Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is an important opportunistic pathogen that causes both healthcare- and community-acquired infections. An increase in the incidence of these infections may lead to a substantial change in the rate of vancomycin usage. Incidence of reduced susceptibility to vancomycin has been increasing worldwide for the last few years, conferring different levels of resistance to vancomycin as well as producing changes in the cell wall structure. The aim of the present study was to determine the effect of vancomycin on cell wall thickening in clinical isolates of vancomycin-tolerant (VT) MRSA obtained from pediatric patients. From a collection of 100 MRSA clinical isolates from pediatric patients, 12% (12/100) were characterized as VT-MRSA, and from them, 41.66% (5/12) exhibited the heterogeneous vancomycin-intermediate S. aureus (hVISA) phenotype. Multiplex-PCR assays revealed 66.66% (8/12), 25% (3/12), and 8.33% (1/12) of the VT-MRSA isolates were associated with agr group II, I, and III polymorphisms, respectively; the II-mec gene was amplified from 83.3% (10/12) of the isolates, and the mecIVa gene was amplified from 16.66% (2/12) of the isolates. Pulsed field electrophoresis (PFGE) fingerprint analysis showed 62% similarity among the VT-MRSA isolates. Thin transverse sections analyzed by transmission electron microscopy (TEM) revealed an average increase of 24 nm (105.55%) in the cell wall thickness of VT-MRSA compared with untreated VT-MRSA isolates. In summary, these data revealed that the thickened cell walls of VT-MRSA clinical isolates with agr type II and SCCmec group II polymorphisms are associated with an adaptive resistance to vancomycin.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is an important opportunistic pathogen associated with nosocomial and community-acquired invasive infections [1–4]. More than 90% of the MRSA isolates are resistant to all available penicillins and other β-lactam antimicrobial drugs, limiting the therapeutic options available to treat serious infections [5]. Vancomycin is the primary drug of choice for the treatment of MRSA infections; however, the excessive use of this antibiotic has led to the emergence of new S. aureus strains, such as vancomycin-intermediate S. aureus (VISA) and heterogeneous-VISA (hVISA). MRSA strains also develop vancomycin tolerance, commonly defined as a minimum bactericidal concentration (MBC)/minimum inhibitory concentration (MIC) ratio of ≥ 32. The infections caused by vancomycin-tolerant MRSA strains (VT-MRSA) are more difficult to treat, particularly when they are associated with endocarditis, meningitis, osteomyelitis, and infections in immunocompromised patients [6–9].
Failures in clinical treatment with vancomycin have been associated with strains having vancomycin susceptibility within the intermediate MIC range (VISA), and two mixed susceptibility subpopulations (hVISA) have also been described. Infections caused by hVISA strains are poorly understood, reflecting the difficulty of detecting low frequency subpopulations resistant to vancomycin [10–14]. Therefore, the population analysis profile (PAP) method has been used for the identification of hVISA strains [15, 16]. Recent reports from many countries have suggested that hVISA strains are responsible for failures in vancomycin therapy and that patients administered vancomycin for prolonged periods of time are potential sources of VISA subclones [17–19].
The accessory gene regulatory (agr) locus is a quorum-sensing operon that coordinates the expression of several housekeeping genes and genes encoding secreted and cell-associated virulence factors [20, 21]. In addition, four major S. aureus agr groups (I, II, III, and IV) associated with distinct clinical manifestations have been described [21–23]. Many VISA strains are highly enriched for the agr group II polymorphism [24, 25]. MRSA strains are hallmarked by the presence of a 2.1-kb mecA gene encoding penicillin-binding protein 2a (78 kDa) with a reduced affinity for β-lactams [26, 27]. Currently, eight major SCCmec types have been associated with the mec gene complex, and they are divided into class A (SCCmec types II, III, and VIII), class B (SCCmec types I, IV, and VI), and class C (SCCmec types V and VII) [28, 29].
Cell wall synthesis in VISA strains affected by metabolic changes influences the cell wall thickening mechanism [8, 30, 31]. Interestingly, cell wall thickening in VT-MRSA clinical isolates when administered with gradually increasing doses of vancomycin has not yet been described; however, both hVISA Mu3 and VISA Mu50 have been shown to use cell wall thickening as a vancomycin tolerance mechanism, suggesting that cell wall thickening is requisite for vancomycin-resistant strains. Therefore, the aim of the present study was to evaluate cell wall thickening in VT-MRSA clinical isolates from pediatric patients as a direct effect of vancomycin stimulation and examine the interrelationships among the susceptibility profiles, agr-group polymorphisms, SCCmec types, and genetic relatedness.
Material and Methods
Ethics statement
The study was reviewed and approved by the Research Committee (Dr. Onofre Muñoz Hernández), Ethics Committee (Dra. Amparo Faure Fontenla), and Biosecurity Committee (Dra. Herlinda Vera Hermosillo) of Hospital Infantil de México Federico Gómez (HIMFG), with permit number HIM/2010/016. The Central Laboratory from HIMFG provided the MRSA isolates for this study. The anonymous clinical information presented in this manuscript prior to analysis was obtained from the patient’s medical record, considering the diagnosis, and sample type. According to the institutional ethical, biosecurity and investigation evaluation an informed consent is not required.
Clinical isolates of S. aureus
A collection of 100 MRSA clinical isolates from pediatric patients (one isolate per patient) kept in the Central Laboratory at the HIMFG was analyzed in this study. The patients’ medical records were reviewed to understand the clinical relevance, sample type and diagnosis. The clinical S. aureus isolates were confirmed in the laboratory using conventional methods, such as colony morphology, Gram staining, catalase activity, human plasma coagulase production, mannitol fermentation, and growth on broth heart infusion agar (BHI; Dibco, México DF, México) supplemented with 15% NaCl. In addition, methicillin resistance in all clinical S. aureus isolates was confirmed through the Kirby-Bauer method using oxacillin (Sigma-Aldrich; MO, USA) at the Clinical and Laboratory Standards Institute (CLSI) 2013 [32].
Determination of vancomycin MIC and MBC
The vancomycin susceptibility profiles of MRSA isolates were determined using the MIC technique via the microdilution method in Mueller-Hinton broth (MHB; Becton Dickinson, México DF, México), according to the CLSI 2013 [32]. Several concentrations (128–0.50 μg/mL) of vancomycin were prepared in MHB, and 100 μL of antibiotic sample was loaded into each well of a microplate. For each dilution, 100 μl of a bacterial suspension (1.5x105 bacteria/mL) was inoculated and grown at 37°C under a CO2 atmosphere for 20 h. The MIC values for each MRSA isolate were calculated when the bacterial colonies were completely inhibited at the lowest concentration after incubation for 20 h. To determine the MBC, 10 μL of the bacterial suspension from the MIC microplate of the well in which bacterial inhibition occurred, was spread onto blood agar, incubated for 24 h, and analyzed to determine the number of colony-forming units. S. aureus strain ATCC 29213 was used as a control. The data were interpreted according to the guidelines of the CLSI 2013 [32].
Identification of clinical isolates of vancomycin-tolerant S. aureus
The vancomycin tolerance test was performed as previously described [7]. In addition, vancomycin tolerance is defined as a MBC/MIC ratio ≥ 32. An MIC test was performed for each clinical S. aureus isolate, followed by the MBC test. The MBC/MIC ratio test was performed as a presumptive concentration screening of the antibiotics used in the present study.
Antimicrobial susceptibility profile for vancomycin-tolerant MRSA
The antibiotic susceptibility profiles for the VT-MRSA isolates were determined using the MIC technique via the microdilution method according to the CLSI 2013 [32], as described in the previous section. MIC tests were performed for ampicillin (Sigma-Aldrich), ceftazidime (Pfizer; México DF, México), ceftriaxone (Roche; Mexico DF, México), ciprofloxacin (Bayer; Levenkusen Westfalia, Germany), erythromycin (MP Biomedicals; Solon, OH, USA), kanamycin (Sigma-Aldrich), meropenem (Astra Zeneca; México DF, Mexico), rifampicin (MP Biomedicals), gentamicin (MP Biomedicals), and trimethoprim-sulfamethoxazole (Sigma-Aldrich).
Population analysis profile
The VT-MRSA isolates were screened to detect the hVISA phenotype using PAP analysis as previously described [33]. From a bacterial suspension adjusted to 1x108 CFU/mL were made serial dilutions (101 to 108), and a 25-μL inoculum of each dilution was expanded in BHI agar supplemented with vancomycin concentrations of 0 to 8 mg/L and incubated at 37°C for 48 h. The hVISA strain Mu3 and the vancomycin-susceptible S. aureus strain (VSSA) ATCC 25923 were used as controls in the present study. An isolate was defined as hVISA if the AUC of the test isolate divided by the AUC of the corresponding strain Mu3 was ≥ 0.9.
DNA extraction
The VT-MRSA clinical isolates were cultured on blood agar for 18 to 24 h and treated with an enzymatic cocktail (2 mg/mL lysozyme, 100 μg/mL proteinase K, and 1 mg/mL lysostaphin [Sigma Aldrich; MO, USA]) for genomic DNA extraction using the commercial Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI, USA).
SCCmec typing using multiplex PCR
To characterize the SCCmec genes (I, II, III, IVa, V, and mecA), six primer pairs were used (Table 1). Briefly, 100 ng of pure DNA was added to a reaction mixture (Promega Corporation, Madison, WI, USA) containing 2.5 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate (dATP, dGTP, dCTP, and dTTP), 1U of Taq DNA polymerase, varying concentrations of the different oligos and nuclease-free water. The genes were amplified under the following conditions: an initial denaturation step at 94°C for 5 min; 10 cycles of denaturation at 94°C for 45 sec, annealing at 65°C for 45 sec, and extension at 72°C for 1.5 min; 25 cycles of denaturation at 94°C for 45 sec, annealing at 65°C for 45 sec, and annealing at 55°C for 45 sec; and a final extension at 72°C for 10 min. The PCR products were separated by electrophoresis on 1.5% agarose gels and subsequently stained with 0.5 mg/mL ethidium bromide dissolved in 0.5X TBE buffer (Tris-borate-EDTA). The stained gels were visualized and analyzed under ultraviolet light. The SCCmec type I (S. aureus 1746), type II (S. aureus 1749), type III (S. aureus 1748), and type IVa (S. aureus USA 300 and MW2) strains were used as positive controls.
Table 1. List of PCR primers and amplicon sizes of the target genes.
| Genes | Primers | Sequences (5´– 3´) | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| agr I | agr I-F | ATGCACATGGTGCACATGC | 441 | [35] |
| agr I-R | GTCACAAGTACTATAAGCTGCGAT | |||
| agr II | agr II-F | ATGCACATGGTGCACATGC | 575 | [35] |
| agr II-R | TATTACTAATTGAAAAGTGGCCATAGC | |||
| agr III | agr III-F | ATGCACATGGTGCACATGC | 323 | [35] |
| agr III-R | GTAATGTAATAGCTTGTAAAAAGTGGCCATAGC | |||
| agr IV | agr IV-F | ATGCACATGGTGCACATGC | 659 | [35] |
| agr IV-R | CGATAATGCCGTAATACCCG | |||
| SCCmec I | mec I-F | GCTTTAAAGAGTGTCGTTACAGG | 613 | [34] |
| mec I-R | GTTCTCTCATAGTATGACGTCC | |||
| SCCmec II | mec II-F | CGTTGAAGATGATGAAGCG | 398 | [34] |
| mec II-R | CGAAATCAATGGTTAATGGACC | |||
| SCCmec III | mec III-F | CCATATTGTGTACGATGCG | 280 | [34] |
| mec III-R | CCTTAGTTGTCGTAACAGATCG | |||
| SCCmec IVa | mec IVa-F | GCCTTATTCGAAGAAACCG | 776 | [34] |
| mec IVa-R | CTACTCTTCTGAAAAGCGTCG | |||
| SCCmec V | mec V-F | GAACATTGTTACTTAAATGAGCG | 325 | [34] |
| mec V-R | TGAAAGTTGTACCCTTGACACC | |||
| mecA | mec 147-F | GTGAAGATATACCAAGTGATT | 147 | [34] |
| mec 147-R | ATGCGCTATAGATTGAAAGGAT |
F = forward and R = reverse
agr typing using multiplex PCR
Multiplex PCR assays were performed to characterize the agr groups using specific oligonucleotides derived from the variable region of the agr operon [34]. In the present study, oligonucleotides for the agr groups (I, II, III, and IV) were synthesized according to Gilot et al., [35]. The primer sequences and GenBank accession numbers are shown in Table 1. The clinical isolates of S. aureus USA300 (type I, 441 bp), S. aureus 1749 (type II, 575 bp), and S. aureus ATCC 25923 (type III, 323 bp) were used as positive controls. Briefly, multiplex assays containing 1.25 U of Taq DNA polymerase, 200 mM dNTPs, 1.5 mM MgCl2, 0.3 mM of each oligonucleotide, 100 ng of DNA, and nuclease-free water were prepared. The DNA amplification was performed using a Thermo Hybaid thermocycler (Hybaid PCR Sprint Thermal Cyclers) under the following thermal cycling conditions: denaturation at 94°C for 5 min, annealing at 55°C for 30 sec, and extension at 72°C for 60 sec; this procedure was performed for 26 cycles. The integrity of the amplified DNA was assessed by electrophoresis on 1.5% agarose gels and visualized after staining with 0.5 mg/mL of ethidium bromide. The length of the PCR fragments was identified using 100-bp molecular weight markers (Fermentas; Vilnius, Lithuania).
Molecular genotyping assays
Pulsed-field gel electrophoresis (PFGE) was performed using a previously described protocol [36]. The chromosomal DNA was digested with SmaI and subjected to electrophoresis on 1% agarose gels (Promega; Madison, WI, USA) using the following parameters: 200 V (6 v/cm) at 14°C for 21.5 h, with an initial change of 5 sec and an end change of 40 sec. The gels were stained with 1.0 mg/mL ethidium bromide solution and visualized using a gel imaging system (ChemiDoc MP System, Biorad; México). The DNA fragment patterns generated by PFGE were analyzed using NTSY program version 2.0 with the Dice coefficient and the unweighted pair group method with an arithmetic mean (UPGMA) clustering system.
Analysis of cell-wall thickening in VT-MRSA clinical isolates
Transmission electron microscopy (MET, JOEL model JEM 10–10) was used to morphometrically visualize cell-wall thickening in the VT-MRSA isolates as an effect of antibiotic concentration. Vancomycin-treated bacteria were grown to the exponential phase in the presence of gradual increments of 1 mg/mL antibiotics to a final concentration of 20 μg/mL. As a control, untreated bacteria were also grown to the exponential phase. The bacteria were harvested, washed, and fixed with a glutaraldehyde/formalin (2.5%/10%) solution in 0.1 M phosphate-buffered saline (PBS), pH 6.0. Subsequently, the bacteria were post-fixed with osmium tetroxide, contrasted with uranyl acetate, and dehydrated in graded concentrations of ethyl alcohol (20, 30, 40, 50, 60, 70, 80, 90, and 100%). Then, transverse thin sections from samples embedded in resin were mounted on grids (300-mesh copper grids; Electron Microscopy Sciences, Hatfield Pennsylvania), followed by treatment with lead citrate. The samples were stained with 1% phosphotungstic acid at pH 7.2 and visualized by TEM. The images were processed at 100,000x and analyzed to determine the cell-wall thickness from an average of 10 cells per bacterial strain. The S. aureus strain ATCC 25923 and the VISA strain Mu50 ATCC 700699 were used as references.
Clinical records of pediatric patients
The clinical analysis of the pediatric patients considered the following parameters: source, sex, age, clinical diagnosis total number of days of vancomycin treatment, and clinical outcome (Table 2).
Table 2. Clinical characteristics of the 12 VT-MRSA isolates.
| Isolate | Source | Sex | Age (Months) | Clinical diagnosis | TVT (days) | Clinical Outcome |
|---|---|---|---|---|---|---|
| 179D | Bloodstream | M | 168 | Chronic renal insufficiency | 21 | Improvement |
| 428H | Bloodstream | F | 4 | Gastroschisis | 18 | Death |
| 74D | Pleural fluid | M | 60 | Pleural effusion | 21 | Improvement |
| 148D | Wound | F | NA | NA | NA | NA |
| 645H | Bloodstream | F | NA | NA | NA | NA |
| 440H | Bloodstream | F | 60 | NA | NA | NA |
| A17 | Bloodstream | F | 2 | Neonatal sepsis | 19 | Improvement |
| 163D | Bronchoaspirate | F | 156 | Iatrogenic gastric perforation | 20 | Death |
| 480H | Bloodstream | F | 2 | Choanal atresia and ear anomalies | 24 | Improvement |
| 250D | Bloodstream | M | 84 | Hydrocephalus and cardiomyopathy | 21 | Improvement |
| 737H | Bloodstream | M | 144 | Chronic renal insufficiency | 18 | No improvement |
| 817H | Bloodstream | F | 2 | Intestinal atresia type II | 18 | Improvement |
TVT: Total number of days of vancomycin treatment; NA: Not available
Results
Identification of VT-MRSA clinical isolates
No vancomycin resistance was observed among the 100 MRSA isolates examined in the present study. The results of the analysis showed that 12% (12/100) of the MRSA clinical isolates were tolerant of vancomycin (Table 3). Further analysis of the 12 clinical isolates showed three profiles of vancomycin tolerance: 25% (3/12) of the MRSA clinical isolates showed a MBC/MIC ratio of ≥ 32, 50% (6/12) showed a MBC/MIC ratio of ≥ 64 μg/mL, and 25% (3/12) showed a MBC/MIC ratio of ≥ 128 μg/mL (Table 3).
Table 3. Phenotypic and genotypic characteristics of the 12 VT-MRSA clinical isolates.
| Isolate | MBCμg/mL | MICμg/mL | MBC/MIC | Genes | |
|---|---|---|---|---|---|
| agr | mec | ||||
| 179D-100406 | 128 | 2 | 64 | II | II |
| 428H-240306 | 128 | 2 | 64 | II | II |
| 74D-130706 | 128 | 2 | 64 | II | II |
| 148D-200706 | 128 | 1 | 128 | II | II |
| 645H-110309 | 32 | 1 | 32 | II | II |
| 440H-140109 | 64 | 2 | 32 | II | II |
| A-17–180506 | 128 | 2 | 64 | II | II |
| 163D-270109 | 128 | 2 | 64 | II | II |
| 480H-140109 | 128 | 1 | 128 | I | II |
| 250D-26–07–06 | 128 | 2 | 64 | I | IVA |
| 737H-200305 | 32 | 1 | 32 | I | IVA |
| 817–050406 | 128 | 1 | 128 | III | II |
| ATCC 25923 | III | II | |||
| Mu50 | II | II | |||
Clinical diagnosis of the 12 patients with nosocomial infections
In the present study, we obtained 100 S. aureus isolates from pediatric patients from 2005 to 2009, with 12% (12/100) identified as VT-MRSA isolates. The VT-MRSA isolates with clinical relevance were further analyzed to determine the effect of vancomycin on cell wall thickening. Briefly, the patients comprised 66.66% (8/12) females and 33.33% (4/12) males ranging in age from 2 to 168 months. Table 3 provides a description of the total number of days of vancomycin treatment and clinical diagnosis of the 12 patients. In addition, the patients showed significant comorbidities, such as chronic renal insufficiency, gastroschisis, pleural effusion, neonatal sepsis, intestinal atresia type II, choanal atresia and ear anomalies, iatrogenic gastric perforation, hydrocephalus, and cardiomyopathy. Notably, the VT-MRSA clinical isolates were acquired from different sources: 75% (9/12) from the bloodstream and 8.33% (1/12) from pleural fluid, bronchoaspirates, and wounds, respectively (Table 2).
Antimicrobial susceptibility testing of VT-MRSA clinical isolates
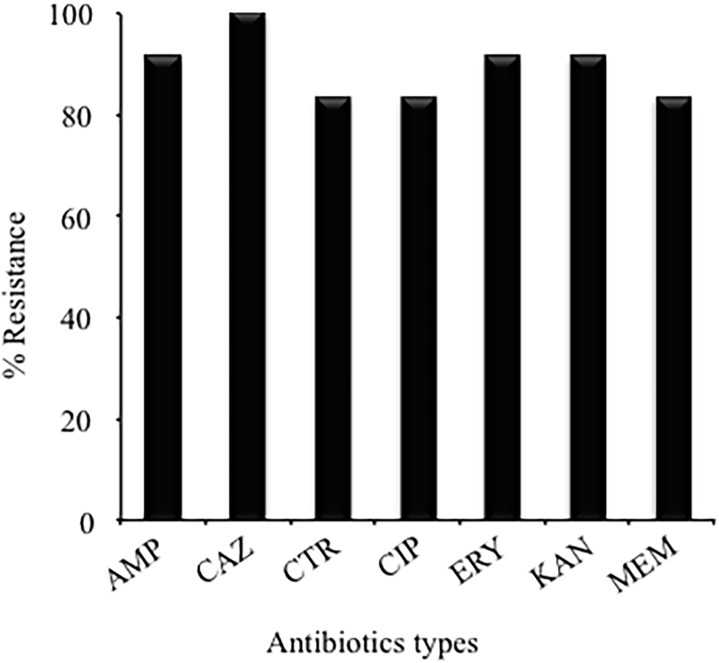
The VT-MRSA clinical isolates were susceptible to the following antibiotics: vancomycin, gentamycin, rifampicin, and trimethoprim/sulfamethoxazole (Fig. 1). In addition, the MIC values indicated that 100% (12/12) of the VT-MRSA clinical isolates were resistant to ceftazidime, 91.66% (11/12) were resistant to ampicillin, erythromycin, and kanamycin, and 83.33% (10/12) were resistant to ciprofloxacin, ceftriaxone, and meropenem (Fig. 1).
Fig 1. Antibiotic resistance of the VT-MRSA clinical isolates obtained from different pediatric patients.
AMP: Ampicillin, CAZ: Ceftazidime, CTR: Ceftriaxone, CIP: Ciprofloxacin, ERY: Erythromycin, KAN: Kanamycin, and MEM: Meropenem.
Identification of the hVISA phenotype among the VT-MRSA clinical isolates
PAP-AUC was used to identify the hVISA phenotype among the VT-MRSA isolates. The results revealed that 41.66% (5/12) of the VT-MRSA isolates showed the hVISA phenotype, growing to 8 mg/mL in the presence of vancomycin concentrations ranging from 0 to 8 mg/mL and with a ratio cutoff value of ≥ 0.9 ± 0.03.
Distribution of mec and agr polymorphisms among VT-MRSA clinical isolates
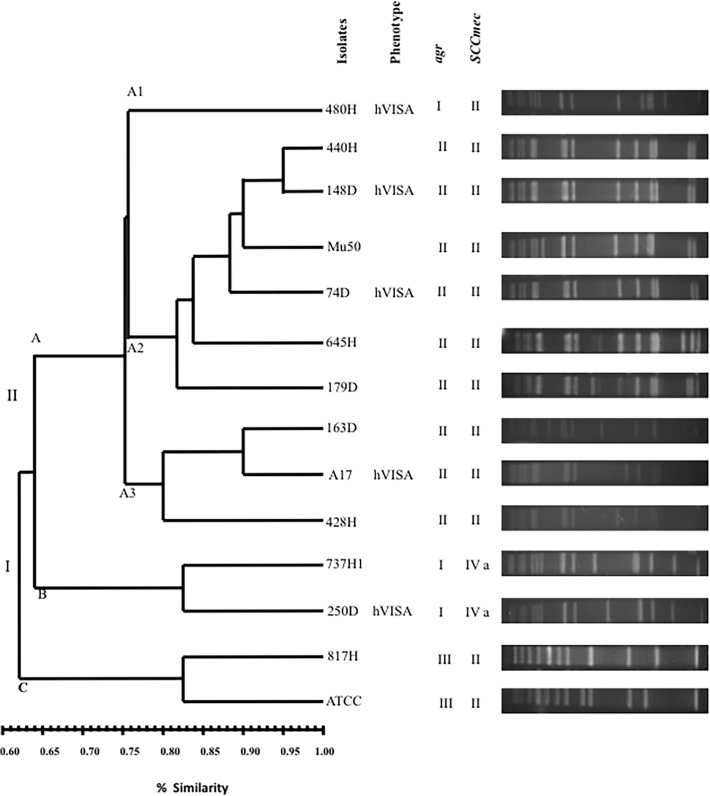
The multiplex-PCR assays revealed the amplification of a 398-bp product corresponding to the mec group II polymorphism in 83.33% (10/12) of the VT-MRSA isolates and an 776-bp product corresponding to the mec group IVa polymorphism in 16.6% (2/12) of the VT-MRSA isolates. In addition, a 575-bp product corresponding to the agr group II polymorphism was amplified in 66.66% (8/12) of the VT-MRSA isolates, a 441-bp product corresponding to the agr group I polymorphism was amplified in 25% (3/12) of the isolates, and a 323-bp product corresponding to the agr III group polymorphism was amplified in 8.33% (1/12) of the isolates. The agr II/SCCmec II polymorphism was observed in 66.66% (8/12) of the MRSA isolates examined. Moreover, the hVISA phenotype identified in the MRSA isolates was associated with three genotypes: agrII/SCCmecII (60%; 3/5), agrI/SCCmecII (20%; 1/5) and agrI/SCCmeIVa (20%; 1/5) (Fig. 2).
Fig 2. Dendrogram analysis of PFGE results showing the genetic relationships among the PFGE profiles and the presence of the agr and SCCmec genes among the 12 VT-MRSA clinical isolates.
Genetic relationship among 12 VT-MRSA clinical isolates
The PFGE analysis revealed two genetic lineages (I and II) with 5 DNA pulsotypes and 62% similarity, showing patterns comprising 10 to 15 DNA fragments ranging in size from 48.5 to 339.5 kb (Fig. 2). In addition, groups A and B were organized into genetic lineage I, and group C was organized into genetic lineage II. However, nine VT-MRSA clinical isolates sharing greater than 75% homology were identified in the three sub-groups A1, A2, and A3 (Fig. 2). In group A, 88.88% (8/9) of the clinical isolates harbored the agr group II polymorphism, and 100% (9/9) of the clinical isolates harbored the SCCmec II polymorphism. Interestingly, four hVISA isolates were also identified in this group, whereas the reference strain Mu50 showed to genes both agrII and SCCmecII. Group B comprised two sub-groups, B1 and B2, each containing a single clinical isolate; both isolates showed genes harboring agr group I and SCCmec group IVa polymorphisms, and one isolate in sub-group B exhibited the hVISA phenotype. Moreover, group C showed a lower genetic correlation with groups A and B (62% similarity); this group comprised a single clinical isolate and the reference strain ATCC 25923.
Determination of cell-wall thickening in VT-MRSA clinical isolates
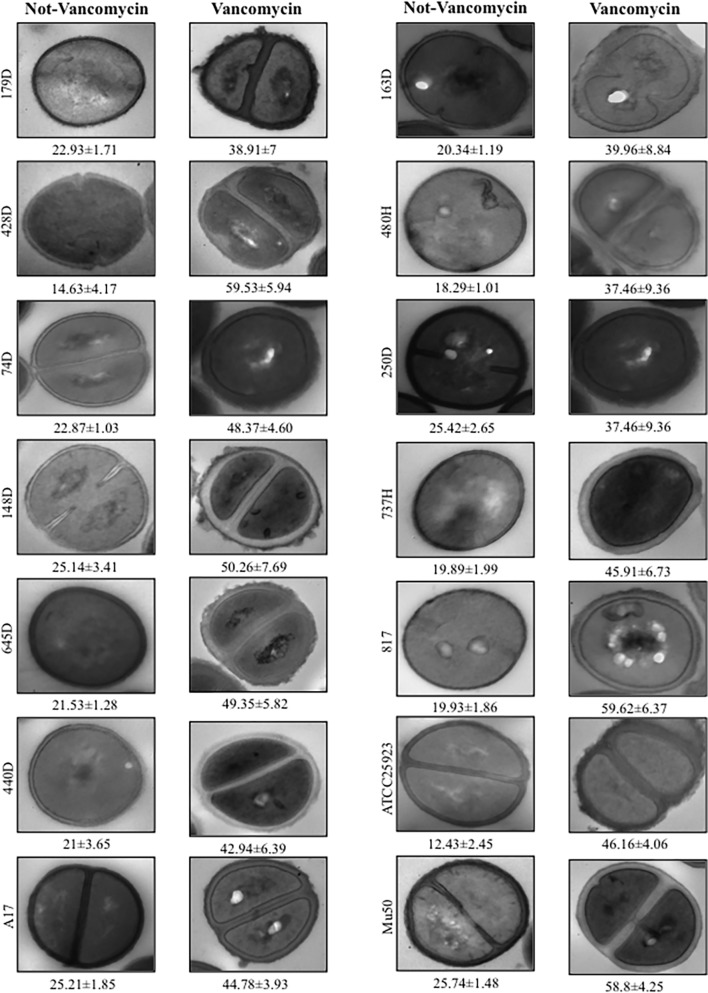
The morphological characteristics of the VT-MRSA clinical isolates are shown in Fig. 3. The 12 VT-MRSA clinical isolates were stimulated with gradual increments of vancomycin, generating cell wall thickening of varying diameters ranging from 37.46 to 59.62 nm (Fig. 3). The unstimulated VT-MRSA isolates showed cell wall diameters ranging from 14.66 to 25.74 nm. Therefore, the average cell wall diameter of the 12 unstimulated (21.46 nm) and stimulated (47.42 nm) VT-MRSA isolates showed a difference of 25.96 nm (Fig. 3 and Table 4). Briefly, the 12 unstimulated VT-MRSA isolates exhibited thinner cell walls than the isolates stimulated with vancomycin.
Fig 3. Comparison of the cell wall thickness in the presence and absence of vancomycin among the VT-MRSA clinical isolates after cultivation in BHI broth.
(A) Untreated VT-MRSA clinical isolates. (B) VT-MRSA clinical isolates treated with vancomycin at concentrations gradually increased by 1 μg until reaching a final concentration of 20 μg/mL. The micrographs of the thin transverse sections were processed to 100,000x magnification. The values shown under each bar represent the means and SDs of the cell wall thickness in nanometers.
Table 4. Comparison of the cell wall thickness among the 12 VT-MRSA clinical isolates in the presence and absence of vancomycin.
| Mean cell wall thickness (nm ± SD a ) | Mean cell wall thickness (nm) | ||||||
|---|---|---|---|---|---|---|---|
| Vancomycin | Vancomycin | ||||||
| Isolates | Untreated | Treated | Increase | Isolates | Untreated | Treated | Increase |
| 179D | 22.93 ± 1.71 | 38.91 ± 7.01 | 15.98 | 163D | 20.34 ± 1.19 | 39.96 ± 8.84 | 19.62 |
| 428H | 14.63 ± 4.17 | 59.53 ± 5.94 | 44.9 | 480H | 18.29 ± 1.01 | 37.46 ± 9.36 | 19.17 |
| 74D | 22.87 ± 1.03 | 48.37 ± 4.60 | 25.5 | 250D | 25.42 ± 2.65 | 52.03 ± 8.92 | 26.61 |
| 148D | 25.54 ± 1.79 | 50.26 ± 7.69 | 24.72 | 737H | 19.89 ± 1.99 | 45.91 ± 6.73 | 26.02 |
| 645H | 21.53 ± 1.28 | 49.35 ± 5.82 | 27.82 | 817H | 19.93 ± 1.86 | 59.62 ± 6.37 | 39.69 |
| 440H | 21 ± 3.65 | 42.94 ± 6.39 | 21.94 | ATCC 25923 | 12.433 ± 2.45 | 46.16 ± 4.065 | 33.73 |
| A17 | 25.21 ± 1.8 | 44.78 ± 3.93 | 19.57 | Mu50 | 25.74 ± 1.48 | 58.87 ± 4.25 | 33.13 |
a The morphometric evaluation of the cell wall thickness was performed using the TEM images obtained at 80,000x.
Discussion
In the present study, 12% of the MRSA clinical isolates were tolerant of vancomycin, with MIC values of 1 and 2 μg/mL; these data are consistent with other reports [6, 37, 38, 39, 40]. Additional studies have shown MRSA strains with vancomycin tolerance rates ranging from 0% to 47% [6, 7, 40, 41, 42, 43]. Our results are consistent with these studies, supporting the relevance of intra-hospital emergent populations such as VT-MRSA clinical isolates associated a nosocomial outbreak. The selection of S. aureus strains that are tolerant of vancomycin likely reflects exposure to suboptimal concentrations of vancomycin in vivo, which could explain the rapid development of hVISA and VISA strains [17, 42]. In the present study, the PAP-AUC analysis revealed 41.66% VT-MRSA strains with the hVISA phenotype. A variable frequency of hVISA strains, ranging from 0 to 50%, has been described [44]. In addition, 29.23% (19/65) of MRSA strains from infective endocarditis patients exhibited the hVISA phenotype [10]. However, among the 100 MRSA strains obtained from Southmead Hospital in the UK, only 7% of them were hVISA strains as detected on gradient plates, and 5% were hVISA strains as detected using the screening method [16]. The hVISA clinical isolates identified in our study are relevant to hospitals due to their ability to acquire vancomycin resistance upon using this antibiotic to treat nosocomial infections.
The agr operon has been identified as a significant factor in the development of reduced vancomycin susceptibility and associated treatment failure [6, 24, 25, 33]. In the present study, 66.66% (8/12) of the VT-MRSA clinical isolates exhibited the agr group II polymorphism, 25% (3/12) exhibited the agr group I polymorphism, and 8.33% (1/12) exhibited the agr group III polymorphism. These results are consistent with those of other studies conducted in the United States and Japan, which have reported a high frequency of the agr group II polymorphism in the majority of S. aureus strains [24, 25]. Our data suggest that VT-MRSA clinical isolates exhibiting the agr group II polymorphism, confer advantages to the bacteria for survival in vancomycin-treated patients in a hospital environment.
The dendrogram analysis using PFGE showed that 88.88% (8/9) of the VT-MRSA isolates exhibiting agr group II polymorphisms and 11.11% (1/9) of the isolates exhibiting agr group I polymorphisms were clustered in group A. Therefore, 66.66% (8/12) of the VT-MRSA clinical isolates displayed a profile of highly related (80%) clustering in group A, whereas 16.66% (2/12) of the VT-MRSA clinical isolates were clustered in group B, with the remaining 8.33% (1/12) clustered in group C. The prevalence of the agr group II polymorphism observed in the present study is consistent with previous studies showing that agr group II isolates are the most prevalent among clinical MRSA strains [45]. Conversely, 100% (9/9) of VT-MRSA clinical isolates displayed a SCCmec group II polymorphism, and consistent with the results of the PFGE analysis, these bacteria were clustered in group A. The presence of both polymorphisms (agr group II and SCCmec group II) was observed in 88.88% (8/9) of the VT-MRSA clinical isolates from the pediatric patients at HIMFG. These data indicate that both polymorphisms identified in the population of VT-MRSA clinical isolates are associated with decreased susceptibility to vancomycin. Interestingly, although 83.33% (10/12) of the VT-MRSA isolates examined in the present study showed the SCCmec group II polymorphism, previous studies have reported a low frequency of this genotype [46]. The high prevalence of the SCCmec group II polymorphism in VT-MRSA isolates found in this study is likely due to their intra-hospital origin. However, other studies have shown SCCmec type IV as the most frequent of the SCCmec types, consistent with reports in other countries showing a frequency of 86.7%. Therefore, SCCmec type IV has been considered one of the most frequent nosocomial SCCmec types observed in several countries [46]. Previous studies have also indicated that the VISA and hVISA clinical isolates from diverse geographic origins with several point mutations show the agr group II polymorphism; however, significant diversity in the agr polymorphism has been observed in hVISA strains [25, 30, 47]. The increased heteroresistance to glycopeptides and the attenuated bactericidal activity at clinically relevant vancomycin concentrations have been associated with isogenic mutations in the agr group II polymorphism [24]. The agr type II polymorphism has been strongly associated with vancomycin treatment failures in MRSA bacteremia, reduced bacterial killing due to reduced autolysis, and decreased agr function in biofilm formation [24, 39].
Cell wall synthesis is a crucial step during the growth and division of bacteria and represents an important target for antibiotics, such as penicillin, vancomycin, and teicoplanin [48]. The use of vancomycin has led to the emergence of hVISA and VISA strains exhibiting cell wall thickness and reduced autolysis [48, 49]. However, the cell wall thickness in vancomycin-tolerant MRSA isolates has not previously been evaluated. The results obtained in the present study suggest that vancomycin induced cell wall thickening in the 12 VT-MRSA isolates examined. The analyzed images of all isolates showed that the average cell wall thickness was 22 nm for untreated VT-MRSA and 46 nm for isolates treated with vancomycin. Furthermore, an average increase in cell wall thickness of 24 nm (105.55%) was observed in the VT-MRSA isolates. A similar increase in cell wall thickness was observed for the vancomycin-sensitive S. aureus strain ATCC 25923 and the VISA strain Mu50. In addition, cell wall thickening has been described in gentamicin- and macrolide-resistant S. aureus clinical strains [50, 51]. The data presented herein strongly indicated that the thickened cell wall in VT-MRSA clinical isolates with agr type II and SCCmec group II polymorphisms is associated with an adaptive resistance to vancomycin, suggesting that this resistance is inducible. Consistently, other studies have shown that S. aureus strains with acquired resistance could be reverted to the original state. The phenotypic profiles obtained from molecular analyses (agr-types, SCCmec-types, susceptibility patterns, and clonal grade by PFGE) and structural studies through TEM (cell wall thickness) on the 12 VT-MRSA isolates did not show a relationship with the clinical data of each pediatric patient.
In conclusion, the results of the present study strongly suggest that cell wall thickness is a major feature of VT-MRSA clinical isolates, conferring resistance at concentrations above the MBC. These findings suggest that the adaptive resistance is inducible and are consistent with other studies suggesting that this adaptive resistance could be reverted to the original state. In addition, the agr type II and SCCmec group II polymorphisms might also positively contribute to vancomycin tolerance through an increase in the high-affinity trapping and cloning of the mesh in the outer layer of peptidoglycan. Combined with preexisting knowledge, these results suggest that these pathogenic attributes mediate cell wall thickening in VT-MRSA clinical isolates.
Acknowledgments
The authors would like to thank Juana Rodríguez Castillo and Ana Karina Espinosa Mazariego for technical assistance. The thin transverse sections were prepared in the Department of Pathology at the Children Hospital of Mexico Federico Gómez and visualized through transmission electron microscopy (JEOL model JEM 10–10) at the National Polytechnic Institute, Mexico.
Data Availability
All data are described in the paper.
Funding Statement
This work was partially supported through a federal grant (HIM/2010/016) from the Children Hospital of Mexico “Federico Gómez” and through additional grants from CONACYT (133451) and the Fondo Sectorial de Investigación en Salud y Seguridad Social, FOSSIS-CONACYT 2012-01-182651. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ahoyo TA, Bankole HS, Adeoti FM, Gbohoun AA, Assavedo S, Amoussou-Guénou M, et al. Prevalence of nosocomial infections and anti-infective therapy in Benin: results of the first nationwide survey in 2012. Antimicrob Resist Infect Control. 2014; 3: 17 10.1186/2047-2994-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aiken AM, Mutuku IM, Sabat AJ, Akkerboom V, Mwangi J, Scott JAG, et al. Carriage of Staphylococcus aureus in Thika Level 5 Hospital, Kenya: a cross-sectional study. Antimicrob Resist Infect Control. 2014; 3: 22 10.1186/2047-2994-3-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appelbaum PC. The emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus . Clin Microbiol Infect. 2006; 12 Suppl 1: 16–23. [DOI] [PubMed] [Google Scholar]
- 4. Tiwari HK, Sen MR. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis. 2006; 6: 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. David MZ, Daum RS. Community-Associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010; 23: 616–687. 10.1128/CMR.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rose WE, Fallon M, Moran JJM, Vanderloo JP. Vancomycin tolerance in methicillin-resistant Staphylococcus aureus: influence of vancomycin, daptomycin, and telavancin on differential resistance gene expression. Antimicrob Agents Chemother. 2012; 56: 4422–4427. 10.1128/AAC.00676-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. May J, Shannon K, King A, French G. Glycopeptide tolerance in Staphylococcus aureus . J Antimicrobi Chemother. 1998; 42: 189–197. [DOI] [PubMed] [Google Scholar]
- 8. Safdar A, Rolston KVI. Vancomycin tolerance, a potential mechanism for refractory gram-positive bacteremia observational study in patients with cancer. Cancer. 2006; 106: 1815–1820. [DOI] [PubMed] [Google Scholar]
- 9. Traczewski MM, Katz BD, Steenbergen JN, Brown SD. Inhibitory and bactericidal activities of daptomycin, vancomycin, and teicoplanin against methicillin-resistant Staphylococcus aureus isolates collected from 1985 to 2007. Antimicrob Agents Chemother. 2009; 53: 1735–1738. 10.1128/AAC.01022-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bae I-G, Federspiel JJ, Miró JM, Woods CW, Park L, Rybak MJ, et al. Heterogeneous vancomycin-intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J Infect Dis. 2009; 200: 1355–1366. 10.1086/606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reipert A, Ehlert K, Kast T, Bierbaum G. Morphological and genetic differences in two isogenic Staphylococcus aureus strains with decreased susceptibilities to vancomycin. Antimicrob Agents Chemother. 2003; 47: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sujatha S, Praharaj I. Glycopeptide resistance in gram-positive cocci: a review. Interdis cip Perspect Infect Dis. 2012: 2012781679. [DOI] [PMC free article] [PubMed]
- 13. Deresinski S. Vancomycin heteroresistance and methicillin-resistant Staphylococcus aureus . J Infect Dis. 2009; 199: 605–609. 10.1086/596630 [DOI] [PubMed] [Google Scholar]
- 14. Tenover FC, Moellering RC. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus . Clin Infect Dis. 2007; 44: 1208–1215. [DOI] [PubMed] [Google Scholar]
- 15. Norazah A, Law NL, Kamel AGM, Salbiah N. The presence of heterogeneous vancomycin-lntermediate Staphylococcus aureus (heteroVISA) in a major Malaysian hospital. Med J Malaysia. 2012; 67: 269–273. [PubMed] [Google Scholar]
- 16. Wootton M, Howe RA, Hillman R, Walsh TR, Bennett PM, MacGowan AP. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J Antimicrob Chemother. 2001; 47: 399–403. [DOI] [PubMed] [Google Scholar]
- 17. Charles PGP, Ward PB, Johnson PDR, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus . Clin Infect Dis. 2004; 38: 448–451. [DOI] [PubMed] [Google Scholar]
- 18. Fong RKC, Low J, Koh TH, Kurup A. Clinical features and treatment outcomes of vancomycin-intermediate Staphylococcus aureus (VISA) and heteroresistant vancomycin-intermediate Staphylococcus aureus (hVISA) in a tertiary care institution in Singapore. Eur J Clin Microbiol Infect Dis. 2009; 28: 983–987. 10.1007/s10096-009-0741-5 [DOI] [PubMed] [Google Scholar]
- 19. Ward PB, Johnson PD, Grabsch EA, Mayall BC, Grayson ML. Treatment failure due to methicillin-resistant Staphylococcus aureus (MRSA) with reduced susceptibility to vancomycin. Med J Aus. 2001; 175: 480–483. [DOI] [PubMed] [Google Scholar]
- 20. Fowler VG, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Stryiewski ME, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004; 190: 1140–1149. [DOI] [PubMed] [Google Scholar]
- 21. Tsuji BT, Rybak MJ, Lau KL, Sakoulas G. Evaluation of accessory gene regulator (agr) group and function in the proclivity towards vancomycin intermediate resistance in Staphylococcus aureus . Antimicrob Agents Chemother. 2007; 51: 1089–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bardiau M, Yamazaki K, Duprez J-N, Taminiau B, Mainil JG, Otei I, et al. Genotypic and phenotypic characterization of methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk of bovine mastitis. Lett Appl Microbiol. 2013; 57: 181–186. 10.1111/lam.12099 [DOI] [PubMed] [Google Scholar]
- 23. Bibalan MH, Shakeri F, Javid N, Ghaemi A, Ghaemi EA. Accessory Gene Regulator Types of Staphylococcus aureus Isolated in Gorgan, North of Iran. J Clin Diagn Res. 2014; 8: DC07–09. 10.7860/JCDR/2014/9713.5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakoulas G, Eliopoulos GM, Moellering RC, Wennersten C, Venkataraman L, Novick RP, et al. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 2002; 46: 1492–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moise-Broder PA, Sakoulas G, Eliopoulos GM, Schentag JJ, Forrest A, Moellering RC. Accessory gene regulator group II polymorphism in methicillin-resistant Staphylococcus aureus is predictive of failure of vancomycin therapy. Clin Infect Dis. 2004; 38: 1700–1705. [DOI] [PubMed] [Google Scholar]
- 26. Hill-Cawthorne GA, Hudson LO, El Ghany MFA, Piepenburg O, Nair M, Dodqson A, et al. Recombinations in staphylococcal cassette chromosome mec elements compromise the molecular detection of methicillin resistance in Staphylococcus aureus . PloS One. 2014; 9: e101419 10.1371/journal.pone.0101419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stojanov M, Blanc DS. Characterization of the staphylococcal cassette chromosome mec insertion site in 108 isolates lacking the mecA gene and identified as methicillin-resistant Staphylococcus aureus by the Xpert MRSA assay. Eur J Clin Microbiol Infect Dis. 2014; 33: 1967–1971. 10.1007/s10096-014-2169-9 [DOI] [PubMed] [Google Scholar]
- 28. Borbón-Esquer EM, Villaseñor-Sierra A, Martínez-López E, Jáuregui-Lomeli JJ, Villaseñor-Martínez R, Ruiz-Briseño Mdel R. SCCmec types and pvl gene in methicillin-resistant Staphylococcus aureus strains from children hospitalized in a tertiary care hospital in Mexico. Scand J Infect Dis. 2014; 46: 523–527. 10.3109/00365548.2014.912349 [DOI] [PubMed] [Google Scholar]
- 29. Zhang K, McClure J-A, Elsayed S, Conly JM. Novel staphylococcal cassette chromosome mec type, tentatively designated type VIII, harboring class A mec and type 4 ccr gene complexes in a Canadian epidemic strain of methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2009; 53: 531–540. 10.1128/AAC.01118-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sakoulas G, Eliopoulos GM, Fowler VG, Moellering RC, Novick RP, Lucindo N, et al. Reduced susceptibility of Staphylococcus aureus to vancomycin and platelet microbicidal protein correlates with defective autolysis and loss of accessory gene regulator (agr) function. Antimicrob Agents Chemother. 2005; 49: 2687–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cui L, Ma X, Sato K, Okuma K, Tenover FC, Mamizuka EM, et al. Cell Wall Thickening Is a Common Feature of Vancomycin Resistance in Staphylococcus aureus . J Clin Microbiol. 2003; 41: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement. M100-S23, January 2013.
- 33. Sakoulas G, Eliopoulos GM, Moellering RC, Novick RP, Venkataraman L, Wennersten C, et al. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance?. J Infect Dis. 2003; 187: 929–938. [DOI] [PubMed] [Google Scholar]
- 34. Zhang K, McClure J-A, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus . J Clin Microbiol. 2005; 43: 5026–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gilot P, Lina G, Cochard T, Poutrel B. Analysis of the genetic variability of genes encoding the RNA III-activating components Agr and TRAP in a population of Staphylococcus aureus strains isolated from cows with mastitis. J Clin Microbiol. 2002; 40: 4060–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover SC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003; 41: 5113–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM, et al. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004; 42: 2398–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soriano A, Marco F, Martínez JA, Pisos E, Almela M, Dimova VP, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008; 46: 193–200. 10.1086/524667 [DOI] [PubMed] [Google Scholar]
- 39. Sakoulas G, Gold HS, Cohen RA, Venkataraman L, Moellering RC, Eliopoulos GM. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J Antimicrob Chemother. 2006; 57: 699–704. [DOI] [PubMed] [Google Scholar]
- 40. Sader HS, Jones RN, Rossi KL, Rybak MJ. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine USA hospitals. J Antimicrob Chemother. 2009; 64: 1024–1028. 10.1093/jac/dkp319 [DOI] [PubMed] [Google Scholar]
- 41. Holmes RL, Jorgensen JH. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob Agents Chemother. 2008; 52: 757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones RN. Key considerations in the treatment of complicated staphylococcal infections. Clin Microbiol Infect. 2008; 14 Suppl 2: 3–9. 10.1111/j.1469-0691.2008.01923.x [DOI] [PubMed] [Google Scholar]
- 43. Biedenbach DJ, Bell JM, Sader HS, Fritsche TR, Jones RN, Turnidge JD. Antimicrobial susceptibility of gram-positive bacterial isolates from the Asia-Pacific region and an in vitro evaluation of the bactericidal activity of daptomycin, vancomycin, and teicoplanin: a SENTRY Program Report (2003–2004). Internat J Antimicrob Agents. 2007; 30: 143–149. [DOI] [PubMed] [Google Scholar]
- 44. Pitz AM, Yu F, Hermsen ED, Rupp ME, Fey PD, Olsen KM. Vancomycin susceptibility trends and prevalence of heterogeneous vancomycin-intermediate Staphylococcus aureus in clinical methicillin-resistant S. aureus isolates. J Clin Microbiol. 2011; 49: 269–274. 10.1128/JCM.00914-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002; 70: 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vindel A, Cuevas O, Cercenado E, Marcos C, Bautista V, Castellares C, et al. Methicillin-Resistant Staphylococcus aureus in Spain: molecular epidemiology and utility of different typing methods. J Clin Microbiol. 2009; 47: 1620–1627. 10.1128/JCM.01579-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rose WE, Rybak MJ, Tsuji BT, Kaatz GW, Sakoulas G. Correlation of vancomycin and daptomycin susceptibility in Staphylococcus aureus in reference to accessory gene regulator (agr) polymorphism and function. J Antimicrob Chemother. 2007; 59: 1190–1193. [DOI] [PubMed] [Google Scholar]
- 48. Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010; 23: 99–139. 10.1128/CMR.00042-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hanaki H, Labischinski H, Inaba Y, Kondo N, Murakami H, Hiramatsu K. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J Antimicrob Chemother. 1998; 42: 315–320. [DOI] [PubMed] [Google Scholar]
- 50. Fukutsuji K, Yamada S, Harada T. Ultrastructural cell wall characteristics of clinical gentamycin-resistant Staphylococcus aureus isolates. Med Mol Morphol. 2013; 46: 70–76. 10.1007/s00795-013-0009-0 [DOI] [PubMed] [Google Scholar]
- 51. Hyo Y, Yamada S, Fukutsuji K, Harada T. Thickening of the cell wall in macrolide-resistant Staphylococcus aureus . Med Mol Morphol. 2013; 46: 217–224. 10.1007/s00795-013-0027-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are described in the paper.