Abstract
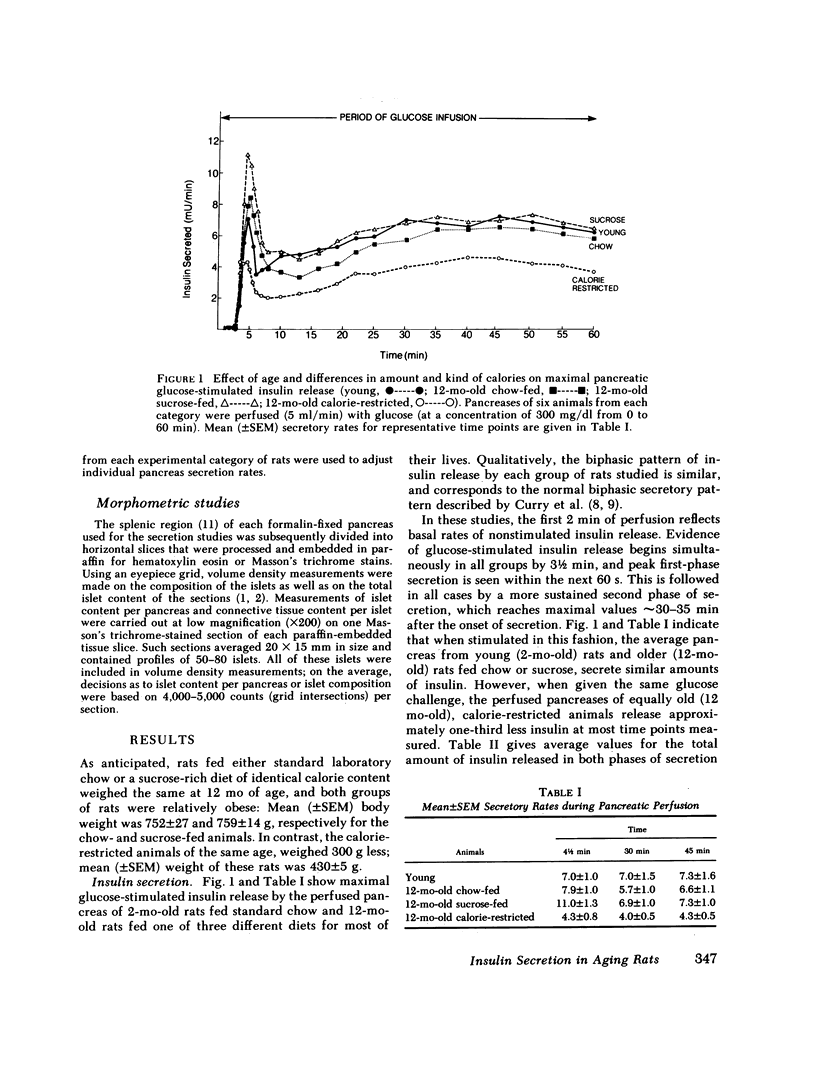
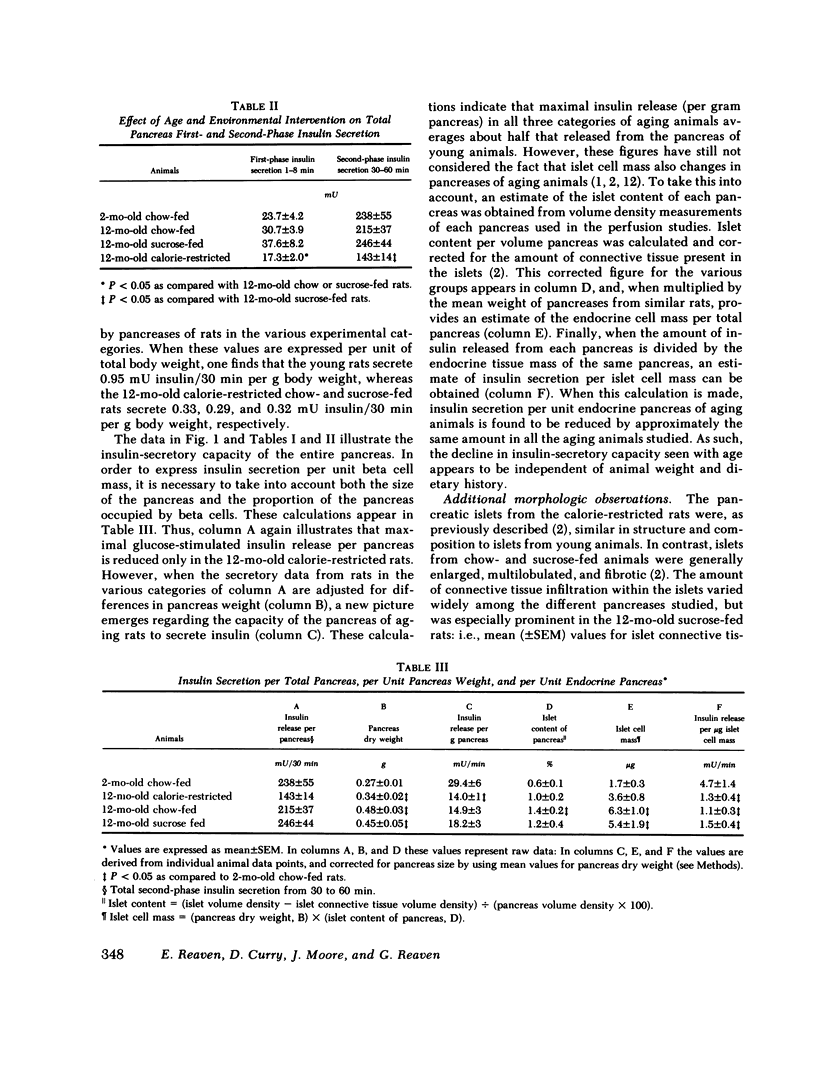
In this study we examined the effect of age and various age-related environmental factors on maximal glucose-stimulated insulin release by the intact perfused pancreas. Male Sprague-Dawley rats were maintained from 40 d to 12 mo of age on standard chow, or on a sucrose-rich or calorie-restricted diet. At 12 mo, studies were carried out on the isolated pancreas of each animal to determine maximal (300 mg/ml) glucose-stimulated insulin secretion. After these studies were completed, each pancreas was perfused with formalin fixative and processed for morphometric estimation of the mass of the endocrine pancreas. Data from these older animals were compared with data from 2-mo-old control rats. The results indicate that maximal glucose-stimulated insulin secretion per unit endocrine pancreas was markedly reduced in all three groups of 12-mo-old rats, and was only 25-33% of that of 2-mo-old rats. Thus, aging led to a decline in insulin secretion per beta cell that was not modifiable by environmental manipulation. On the other hand, environmental factors can influence the development of endocrine tissue within the pancreas, and in so doing, modify total pancreatic insulin secretion. The mass of the endocrine pancreas of 12-mo-old rats fed either sucrose or chow was between three and four times that of 2-mo-old control rats, and these older rats were able to maximally secrete as much insulin per total pancreas as the young rats. In contrast, the endocrine cell mass of the calorie-restricted rats had not enlarged to this extent, and the maximally stimulated perfused pancreas from these rats secreted less insulin. These data suggest that the aging animal, challenged in vivo to secrete insulin, can overcome the loss of the beta cell response by expanding its pancreatic pool of beta cells. Although this compensation is successful in the 12-mo-old, obese, middle-aged rat, it is not yet clear what effect further aging would have on these events.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres R. Aging and diabetes. Med Clin North Am. 1971 Jul;55(4):835–846. doi: 10.1016/s0025-7125(16)32479-8. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Joy R. M., Holley D. C., Bennett L. L. Magnesium modulation of glucose-induced insulin secretion by the perfused rat pancreas. Endocrinology. 1977 Jul;101(1):203–208. doi: 10.1210/endo-101-1-203. [DOI] [PubMed] [Google Scholar]
- Davidson M. B. The effect of aging on carbohydrate metabolism: a review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism. 1979 Jun;28(6):688–705. doi: 10.1016/0026-0495(79)90024-6. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Glucose intolerance and aging. Diabetes Care. 1981 Jul-Aug;4(4):493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M. A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J Clin Invest. 1972 Aug;51(8):2047–2059. doi: 10.1172/JCI107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLMAN B. The total volume of the pancreatic islet tissue at different ages of the rat. Acta Pathol Microbiol Scand. 1959;47:35–50. doi: 10.1111/j.1699-0463.1959.tb03419.x. [DOI] [PubMed] [Google Scholar]
- Kitahara A., Adelman R. C. Altered regulation of insulin secretion in isolated islets of different sizes in aging rats. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1207–1213. doi: 10.1016/s0006-291x(79)80035-2. [DOI] [PubMed] [Google Scholar]
- Lipson L. G., Bobrycki V. A., Bush M. J., Tietjen G. E., Yoon A. Insulin release in aging: studies on adenylate cyclase, phosphodiesterase, and protein kinase in isolated islets of Langerhans of the rat. Endocrinology. 1981 Feb;108(2):620–624. doi: 10.1210/endo-108-2-620. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Gold G., Reaven G. M. Effect of age on glucose-stimulated insulin release by the beta-cell of the rat. J Clin Invest. 1979 Aug;64(2):591–599. doi: 10.1172/JCI109498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E. P., Reaven G. M. Structure and function changes in the endocrine pancreas of aging rats with reference to the modulating effects of exercise and caloric restriction. J Clin Invest. 1981 Jul;68(1):75–84. doi: 10.1172/JCI110256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E., Gold G., Reaven G. Effect of age on leucine-induced insulin secretion by the beta-cell. J Gerontol. 1980 May;35(3):324–328. doi: 10.1093/geronj/35.3.324. [DOI] [PubMed] [Google Scholar]
- Reaven E., Solomon R., Azhar S., Reaven G. Functional homogeneity of pancreatic islets of aging rats. Metabolism. 1982 Sep;31(9):859–860. doi: 10.1016/0026-0495(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Reaven E. P. Effects of age on various aspects of glucose and insulin metabolism. Mol Cell Biochem. 1980 May 28;31(1):37–47. doi: 10.1007/BF00817889. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Risser T. R., Chen Y. D., Reaven E. P. Characterization of a model of dietary-induced hypertriglyceridemia in young, nonobese rats. J Lipid Res. 1979 Mar;20(3):371–378. [PubMed] [Google Scholar]



