Abstract
A simple venous thrombosis model in rabbits was used for the quantitative evaluation of the thrombolytic effect of human extrinsic (tissue-type) plasminogen activator as compared with urokinase.
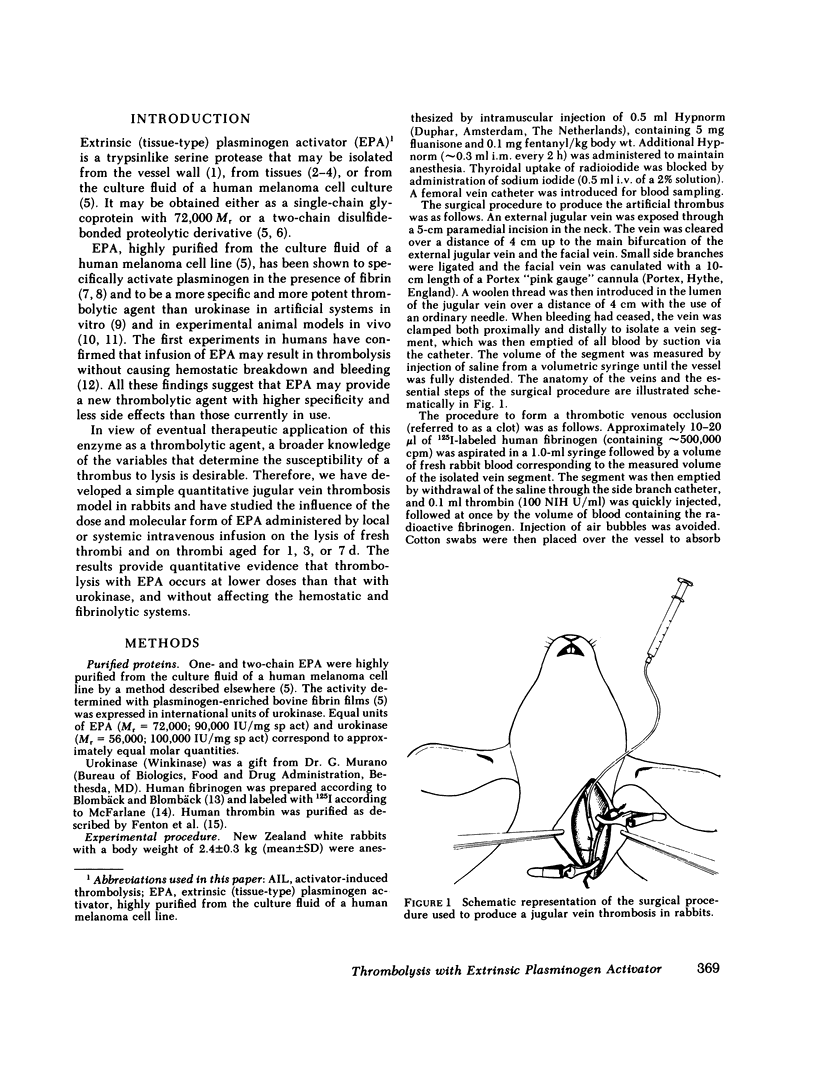
A thrombus was formed in an isolated segment of the jugular vein from a mixture of 125I-labeled fibrinogen, whole rabbit blood, and thrombin. In order to immobilize the thrombus during lysis, it was formed around a woolen thread introduced longitudinally in the lumen of the vein. Thrombotic extension of the clot was prevented by subcutaneous injection of heparin. The extent of thrombolysis was measured as the difference between the radioactivity introduced in the clot and that recovered in the vein segment at the end of the experiment. In control animals the extent of thrombolysis was 5.6±1.4% (n = 5) after 6 h, 14.5±1.7% (n = 10) after 30 h, 16.0±1.5% (n = 11) after 78 h, and 48.1±2.7% (n = 10) after 174 h (mean±SEM).
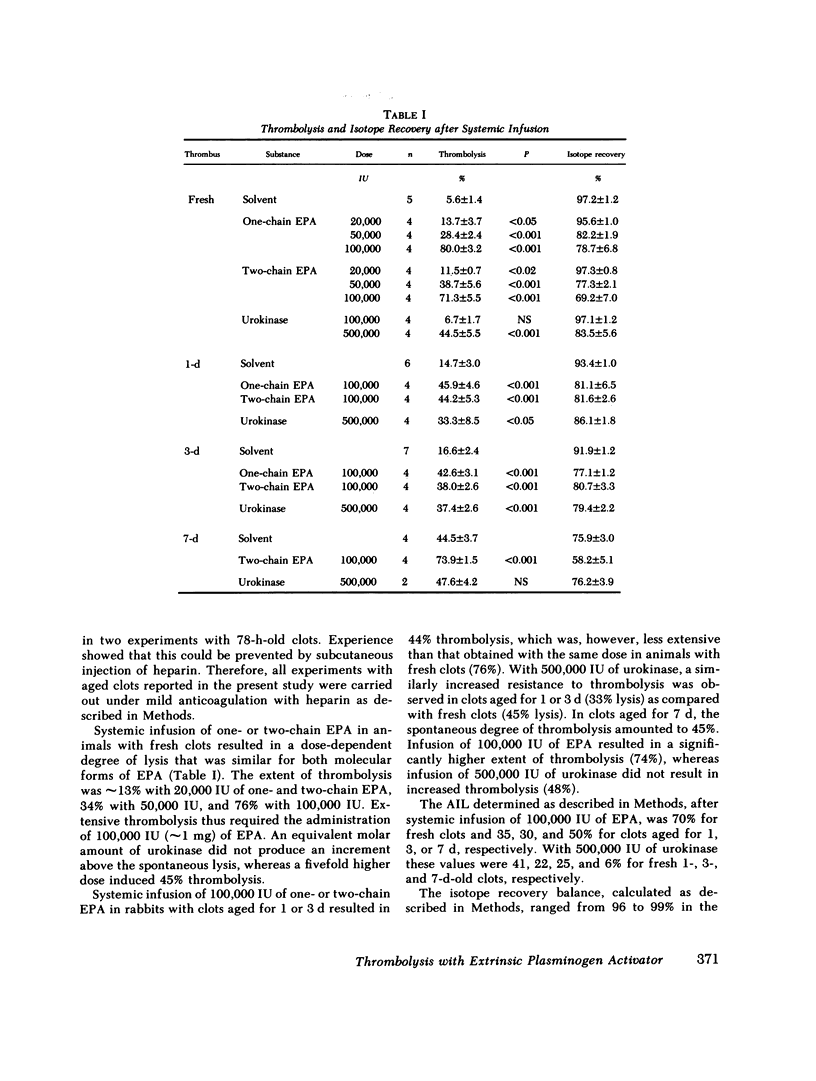
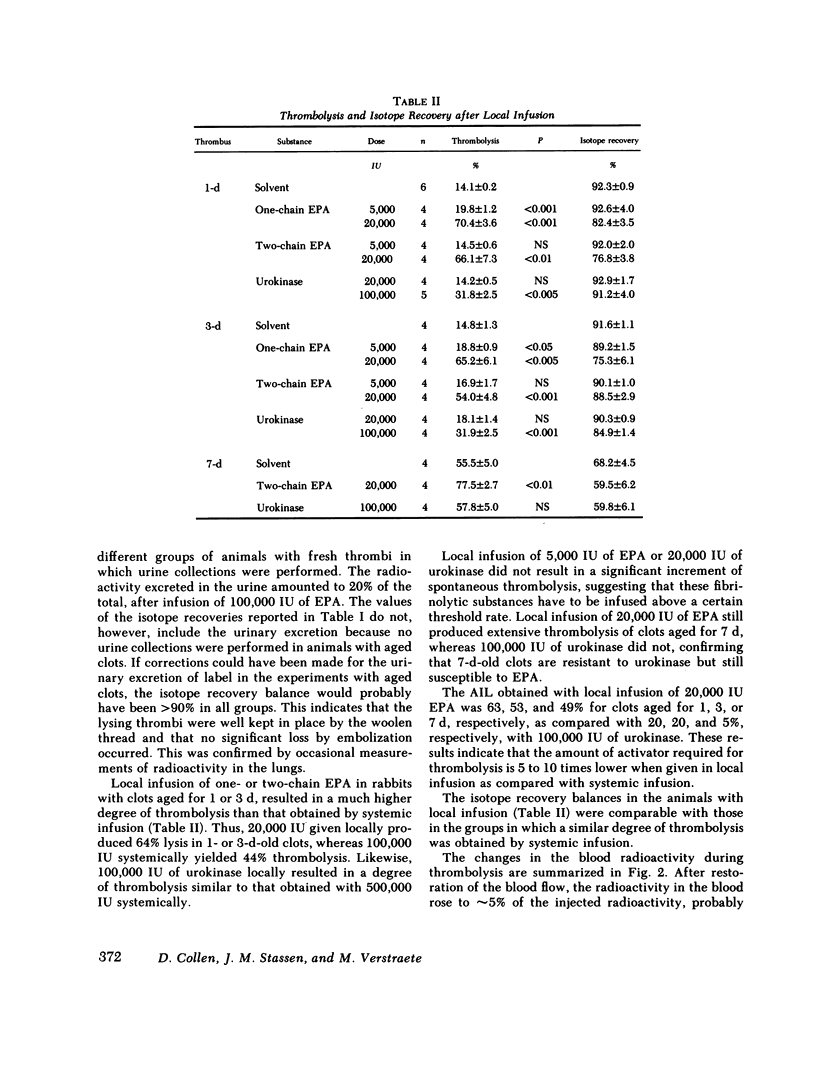
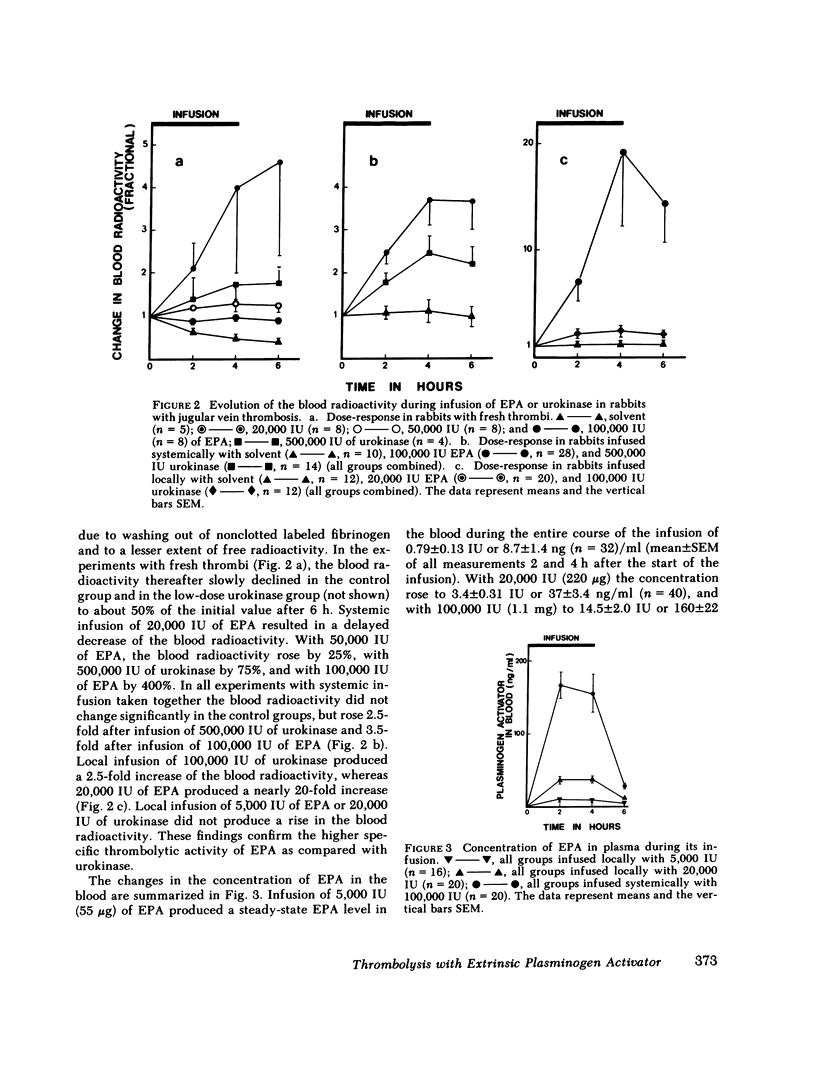
Extrinsic (tissue-type) plasminogen activator, highly purified from the culture fluid of a human melanoma cell line, was administered systemically or locally over a time period of 4 h and the percent thrombolysis measured 2 h after the end of the infusion. One- and two-chain extrinsic plasminogen activator had very similar thrombolytic potency. Systemic infusion resulted in a dose-dependent degree of thrombolysis. The activator-induced thrombolysis, after infusion of 100,000 IU (≅1 mg protein), was ∼75% for fresh clots, 35% for 1-d-old clots, 30% for 3-d-old clots, and 50% for 7-d-old clots. The thrombolytic activity of urokinase was more than five times lower than that of extrinsic plasminogen activator: Infusion of 500,000 IU resulted in ∼40% lysis of fresh clots and 25% of 1-3-d-old clots, while 7-d-old clots appeared to have become resistent to urokinase. Local infusion resulted in a 5-10 times higher thrombolytic effect of both extrinsic plasminogen activator and urokinase.
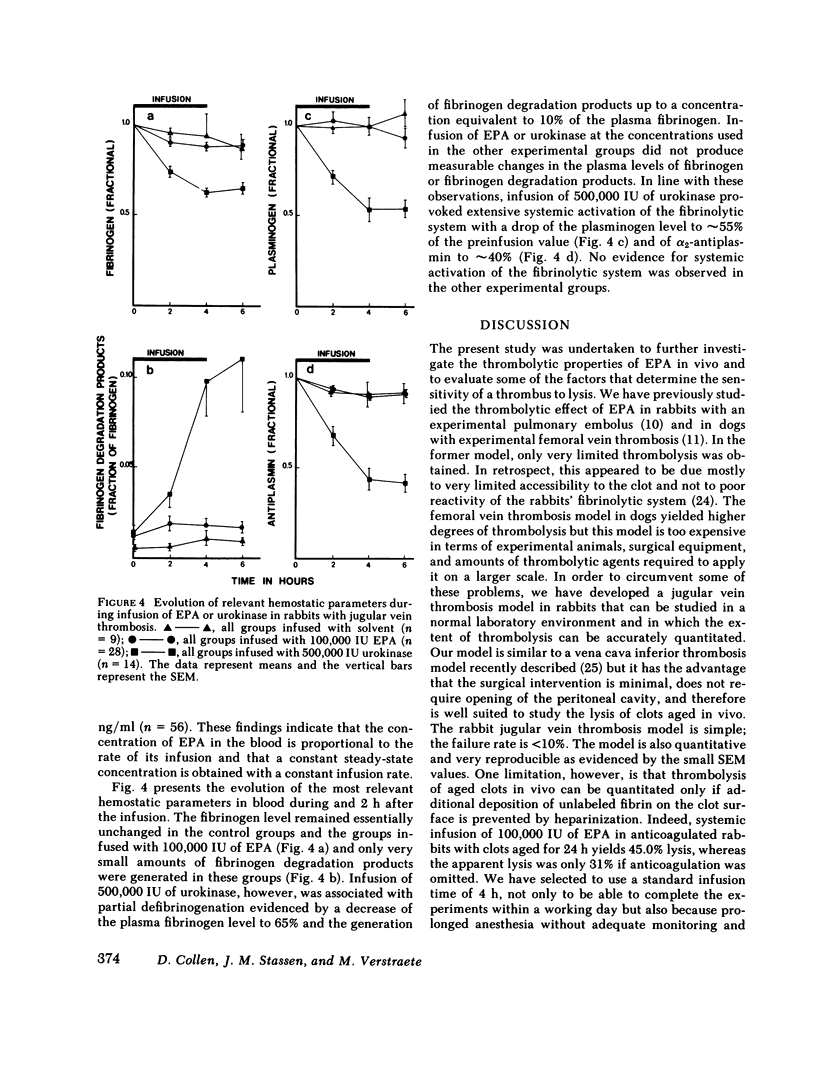
Thrombolysis with extrinsic plasminogen activator was not associated with systemic activation of the fibrinolytic system as evidenced by unaltered plasma levels of fibrinogen, plasminogen, and α2-antiplasmin. Systemic infusion of urokinase resulted in significant thrombolysis only at doses that were associated with disseminated plasminogen activation. Local infusion of urokinase required a 5-10-fold higher dose than extrinsic plasminogen activator to obtain a similar degree of thrombolysis, which also occurred in the absence of systemic activation of the fibrinolytic system.
It is concluded that the extent of thrombolysis by extrinsic plasminogen activator is mainly determined by the dose of activator and its delivery in the vicinity of the thrombus and much less by the age of the thrombus or the molecular form of the activator. Extrinsic plasminogen activator appears to be superior to urokinase because of its higher (5-10-fold) specific thrombolytic activity and the absence of systemic activation of the fibrinolytic system, which results in defibrinogenation and a bleeding tendency.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkjaersig N. The purification and properties of human plasminogen. Biochem J. 1964 Oct;93(1):171–182. doi: 10.1042/bj0930171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N., Von Kaulla K. N. The extraction of vascular plasminogen activator from human cadavers and a description of some of its properties. Am J Clin Pathol. 1971 Feb;55(2):171–179. doi: 10.1093/ajcp/55.2.171. [DOI] [PubMed] [Google Scholar]
- Cole E. R., Bachmann F. W. Purification and properties of a plasminogen activator from pig heart. J Biol Chem. 1977 Jun 10;252(11):3729–3737. [PubMed] [Google Scholar]
- Dupe R. J., English P. D., Smith R. A., Green J. The evaluation of plasmin and streptokinase activator complexes in a new rabbit model of venous thrombosis. Thromb Haemost. 1981 Aug 28;46(2):528–534. [PubMed] [Google Scholar]
- Edy J., De Cock F., Collen D. Inhibition of plasmin by normal and antiplasmin-depleted human plasma. Thromb Res. 1976 Apr;8(4):513–518. doi: 10.1016/0049-3848(76)90229-2. [DOI] [PubMed] [Google Scholar]
- FLETCHER A. P., BIEDERMAN O., MOORE D., ALKJAERSIG N., SHERRY S. ABNORMAL PLASMINOGEN-PLASMIN SYSTEM ACTIVITY (FIBRINOLYSIS) IN PATIENTS WITH HEPATIC CIRRHOSIS: ITS CAUSE AND CONSEQUENCES. J Clin Invest. 1964 Apr;43:681–695. doi: 10.1172/JCI104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Kok P., Astrup T. Isolation and purification of a tissue plasminogen activator and its comparison with urokinase. Biochemistry. 1969 Jan;8(1):79–86. doi: 10.1021/bi00829a013. [DOI] [PubMed] [Google Scholar]
- Korninger C., Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981 Aug 28;46(2):561–565. [PubMed] [Google Scholar]
- Korninger C., Matsuo O., Suy R., Stassen J. M., Collen D. Thrombolysis with human extrinsic (tissue-type) plasminogen activator in dogs with femoral vein thrombosis. J Clin Invest. 1982 Mar;69(3):573–580. doi: 10.1172/JCI110483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo O., Rijken D. C., Collen D. Comparison of the relative fibrinogenolytic, fibrinolytic and thrombolytic properties of tissue plasminogen activator and urokinase in vitro. Thromb Haemost. 1981 Jun 30;45(3):225–229. [PubMed] [Google Scholar]
- Matsuo O., Rijken D. C., Collen D. Thrombolysis by human tissue plasminogen activator and urokinase in rabbits with experimental pulmonary embolus. Nature. 1981 Jun 18;291(5816):590–591. doi: 10.1038/291590a0. [DOI] [PubMed] [Google Scholar]
- Merskey C., Lalezari P., Johnson A. J. A rapid, simple, sensitive method for measuring fibrinolytic split products in human serum. Proc Soc Exp Biol Med. 1969 Jul;131(3):871–875. doi: 10.3181/00379727-131-33998. [DOI] [PubMed] [Google Scholar]
- PROCTOR R. R., RAPAPORT S. I. The partial thromboplastin time with kaolin. A simple screening test for first stage plasma clotting factor deficiencies. Am J Clin Pathol. 1961 Sep;36:212–219. doi: 10.1093/ajcp/36.3.212. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Rijken D. C., Hoylaerts M., Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982 Mar 25;257(6):2920–2925. [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Zaal-de Jong M., Welbergen J. Purification and partial characterization of plasminogen activator from human uterine tissue. Biochim Biophys Acta. 1979 Sep 29;580(1):140–153. doi: 10.1016/0005-2795(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Tytgat G., Collen D., De Vreker R., Verstraete M. Investigations on the fibrinolytic system in liver cirrhosis. Acta Haematol. 1968;40(5):265–274. doi: 10.1159/000208914. [DOI] [PubMed] [Google Scholar]
- VERMYLEN C., DE VREKER R. A., VERSTRAETE M. A rapid enzymatic method for assay of fibrinogen fibrin polymerization time (FPT test). Clin Chim Acta. 1963 May;8:418–424. doi: 10.1016/0009-8981(63)90080-9. [DOI] [PubMed] [Google Scholar]
- Weimar W., Stibbe J., van Seyen A. J., Billiau A., De Somer P., Collen D. Specific lysis of an iliofemoral thrombus by administration of extrinsic (tissue-type) plasminogen activator. Lancet. 1981 Nov 7;2(8254):1018–1020. doi: 10.1016/s0140-6736(81)91217-4. [DOI] [PubMed] [Google Scholar]



