Abstract
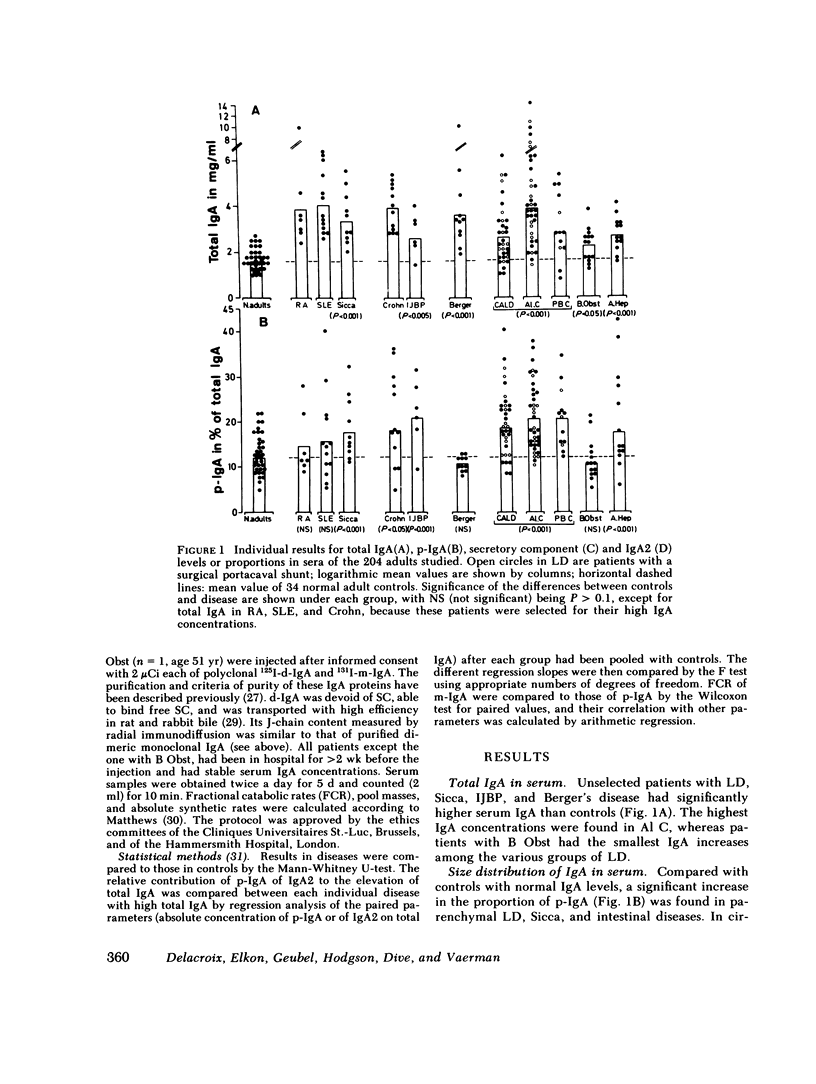
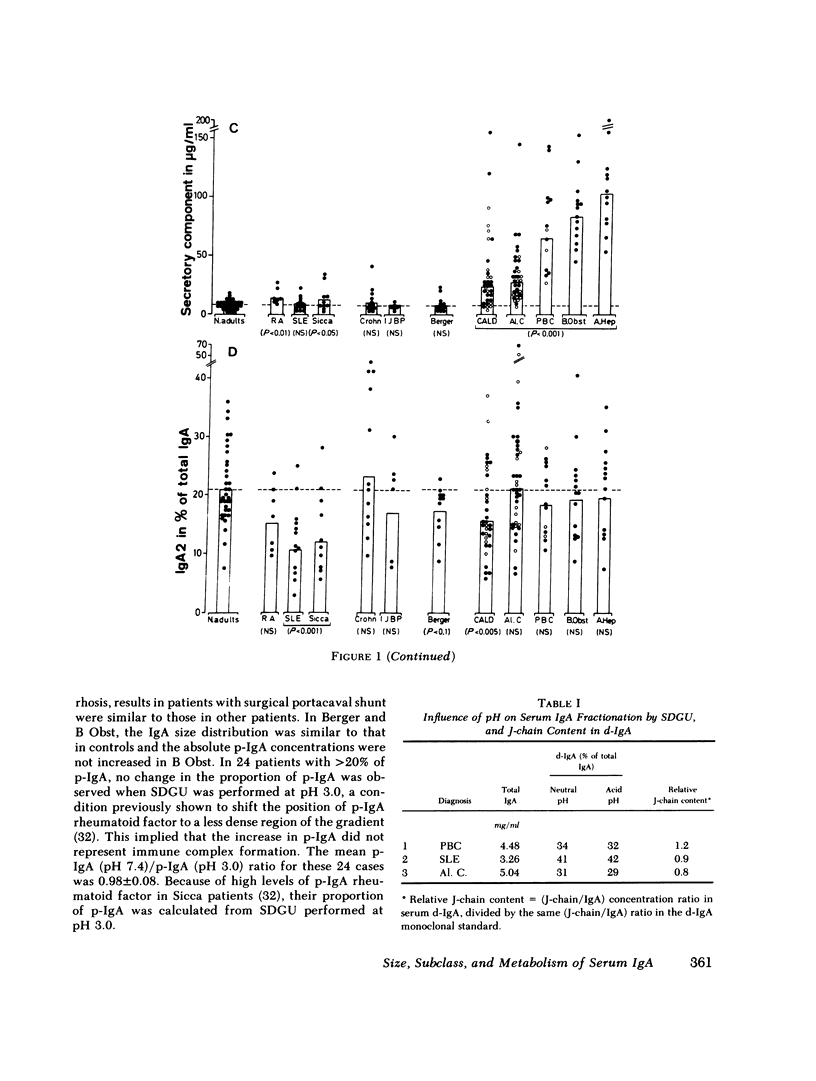
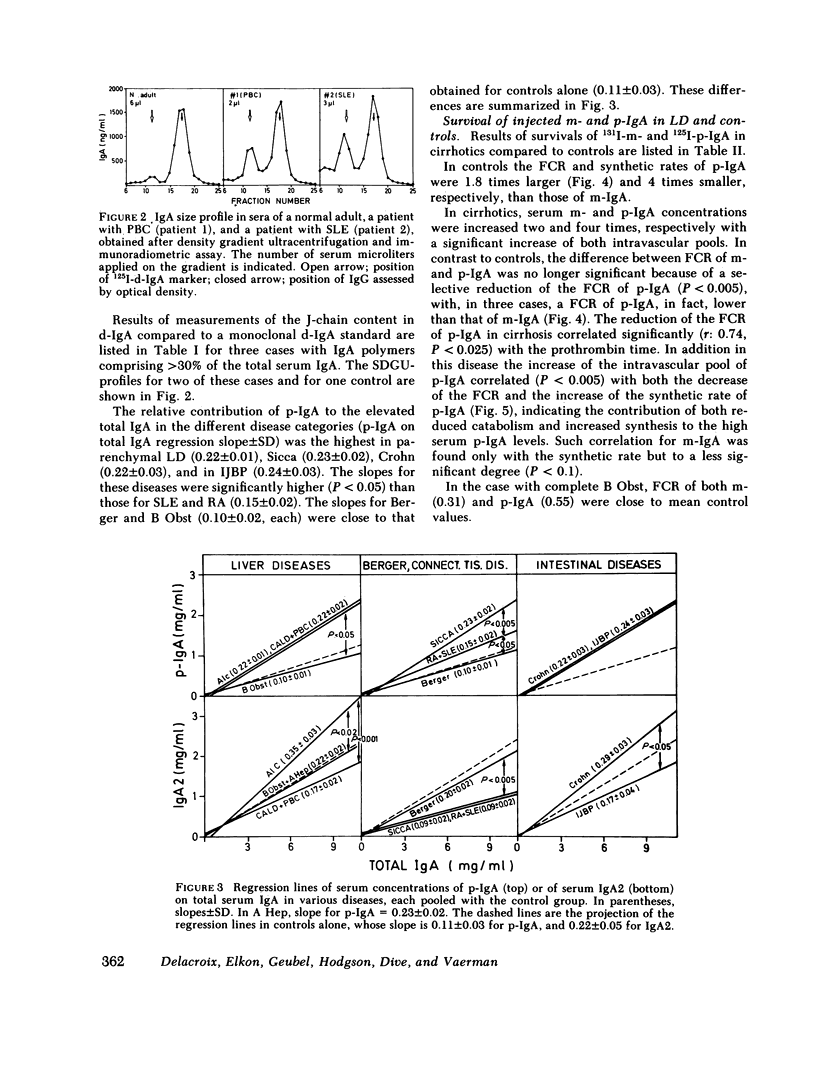
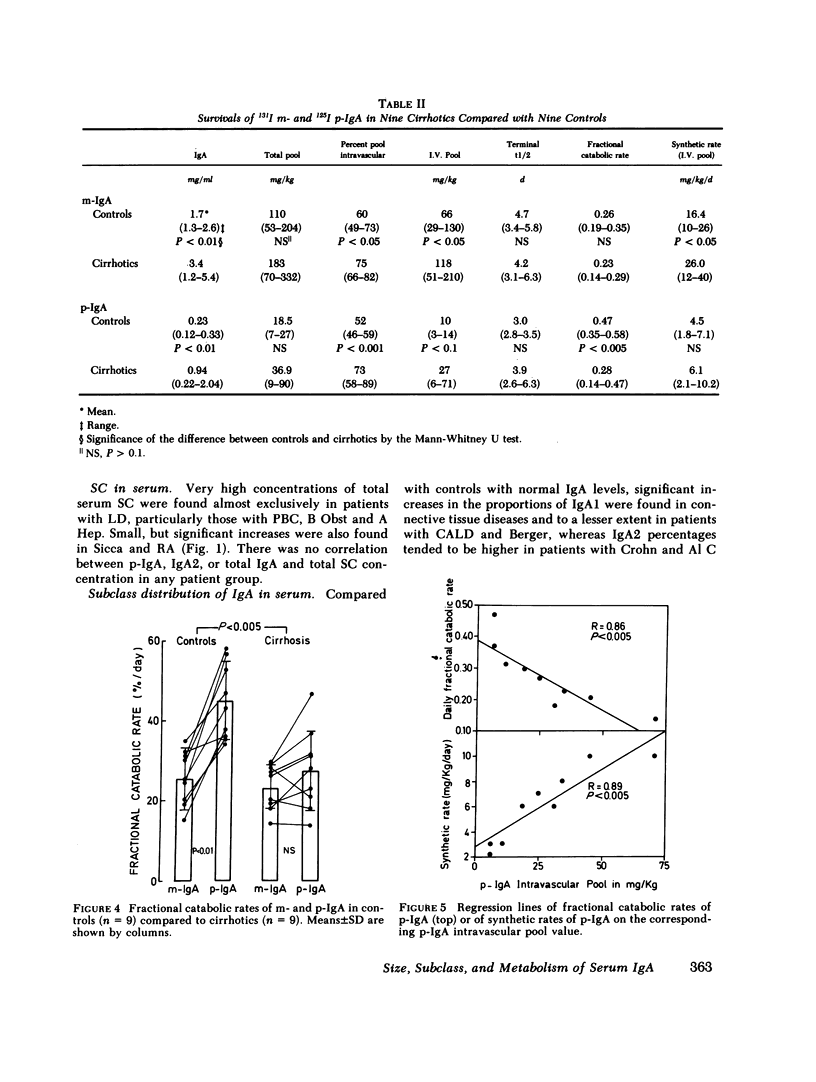
We have studied the relative contributions of monomeric (m-) and polymeric IgA (p-IgA) and of IgA1 and IgA2 to total serum IgA in healthy adults and patients with liver disease (LD) or with other diseases and high serum IgA. Serum concentration of total secretory component (SC) was also determined. In addition, fractional catabolic rates (FCR) and synthetic rates for both m- and p-IgA were measured in nine controls and nine cirrhotics. Our results support four main conclusions: (a) In healthy adults, intravascular p-IgA contributes to only 4-22% (mean 12%) of serum IgA, because its FCR and synthetic rate are approximately two times higher and four times smaller, respectively, than those of intravascular m-IgA. (b) in LD, biliary obstruction does not result in a significant increase in serum p-IgA unlike in rats and rabbits, indicating that in humans the SC-dependent biliary transport of p-IgA plays a much less significant role in selective removal of p-IgA from plasma than in rats and rabbits. (c) In contrast to biliary obstruction, parenchymal LD results in a significant and preferential increase in serum p-IgA, which in cirrhotics correlates with a selective reduction of the p-IgA-FCR. This supports a role for the human liver in selective removal of p-IgA from plasma, but another mechanism than the SC-dependent biliary transport should be considered. (d) Total SC, p-IgA, and IgA2 in serum are unlinked parameters, not necessarily reflecting mucosal events. A marked increase in serum SC occurs almost selectively in LD. Although a shift to IgA2 is suggested in Crohn's disease and alcoholic cirrhosis, a shift to IgA1 frequently associated to a shift to p-IgA occurs in chronic active LD, primary Sicca, and connective tissue diseases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., André F., Fargier C. Distribution of IgA 1 and IgA 2 plasma cells in various normal human tissues and in the jejunum of plasma IgA-deficient patients. Clin Exp Immunol. 1978 Aug;33(2):327–331. [PMC free article] [PubMed] [Google Scholar]
- André C., Berthoux F. C., André F., Gillon J., Genin C., Sabatier J. C. Prevalence of IgA2 deposits in IgA nephropathies: a clue to their pathogenesis. N Engl J Med. 1980 Dec 4;303(23):1343–1346. doi: 10.1056/NEJM198012043032306. [DOI] [PubMed] [Google Scholar]
- André F., André C. Participation de l'immunoglobuline A monomérique et dimérique à l'hypergammaglobulinémie de la cirrhose éthylique. Biol Gastroenterol (Paris) 1976 Apr-May;9(2):147–150. [PubMed] [Google Scholar]
- BLOCH K. J., BUCHANAN W. W., WOHL M. J., BUNIM J. J. SJOEGREN'S SYNDROME. A CLINICAL, PATHOLOGICAL, AND SEROLOGICAL STUDY OF SIXTY-TWO CASES. Medicine (Baltimore) 1965 May;44:187–231. [PubMed] [Google Scholar]
- Baklien K., Brandtzaeg P. Comparative mapping of the local distribution of immunoglobulin-containing cells in ulcerative colitis and Crohn's disease of the colon. Clin Exp Immunol. 1975 Nov;22(2):197–209. [PMC free article] [PubMed] [Google Scholar]
- Benner R., Hijmans W., Haaijman J. J. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol. 1981 Oct;46(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Transport models for secretory IgA and secretory IgM. Clin Exp Immunol. 1981 May;44(2):221–232. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Two types of IgA immunocytes in man. Nat New Biol. 1973 May 30;243(126):142–143. doi: 10.1038/newbio243142a0. [DOI] [PubMed] [Google Scholar]
- Conley M. E., Cooper M. D., Michael A. F. Selective deposition of immunoglobulin A1 in immunoglobulin A nephropathy, anaphylactoid purpura nephritis, and systemic lupus erythematosus. J Clin Invest. 1980 Dec;66(6):1432–1436. doi: 10.1172/JCI109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Dehennin J. P., Vaerman J. P. Influence of molecular size of IgA on its immunoassay by various techniques. II. Solid-phase radioimmunoassays. J Immunol Methods. 1982;48(3):327–337. doi: 10.1016/0022-1759(82)90333-7. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Denef A. M., Acosta G. A., Montgomery P. C., Vaerman J. P. Immunoglobulins in rabbit hepatic bile: selective secretion of IgA and IgM and active plasma-to-bile transfer of polymeric IgA. Scand J Immunol. 1982 Oct;16(4):343–350. doi: 10.1111/j.1365-3083.1982.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Dive C., Rambaud J. C., Vaerman J. P. IgA subclasses in various secretions and in serum. Immunology. 1982 Oct;47(2):383–385. [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Hodgson H. J., McPherson A., Dive C., Vaerman J. P. Selective transport of polymeric immunoglobulin A in bile. Quantitative relationships of monomeric and polymeric immunoglobulin A, immunoglobulin M, and other proteins in serum, bile, and saliva. J Clin Invest. 1982 Aug;70(2):230–241. doi: 10.1172/JCI110610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Jonard P., Dive C., Vaerman J. P. Serum IgM-bound secretory component (sIgM) in liver diseases: comparative molecular state of the secretory component in serum and bile. J Immunol. 1982 Jul;129(1):133–138. [PubMed] [Google Scholar]
- Delacroix D. L., Meykens R., Vaerman J. P. Influence of molecular size of IgA on its immunoassay by various techniques--I. Direct and reversed single radial immunodiffusion. Mol Immunol. 1982 Feb;19(2):297–305. doi: 10.1016/0161-5890(82)90343-1. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Reynaert M., Pauwels S., Geubel A. P., Vaerman J. P. High serum levels of secretory IgA in liver disease: possible liver origin of the circulating secretory component. Dig Dis Sci. 1982 Apr;27(4):333–340. doi: 10.1007/BF01296753. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Vaerman J. P. A solid phase, direct competition, radioimmunoassay for quantitation of secretory IgA in human serum. J Immunol Methods. 1981;40(3):345–358. doi: 10.1016/0022-1759(81)90366-5. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Vaerman J. P. Influence of molecular size of IgA on its immunoassay by various techniques. III. Immunonephelometry. J Immunol Methods. 1982;51(1):49–55. doi: 10.1016/0022-1759(82)90381-7. [DOI] [PubMed] [Google Scholar]
- Delacroix D. L., Vaerman J. P. Secretory component (SC): preferential binding to heavy (greater than 11S) IgA polymers and IgM in serum, in contrast to predominance of 11S and free SC forms in secretions. Clin Exp Immunol. 1982 Sep;49(3):717–724. [PMC free article] [PubMed] [Google Scholar]
- Delacroix D., Vaerman J. P. Reassessment of levels of secretory IgA in pathological sera using a quantitative radioimmunoassay. Clin Exp Immunol. 1981 Mar;43(3):633–640. [PMC free article] [PubMed] [Google Scholar]
- Dooley J. S., Potter B. J., Thomas H. C., Sherlock S. A comparative study of the biliary secretion of human dimeric and monomeric IgA in the rat and in man. Hepatology. 1982 May-Jun;2(3):323–327. doi: 10.1002/hep.1840020306. [DOI] [PubMed] [Google Scholar]
- Drenick E. J., Ament M. E., Finegold S. M., Corrodi P., Passaro E. Bypass enteropathy. Intestinal and systemic manifestations following small-bowel bypass. JAMA. 1976 Jul 19;236(3):269–272. doi: 10.1001/jama.236.3.269. [DOI] [PubMed] [Google Scholar]
- EISENMENGER W. J. Hepatic function and protein metabolism in cirrhosis of the liver. Med Clin North Am. 1955 May;NEW:719–734. doi: 10.1016/s0025-7125(16)34677-6. [DOI] [PubMed] [Google Scholar]
- Elkon K. B., Caeiro F., Gharavi A. E., Patel B. M., Ferjencik P. P., Hughes G. R. Radioimmunoassay profile of antiglobulins in connective tissue diseases: elevated level of IgA antiglobulin in systemic sicca syndrome. Clin Exp Immunol. 1981 Dec;46(3):547–556. [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Delacroix D. L., Gharavi A. E., Vaerman J. P., Hughes G. R. Immunoglobulin A and polymeric IgA rheumatoid factors in systemic sicca syndrome: partial characterization. J Immunol. 1982 Aug;129(2):576–581. [PubMed] [Google Scholar]
- Grey H. M., Abel C. A., Yount W. J., Kunkel H. G. A subclass of human gamma-A-globulins (gamma-A2) which lacks the disulfied bonds linking heavy and light chains. J Exp Med. 1968 Dec 1;128(6):1223–1236. doi: 10.1084/jem.128.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEREMANS J. F., HEREMANS M. T., SCHULTZE H. E. Isolation and description of a few properties of the beta 2A-globulin of human serum. Clin Chim Acta. 1959 Jan;4(1):96–102. doi: 10.1016/0009-8981(59)90088-9. [DOI] [PubMed] [Google Scholar]
- Hopf U., Brandtzaeg P., Hütteroth T. H., Meyer zum Büschenfelde K. H. In vivo and in vitro binding of IgA to the plasma membrane of hepatocytes. Scand J Immunol. 1978;8(6):543–549. doi: 10.1111/j.1365-3083.1978.tb00554.x. [DOI] [PubMed] [Google Scholar]
- Jackson G. D., Lemaître-Coelho I., Vaerman J. P., Bazin H., Beckers A. Rapid disappearance from serum of intravenously injected rat myeloma IgA and its secretion into bile. Eur J Immunol. 1978 Feb;8(2):123–126. doi: 10.1002/eji.1830080210. [DOI] [PubMed] [Google Scholar]
- Kunkel H. G., Prendergast R. A. Subgroups of gamma-A immune globulins. Proc Soc Exp Biol Med. 1966 Jul;122(3):910–913. doi: 10.3181/00379727-122-31287. [DOI] [PubMed] [Google Scholar]
- Kutteh W. H., Prince S. J., Mestecky J. Tissue origins of human polymeric and monomeric IgA. J Immunol. 1982 Feb;128(2):990–995. [PubMed] [Google Scholar]
- Kutteh W. H., Prince S. J., Phillips J. O., Spenney J. G., Mestecky J. Properties of immunoglobulin A in serum of individuals with liver diseases and in hepatic bile. Gastroenterology. 1982 Feb;82(2):184–193. [PubMed] [Google Scholar]
- Kühn L. C., Kraehenbuhl J. P. The membrane receptor for polymeric immunoglobulin is structurally related to secretory component. Isolation and characterization of membrane secretory component from rabbit liver and mammary gland. J Biol Chem. 1981 Dec 10;256(23):12490–12495. [PubMed] [Google Scholar]
- Lemaître-Coelho I., Jackson G. D., Vaerman J. P. High levels of secretory IgA and free secretory component in the serum of rats with bile duct obstruction. J Exp Med. 1978 Mar 1;147(3):934–939. doi: 10.1084/jem.147.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesavre P., Digeon M., Bach J. F. Analysis of circulating IgA and detection of immune complexes in primary IgA nephropathy. Clin Exp Immunol. 1982 Apr;48(1):61–69. [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Seiden M. V., Bragdon M. J., Beale M. G. Alterations of IgM, IgG, and IgA Synthesis and secretion by peripheral blood and intestinal mononuclear cells from patients with ulcerative colitis and Crohn's disease. Gastroenterology. 1981 Nov;81(5):844–852. [PubMed] [Google Scholar]
- Nagura H., Nakane P. K., Brown W. R. Translocation of dimeric IgA through neoplastic colon cells in vitro. J Immunol. 1979 Nov;123(5):2359–2368. [PubMed] [Google Scholar]
- Nagura H., Smith P. D., Nakane P. K., Brown W. R. IGA in human bile and liver. J Immunol. 1981 Feb;126(2):587–595. [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radl J., Schuit H. R., Mestecky J., Hijmans W. The origin of monomeric and polymeric forms of IgA in man. Adv Exp Med Biol. 1974;45(0):57–65. doi: 10.1007/978-1-4613-4550-3_7. [DOI] [PubMed] [Google Scholar]
- Rifai A., Small P. A., Jr, Teague P. O., Ayoub E. M. Experimental IgA nephropathy. J Exp Med. 1979 Nov 1;150(5):1161–1173. doi: 10.1084/jem.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho J., Egido J., Sánchez-Crespo M., Blasco R. Detection of monomeric and polymeric IgA containing immune complexes in serum and kidney from patients with alcoholic liver disease. Clin Exp Immunol. 1982 Feb;47(2):327–335. [PMC free article] [PubMed] [Google Scholar]
- Skvaril F., Morell A. Distribution of IgA subclasses in sera and bone marrow plasma cells of 21 normal individuals. Adv Exp Med Biol. 1974;45(0):433–435. doi: 10.1007/978-1-4613-4550-3_51. [DOI] [PubMed] [Google Scholar]
- Socken D. J., Jeejeebhoy K. N., Bazin H., Underdown B. J. Identification of secretory component as an IgA receptor on rat hepatocytes. J Exp Med. 1979 Dec 1;150(6):1538–1548. doi: 10.1084/jem.150.6.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W., Wochner R. D., Barlow M. H., McFarlin D. E., Waldmann T. A. Immunoglobulin metabolism in ataxia telangiectasia. J Clin Invest. 1968 Aug;47(8):1905–1915. doi: 10.1172/JCI105881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Nakane P. K., Brown W. R. Ultrastructural events in the translocation of polymeric IgA by rat hepatocytes. J Immunol. 1982 Mar;128(3):1181–1187. [PubMed] [Google Scholar]
- Thompson R. A., Asquith P. Quantitation of exocrine IgA in human serum in health and disease. Clin Exp Immunol. 1970 Oct;7(4):491–500. [PMC free article] [PubMed] [Google Scholar]
- Thompson R. A., Carter R., Stokes R. P., Geddes A. M., Goodall J. A. Serum immunoglobulins, complement component levels and autoantibodies in liver disease. Clin Exp Immunol. 1973 Jul;14(3):335–346. [PMC free article] [PubMed] [Google Scholar]
- Tomasi T. B., Grey H. M. Structure and function of immunoglobulin A. Prog Allergy. 1972;16:81–213. [PubMed] [Google Scholar]
- Trascasa M. L., Egido J., Sancho J., Hernando L. IgA glomerulonephritis (Berger's disease): evidence of high serum levels of polymeric IgA. Clin Exp Immunol. 1980 Nov;42(2):247–254. [PMC free article] [PubMed] [Google Scholar]
- Unsworth D. J., Payne A. W., Leonard J. N., Fry L., Holborow E. J. IgA in dermatitis-herpetiformis skin is dimeric. Lancet. 1982 Feb 27;1(8270):478–479. doi: 10.1016/s0140-6736(82)91452-0. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., Heremans J. F. Subclasses of human immunoglobulin a based on differences in the alpha polypeptide chains. Science. 1966 Aug 5;153(3736):647–649. doi: 10.1126/science.153.3736.647. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]



