Abstract
Acute Charcot neuroarthropathy of the foot and ankle presents with the insidious onset of a unilateral acutely edematous, erythematous, and warm lower extremity. The acute stages are typically defined as Eichenholtz Stage 1, or Stage 0, which was first described by Shibata et al. in 1990. The ultimate goal of treatment is maintenance of a stable, plantigrade foot which can be easily shod, minimizing the risk of callus, ulceration, infection, and amputation. The gold standard of treatment is non-weight-bearing immobilization in a total contact cast. Surgical intervention remains controversial. A review of the literature was performed to provide an evidenced-based approach to the conservative and surgical management of acute Charcot neuroarthropathy of the foot and ankle.
Keywords: foot collapse, neuroarthropathy, peripheral neuropathy, swollen foot, total contact cast
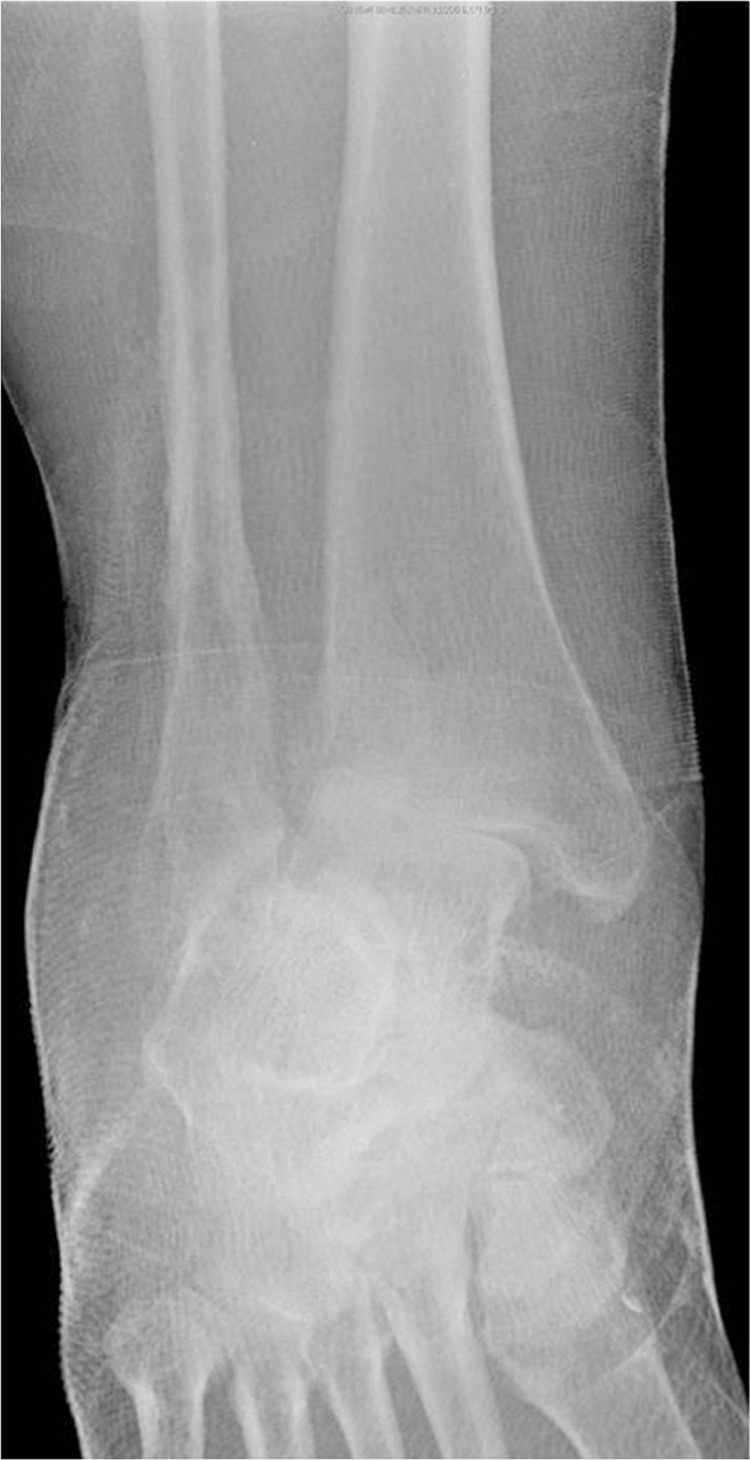
Eichenholtz proposed a three stage radiographic classification system for Charcot neuroarthropathy affecting the foot in 1966 (1–6). Stage I, the ‘destruction’ phase, is defined by capsular distention, osseous fragmentation, peri-articular debris formation, subluxations/dislocation, and fracture. Stage II, the ‘coalescence’ phase, is defined by osseous resorption and evidence of consolidation. Stage III, the ‘reconstruction’ phase, involves maturation of bone consolidation (1–6). Stage 0 was first coined by Shibata et al. in 1990 (7). Since that time, several authors have reported on the complexities of diagnosing and treating Stage 0 Charcot (6, 8–17). This phase is characterized more by physical examination findings consisting of a unilateral red, hot, swollen foot and leg (1, 7–13, 18–21) (Fig. 1). Radiographs are typically normal except for increased soft tissue volume (7, 9–13, 17, 18, 22, 23). Subtle subluxations and/or small avulsion fractures may be seen but are often overlooked or felt to be an incidental finding (12) (Figs. 2–5). The reported incidence of acute Charcot ranges from 9 to 13% of Charcot cases, although the true incidence may be higher due to the number of cases that are initially misdiagnosed (4–6, 8, 10, 11, 14–16, 18, 19, 22–30). The average delay in the diagnosis of acute Charcot has been reported to be around 29 weeks (12, 15, 27, 31). Common misdiagnoses include cellulitis, erysipelas, deep vein thrombosis (DVT), venous insufficiency, gout, pseudogout, acute inflammatory arthritis, fracture, sprain, tumor, septic arthritis, osteomyelitis, Sudeck's atrophy, and rheumatoid arthritis (8, 9, 13, 15, 16, 18, 23, 32–34).
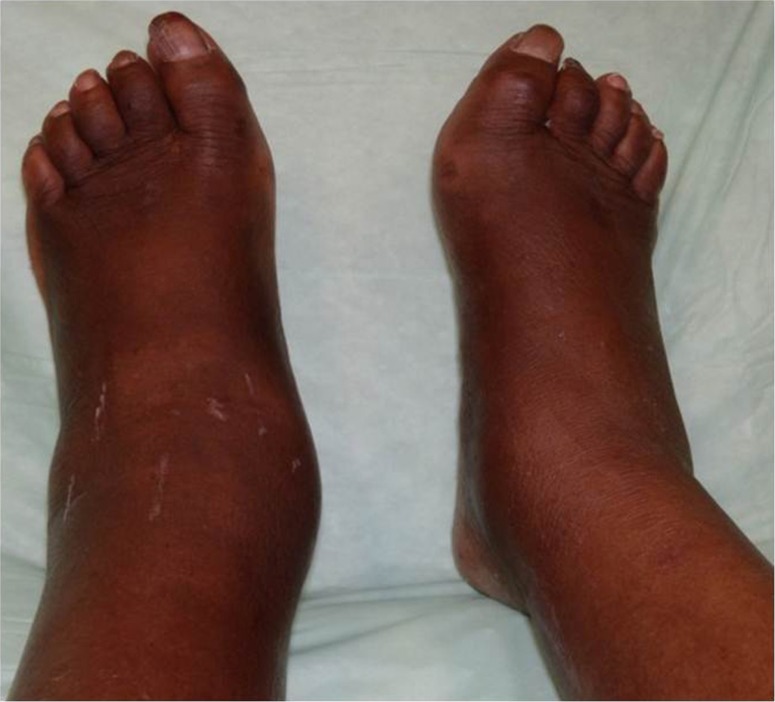
Fig. 1.
Increased edema of the left lower extremity secondary to Stage 1 acute Charcot.
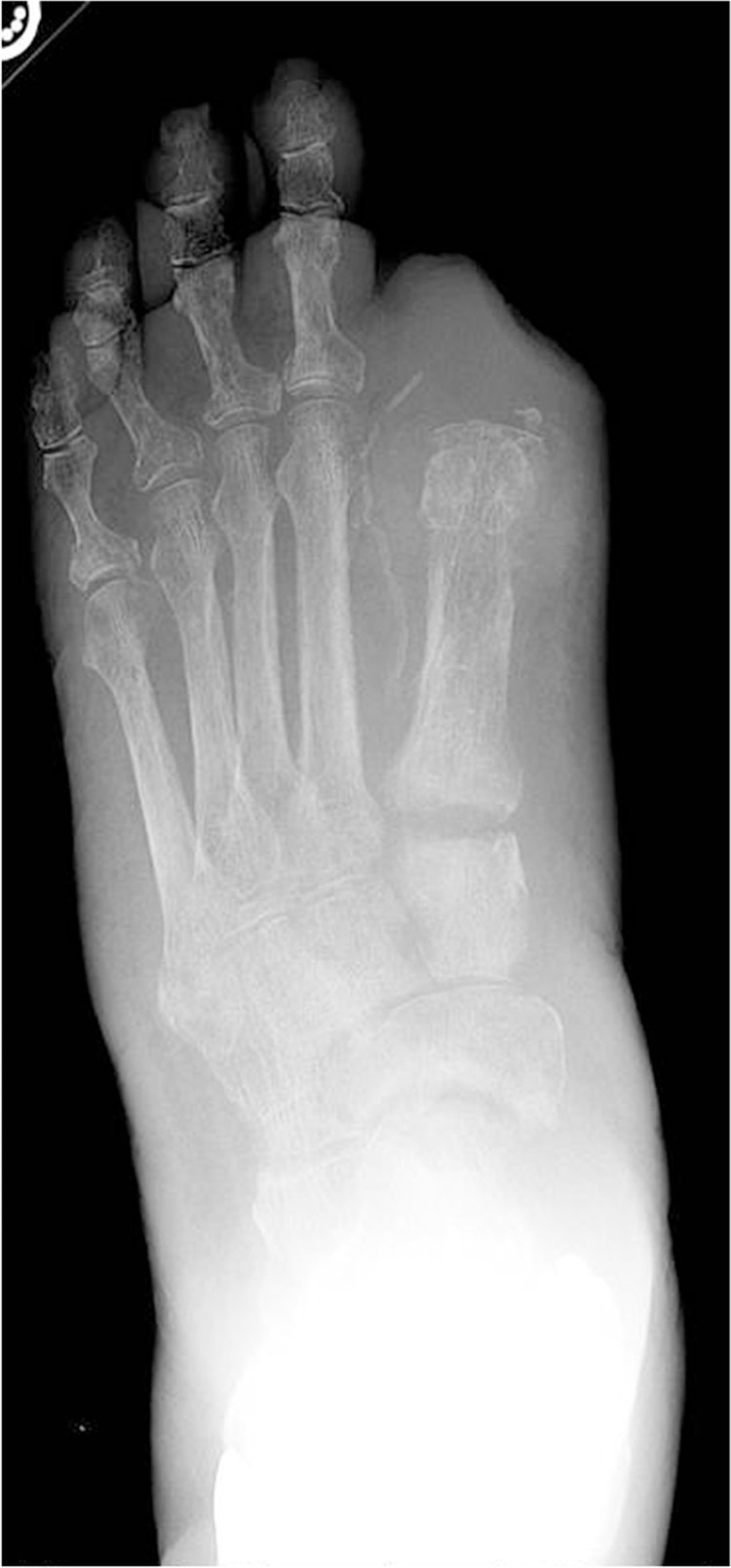
Fig. 2.
Bilateral foot radiographs reveal increased soft tissue volume and irregularities about the tarsometatarsal joint of the left foot secondary to Stage 0 acute Charcot.
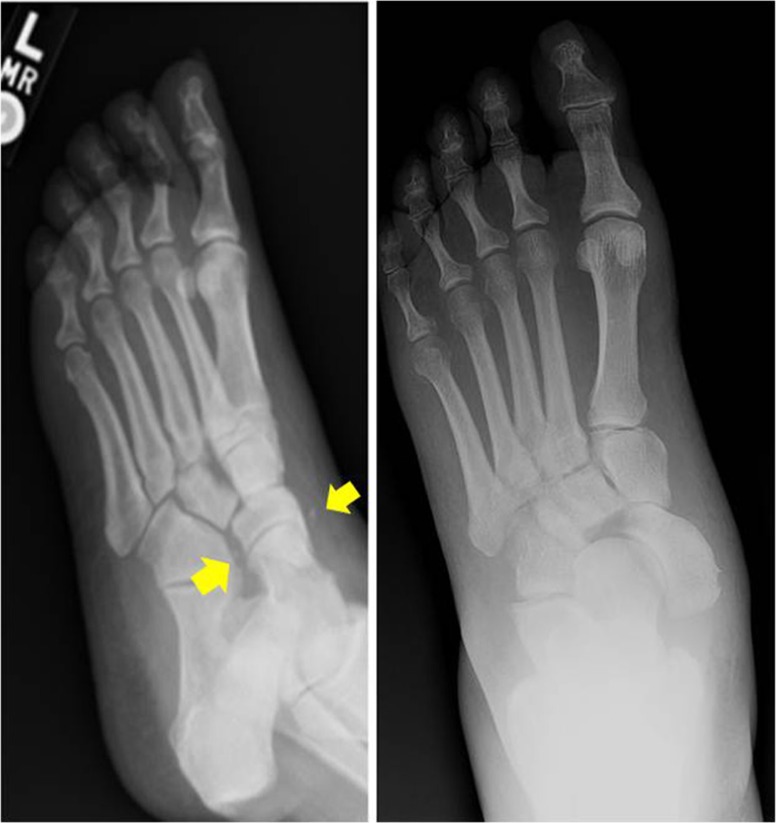
Fig. 3.
Oblique radiographs of a Stage 0 acute Charcot foot initially read as ‘normal’, yellow arrows identify ligamentous avulsion fractures with subsequent medial dislocation of the navicular.
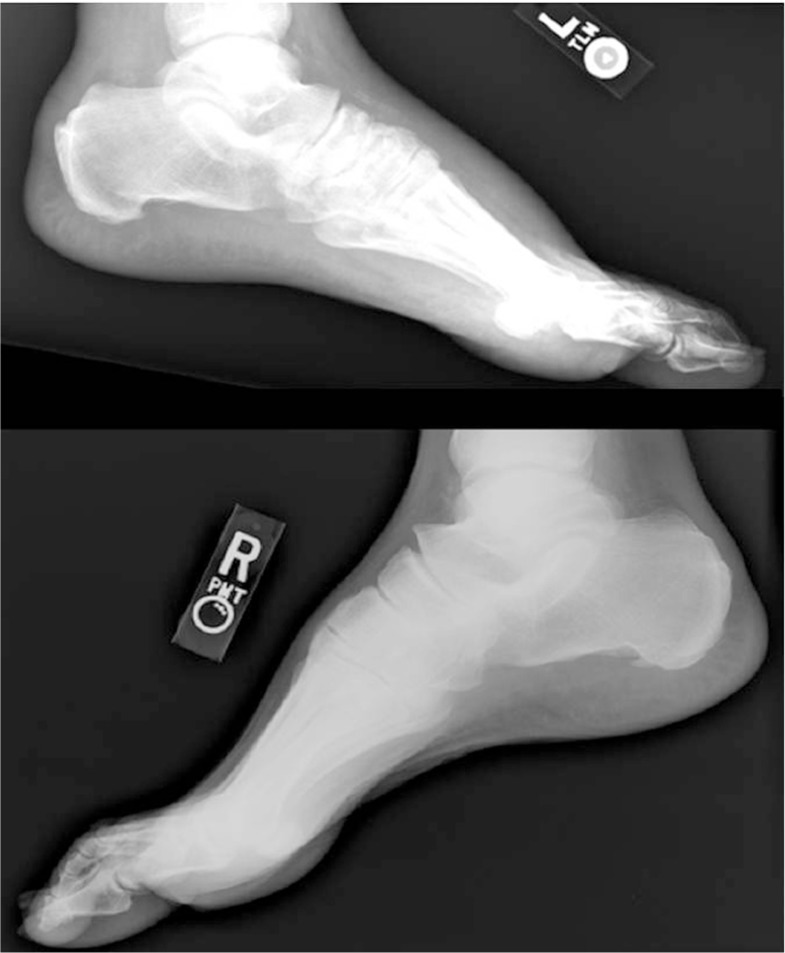
Fig. 4.
Stage 1 acute Charcot of the first metatarsal-cuneiform joint.
Fig. 5.
Stage 1 acute Charcot of the ankle joint.
Delay in prompt diagnosis and initiation of treatment results in progression of the pathology resulting in rigid osseous deformity of the foot increasing the risk of callus formation, ulceration, infection, and lower extremity amputation by 15- to 40-fold (8–16, 18, 19, 22, 23, 34, 35). Chantelau reported on two groups of patients with Stage 0 Charcot (13). Group 1 consisted of 11 patients who were diagnosed within 1 month from onset of symptoms. Group 2 consisted of 13 patients who were diagnosed approximately 3 months after symptom onset. Both groups were treated conservatively with a weight-bearing (WB) total contact cast (TCC). Group 1 was immobilized for an average of 3 months compared to 5 months for Group 2. Only one patient in Group 1 progressed to fracture of the foot and development of a pes planovalgus foot type which was attributed to patient non-compliance. All 13 patients in Group 2 progressed to fracture of the foot with a resultant deformity: rocker bottom foot deformity (18), pes planovalgus abductus (7), and pes planovalgus (7, 13). Chantelau also reported on 12 patients with Stage 0 Charcot deformity (33). Eleven (91.7%) patients had initiation of treatment with a WB-TCC within 1–12 weeks from the onset of symptoms. One patient was not diagnosed and thus treatment was not initiated until 22 weeks following symptom onset. The average duration of casting for all patients was 17.8 weeks (range: 8–52). All 11 patients who were diagnosed and immobilized early did not progress to fracture formation during the follow-up time (range: 12–26 months). The one patient who was diagnosed late progressed to Charcot fracture of the foot during their 3-month follow-up time (33). Wukich et al. performed a similar study on 20 patients (22 feet) (34). Group 1 consisted of seven patients who were diagnosed an average of 4.1 weeks from onset of symptoms. Group 2 consisted of 15 patients who were diagnosed an average of 6.8 weeks from onset of symptoms. All patients were treated with a non-weight-bearing (NWB) TCC. None of the patients in Group 1 progressed to the further stages of Charcot during the follow-up time of 49.9 weeks. One patient who already had deformity of the foot upon presentation did develop a midfoot ulceration which was treated surgically with resolution. All 15 patients progressed to the further stages of Charcot at an average of 10.9 weeks. Ten of these patients required surgical intervention, averaging 2.9 surgeries per limb. The following postoperative complications were reported: ulceration (11), cellulitis (9), postsurgical wound dehiscence (8), septic non-union/osteomyelitis (7), hardware complication (7), and a tibial shaft fracture (7). Group 2 required a longer follow-up time of 114.4 weeks presumably due to these complications which were noted to be statistically more significant than those for Group 1 (34).
With the propensity for misdiagnosis and high potential for progression to a rigid foot deformity, early recognition and prompt initiation of treatment is paramount (9–15, 23, 27–29, 33, 35). Certain components of a patient's history, physical examination, laboratory and imaging studies can facilitate the provider in making the diagnosis of an acute Charcot foot. The majority of patients presenting with this diagnosis are ≥50 years of age and have been diagnosed with type 1 or type 2 diabetes for ≥10 years (5, 8, 10, 11, 13–18, 20, 22–25, 27, 29–31, 34–37). The patient often has no recollection of an inciting event or reports only a minor injury such as a sprain or twisting injury (17.7–55.6% of patients) (9–12, 14–16, 18, 22, 23, 31–33, 35, 37). Up to 75% of patients will complain of pain described as a constant discomfort which does not hinder ambulation (8–10, 12, 14, 22, 33). Vital signs are typically stable (9–11). The primary complaint is often unilateral edema which hinders the ability to wear shoe gear (8–13, 19, 22, 23, 31). The patient may have already been treated for ‘recurrent cellulitis’, with or without hospital admission, and may have had a venous duplex ultrasound performed which is often negative for DVT. Physical examination will reveal a unilateral edematous limb (8–13, 19, 22, 23, 31). Concurrent bilateral acute Charcot is less common with reports ranging from 9 to 31%. The edematous limb is often without any open wounds and has increased warmth and erythema which resolves with elevation (8, 10–13, 23, 29, 31, 33). Radiographs are often normal apart from an increase in soft tissue volume. Bilateral weight-bearing radiographs are recommended to allow for assessment of joint instability in the form of small avulsion fractures or subtle subluxations (9–13, 15, 18, 19, 23). Laboratory markers of infection, that is, white blood cell count (WBC), C-reactive protein, and erythrocyte sedimentation rate, are typically within normal limits (9, 11).
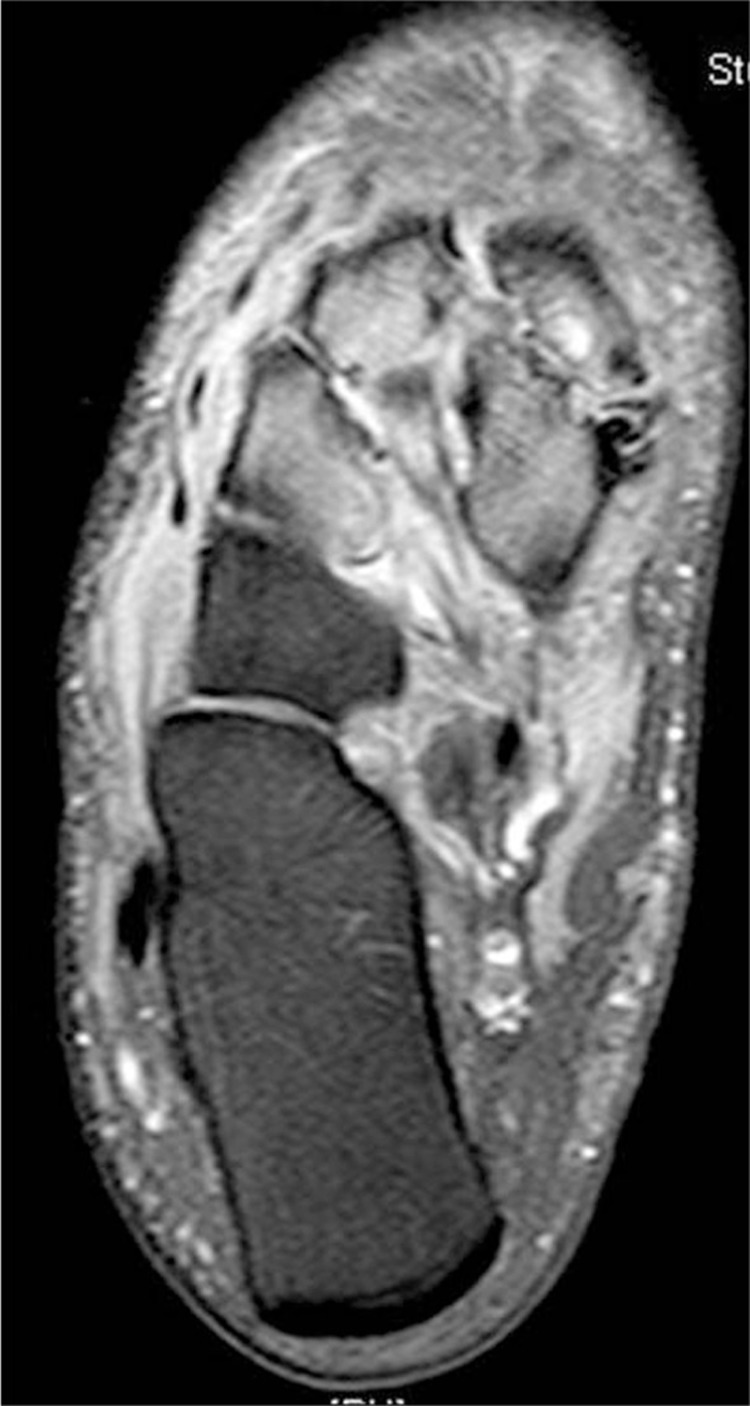
Several authors have recently advocated the early use of magnetic resonance imaging (MRI) to aid in diagnosis of acute Charcot (5, 6, 12, 13, 15, 17, 19, 20, 33, 38, 39) (Fig. 6). Chantelau and Poll performed an MRI on seven patients with Stage 0 Charcot within 2 weeks of obtaining radiographs which were read as negative for any osseous abnormalities (17). MRI revealed advanced stress bone injuries, microtrabecular fractures of the talus or calcaneus, edema of the adjacent soft tissues, and joint effusion with an average of four (range: 1–8) bones and five (range: 0–8) joints affected. These findings resolved after cast immobilization for 6 months (17). Schlossbauer confirmed these findings and correlated them with the amount of edema and pain found on physical examination (5). As these physical examination findings decreased with cast immobilization, the MRI findings decreased as well. Given these findings, the use of MRI was recommended to aid in determination of the appropriate duration of immobilization for conservative management of acute Charcot (5). Zampa performed a prospective study of 40 patients with acute Charcot foot that further expounded on this finding (20). Each patient had an MRI performed every 3 months until deemed healed or at a maximum of 12 months from initiation of immobilization. The average follow-up time was 8.2 months. The mean time to clinical healing was 6.8 months as determined by resolution of edema, erythema, and warmth. The mean time to resolution of MRI findings was 8.3 months. The recommendation was made to obtain an MRI at baseline and every 3 months until the patient is deemed clinically stable to correlate clinical healing with objective healing on MRI (20). Chantelau and Richter performed a retrospective, observational, exploratory cohort study of 59 patients (71 acute Charcot feet) whose management was based on MRI findings (21). The patients were followed over a 12-year time period. Early MRI was found to be instrumental in prompt diagnosis and initiation of treatment minimizing the risk for development of rigid foot deformities. When Stage 0 Charcot was diagnosed and immobilization initiated within 1 month of symptom onset, approximately 70% of patients healed without foot deformity with an average immobilization time of 4 months. This is in contrast to patients diagnosed in Stage 1 approximately 2 months after symptom onset who needed an average immobilization time of 5 months with approximately 30% healing without deformity of the foot. Results of this study lead to the conclusion that: 1) radiographs were insufficient in diagnosing midfoot fractures, 2) any bone marrow edema in the foot on MRI in a patient with peripheral neuropathy of any etiology was likely to progress to cortical fracture with continued unprotected ambulation, 3) the presence of cortical fractures was a significant marker for impending deformity development, 4) both Stage 0 and Stage 1 Charcot involving the tarsometatarsal and midtarsal joints required longer immobilization times, and 5) patients with type 1 diabetes were slightly younger, less obese, had a longer duration of diagnosis, and were more prone to recurrence of Charcot events than patients with type 2 diabetes (21). Given the ability of MRI to detect acute Charcot before any radiographic findings are visualized, the authors felt that the radiograph-based Eichenholtz classification does not encompass the full spectrum of the disease process and proposed a new classification for patients with peripheral neuropathy and an acutely swollen foot consisting of two stages and two grades. This classification system correlates clinical, computed tomography and MRI, and histopathology findings of Charcot and can be used to determine time for initiation and duration of treatment (6) (Table 1).
Fig. 6.
Increased bone marrow edema consistent with Stage 0 acute Charcot of the midfoot.
Table 1.
Chantelau and Richter Charcot classification system (21)
| Grade | |||
|---|---|---|---|
| Stage | 0 (low severity) | 1 (high severity) | |
| Active (acute) | Mild edema, erythema, and warmth Possible pain No deformity |
Severe edema, erythema, and warmth Possible pain Gross deformity |
Clinical |
| No osseous abnormality | Macrofractures | Radiographs | |
| MRI – bone marrow edema, microfractures, no cortical disruption | MRI – bone marrow edema, macrofractures, cortical disruption | MRI | |
| Lamellar bone with active surface Trabeculae remodeling associated with microfracture Marrow space replaced by loose spindle cells |
Increased marrow space vascularity Active remodeling of woven bone Osteonecrosis Invasion of inflammatory cells Thickened synovium Fragmented cartilage and subchondral bone |
Histopathology | |
| Inactive (chronic) | No inflammation No deformity |
No inflammation Gross deformity |
Clinical |
| No osseous abnormality | Osseous abnormality | Radiographs | |
| MRI – no significant bone marrow edema | MRI – no significant bone marrow edema | MRI | |
| Sclerosis of bone, broad lamellar trabeculae with collagenous replacement; low vascularity of the marrow space | Woven bone, immature and structurally disorganized, fibrosis | Histopathology | |
Once the diagnosis of acute Charcot is made the focus is on treatment for resolution. The goal of both conservative and surgical treatment is a stable and plantigrade foot which can be protected in an extra depth or custom shoe with a rocker bottom sole and custom orthotic with or without specialized bracing. In the literature, both Stage 0 and Stage I are commonly defined as acute Charcot with the majority focused on conservative or surgical treatment of Stage 1 (1, 3–6, 11, 13–15, 17, 20–22, 25, 30, 33, 34, 40–45). This is most likely due to the routine delay in the diagnosis of Stage 0 Charcot as mentioned above.
Conservative management of the acute Charcot foot and ankle
The mainstay treatment of acute Charcot has been conservative in the form of immobilization. Immobilization can consist of the use of a removable or non-removable device with instruction to be NWB, WB, or partial WB. A TCC was designed to allow a patient to be ambulatory, either fully or partially, while still immobilized (2, 40, 41, 46). This device works by increasing the total surface area of contact to the entire lower extremity evenly distributing vertical forces of ambulation thereby minimizing pressure distribution to the foot. The duration of immobilization is commonly dependent upon resolution of edema and warmth of the extremity and evidence of osseous consolidation on serial radiographs typically averaging 14 weeks (range: 8–52 weeks) (9–15, 23, 27–29, 33, 35).
Myerson et al. studied 18 patients with Eichenholtz Stage 1 midfoot Charcot (42). The average patient age was 54 years. The average duration and type of diabetes was not stated. Nine (50%) patients were treated with TCC immobilization. The WB restriction was not stated. Of these nine patients, eight (88.9%) remained with a stable, plantigrade foot at 32 months following treatment. One (11.1%) patient developed Charcot deformity in a new area 40 months following successful treatment of the initial Charcot event. This ultimately required a triple arthrodesis for stabilization. This study included midfoot Charcot of various stages. The authors found that TCC immobilization provided effective resolution with maintenance of a stable, plantigrade foot in 75% of cases, concluding that TCC immobilization remains the mainstay of treatment for midfoot Charcot (42).
Armstrong et al. performed a retrospective review of 55 patients who presented with acute Charcot over a 36-month time frame (14). Although it is mentioned that these patients had acute Charcot, the differentiation between Stage 0 and Stage 1 was never clearly defined. As 22 (40%) patients presented with ulceration, an assumption can be made that these patients were in Stage 1 as opposed to Stage 0 as collapse of the foot had already occurred. Ulceration was present at the cuboid-metatarsal articulation (40), the talonavicular joint (11), the medial malleolus (11), and the hallux interphalangeal joint (7). Mean patient age was 58.6±8.5 years. All patients had a diagnosis of type 1 or type 2 diabetes for a mean of 15.9±5.7 years. Five (11.0%) patients presented with bilateral acute Charcot. All patients were treated with TCC immobilization. The weight-bearing restriction was not stated. The mean duration of immobilization was 18.5 weeks. The mean time of return to shoe gear was 28.3 weeks. Male patients required a longer period of immobilization and took longer to return to shoe gear compared to women, 21.8 versus 15.2 weeks and 30.2 versus 26.4 weeks, respectively. Patients with bilateral acute Charcot also had a significantly increased time of immobilization and return to shoe gear at 28 weeks and 48 weeks, respectively. Eight (14.5%) patients developed a transient increase in temperature of the affected foot when transitioned to normal shoe gear with seven experiencing this within the first month. The remaining patient developed this at 35 months following transition to normal shoe gear. None of these patients developed radiographic signs of destruction and had complete resolution with an average of 3 weeks of immobilization in a TCC. Four (7.3%) patients developed a new ulceration at a mean of 10 months following return to normal shoe gear. All four of these patients had a midfoot ulceration on initial presentation with new ulceration occurring at the forefoot. The reason for development of these new ulcerations was not stated, that is, unprotected ambulation, rigid plantarflexed forefoot secondary to midfoot collapse resulting in callus formation, lack of routine foot care, and so on. No patient developed a Charcot event on the contralateral extremity during the study time. No instances of recurrent Charcot to the ipsilateral foot were reported (14).
Sella et al. reported on 40 patients (51 feet) of which 10 presented with Stage 0 Charcot and 6 with Stage 1 Charcot (22). The average age at onset was 58 years. The average age when diabetes, type not stated, was diagnosed was 38 years and the average duration was 20 years. Follow-up time ranged from 2 to 6 years. Initial radiographs of patients with Stage 0 Charcot revealed only increased soft tissue volume. Three phase bone scan was positive for increased uptake in the area of the affected joints. Indium/Gallium WBC tagged scans were negative. Initial radiographs for patients with Stage 1 Charcot were positive for osseous cysts, erosion, and diastasis. Results of three phase and Indium/Gallium WBC tagged scan were the same as for Stage 0. The majority of patients with Stage 0 Charcot presented with new onset of pain. All patients were treated with partial weight-bearing (PWB) in a TCC for 6 weeks. Of the 10 Stage 0 Charcot feet, 3 stabilized at Stage 1, 2 stabilized at Stage 2, and 3 stabilized at Stage 3. Two of the patients were lost to follow-up. Of the six Stage 1 Charcot feet, one stabilized at Stage 1, three stabilized at Stage 2, and two stabilized at Stage 3. No complications were reported. The authors found that early recognition and treatment via TCC immobilization resulted in stabilization without deformity in 50% of patients presenting with Stage 0 or Stage 1 Charcot. Given these findings, conservative treatment was recommended for treatment of Stage 0 and Stage 1 Charcot.
Although the expert consensus remains NWB immobilization in a TCC, literature exists that suggests that continued WB while immobilized does not hinder the resolution of acute Charcot with a stable, plantigrade foot (2–4, 10, 12, 16, 21, 22, 27, 40, 43). Pinzur et al. reported on nine patients who were allowed to be full WB in a TCC for treatment of Stage 1 Charcot (3). Cast change occurred every 2 weeks. Once the patient was deemed clinically stable and the objective measure of water displacement revealed a decrease of <10% in edema compared to the most recent visit, subjects were transitioned to full WB in a removable walking boot. Once the size and shape of the foot was deemed clinically stable, the subject was then transitioned to commercial depth-inlay shoes and custom accommodative orthotics. The type of diabetes was not stated. The average patient was 58.2 years (range: 39–72). The average duration of the diagnosis was 16.4 years (range: 7–30). All patients progressed to healing and remained independent community ambulators. The average time spent in a TCC was 5.8 weeks (range: 4–10) with progression to shoe gear at an average of 12.0 weeks (range: 6–16). Only one patient developed a superficial ulceration once transitioned to orthotic and shoe gear use which resolved with orthotic modification. Conclusion of the study found that full WB did not appear to negatively affect the resolution of Stage 1 Charcot with a stable, plantigrade foot (3). de Souza et al. reported on 27 patients (34 feet) treated with cast immobilization for acute Charcot (4). Patients were initially instructed to be NWB. However, the authors found that patients often did not comply with this instruction. This was initially thought to be due to non-compliance but upon further investigation was found to be due to: 1) a lack of proprioception and inability to determine how much weight was being placed on the foot due to peripheral neuropathy, 2) poor eyesight secondary to diabetic retinopathy, and 3) poor strength and coordination which made the use of ambulation assistive devices difficult. The authors found that despite the patients being WB more often than not, only one progressed to deformity of the foot during the treatment period. Thus, they allowed all subsequent patients to be WB as tolerated (WBAT) while immobilized (4). Sinacore found the same issue in a retrospective review of 30 subjects (35 feet) with acute Charcot (27). All subjects were instructed to be PWB. Only 28% of the study subjects complied with this instruction. Despite >70% of the study subjects being full WB in their TCC, all progressed to healing without development of foot deformity at an average of 86±45 days. Subjects who were full WB did require an average of 5 more weeks of immobilization prior to resolution of symptoms compared to those who complied with the PWB instruction (27).
The use of removable high profile orthotic devices, that is, Charcot Restraint Orthotic Walker, patellar tendon bearing brace, removable walking boots or modifications thereof, have been reported to be advantageous in that the patients are more compliant with NWB instruction with the use of these devices and the potential avoidance of iatrogenic complications related to TCC use are avoided. Improved patient compliance has been postulated to be due to the less bulky nature of the appliance and the ability to remove it during periods of inactivity (10, 44). However, immobilization times with these devices are longer compared to those of non-removable devices as patients may remove the device and ambulate without them (10, 27, 45). Guyton performed a retrospective review over a 28-month period of 70 patients with peripheral neuropathy who underwent TCC immobilization for treatment of a diabetic foot ulcer or a Charcot event of the foot with or without ulceration present (47). Patients with osteomyelitis or vascular compromise were excluded. In total, 398 consecutive cast applications were included in the study. Twenty-seven (38.6%) patients underwent surgery for deformity correction. All casts were changed at an average of 7.69 days (range: 3–14). Each patient received an average of 5.69 casts. The total complication rate was 5.52% (22 patients). All complications consisted of development of a new ulceration: pretibial (11), midfoot (11), hindfoot (1), forefoot/toes (10), and malleolar (7). None of the pre-existing ulcerations worsened. All new ulcerations healed with discontinuation of casting and local wound care. Only one permanent complication consisting of amputation of a second digit secondary to a wound sustained to the dorsum of the proximal interphalangeal joint occurred for a rate of permanent complications related to TCC use of 0.25% per cast or 1.4% per patient. The odds ratio for risk of complications in descending order were: 1) presence of Charcot deformity – 1.46, 2) peripheral neuropathy secondary to type 1 or type 2 diabetes – 1.34, 3) neuropathic ulceration – 0.69, 4) casting following deformity corrective surgery – 0.44, and 5) adherence to NWB instructions – 0.27. Given these findings, use of a TCC for the treatment of diabetic foot ulcerations when applied by an experienced provider was found to have a high rate of reversible minor complication in the form of iatrogenic wounds in a new area that responded to discontinuation of casting and local wound care. The primary wound prompting TCC use did not worsen (47).
Surgical management of the acute Charcot foot and ankle
Surgery during the acute phase of Charcot has historically been a relative contraindication. Concerns lie with the potential for wound healing complications and infection that can occur when operating on an edematous limb (1, 10, 15, 18, 39, 40, 46, 48, 49). The primary concern, however, is with the quality of the bone as it is undergoing a pathogenic process of osteoclastic resorption and fragmentation, and its quality is feared to be less than optimal for adequate fixation purchase. This could lead to complications such as hardware failure, pseudoarthrosis, delayed unions, or non-unions (1, 10, 46, 48). Stage II has been reported to be the optimal stage for surgical intervention in the form of open reduction and internal fixation or arthrodesis as the deformity is still reducible (22). Once the process has reached Stage III, arthrodesis may be more difficult as bone must be resected to allow for deformity correction. Exostectomy or ostectomy has been recommended during this stage to remove osseous prominences that could result in ulceration (14, 22).
Shibata et al. reported on four patients who underwent ankle arthrodesis due to acute ankle Charcot secondary to leprosy (7). The average patient age was 47.5 years. The average follow-up time was 61.25 months. Complete arthrodesis was achieved in all patients at an average of 6 months. Of the four patients, two developed a transient ulcer during the duration of follow-up. The authors felt that if plantigrade WB was possible in the early stages of Charcot, the use of a brace or rest was all that was necessary for treatment. The single indication for surgery in the early stages of Charcot was reported to be repeated infection and deep ulcerations of the ankle or forefoot (7).
In the same retrospective review performed by Myerson et al. in 1994, nine patients with Stage 1 Charcot foot underwent surgical intervention (42). The mean duration of postoperative immobilization utilizing a TCC was 5 months (range: 4–9) with the initial 8–10 weeks of this being NWB followed by WBAT. Eight (88.9%) patients remained with a stable, plantigrade foot at 28 months postoperative. One patient death, cause not reported, occurred during the study time and was not included in the final analysis. No complications were reported. Surgery was only performed if a severe, unstable, reducible deformity was present. Surgery was felt to be contraindicated if bone resorption or fragmentation was seen on radiographs due to the concern for adequate bone stock present for secure internal fixation. Bone stock was felt to be adequate in early Charcot if these radiographic findings were not present (42).
In the same retrospective review of conservative care performed by Armstrong et al. in 1997, 14 patients underwent surgical intervention (14). Surgery was only performed if deformity that could result in ulceration existed. All surgery was performed in the post-acute phase after radiographic coalescence was observed. In total, 14 surgeries were performed; nine exostectomies and five fusions. The average time of casting and return to shoe gear for those who underwent exostectomy was 15.4 and 27.6 weeks, respectively. The average time of casting and return to shoe gear for those who had a fusion performed was 29.0 and 48.1 weeks, respectively. Of the five fusions performed, one pseudarthrosis of the ankle developed which was subsequently managed conservatively with an ankle foot orthosis. Patients who underwent a fusion had a significantly increased time of immobilization and return to shoe gear compared to patients treated conservatively or with an exostectomy (14).
Simon et al. has the article most cited when advocating for early surgical intervention of acute Charcot. The results of 14 patients with diabetes, type not stated, and Stage 1 Charcot who underwent surgical intervention were reported (1). These patients elected to have surgery for the following reasons: 1) concern for potential complications if a foot deformity developed (two patients), 2) the need for a kidney transplant and the effect of this on casting (seven patients), and 3) the functional and occupational difficulties associated with prolonged immobilization with conservative treatment (five patients). The majority of patients cited more than one of these reasons. All patients underwent arthrodesis of the affected joint. Patients were instructed to be NWB until radiographic evidence of consolidation was noted. Patients were then transitioned to a WB short leg cast (SLC) for a mean time of 10±3.3 weeks of assisted WB followed by 15±8.8 weeks of unassisted WB. The mean time to return to normal shoe gear was 27±14.4 weeks. No complications were reported during the mean follow-up time of 41 months (1).
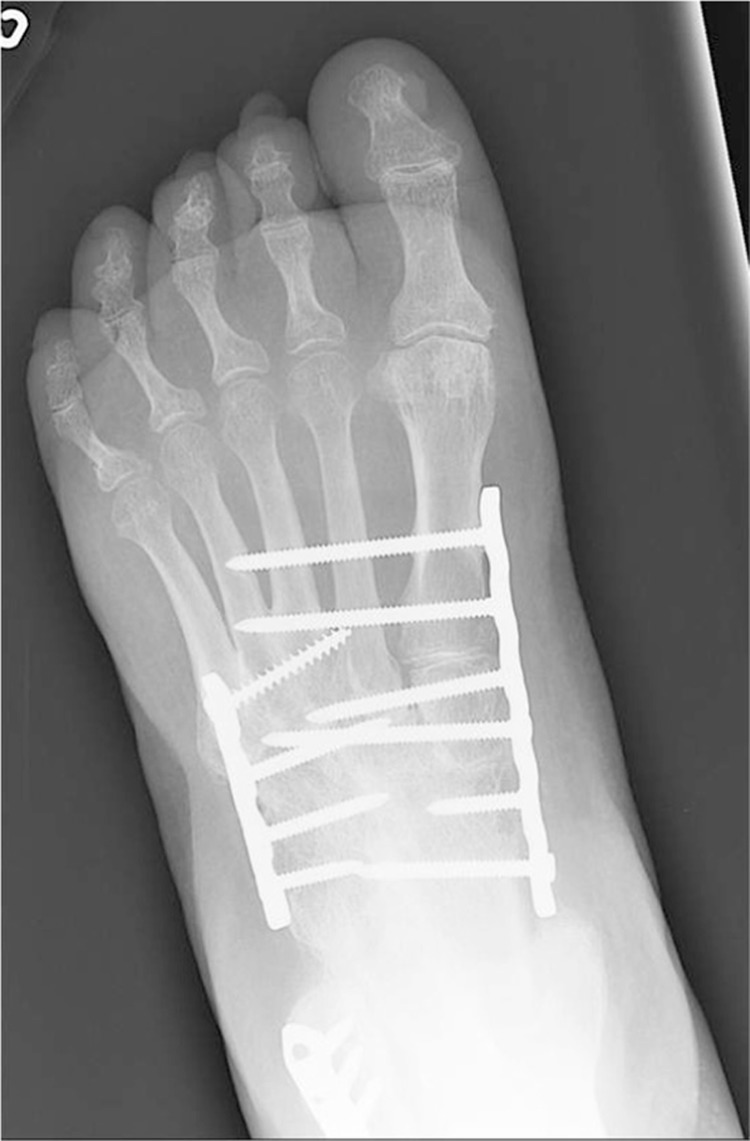
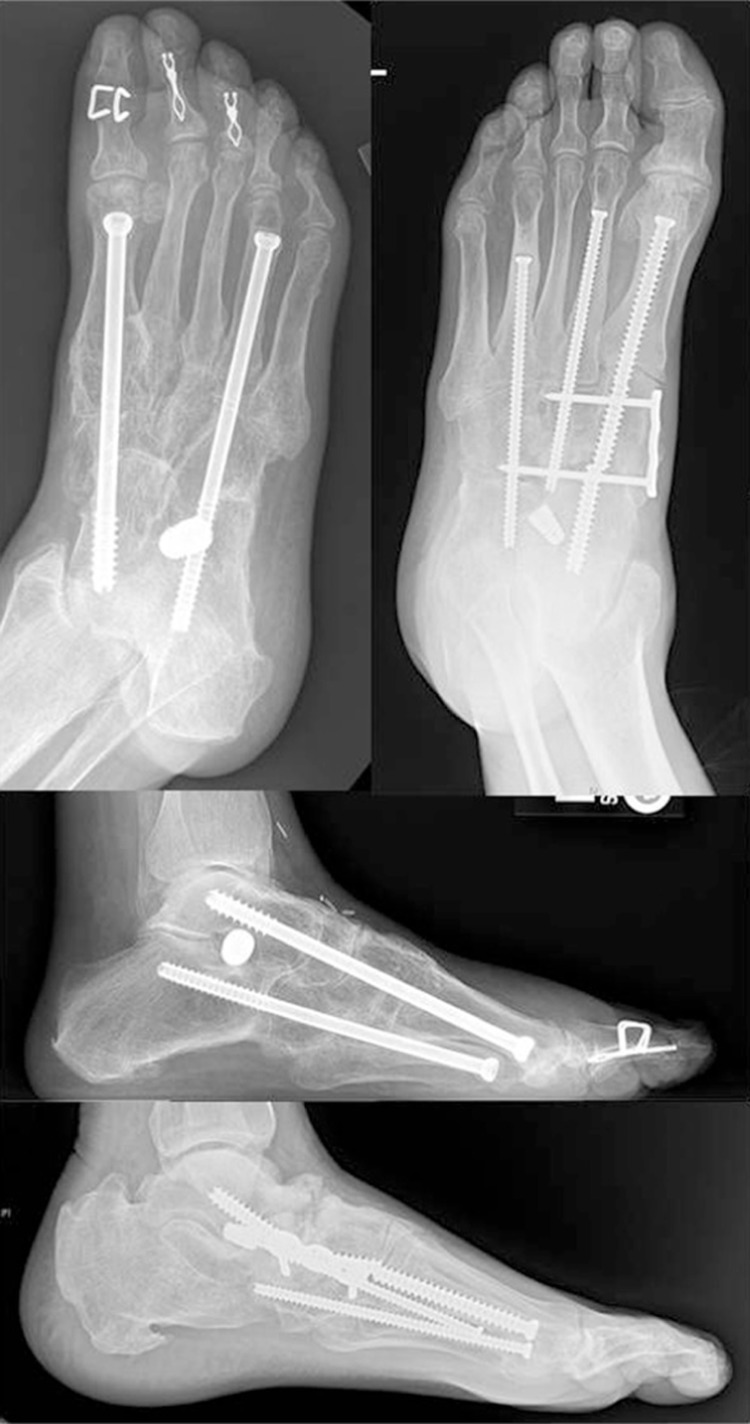
The current primary indications for surgery in the acute phase of Charcot are: severe dislocation or instability, concern for skin breakdown, and failure of conservative treatment to obtain a stable, plantigrade foot (10, 12, 15, 18, 40, 46, 48). Other factors to consider when surgical intervention is indicated are: the vascular supply to the affected limb, the anatomic location of breakdown, the patient's comorbidities and medical stability, the patient's history of alcohol and tobacco use, the patient's ability to comply with postoperative weight-bearing restrictions, the patient's preoperative ambulatory status, the patient's life expectancy, the patient's support network, the ability of the contralateral foot to be the primary WB limb, and the type of fixation to use (50–52). Several fixation methods exist for the treatment of acute Charcot, including: non-locking and locking plate and screw fixation, intramedullary screw or nail fixation, external fixation, and a combination of external and internal fixation (51, 53) (Figs. 7 and 8). The often increased time to osseous healing, decreased bone mineral density, and potential for loss of fixation must be considered when choosing what type of fixation to use (52).
Fig. 7.
Superconstruct with locking plate fixation for acute Charcot that had an unstable dislocation.
Fig. 8.
Superconstruct with intramedullary screw fixation: broken screw upper left and bottom radiographs.
Because of poor bone quality, the use of internal fixation alone has most recently been in the form of a ‘superconstruct’. The superconstruct technique was first described by Sammarco et al. and involves four tenants: 1) osseous resection for deformity correction and reduction of tension on the soft tissue envelope, 2) arthrodesis extending beyond the affected joints, 3) utilization of hardware deemed to be the strongest that the soft tissues will allow, and 4) use of this hardware in a novel position that maximizes mechanical function (54). Hardware thus spans the affected joint to include non-affected joints in an effort to gain purchase into bone of adequate stock. Use of spanning plates for internal fixation increases the risk for wound healing and infection given the large incisions necessary to place this fixation, and the risk of hardware exposure should soft tissue healing complications occur (53). Intramedullary screws and nails limit this risk as they can be placed either percutaneously or with a limited open approach and are inserted in such a way as to minimize the risk of hardware exposure should soft tissue healing complications occur. The osseous vascular supply is also preserved due to the minimal periosteal stripping required with the use of this type of fixation. Other advantages of intramedullary fixation include: the elimination of stress risers in already weakened bone, and the ability to correct and realign deformities and place temporary fixation via guide wires for maintenance and confirmation of the reduction under intra-operative fluoroscopy prior to final fixation placement (52, 54–58). Complications reported specific to the use of intramedullary screws and nails are: hardware failure, hardware migration that may necessitate removal, and the potential lack of compression or loss of purchase resulting in non-union (52, 54, 55, 57, 59–61) (Fig. 8).
External fixation can be used on its own or in conjunction with internal fixation when severe instability or gross deformity is present (62). Bone quality is less of a concern with the use of external fixation as it does not rely on cortical purchase for stabilization. The use of tensioned wires, or the ‘bent wire technique’, allows for interfragmentary compression and stabilization in multiple planes with minimal risk of implant loosening. Other advantages of external fixation are: neutralization of stresses placed across the foot, the ability to allow weight sharing with a static construct and deter inappropriate or excessive WB, assistance in deformity correction when dynamic constructs are used, lower extremity stabilization when large bone voids exist, and the ability to perform bone transport if needed, all via minimally invasive surgical incisions that preserve soft tissue and osseous vascular supply (53). External fixation is also useful for monitoring the limb when advanced soft tissue plastic surgery techniques are used for closure. When used in conjunction with internal fixation, external fixation bridges the arthrodesis site neutralizing stresses applied, increases the bending stiffness and torsional resistance of the construct enhancing mechanical stability, stabilizes adjacent joints, and functions as a splint to maintain any soft tissue equinus correction performed (52, 63). Internal fixation can be placed once osseous consolidation has been achieved with the use of external fixation alone if concern for motion still exists (62). Patients with diabetes have been shown to have significantly more complications with the use of external fixation compared to patients without diabetes (65). Complications associated with external fixation are: mild to severe pin tract infections, pin loosening, pin breakage necessitating exchange, pin failure requiring revision or early removal, stress fractures, osteomyelitis, and difficulty of the patient to psychologically adjust to the device. Pin tract infections are the most commonly reported complication with a reported rate ranging from 0.9 to 100% (52, 62, 64, 65). The rate of this complication has been shown to decrease with weekly physician-directed pin care as opposed to self-care by the patient or their support network (66).
When surgical correction necessitates arthrodesis, bone grafting may be required. Autogenic or allogenic bone grafts, bone graft substitutes, or a combination of these can be used. Autogenic bone grafting remains the gold standard. Autogenic cancellous bone graft is most often used to fill osseous voids; if structural support is necessary, then a cortical autograft should be utilized (66). Harvest sites of autogenic bone graft are the iliac crest, the distal tibia, the fibula, and the calcanus (67–70). When the deformity requires arthrodesis due to osseous dislocation, the dislocated bone can be prepared and used as its own autogenic bone grafting material (68). The major disadvantage of autogenic bone graft is the limited availability, donor site morbidity, and pain (70, 71). Allogenic grafts also come as cancellous or cortical grafts. Use of allogenic bone grafts avoids the concern of availability, donor site morbidity, and the increased operating time associated with obtaining autogenic bone grafts. Bone graft substitutes, that is, demineralized bone matrix, inorganic bioceramics, calcium phosphate, calcium sulfate, and hydroxyapatites are available in vast quantities and are used to fill osseous voids or augment autogenic or allogenic bone grafts being used. Calcium sulfate and hydroxyapatites can be also used for local delivery of antibiotics (58, 71).
Orthobiologic agents such as bone morphogenic proteins, bone marrow aspirate, and platelet-rich plasma have all been used to augment allogenic bone grafts or bone graft substitutes due to growth factors present and osteoinductive properties which have been shown to enhance both wound and osseous healing (58, 71–73). Bone marrow aspirate can be obtained from the iliac crest, proximal tibia, or the calcaneus in a safe and minimally invasive manner (67, 74–76). Platelet-rich plasma is also obtained in a safe and minimally invasive manner at a cost similar to other orthobiologics used to augment osseous healing (72). A study of 44 patients (46 feet) determined to be high risk due to the presence of immunodeficiency, recurrent infections, morbid obesity, chronic draining osteomyelitis, or multiple comorbidities underwent arthrodesis for correction of Charcot foot deformities and had the arthrodesis site supplemented with bone marrow aspirate obtained from the iliac crest and platelet-rich plasma. More than 90% of the feet had radiographic evidence of arthrodesis by the 16-week postoperative visit and remained free of osseous infection at 26 months postoperative. Complications were related to use of external fixation (three tibial stress fractures), recurrent ulcerations (seven, six of which healed with exostectomy), gangrene (one fifth ray resection), continued infection necessitating amputation (eight), and one unrelated death. The authors concluded that the combination of bone marrow aspirate and platelet-rich plasma to arthrodesis sites for Charcot deformity correction in high-risk patients appeared to achieve favorable outcomes (77).
Any encounter with a patient with acute Charcot in which surgical intervention is necessary should involve a frank discussion with the patient and their support network. This discussion should focus on the extended postoperative recovery time, to include the protracted period where WB restrictions will be necessary, and the high potential for postoperative complications to occur. The potential for postoperative infection; delayed skin, soft tissue, and osseous healing; hardware failure; the need for further surgery; and major lower extremity amputation should be highlighted. The patient's psychosocial state should also be taken into consideration, especially if external fixation is being considered for use. What the device entails and the extended period of time that it will be in place should be relayed to the patient and their support network in efforts to minimize the depression and anxiety that patients often experience with an external fixator in place (51, 53, 64, 78, 79).
Adjunctive treatments in the management of the acute Charcot foot and ankle
Bisphosphonates
Richard et al. performed a systematic review of bisphosphonate efficacy and safety in the adjunctive treatment of acute Charcot (45). Inclusion criteria consisted of studies that reported on the number of patients who received bisphosphonates during conservative treatment of acute Charcot neuroarthropathy of any etiology. Outcome measures were reduction of pain, edema, and temperature on physical examination; reduction in bone turnover markers; and bone mineral density and rate of osseous consolidation on radiographs. In total, 10 studies met the inclusion criteria with the majority being of a low level evidence (Grade 3 and 4). Only three studies were randomized controlled trials. Comparison between studies was difficult due to their heterogeneity in bisphosphonate treatment, outcome measures reported, and the low numbers of patients studied. No serious adverse events were reported with bisphosphonate use. A more rapid reduction in skin temperature was noted with bisphosphonate use; however, this reduction was not sustained over time. Pain reduction was not consistently reported. One study reported a reduction of pain whereas another did not. Reduction of bone turnover markers was noted, however, this was most consistently seen for bone specific alkaline phosphatase (BALP) a marker of osteoblast activity. No long-term data on the minimization of the development of rigid foot deformities were reported. In addition, two studies reported longer immobilization times with the use of bisphosphonates. The overall conclusion of the systematic review was that the use of bisphosphonates as an adjunct to conservative management of acute Charcot is not supported (45).
Intranasal calcitonin
A single randomized controlled trial has been performed on the use of intranasal calcitonin in adjunct to cast immobilization for the treatment of acute Charcot (80). The effect of intranasal calcitonin on bone metabolism and the disease process was evaluated over a period of 6 months. Calcitonin is felt to be more advantageous than bisphosphonate use as an adjunctive treatment as it acts directly on the osteoclast inhibition pathway as opposed to bisphosphonates which act indirectly on this pathway; it does not decrease osteoblast activity as bisphosphonate use can, and it can be used in patients with renal insufficiency whereas bisphosphonate use in these patients must be done with caution. In total, 32 consecutive patients with acute Charcot were enrolled, including patients with renal insufficiency. Each subject had the diagnosis of acute Charcot confirmed due to the presence of unilateral edema and ≥2°C increase in temperature compared to the contralateral limb as well as positive findings on radiographs and bone scans. Subjects were randomized to a study group or a control group. Both groups were treated with cast immobilization. The study group took 200 international units of salmon calcitonin via nasal spray and oral calcium supplementation daily. The control group took only daily oral calcium supplementation. No difference was noted in age, type of diabetes, sex, or severity of foot deformity between the two groups. The markers for bone turnover, COOH-terminal telopeptide region of type I collagen (ICTP) and BALP, were measured at 3 and 6 months. A significantly greater reduction in ICTP and BALP was noted in the study group at 3 months. Reduction in BALP was not seen at 6 months. Findings of this study suggest that daily nasal calcitonin may be an effective adjunctive treatment modality in the treatment of acute Charcot (80).
Bone stimulation
Hanft et al. performed an expanded pilot study on 31 patients with Stage 1 Charcot who were followed for an average of 23.3 weeks (range: 0.5–108) (43). All patients had no open wound, active infection, previous history of Charcot, or renal failure. All patients were treated with a TCC or a compression sock and a fixed ankle walker with a contact molded multi-density thermoplastic insole and instructed to reduce their WB by 50%. Patients were then divided into a control and a study group. The study group had the additional treatment of application of a combined magnetic field bone growth stimulator for 30 min daily. Subjects had to use this adjunctive treatment for a minimum of 75% of the study period to be included in the study group. Resolution of the acute phase was measured by reduction in temperature measured by a dermal thermometer and reduction in edema measured by water displacement. The control group consisted of 11 patients. The study group consisted of 20 patients. No statistically significant difference was found in the rate of resolution of Charcot based on: duration of the acute Charcot event prior to initiation of treatment, sex, age, obesity, insulin dependent versus non-insulin dependent diabetes, or the type of immobilization used. Bone stimulator use was the only factor found to result in a statistically significant reduction with a mean time to osseous consolidation occurring in the study group 12.8±7.7 weeks before the control group. All patients were found to have a stable, plantigrade foot free of deformity upon completion of the study. Findings of this study suggest that the use of a combined magnetic field bone growth stimulator may be an effective adjunctive modality in the treatment of acute Charcot (43).
Discussion
Insidious onset of an acutely swollen foot in a patient with peripheral neuropathy of any etiology should be considered an acute Charcot event until proven otherwise (6, 9, 11, 15, 18, 21, 23, 32). Components of a patient's history, physical examination, laboratory and imaging findings should be utilized to make a prompt diagnosis for initiation of treatment. Plain film radiographs are often normal emphasizing the importance of early utilization of advanced imaging (9–11, 13, 18, 23). Literature demonstrating that outcomes are significantly better if patients are diagnosed and treated within the first month supports a policy of obtaining advanced imaging, preferably MRI, in patients with an insensate foot and insidious onset of pain and swelling >3 weeks duration (5, 6, 9–15, 17, 19–21, 23, 27–29, 33, 35, 39). Serial MRIs can also serve as a guide for determining the duration of immobilization (5, 17, 20).
Limitations of this study consist of the inconsistent nature in how acute Charcot is defined and the paucity of literature that exists regarding the surgical management of acute Charcot. One must carefully glean the literature as no consensus exists on the true definition of acute Charcot. Stage 0 and Stage 1 have been used interchangeably as the designation of acute Charcot in the literature. Differences in treatment of these two stages can exist as some component of deformity may already be present in patients with Stage 1 Charcot (1, 3–5, 11, 13–15, 17, 20–22, 25, 30, 33, 34, 38, 40–45).
Overall, the expert consensus on the mainstay of treatment remains immobilization in a non-removable device with preference for instruction to be NWB. However, strict adherence to this expert consensus does not appear to be reality in clinical practice (2, 37). In a questionnaire sent to members of the Diabetes Committee of the American Orthopaedic Foot and Ankle Society who treat patients with Charcot and physicians interested in forming the American Orthopaedic Foot and Ankle Society Charcot Study Group found that 21.6% of respondents used removable devices for immobilization and 40.5% allowed WB during immobilization (2). In a web-based survey of centers treating acute Charcot in the United Kingdom and Ireland conducted over a 20-month period, initial offloading with a non-removable device was used initially in only 35.4% of patients and upwards of 40.1% for some duration of immobilization. A removable device was initially used for offloading in 50% of patients reaching 65.2% for some duration of immobilization (37).
Surgical intervention in the early stages of Charcot remains controversial. The majority of literature involving surgical treatment of acute Charcot reports on the outcomes of procedures performed in Stage 1 where some form of osseous collapse has already occurred (1, 3, 15, 41, 42). Several inconsistencies are noted in these studies. Myerson et al. reported that early surgical intervention was indicated only if a severe, unstable, and reducible deformity was present and was contraindicated if bone resorption or fragmentation was noted on radiographs (42). This contradicts the true definition of Stage 1 Charcot as bone fragmentation is one of the characteristic radiographic findings of this stage (1–6). In the paper by Armstrong et al. regarding treatment of acute Charcot, surgery was only performed in the post-acute phase when osseous consolidation had occurred (14). In the study most cited purporting early surgical intervention for acute Charcot, one of the advantages touted was a shorter period of immobilization compared to conservative treatment. However, the total time for return to shoe gear following surgical intervention was 27 weeks which is similar to the findings following conservative treatment reported by Armstrong et al. at 26.4 weeks with the exclusion of patients with bilateral acute Charcot. In addition, this study involved a total of 43 patients with only 14 (32.6%) electing for surgery. Twenty-nine (67.4%) patients were treated conservatively with immobilization and activity restriction. No results were reported for these 29 patients. The paper goes on to compare clinical results, financial costs, walking patterns, and the presence or absence of abnormal foot pressures of patients who underwent early surgical intervention to patients with type 1 or 2 diabetes and peripheral neuropathy without a history of Charcot and patients with below-the-knee amputees and not the 29 patients who underwent conservative treatment (1).
Conclusion
Review of the literature is helpful in establishing an evidence-based approach to the management of the acute Charcot foot and ankle. An understanding of the importance of early diagnosis and treatment of Stage 0 is critical. Any patient with peripheral neuropathy and pain and swelling to the foot and or ankle should be considered to have Charcot until proven otherwise (6, 9, 11, 15, 18, 21, 23, 32). As plain film radiographs are often normal, obtaining advanced imaging is recommended (5, 6, 9–13, 15, 17–21, 23, 33, 39). The mainstay of treatment remains lower extremity immobilization (2, 40, 41, 46). Immobilization can be with either a non-removable or removable device, although shorter periods of immobilization are seen with non-removable devices. The allowance of WB has not been shown to result in increased development of deformities; however, immobilization times can be increased compared to adherence with NWB restriction (10, 27, 44, 45). Early surgical intervention should be considered in cases of severe dislocation, unstable deformity, non-reducible deformity, or failure of conservative treatment to maintain a stable, plantigrade foot (10, 12, 15, 18, 40, 46, 48).
Disclaimer
The views expressed are those of the author(s) and do not reflect the official policy of the Department of the Army, the Department of Defense, or the US government.
Conflict of interest and funding
There are no known conflicts of interest or any funding provided associated with this paper.
References
- 1.Simon SR, Tejwani SG, Wilson DL, Santner TJ, Denniston NL. Arthrodesis as an early alternative to nonoperative management of Charcot arthropathy of the diabetic foot. J Bone Joint Surg Am. 2000;82-A:939–50. doi: 10.2106/00004623-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Pinzur MS, Shields N, Trepman E, Dawson P, Evans A. Current practice patterns in the treatment of Charcot foot. Foot Ankle Int. 2000;21:916–20. doi: 10.1177/107110070002101105. [DOI] [PubMed] [Google Scholar]
- 3.Pinzur MS, Lio T, Posner M. Treatment of Eichenholtz Stage I Charcot foot arthropathy with a weightbearing total contact cast. Foot Ankle Int. 2006;27:324–9. doi: 10.1177/107110070602700503. [DOI] [PubMed] [Google Scholar]
- 4.de Souza LJ. Charcot arthropathy and immobilization in a weight-bearing total contact cast. J Bone Joint Surg Am. 2008;90:754–9. doi: 10.2106/JBJS.F.01523. [DOI] [PubMed] [Google Scholar]
- 5.Schlossbauer T, Mioc T, Sommerey S, Kessler SB, Reiser MF, Pfeifer KJ. Magnetic resonance imaging in early stage Charcot arthropathy: correlation of imaging findings and clinical symptoms. Eur J Med Res. 2008;13:409–14. [PubMed] [Google Scholar]
- 6.Chantelau EA, Grützner G. Is the Eichenholtz classification still valid for the diabetic Charcot foot? Swiss Med Wkly. 2014;144:w13948. doi: 10.4414/smw.2014.13948. [DOI] [PubMed] [Google Scholar]
- 7.Shibata T, Tada K, Hashizume C. The results of arthrodesis of the ankle for leprotic neuroarthropathy. J Bone Joint Surg Am. 1990;72:749–56. [PubMed] [Google Scholar]
- 8.Giurini JM, Chrzan JS, Gibbons GW, Habershaw GM. Charcot's disease in diabetic patients. Correct diagnosis can prevent progressive deformity. Postgrad Med. 1991;89:163–9. doi: 10.1080/00325481.1991.11700869. [DOI] [PubMed] [Google Scholar]
- 9.Caputo GM, Ulbrecht J, Cavanagh PR, Juliano P. The Charcot foot in diabetes: six key points. Am Fam Physician. 1998;57:2705–10. [PubMed] [Google Scholar]
- 10.Sinacore DR, Withrington NC. Recognition and management of acute neuropathic (Charcot) arthropathies of the foot and ankle. J Orthop Sports Phys Ther. 1999;29:736–46. doi: 10.2519/jospt.1999.29.12.736. [DOI] [PubMed] [Google Scholar]
- 11.Sommer TC, Lee TH. Charcot foot: the diagnostic dilemma. Am Fam Physician. 2001;64:1591–8. [PubMed] [Google Scholar]
- 12.Yu GV, Hudson JR. Evaluation and treatment of stage 0 Charcot's neuroarthropathy of the foot and ankle. J Am Podiatr Med Assoc. 2002;92:210–20. doi: 10.7547/87507315-92-4-210. [DOI] [PubMed] [Google Scholar]
- 13.Chantelau E. The perils of procrastination: effects of early vs. delayed detection and treatment of incipient Charcot fracture. Diabet Med. 2005;22:1707–12. doi: 10.1111/j.1464-5491.2005.01677.x. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong DG, Todd WF, Lavery LA, Harkless LB, Bushman TR. The natural history of acute Charcot's arthropathy in a diabetic foot specialty clinic. Diabet Med. 1997;14:357–63. doi: 10.1002/(SICI)1096-9136(199705)14:5<357::AID-DIA341>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Pakarinen TK, Laine HJ, Honkonen SE, Peltonen J, Oksala H, Lahtela J. Charcot arthropathy of the diabetic foot. Current concepts and review of 36 cases. Scand J Surg. 2002;91:195–201. doi: 10.1177/145749690209100212. [DOI] [PubMed] [Google Scholar]
- 16.Foltz KD, Fallat LM, Schwartz S. Usefulness of a brief assessment battery for early detection of Charcot foot deformity in patients with diabetes. J Foot Ankle Surg. 2004;43:87–92. doi: 10.1053/j.jfas.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Chantelau E, Poll LW. Evaluation of the diabetic Charcot foot by MR imaging or plain radiography – an observational study. Exp Clin Endocrinol Diabetes. 2006;114:428–31. doi: 10.1055/s-2006-924229. [DOI] [PubMed] [Google Scholar]
- 18.Lee L, Blume PA, Sumpio B. Charcot joint disease in diabetes mellitus. Ann Vasc Surg. 2003;17:571–80. doi: 10.1007/s10016-003-0039-5. [DOI] [PubMed] [Google Scholar]
- 19.Schoots IG, Slim FJ, Busch-Westbroek TE, Maas M. Neuro-osteoarthropathy of the foot-radiologist: friend or foe? Semin Musculoskelet Radiol. 2010;14:365–76. doi: 10.1055/s-0030-1254525. [DOI] [PubMed] [Google Scholar]
- 20.Zampa V, Bargellini I, Rizzo L, Turini F, Ortori S, Piaggesi A, Bartolozzi C. Role of dynamic MRI in the follow-up of acute Charcot foot in patients with diabetes mellitus. Skeletal Radiol. 2011;40:991–9. doi: 10.1007/s00256-010-1092-0. [DOI] [PubMed] [Google Scholar]
- 21.Chantelau EA, Richter A. The acute diabetic Charcot foot managed on the basis of magnetic resonance imaging – a review of 71 cases. Swiss Med Wkly. 2013;143:w13831. doi: 10.4414/smw.2013.13831. [DOI] [PubMed] [Google Scholar]
- 22.Sella EJ, Barrette C. Staging of Charcot neuroarthropathy along the medial column of the foot in the diabetic patient. J Foot Ankle Surg. 1999;38:34–40. doi: 10.1016/s1067-2516(99)80086-6. [DOI] [PubMed] [Google Scholar]
- 23.Shah MK, Hugghins SY. Charcot's joint: an overlooked diagnosis. J La State Med Soc. 2002;154:246–50. discussion 250. [PubMed] [Google Scholar]
- 24.Sinha SB, Munichoodappa CS, Kozak GP. Neuro-arthropathy (Charcot joints) in diabetes mellitus: clinical study of 101 cases. Medicine (Baltimore) 1972;51:191–210. doi: 10.1097/00005792-197205000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Cofield RH, Morrison MJ, Beabout JW. Diabetic neuroarthropathy in the foot: patient characteristics and patterns of radiographic change. Foot Ankle. 1983;4:15–22. doi: 10.1177/107110078300400104. [DOI] [PubMed] [Google Scholar]
- 26.Cavanagh PR, Young MJ, Adams JE, Vickers KL, Boulton AJ. Radiographic abnormalities in the feet of patients with diabetic neuropathy. Diabetes Care. 1994;17:201–9. doi: 10.2337/diacare.17.3.201. [DOI] [PubMed] [Google Scholar]
- 27.Sinacore DR. Acute Charcot arthropathy in patients with diabetes mellitus: healing times by foot location. J Diabetes Complications. 1998;12:287–93. doi: 10.1016/s1056-8727(98)00006-3. [DOI] [PubMed] [Google Scholar]
- 28.Gierbolini R. Charcot's foot: often overlooked complication of diabetes. JAAPA. 1999;12:62–8. [PubMed] [Google Scholar]
- 29.Choksi P, Thomas R, Simmons DL. Charcot arthropathy. An often overlooked complication of diabetes mellitus. J Ark Med Soc. 2007;103:229–31. [PubMed] [Google Scholar]
- 30.Christensen TM, Gade-Rasmussen B, Pedersen LW, Hommel E, Holstein PE, Svendsen OL. Duration of off-loading and recurrence rate in Charcot osteo-arthropathy treated with less restrictive regimen with removable walker. J Diabetes Complications. 2012;26:430–4. doi: 10.1016/j.jdiacomp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Fabrin J, Larsen K, Holstein PE. Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care. 2000;23:796–800. doi: 10.2337/diacare.23.6.796. [DOI] [PubMed] [Google Scholar]
- 32.Gill G, Benbow S, Tesfaye S, Kaczmarczyk E, Kaye L. Painless stress fractures in diabetic neuropathic feet. Postgrad Med J. 1997;73:241–2. doi: 10.1136/pgmj.73.858.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chantelau E, Richter A, Ghassem-Zadeh N, Poll LW. “Silent” bone stress injuries in the feet of diabetic patients with polyneuropathy: a report on 12 cases. Arch Orthop Trauma Surg. 2007;127:171–7. doi: 10.1007/s00402-006-0271-x. [DOI] [PubMed] [Google Scholar]
- 34.Wukich DK, Sung W, Wipf SA, Armstrong DG. The consequences of complacency: managing the effects of unrecognized Charcot feet. Diabet Med. 2011;28:195–8. doi: 10.1111/j.1464-5491.2010.03141.x. [DOI] [PubMed] [Google Scholar]
- 35.Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJ. Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care. 1995;18:34–8. doi: 10.2337/diacare.18.1.34. [DOI] [PubMed] [Google Scholar]
- 36.Tisdel CL, Marcus RE, Heiple KG. Triple arthrodesis for diabetic peritalar neuroarthropathy. Foot Ankle Int. 1995;16:332–8. doi: 10.1177/107110079501600604. [DOI] [PubMed] [Google Scholar]
- 37.Game FL, Catlow R, Jones GR, Edmonds ME, Jude EB, Rayman G, et al. Audit of acute Charcot's disease in the UK: the CDUK study. Diabetologia. 2012;55:32–5. doi: 10.1007/s00125-011-2354-7. [DOI] [PubMed] [Google Scholar]
- 38.Chantelau E, Richter A, Schmidt-Grigoriadis P, Scherbaum WA. The diabetic Charcot foot: MRI discloses bone stress injury as trigger mechanism of neuroarthropathy. Exp Clin Endocrinol Diabetes. 2006;114:118–23. doi: 10.1055/s-2006-924026. [DOI] [PubMed] [Google Scholar]
- 39.Ulbrecht JS, Wukich DK. The Charcot foot: medical and surgical therapy. Curr Diab Rep. 2008;8:444–51. doi: 10.1007/s11892-008-0077-z. [DOI] [PubMed] [Google Scholar]
- 40.Pinzur M. Surgical versus accommodative treatment for Charcot arthropathy of the midfoot. Foot Ankle Int. 2004;25:545–9. doi: 10.1177/107110070402500806. [DOI] [PubMed] [Google Scholar]
- 41.Mittlmeier T, Klaue K, Haar P, Beck M. Should one consider primary surgical reconstruction in Charcot arthropathy of the feet? Clin Orthop Relat Res. 2010;468:1002–11. doi: 10.1007/s11999-009-0972-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myerson MS, Henderson MR, Saxby T, Short KW. Management of midfoot diabetic neuroarthropathy. Foot Ankle Int. 1994;15:233–41. doi: 10.1177/107110079401500502. [DOI] [PubMed] [Google Scholar]
- 43.Hanft JR, Goggin JP, Landsman A, Surprenant M. The role of combined magnetic field bone growth stimulation as an adjunct in the treatment of neuroarthropathy/Charcot joint: an expanded pilot study. J Foot Ankle Surg. 1998;37:510–15. doi: 10.1016/s1067-2516(98)80028-8. discussion 550–1. [DOI] [PubMed] [Google Scholar]
- 44.Hastings MK, Sinacore DR, Fielder FA, Johnson JE. Bone mineral density during total contact cast immobilization for a patient with neuropathic (Charcot) arthropathy. Phys Ther. 2005;85:249–56. [PMC free article] [PubMed] [Google Scholar]
- 45.Richard JL, Almasri M, Schuldiner S. Treatment of acute Charcot foot with bisphosphonates: a systematic review of the literature. Diabetologia. 2012;55:1258–64. doi: 10.1007/s00125-012-2507-3. [DOI] [PubMed] [Google Scholar]
- 46.Rogers LC, Frykberg RG, Armstrong DG, Boulton AJ, Edmonds M, Van GH, et al. The Charcot foot in diabetes. Diabetes Care. 2011;34:2123–9. doi: 10.2337/dc11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guyton GP. An analysis of iatrogenic complications from the total contact cast. Foot Ankle Int. 2005;26:903–7. doi: 10.1177/107110070502601101. [DOI] [PubMed] [Google Scholar]
- 48.Trepman E, Nihal A, Pinzur MS. Current topics review: Charcot neuroarthropathy of the foot and ankle. Foot Ankle Int. 2005;26:46–63. doi: 10.1177/107110070502600109. [DOI] [PubMed] [Google Scholar]
- 49.Wu T, Chen PY, Chen CH, Wang CL. Doppler spectrum analysis: a potentially useful diagnostic tool for planning the treatment of patients with Charcot arthropathy of the foot? J Bone Joint Surg Br. 2012;94:344–7. doi: 10.1302/0301-620X.94B3.27122. [DOI] [PubMed] [Google Scholar]
- 50.Roukis TS, Stapleton JJ, Zgonis T. Addressing psychosocial aspects of care for patients with diabetes undergoing limb salvage surgery. Clin Podiatr Med Surg. 2007;24:601–10. doi: 10.1016/j.cpm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Zgonis T, Stapleton JJ, Jeffries LC, Girard-Powell VA, Foster LJ. Surgical treatment of Charcot neuropathy. AORN J. 2008;87:971–86. doi: 10.1016/j.aorn.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Stapleton JJ, Zgonis T. Surgical reconstruction of the diabetic Charcot foot: internal, external or combined fixation? Clin Podiatr Med Surg. 2012;29:425–33. doi: 10.1016/j.cpm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Zgonis T, Roukis TS, Lamm BM. Charcot foot and ankle reconstruction: current thinking and surgical approaches. Clin Podiatr Med Surg. 2007;24:505–17. doi: 10.1016/j.cpm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Sammarco VJ. Superconstructs in the treatment of Charcot foot deformity: plantar plating, locked plating, and axial screw fixation. Foot Ankle Clin N Am. 2009;14:393–407. doi: 10.1016/j.fcl.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Sammarco VJ, Sammarco GJ, Walker EW, Guiao RP. Midtarsal arthrodesis in the treatment of Charcot midfoot arthropathy. J Bone Joint Surg Am. 2009;91:80–91. doi: 10.2106/JBJS.G.01629. [DOI] [PubMed] [Google Scholar]
- 56.Assal M, Stern R. Realignment and extended fusion with use of a medial column screw for midfoot deformities secondary to diabetic neuropathy. J Bone Joint Surg Am. 2009;91:812–20. doi: 10.2106/JBJS.G.01396. [DOI] [PubMed] [Google Scholar]
- 57.Grant WP, Garcia-Lavin SE, Sabo RT, Tam HS, Jerlin E. A retrospective analysis of 50 consecutive Charcot diabetic salvage reconstructions. J Foot Ankle Surg. 2009;48:30–8. doi: 10.1053/j.jfas.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Pappalardo J, Fitzgerald R. Utilization of advanced modalities in the management of diabetic Charcot neuroarthropathy. J Diabetes Sci Technol. 2010;4:1114–20. doi: 10.1177/193229681000400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grant WP, Garcia-Lavin S, Sabo R. Beaming the columns for Charcot diabetic reconstruction: a retrospective analysis. J Foot Ankle Surg. 2011;50:182–9. doi: 10.1053/j.jfas.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Wiewiorski M, Valderrabano V. Intramedullary fixation of the medial column of the foot with a solid bolt in Charcot midfoot arthropathy: a case report. J Foot Ankle Surg. 2012;51:379–81. doi: 10.1053/j.jfas.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 61.Wiewiorski M, Yasui T, Miska M, Frigg A, Valderrabano V. Solid bolt fixation of the medial column in Charcot midfoot arthropathy. J Foot Ankle Surg. 2013;52:88–94. doi: 10.1053/j.jfas.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 62.Capobianco CM, Ramanujam CL, Zgonis T. Charcot foot reconstruction with combined internal and external fixation: case report. J Orthop Surg Res. 2010;5:7. doi: 10.1186/1749-799X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jolly GP, Zgonis T, Polyzois V. External fixation in the management of Charcot neuroarthropathy. Clin Podiatr Med Surg. 2003;20:741–56. doi: 10.1016/S0891-8422(03)00071-5. [DOI] [PubMed] [Google Scholar]
- 64.Roukis TS, Zgonis T. The management of acute Charcot fracture-dislocations with the Taylor's spatial external fixation system. Clin Podiatr Med Surg. 2006;23:467–83. doi: 10.1016/j.cpm.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Wukich DK, Belczyk RJ, Burns PR, Frykberg RG. Complications encountered with circular ring fixation in persons with diabetes mellitus. Foot Ankle Int. 2008;29:994–1000. doi: 10.3113/FAI.2008.0994. [DOI] [PubMed] [Google Scholar]
- 66.Schweinberger MH, Roukis TS. The effectiveness of physician-directed external fixation pin site care in preventing pin site infection in a high-risk patient population. Foot Ankle Spec. 2008;1:218–21. doi: 10.1177/1938640008318176.. [DOI] [PubMed] [Google Scholar]
- 67.Roukis T. A simple technique for harvesting autogenous bone grafts from the calcaneus. Foot Ankle Int. 2006;27:998–9. doi: 10.1177/107110070602701123. [DOI] [PubMed] [Google Scholar]
- 68.Deresh GM, Cohen M. Reconstruction of the diabetic Charcot foot incorporating bone grafts. J Foot Ankle Surg. 1996;35:474–88. doi: 10.1016/s1067-2516(96)80069-x. [DOI] [PubMed] [Google Scholar]
- 69.Fitzgibbons TC, Hawks MA, McMullen ST, Inda DJ. Bone grafting in surgery about the foot and ankle: indications and techniques. J Am Acad Orthop Surg. 2011;19:112–20. doi: 10.5435/00124635-201102000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Baumhauer J, Pinzur MS, Donahue R, Beasley W, DiGiovanni C. Site selection and pain outcome after autologous bone graft harvest. Foot Ankle Int. 2014;35:104–7. doi: 10.1177/1071100713511434. [DOI] [PubMed] [Google Scholar]
- 71.Ramanujam CL, Facaros Z, Zgonis T. An overview of bone grafting techniques for the diabetic Charcot foot and ankle. Clin Podiatr Med Surg. 2012;29:589–95. doi: 10.1016/j.cpm.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Smith SE, Roukis TS. Bone and wound healing augmentation with platelet-rich plasma. Clin Podiatr Med Surg. 2009;26:559–88. doi: 10.1016/j.cpm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Roukis TS, Zgonis T, Tiernan B. Autologous platelet-rich plasma for wound and osseous healing: a review of the literature and commercially available products. Adv Ther. 2006;23:218–37. doi: 10.1007/BF02850128. [DOI] [PubMed] [Google Scholar]
- 74.Roukis TS, Hyer CF, Philbin TM, Berlet GC, Lee TH. Complications associated with autogenous bone marrow aspirate harvest from the lower extremity: an observational cohort study. J Foot Ankle Surg. 2009;48:668–71. doi: 10.1053/j.jfas.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 75.Schade VL, Roukis TS. Percutaneous bone marrow aspirate and bone graft harvesting techniques in the lower extremity. Clin Podiatr Med Surg. 2008;25:733–42. doi: 10.1016/j.cpm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 76.Schweinberger MH, Roukis TS. Percutaneous autologous bone marrow harvest from the calcaneus and proximal tibia: surgical technique. J Foot Ankle Surg. 2007;46:411–14. doi: 10.1053/j.jfas.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 77.Pinzur MS. Use of platelet-rich concentrate and bone marrow aspirate in high-risk patients with Charcot arthropathy of the foot. Foot Ankle Int. 2009;30:124–7. doi: 10.3113/FAI-2009-0124. [DOI] [PubMed] [Google Scholar]
- 78.Limb M. Psychosocial issues relating to external fixation of fractures. Nurs Times. 2003;99:28–30. [PubMed] [Google Scholar]
- 79.Santy J, Vincent M, Duffield B. The principles of caring for patients with Ilizarov external fixation. Nurs Stand. 2009;23:50–5. doi: 10.7748/ns2009.03.23.26.50.c6835. [DOI] [PubMed] [Google Scholar]
- 80.Bem R, Jirkovská A, Fejfarová V, Skibová J, Jude EB. Intranasal calcitonin in the treatment of acute Charcot neuroosteoarthropathy: a randomized controlled trial. Diabetes Care. 2006;29:1392–4. doi: 10.2337/dc06-0376. [DOI] [PubMed] [Google Scholar]