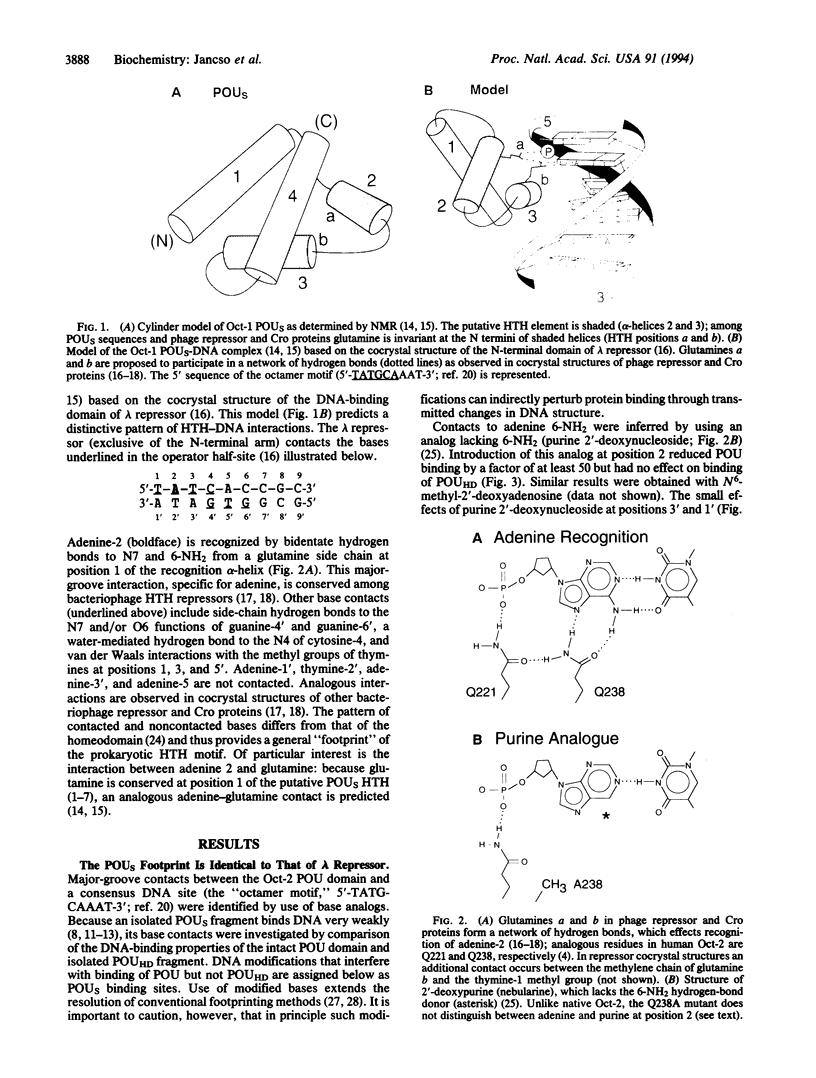
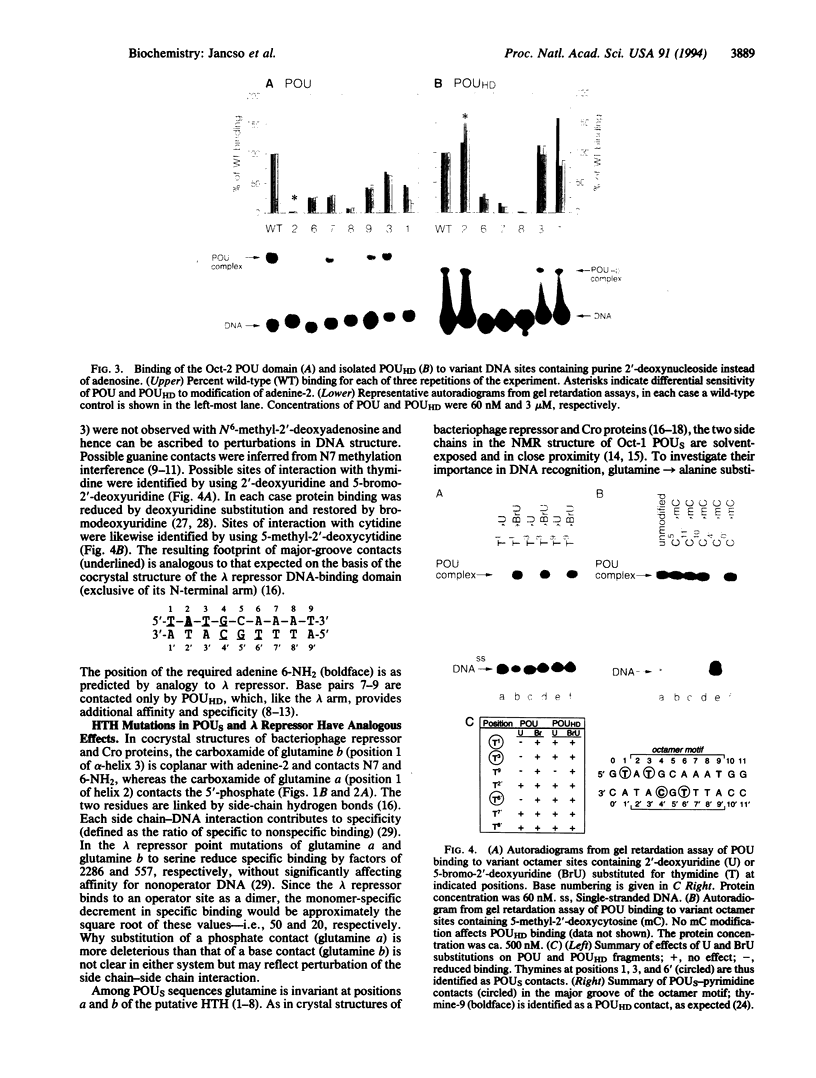
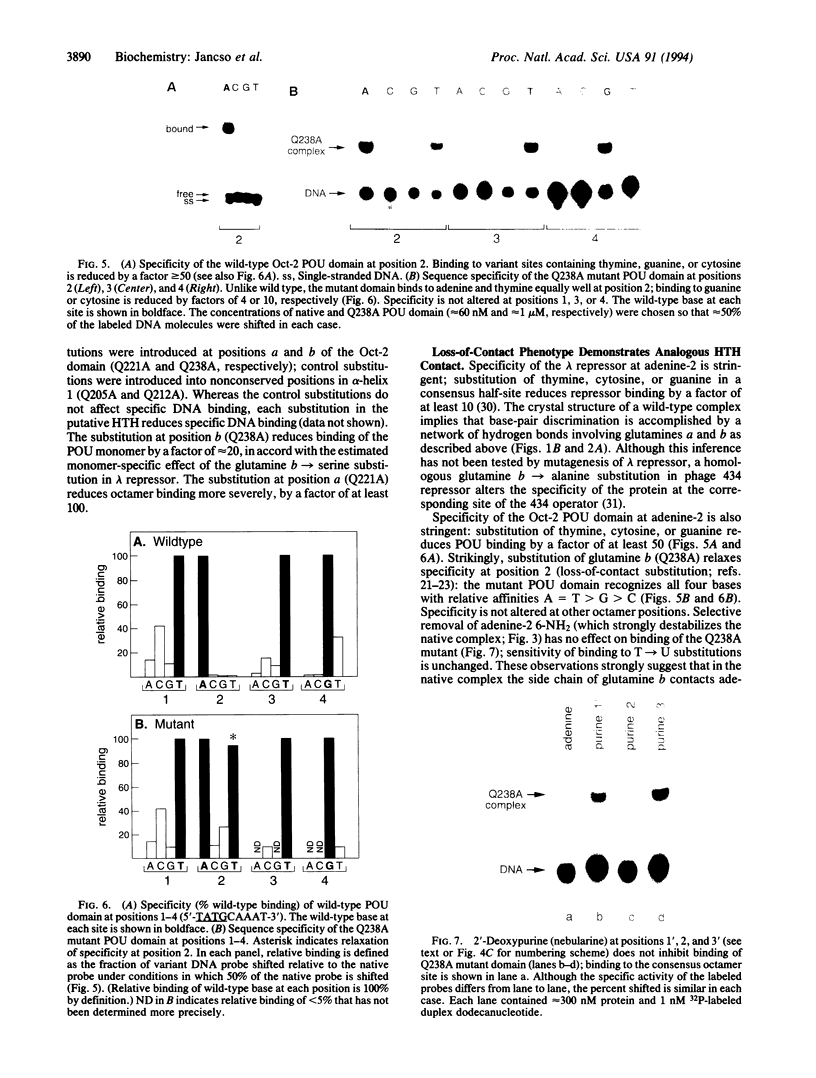

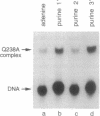
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A. K., Rodgers D. W., Drottar M., Ptashne M., Harrison S. C. Recognition of a DNA operator by the repressor of phage 434: a view at high resolution. Science. 1988 Nov 11;242(4880):899–907. doi: 10.1126/science.3187531. [DOI] [PubMed] [Google Scholar]
- Assa-Munt N., Mortishire-Smith R. J., Aurora R., Herr W., Wright P. E. The solution structure of the Oct-1 POU-specific domain reveals a striking similarity to the bacteriophage lambda repressor DNA-binding domain. Cell. 1993 Apr 9;73(1):193–205. doi: 10.1016/0092-8674(93)90171-l. [DOI] [PubMed] [Google Scholar]
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Boelens R., Scheek R. M., van Boom J. H., Kaptein R. Complex of lac repressor headpiece with a 14 base-pair lac operator fragment studied by two-dimensional nuclear magnetic resonance. J Mol Biol. 1987 Jan 5;193(1):213–216. doi: 10.1016/0022-2836(87)90638-3. [DOI] [PubMed] [Google Scholar]
- Botfield M. C., Jancso A., Weiss M. A. Biochemical characterization of the Oct-2 POU domain with implications for bipartite DNA recognition. Biochemistry. 1992 Jun 30;31(25):5841–5848. doi: 10.1021/bi00140a020. [DOI] [PubMed] [Google Scholar]
- Brennan C. A., Van Cleve M. D., Gumport R. I. The effects of base analogue substitutions on the cleavage by the EcoRI restriction endonuclease of octadeoxyribonucleotides containing modified EcoRI recognition sequences. J Biol Chem. 1986 Jun 5;261(16):7270–7278. [PubMed] [Google Scholar]
- Clerc R. G., Corcoran L. M., LeBowitz J. H., Baltimore D., Sharp P. A. The B-cell-specific Oct-2 protein contains POU box- and homeo box-type domains. Genes Dev. 1988 Dec;2(12A):1570–1581. doi: 10.1101/gad.2.12a.1570. [DOI] [PubMed] [Google Scholar]
- Dekker N., Cox M., Boelens R., Verrijzer C. P., van der Vliet P. C., Kaptein R. Solution structure of the POU-specific DNA-binding domain of Oct-1. Nature. 1993 Apr 29;362(6423):852–855. doi: 10.1038/362852a0. [DOI] [PubMed] [Google Scholar]
- Ebright R. H. Evidence for a contact between glutamine-18 of lac repressor and base pair 7 of lac operator. Proc Natl Acad Sci U S A. 1986 Jan;83(2):303–307. doi: 10.1073/pnas.83.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright R. H. Use of "loss-of-contact" substitutions to identify residues involved in an amino acid-base pair contact: effect of substitution of Gln18 of lac repressor by Gly, Ser, and Leu. J Biomol Struct Dyn. 1985 Oct;3(2):281–297. doi: 10.1080/07391102.1985.10508417. [DOI] [PubMed] [Google Scholar]
- Eritja R., Horowitz D. M., Walker P. A., Ziehler-Martin J. P., Boosalis M. S., Goodman M. F., Itakura K., Kaplan B. E. Synthesis and properties of oligonucleotides containing 2'-deoxynebularine and 2'-deoxyxanthosine. Nucleic Acids Res. 1986 Oct 24;14(20):8135–8153. doi: 10.1093/nar/14.20.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney M., Ruvkun G., Horvitz H. R. The C. elegans cell lineage and differentiation gene unc-86 encodes a protein with a homeodomain and extended similarity to transcription factors. Cell. 1988 Dec 2;55(5):757–769. doi: 10.1016/0092-8674(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Douhan J., 3rd, Ptashne M. How lambda repressor and lambda Cro distinguish between OR1 and OR3. Cell. 1986 Dec 5;47(5):807–816. doi: 10.1016/0092-8674(86)90523-4. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Ptashne M. Homologous interactions of lambda repressor and lambda Cro with the lambda operator. Cell. 1986 Mar 28;44(6):925–933. doi: 10.1016/0092-8674(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Jordan S. R., Pabo C. O. Structure of the lambda complex at 2.5 A resolution: details of the repressor-operator interactions. Science. 1988 Nov 11;242(4880):893–899. doi: 10.1126/science.3187530. [DOI] [PubMed] [Google Scholar]
- Kissinger C. R., Liu B. S., Martin-Blanco E., Kornberg T. B., Pabo C. O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 A resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990 Nov 2;63(3):579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- Ko H. S., Fast P., McBride W., Staudt L. M. A human protein specific for the immunoglobulin octamer DNA motif contains a functional homeobox domain. Cell. 1988 Oct 7;55(1):135–144. doi: 10.1016/0092-8674(88)90016-5. [DOI] [PubMed] [Google Scholar]
- Kristie T. M., Sharp P. A. Interactions of the Oct-1 POU subdomains with specific DNA sequences and with the HSV alpha-trans-activator protein. Genes Dev. 1990 Dec;4(12B):2383–2396. doi: 10.1101/gad.4.12b.2383. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., Kobayashi T., Staudt L., Baltimore D., Sharp P. A. Octamer-binding proteins from B or HeLa cells stimulate transcription of the immunoglobulin heavy-chain promoter in vitro. Genes Dev. 1988 Oct;2(10):1227–1237. doi: 10.1101/gad.2.10.1227. [DOI] [PubMed] [Google Scholar]
- Levy D. The wide world of geography turns in Washington. Science. 1992 Aug 28;257(5074):1210–1210. doi: 10.1126/science.1519056. [DOI] [PubMed] [Google Scholar]
- Nelson H. C., Sauer R. T. Interaction of mutant lambda repressors with operator and non-operator DNA. J Mol Biol. 1986 Nov 5;192(1):27–38. doi: 10.1016/0022-2836(86)90461-4. [DOI] [PubMed] [Google Scholar]
- Newman P. C., Nwosu V. U., Williams D. M., Cosstick R., Seela F., Connolly B. A. Incorporation of a complete set of deoxyadenosine and thymidine analogues suitable for the study of protein nucleic acid interactions into oligodeoxynucleotides. Application to the EcoRV restriction endonuclease and modification methylase. Biochemistry. 1990 Oct 23;29(42):9891–9901. doi: 10.1021/bi00494a020. [DOI] [PubMed] [Google Scholar]
- Sarai A., Takeda Y. Lambda repressor recognizes the approximately 2-fold symmetric half-operator sequences asymmetrically. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6513–6517. doi: 10.1073/pnas.86.17.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Cromlish J. A., Gerster T., Kawakami K., Balmaceda C. G., Currie R. A., Roeder R. G. A human lymphoid-specific transcription factor that activates immunoglobulin genes is a homoeobox protein. Nature. 1988 Dec 8;336(6199):551–557. doi: 10.1038/336551a0. [DOI] [PubMed] [Google Scholar]
- Schultz S. C., Shields G. C., Steitz T. A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991 Aug 30;253(5023):1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- Seeman N. C., Rosenberg J. M., Rich A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):804–808. doi: 10.1073/pnas.73.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Sturm R. A., Das G., Herr W. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 1988 Dec;2(12A):1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- Sturm R. A., Herr W. The POU domain is a bipartite DNA-binding structure. Nature. 1988 Dec 8;336(6199):601–604. doi: 10.1038/336601a0. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., Alkema M. J., van Weperen W. W., Van Leeuwen H. C., Strating M. J., van der Vliet P. C. The DNA binding specificity of the bipartite POU domain and its subdomains. EMBO J. 1992 Dec;11(13):4993–5003. doi: 10.1002/j.1460-2075.1992.tb05606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrijzer C. P., Kal A. J., van der Vliet P. C. The oct-1 homeo domain contacts only part of the octamer sequence and full oct-1 DNA-binding activity requires the POU-specific domain. Genes Dev. 1990 Nov;4(11):1964–1974. doi: 10.1101/gad.4.11.1964. [DOI] [PubMed] [Google Scholar]
- Wharton R. P., Ptashne M. A new-specificity mutant of 434 repressor that defines an amino acid-base pair contact. 1987 Apr 30-May 6Nature. 326(6116):888–891. doi: 10.1038/326888a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. P., Ebright R. H. Identification of a contact between arginine-180 of the catabolite gene activator protein (CAP) and base pair 5 of the DNA site in the CAP-DNA complex. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4717–4721. doi: 10.1073/pnas.87.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]