Abstract
Sepsis is defined as a systemic inflammatory response syndrome that disorders the functions of host immune system, including the imbalance between pro- and anti-inflammatory responses mediated by immune macrophages. Sepsis could also induce acute hyperglycemia. Studies have shown that the silent mating type information regulation 2 homolog 1 (SIRT1), an NAD+-dependent deacetylase, mediates NF-κb deacetylation and inhibits its function. Therefore, SIRT1 is likely to play an important role in high glucose-mediated inflammatory signalings. Here we demonstrate that high glucose significantly downregulates both the mRNA and protein levels of SIRT1 and upregulates the mRNA level and the release of two pro-inflammatory cytokines, IL-1β and TNF-α, in RAW264.7 macrophages. Interestingly, the reduced level of SIRT1 by high glucose is remarkably upregulated by SIRT1 activator SRT1720, while the level and the release of IL-1β and TNF-α significantly decrease with the use of SRT1720. However, when the function of SIRT1 is inhibited by EX527 or its expression is suppressed by RNAi, the upregulated level and release of IL-1β and TNF-α by high glucose are further increased. Taken together, these findings collectively suggest that SIRT1 is an important regulator in many high glucose-related inflammatory diseases such as sepsis.
Introduction
Sepsis is defined as a systemic inflammatory response syndrome mediated by a harmful host immune response to infection. Lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, is a common cause of sepsis via various immune cells, including monocytes and macrophages [1,2]. Sepsis is known to induce acute hyperglycemia [3,4], and its progression is usually accompanied with the change of glycemia levels. Especially, the concentration of glucose has shown to accelerate the aggravation of sepsis [3,5].
Macrophages are important cells involved in inflammation, which have been implicated in the initiation of inflammatory response and play critical roles in the pathogenesis of numerous inflammatory diseases by secreting various inflammatory mediators/cytokines. The nuclear factor kappa B (NF-κB), a proinflammatory transcription factor, is a heterodimer composed of p50 and RelA/p65 subunits and is a key mediator of the immune response in macrophages [6]. In unstimulated cells, NF-κB resides in the cytoplasm bound to inhibitory proteins of the inhibitor of κB (IκB) family [7,8]. Stimulation of cells by environmental factors liberates NF-κB, allowing it to translocate to the nucleus, where it further mediates the transcription of targeting genes, including IL-1β and TNF-α.
The mammalian sirtuin members, named SIRT1 to SIRT7, are highly conserved NAD+-dependent deacetylases that have emerged as key factors involved in aging and metabolism, including to adapt gene expression and metabolism to the cellular energy state [9,10]. SIRT1, the leading family member, has been reported to promote longevity in species ranging from yeast to flies [11,12]. SIRT1 is a known suppressor of NF-κB activity, it deacetylates the NF-κB subunit RelA/p65 at lysine310 and thereby inhibits transcription[7,13].
Our study aims at examining the effect of high glucose on the expression levels of SIRT1 and related proinflammatory cytokines in RAW264.7 macrophages, and also to investigate the potential role of SIRT1 in inflammatory response with the use of SIRT1 activator, inhibitor or SIRT1 siRNA.
Materials and Methods
Cell Cultures
The immortal mouse macrophage cell line RAW264.7 was obtained from American Type Culture Collection (Livingstone, MT). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Gaithersburg, MD) containing 10% fetal bovine serum (Gibco), 100 IU/ml penicillin and 100 mg/ml streptomycin (Beyotime, Shanghai, China), and maintained at 37°C in a humidified incubator with 5% CO2. To harvest RAW264.7 cells, they were first trypsinized with 0.25% trypsin/EDTA in phosphate-buffered saline (PBS) and partly collected, then the remaining undetached cells were further collected by scraping. Cells were then centrifuged at 400 g for 5 min, and then resuspended in serum-free DMEM. Cells were seeded in six-well plates (approximately 3.0 × 104 cells/cm2) before further treatments.
Western Blot
Cells were washed twice with precooled PBS and then lysed in RIPA buffer (50 mM Tris-HCl/pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS). The protein concentration was measured using the BCA Protein Assay Kit (Biyotime). 50 μg of total protein extracts were resolved by SDS-PAGE and transfered onto PVDF membrane. The membrane was blocked with 5% non-fat milk and incubated with mouse anti-SIRT1 monoclonal antibody (Catalog# ab110304, clone# 19A7AB4; Abcam, Cambridge, UK) or rabbit anti-β-ACTIN polyclonal antibody (Catalog# CW0097; Cwbiotech, Beijing, China) overnight at 4°C. The next day, the membrane was washed with 1 × TBST (13.7 mM NaCl, 0.27 mM KCl, 2.5 mM Tris, 0.1% Tween-20, pH 7.8) and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG or goat anti-rabbit IgG (Cwbiotech) for 1 h at room temperature. Immunoreactive proteins were detected using Chemiluminescent HRP Substrate (Millipore, Billerica, MA). Bands were quantitated by ImageJ software and the fold expression was indicated as the relative protein level.
Cell Cytotoxicity Assay
Cells were seeded in 96-well dishes at a density of 3.0 × 103 cells/cm2 and treated with high glucose at the concentrations of 5.6, 11.1, 25 and 30 mM, alone or with SRT1720 (1 μM; Catalog# 1001645–58–4; Selleck, Houston, TX) or EX527(10 μM; Catalog# 49843–98–3; Cayman Chemical, Ann Arbor, MI) for 24 h. The stock solution of SRT1720 or EX527 was prepared by dissolving each of them (in powder form) respectively in DMSO yielding a concentration of 100 μM and then stored at -80°C. MTT solution (0.5 mg/ml) was then added to each well and cells were incubated for 4 h at 37°C in a 5% CO2 incubator. Subsequently, the supernatant was removed, the formation of farmazan was solubilized with dimethyl sulfoxide (DMSO) and measured at 540 nm with a Bio-Rad Model 680 Plate Reader (Bio-Rad Laboratories, Hercules, CA).
Enzyme-Linked Immunosorbent Assay (ELISA)
Supernatants from RAW264.7 macrophage cultures were harvested at indicated time points. Mouse TNF-α and mouse IL-1β were detected using ELISA kit (RayBio, GA, USA; NeoBioscience, Shenzhen, China) respectively according to the manufacturer’s instructions. Concentration was calculated by regression analysis of a standard curve.
Real-time PCR
Total RNA was extracted from RAW264.7 cells by using RisoPlus (TaKaRa, Shiga, Japan) according to the manufacturer’s protocol. Obtained RNA was reverse transcribed into cDNA using the PrimeScript RT Master Mix Kit (Perfect Real Time) (TaKaRa). The primer sequences for each gene were listed as following:
SIRT1: Sense, 5'-CAGACCCTCAAGCCATGTTTGATA-3'; Anti-sense, 5'-TTGGATTCCTGCAACCTGCTC-3'. TNF-α: Sense, 5'-CGTCAGCCGATTTGCTATCT-3'; Anti-sense, 5'-CGGACTCCGCAAAGTCTAAG-3'. IL-1β: Sense, 5'-GCCCATCCTCTGTGACTCAT-3'; Anti-sense, 5'-AGGCCACAGGTATTTTGTCG-3'. GAPDH: Sense, 5'-TGTGTCCGTCGTGGATCTGA-3'; Anti-sense, 5'-TTGCTGTTGAAGTCGCAGGAG-3'.
RNA Interference
The siRNA duplexes for SIRT1 or scramble control were purchased from GenePharma (Shanghai, China), sequences were listed as following:
Sirt1-siRNA-2195: Sense, 5'-GGGAUCAAGAGGUUGUUAATT-3'; Anti-sense, 5'-UUAACAACCUCUUGAUCCCTT-3'. Sirt1-siRNA-1003: Sense, 5'-CCGUCUCUGUGUCACAAAUTT-3'; Anti-sense, 5'-AUUUGUGACACAGAGACGGTT-3'. Sirt1-siRNA-576: Sense, 5'-GCGGAUAGGUCCAUAUACUTT-3'; Anti-sense, 5'-AGUAUAUGGACCUAUCCGCTT-3'. Scramble siRNA: Sense, 5'-UUCUCCGAACGUGUCACGUTT-3'; Anti-sense, 5'-ACGUGACACGUUCGGAGAATT-3'.
siRNAs were transfected into RAW264.7 cells with Lipofectamine 2000 Transfection Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The culture medium containing the transfection mixture was removed 6 h later and repleaced with fresh miedium. The transfected cells were continued to be incubated at 37°C in a 5% CO2 incubator for another 42 h and used for further assays.
Statistical Analysis
All experiments were performed at least three times using different batches of cells. Results were presented as mean ± SEM. Statistical analysis of the data was performed using SPSS17.0 software (SPSS, Chicago, IL) by one-way analysis of variance (ANOVA) with LSD’s or S-N-K’s post-hoc analysis. Values of p < 0.05 were considered to be statistically significant.
Results
High Glucose Mitigates SIRT1 Levels in RAW264.7 Cells without Comprising Cell Viability
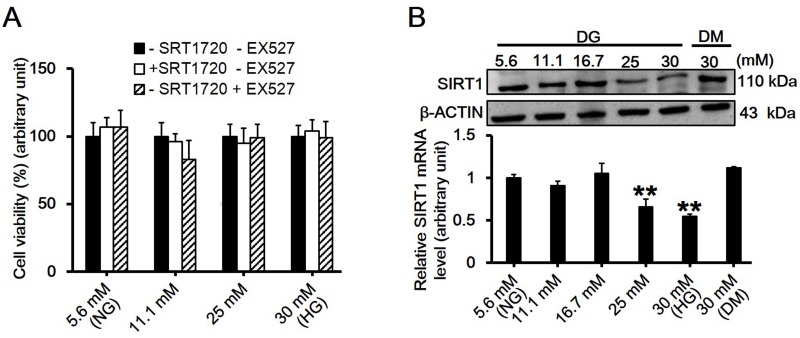
To determine the cytotoxicity of high glucose, alone or with its activator SRT1720 or inhibitor EX527, MTT assay was performed. Results showed that neither high glucose up to 30 mM nor SRT1720 or EX527 reduced the viability of RAW264.7 macrophage cells (Fig. 1A). The protein level of SIRT1 in RAW264.7 cells treated with different concentrations of high glucose was assessed by immunobloting. Results showed that in vitro stimulation of RAW264.7 macrophages submitted to glucose at various concentrations, and 30 mM high glucose exerted the maximum suppressive effect on SIRT1 level (Fig. 1B; df = 5, f = 15.3, p = 0.002).
Fig 1. Assessment of the effects of high glucose on cell viability and SIRT1 expression in RAW264.7 cells.
(A) RAW264.7 cells were treated with D-glucose at gradually increased concentrations of 5.6, 11.1, 25 and 30 mM at the presence of SRT1720 (1 μM, SIRT1 activator) or EX527 (10 μM, SIRT1 inhibitor) for 24h, and the cell viability was evaluated by MTT assay. Each data point is the mean ± SEM of n = 3 with the value in 5.6 mM D-glucose without SRT1720 and EX527 group arbitrarily set as 100%. (B) The expression levels of SIRT1 in RAW264.7 cells treated with different concentrations of D-glucose (DG) at 5.6, 11.1, 16.7, 25 and 30 mM for 24h were evaluated by western blot. 30 mM of D-mannitol (DM) served as an osmotic control. Each data point is the mean ± SEM of n = 3 and normalized against corresponding β-ACTIN protein level with the value in 5.6 mM group arbitrarily set as 1 (df = 5, f = 15.3, p = 0.002). **p < 0.01. ‘NG’, normal glucose, standing for 5.6 mM D-glucose throughout the whole study; ‘HG’, high glucose, standing for 30 mM D-glucose throughout the whole study.
High Glucose Transiently Downregulates Both the mRNA and Protein Levels of SIRT1 in RAW264.7 Cells
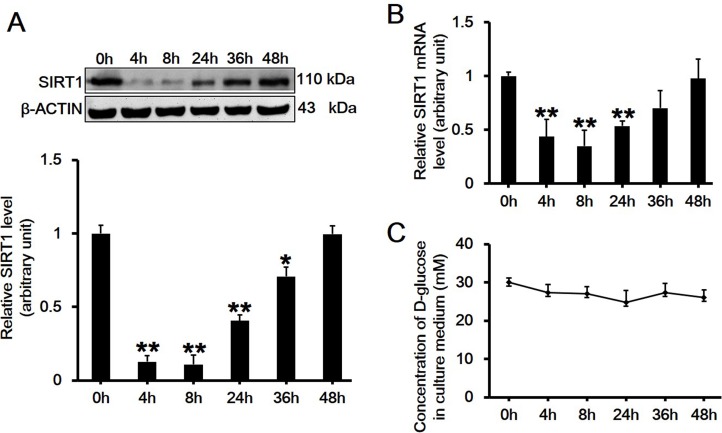
RAW264.7 cells were treated with 30 mM D-glucose for different time periods, then the protein or mRNA level of SIRT1 was assessed by western blot or quantitative real-time PCR, respectively. Results showed that both the protein (Fig. 2A; df = 5, f = 134.651, p < 0.001) and mRNA levels (Fig. 2B; df = 5, f = 127.339, p < 0.001) of SIRT1 showed significant transient downregulation after 30 mM high glucose treatment. The maximum suppressive effect appeared between 4 to 8 h, and then the SIRT1 level gradually increased from 24 h and showed no difference at 48 h post-treatment (Fig. 2A-B). To determine whether the level change of SIRT1 was associated with D-glucose consumption in the culture medium, we then measured the glucose concentration in the supernatant at selected time points. Results showed that the concentration of D-glucose in culture medium did not change over time (Fig. 2C).
Fig 2. Assessment of the protein and mRNA level changes of SIRT1 in RAW264.7 cells treated with 30 mM high glucose over time.
(A-B) Immunoblotting (A) or quantatitive RT-PCR (B) assessing the change in SIRT1 protein or mRNA level after treatment of RAW264.7 cells with 30 mM high glucose at indicated time points (0, 4, 8, 24, 36 and 48h), respectively. Each data point is the mean ± SEM of n = 3 and normalized against corresponding β-ACTIN protein level (A, df = 5, f = 134.651, p < 0.001) or GAPDH mRNA level (B, df = 5, f = 127.339, p < 0.001) with the value at 0h arbitrarily set as 1. *p < 0.05; **p < 0.01. (C) The concentration of D-glucose in the culture medium was measured over time using ONETOUCH Ultra at indicated time points (0, 4, 8, 24, 36 and 48h). Each data point is the mean ± SEM of n = 3.
High Glucose Induces the Upregulation of the mRNA levels of IL-1β and TNF-α in RAW264.7 Cells
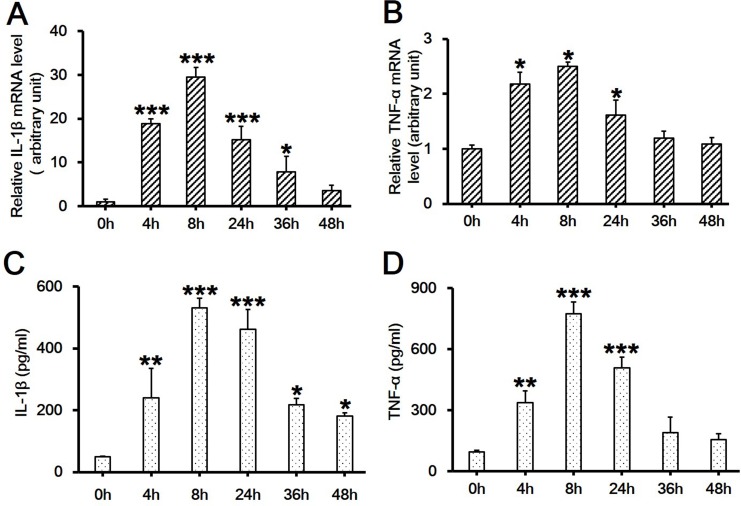
It is known that tissue macrophages release cytokines that further activate the inflammatory program in neighboring adipocytes, exacerbating inflammation and insulin resistance[14]. It is also known that the proinflammatory cytokines, such as IL-1β and TNF-α, play key roles in the pathogenesis of several inflammatory diseases. Therefore, in order to elucidate whether high glucose would cause inflammatory response, we measured the mRNA levels of IL-1β and TNF-α in RAW264.7 cells stimulated with 30 mM D-glucose. Results showed that the mRNA levels of both IL-1β (Fig. 3A; df = 5, f = 62.812, p < 0.001) and TNF-α (Fig. 3B; df = 5, f = 5.492, p = 0.03) were significantly upregulated as early as 4 h post-high glucose treatment and peaked at 8 h. The tendency was maintained up to 48 h post-treatment for IL-1β (Fig. 3A) and 24 h for TNF-α (Fig. 3B). 30 mM D-glucose also induced more release of IL-1β up to 48 h (Fig. 3C; df = 5, f = 27.324, p < 0.001) and TNF-α up to 24 h (Fig. 3D; df = 5, f = 48.74, p < 0.001). The time course of the upregulation of cytokine levels showed great synchronization with the downregulation of SIRT1 levels in Fig. 2A-B.
Fig 3. Assessment of the mRNA level and the release of IL-1β and TNF-α in RAW264.7 cell culture treated with 30 mM high glucose over time.
(A-B) RAW264.7 cells were exposed to 30 mM D-glucose and cultured for indicated time periods of 0, 4, 8, 24, 36 and 48h. The mRNA levels of two inflammatory cytokines, IL-1β (A, df = 5, f = 62.812, p < 0.001) and TNF-α (B, df = 5, f = 5.492, p = 0.03), were assessed by real-time PCR. Each data point is the mean ± SEM of n = 3 and normalized against corresponding GAPDH mRNA level with the value at 0h arbitrarily set as 1. (C-D) The release of IL-1β (C, df = 5, f = 27.324, p < 0.001) and TNF-α (D, df = 5, f = 48.74, p < 0.001) in culture medium was assessed by ELISA. Each data point is the mean ± SEM of n = 3. *p < 0.05; **p < 0.01; ***p < 0.001.
SIRT1 Mediates the Production and Secretion of Two Proinflammatory Cytokines Induced by High Glucose
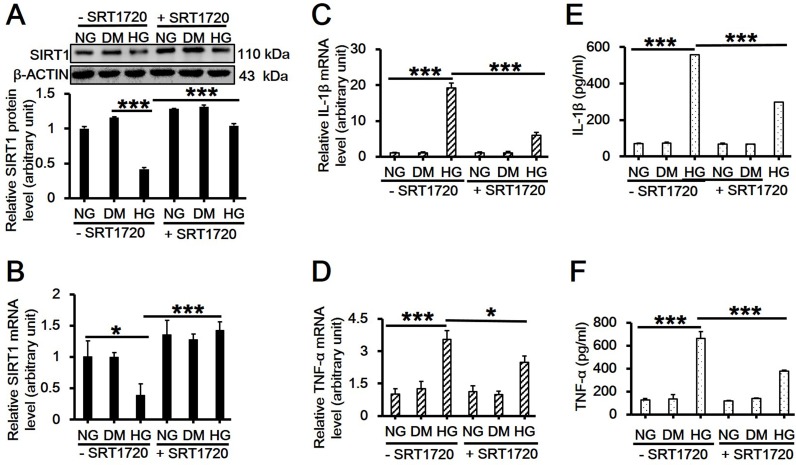
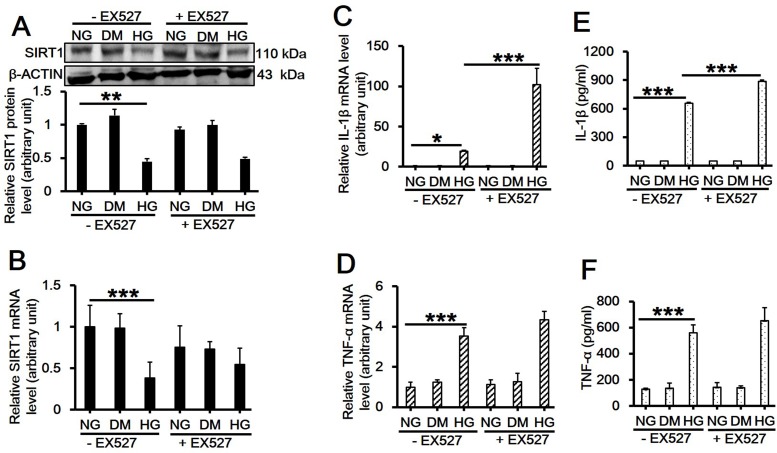
Previous reports have demonstrated that artificial overexpression of SIRT1 in hepatocytes led to the suppression of inflammatory response, whereas deletion of SIRT1 resulted in enhanced local inflammation [15,16]. In order to investigate the role of SIRT1 in inflammatory response induced by high glucose, RAW264.7 cells were pretreated with SRT1720, a putative SIRT1 activator, or EX527, its inhibitor, for 6 h and then received 30 mM D-glucose treatment for 8 h followed by immunoblotting and qPCR. Results showed that the reduced SIRT1 protein (Fig. 4A; df = 5, f = 53.307, p < 0.001; 5A; df = 5, f = 6.194, p = 0.023) or mRNA levels (Fig. 4B; df = 5, f = 16.042, p = 0.002; 5B; df = 5, f = 7.118, p = 0.003) by high glucose was significantly upregulated by SRT1720 (Fig. 4A-B), while had no further change with the use of EX527 (Fig. 5A-B). In the presence of SRT1720, high glucose-induced upregulation on the mRNA level and the release of IL-1β (Fig. 4C; df = 5, f = 328.474, p < 0.001; Fig. 4E; df = 5, f = 5739.982, p < 0.001) and TNF-α (Fig. 4D; df = 5, f = 43.581, p < 0.001; Fig. 4F; df = 5, f = 108.365, p < 0.001) were remarkably inhibited (Fig. 4C-F). Although EX527 did not further suppress the SIRT1 level, it did significantly increase IL-1β mRNA level (Fig. 5C; df = 5, f = 71.26, p < 0.001) and its release (Fig. 5E; df = 5, f = 6057.757, p < 0.001), and slightly upregulated TNF-α level (Fig. 5D; df = 5, f = 50.638, p < 0.001) and its release although showing no significant difference with high glucose treatment alone (Fig. 5F; df = 5, f = 47.79, p < 0.001).
Fig 4. Effects of SIRT1 activator SRT1720 on the expression and/or release of SIRT1, IL-1β and TNF-α in RAW264.7 cell culture treated with 30 mM high glucose.
(A-D) RAW264.7 cells were pretreated with 1 μM SRT1720 for 6h and then cultured in 30 mM D-glucose-containing medium for additional 8h. Cells were then harvested and used for western blot analysis for SIRT1protein level (A, df = 5, f = 53.307, p < 0.001), and real-time PCR analysis for SIRT1 (B, df = 5, f = 16.042, p = 0.002), IL-1β (C, df = 5, f = 328.474, p < 0.001) or TNF-α mRNA level (D, df = 5, f = 43.581, p < 0.001). Each data point is the mean ± SEM of n = 3 and normalized against corresponding β-ACTIN protien level or GAPDH mRNA level with the value in NG without SRT1720 group arbitrarily set as 1. (E-F) The culture medium was also collected and used for ELISA for detecting the release of IL-1β (E, df = 5, f = 5739.982, p < 0.001) and TNF-α (F, df = 5, f = 108.365, p < 0.001). Each data point is the mean ± SEM of n = 3. *p < 0.05; ***p < 0.001. 30 mM of D-mannitol served as an osmotic control. ‘NG’, 5.6 mM normal glucose; ‘HG’, 30 mM high glucose; ‘DM’, 30 mM D-mannitol.
Fig 5. Effects of SIRT1 inhibitor EX527 on the expression and/or release of SIRT1, IL-1β and TNF-α in RAW264.7 cell culture treated with 30 mM high glucose.
(A-D) RAW264.7 cells were pretreated with 10 μM EX527 for 6h and then cultured in 30 mM D-glucose-containing medium for additional 8h. Cells were then harvested and used for western blot analysis for SIRT1 protein level (A, df = 5, f = 6.194, p = 0.023), and real-time PCR analysis for SIRT1 (B, df = 5, f = 7.118, p = 0.003), IL-1β (C, df = 5, f = 71.26, p < 0.001) or TNF-α mRNA level (D, df = 5, f = 50.638, p < 0.001). Each data point is the mean ± SEM of n = 3 and normalized against corresponding β-ACTIN protein level or GAPDH mRNA level with the value in NG without EX527 group arbitrarily set as 1. (E-F) The culture medium was also collected and used for ELISA for detecting the release of IL-1β (E, df = 5, f = 6057.757, p < 0.001) and TNF-α (F, df = 5, f = 47.79, p < 0.001). Each data point is the mean ± SEM of n = 3. *p < 0.05; **p < 0.01; ***p < 0.001. 30 mM of D-mannitol served as an osmotic control. ‘NG’, 5.6 mM normal glucose; ‘HG’, 30 mM high glucose; ‘DM’, 30 mM D-mannitol.
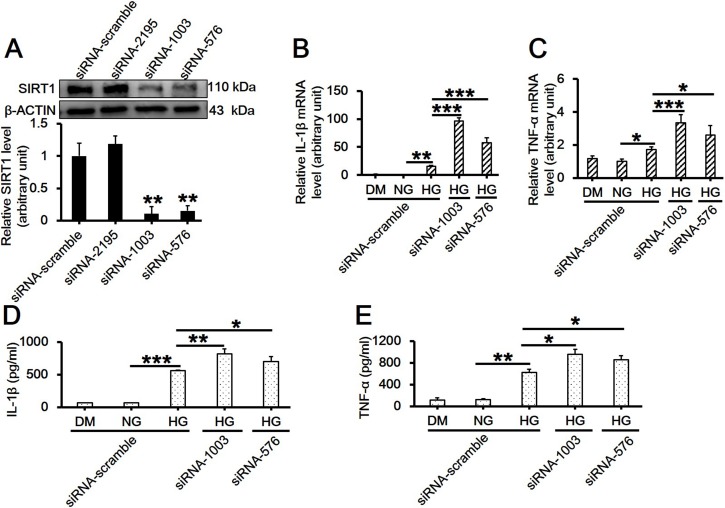
Loss of SIRT1 in Macrophages further Promotes the mRNA Level of Proinflammatory Cytokines Induced by High Glucose
To further confirm the regulative role of SIRT1 in inflammatory response, RAW264.7 cells were subjected to RNAi followed by high glucose exposure. The mRNA levels of IL-1β and TNF-α were measured by real-time PCR, and their release were tested by ELISA. Results showed that both siRNA-1003 and siRNA-576 potently suppressed SIRT1 protein level (Fig. 6A; df = 3, f = 38.663, p = 0.002), and also significantly upregulated the mRNA level and the release of IL-1β (Fig. 6B; df = 4, f = 250.541, p < 0.001; 6D; df = 4, f = 110.68, p < 0.001) and TNF-α (Fig. 6C; df = 4, f = 24.145, p < 0.001; 6E; df = 4, f = 80.041, p < 0.001) in RAW264.7 cells treated with high glucose. This finding was highly consistent with the result using SIRT1 inhibitor EX527 as shown in Fig. 5C-F.
Fig 6. Effects of SIRT1 knockdown by RNAi on the mRNA level and the release of IL-1β and TNF-α in RAW264.7 cell culture treated with 30 mM high glucose.
(A) Cells cultured in NG medium were transfected with 33 nM scramble or SIRT1-specific siRNA duplex (serial number: 2195, 1003, and 576) for 6h, incubated for another 42h, and then harvested for the detection of SIRT1 suppression by western blot (df = 3, f = 38.663, p = 0.002). (B-C) Cells receiving RNAi treatment were continued to be cultured in 30 mM D-glucose-containing medium for additional 8h, mRNA levels of IL-1β (B, df = 4, f = 250.541, p < 0.001) and TNF-α (C, df = 4, f = 24.145, p < 0.001) were assessed by real-time PCR. Each data point is the mean ± SEM of n = 3 and normalized against corresponding β-ACTIN protein level (A) or GAPDH mRNA level (B-C) with the value in NG with scramble siRNA group arbitrarily set as 1. (D-E) The culture medium was also collected and used for ELISA for detecting the release of IL-1β (D, df = 4, f = 110.68, p < 0.001) and TNF-α (E, df = 4, f = 80.041, p < 0.001). Each data point is the mean ± SEM of n = 3. *p < 0.05; **p < 0.01; ***p < 0.001. 30 mM of D-mannitol served as an osmotic control. ‘NG’, 5.6 mM normal glucose; ‘HG’, 30 mM high glucose; ‘DM’, 30 mM D-mannitol.
Discussion
The pathogenesis of various inflammatory diseases such as sepsis is a complex process mediated by immune cells such as macrophages and monocytes [17,18]. Major efforts have been made to understand the proinflammatory process, especially focused on the early stage involving the gene expression of key proinflammatory mediators/cytokines [31]. Studies have shown that pretreatment of RAW264.7 macrophages with SRT1720 could downregulate LPS-induced release of proinflammatory factors, such as NO, PGE2, iNOS and COX-2, and proinflammatory cytokines, including TNF-α and IL-1β [19,20,21]. The progression of inflammatory diseases is usually associated with the irritability of high blood glucose [22]. Our study showed that high glucose significantly increased the production and secretion of IL-1β and TNF-α in RAW264.7 cells (Fig. 3A-D), which was consistent with a previous finding that high glucose increased the levels of TNF-α and IL-6 in the podocytes and thus induced subsequent inflammation and kidney fibrosis [23]. However, the signaling cascades mediating the effects of high glucose on the production of proinflammatory factors have not been clearly characterized.
It is well established that SIRT1 is a regulator of longevity and is associated with caloric restriction, energy metabolism, cell cycle and differentiation, however, its role in endotoxic or septic lethality has yet to be fully understood. There is accumulating evidence that SIRT1 could mediate the inhibition of resveratrol-induced release of proinflammatory cytokines [24,25,26]. A recent study has indicated that SIRT1 acted as a positive modulator in insulin signalings through PI3K in muscle cells [27,28]. Therefore, we hypothesized that SIRT1 might play a role in mediating high glucose-induced inflammatory response. In the present study, we first observed that high glucose significantly downregulated SIRT1 expression on both mRNA and protein levels in RAW264.7 cells in a dose- and time-dependent manner (Figs. 1B and 2A-B). Also, the concentration of D-glucose in culture medium did not change over time (Fig. 2C), suggesting the independence of SIRT1 reduction with extracellular glucose concentration. Next, we pretreated cells with SIRT1 activator SRT1720 or its inhibitor EX527 followed by high glucose exposure. Interestingly, SRT1720 abolished the downregulation of SIRT1 level induced by high glucose, and also suppressed the increased mRNA level and release of TNF-α and IL-1β (Fig. 4). On the contrary, EX527 exerted an inhibitory effect on SIRT1 activity without affecting SIRT1 expression on both mRNA and protein levels, which was consistent with the findings from Charles E. McCall’s lab [29,30], while it further upregulated the increased mRNA level and release of IL-1β induced by high glucose (Fig. 5). Importantly, results from the knockdown of SIRT1 by RNAi further confirmed above conclusion (Fig. 6). Our results showed a more than 120-fold increase on IL-1β mRNA level in SIRT1 RNAi cells under high glucose over that in scramble siRNA-transfected cells under normal glucose condition, this was consistent with a study showing that LPS increased IL-1β mRNA level more than 200 folds in SIRT1-knockdowned cells compared to that in untreated and scramble siRNA-transfected cells[31]. Therefore, SIRT1 appears to be a negative regulator for IL-1β at the early stage of inflammation. Taken together, our findings suggested a possible mechanism regulating the high glucose-induced inflammatory response in RAW264.7 cells, that is, high glucose inhibits SIRT1 expression, which subsequently stimulates the production of IL-1β and TNF-α and thus promotes inflammation process. However, the precise mechanism by which high glucose exerts inhibitory effects on SIRT1 expression is largely unknown. It is possible that high glucose may trigger a common signaling cascade that ultimately disturbs SIRT1 level [32,33].
While a number of studies have demonstrated that SIRT1 exhibits pronounced anti-inflammatory properties [34,35,36], the present study again provides evidences supporting its central role in the inhibition of high glucose-induced inflammatory response in RAW264.7 cells. To this end, our study attempts to reveal, at least partly, the molecular mechanism of high glucose-induced macrophage activation, which is desirable in delineating the molecular therapeutic target to reduce inflammatory response in various inflammation-related diseases such as sepsis. Nonetheless, it is noteworthy that our findings were mainly based on an in vitro high glucose model, which cannot fully simulate the in vivo condition in sepsis. Moreover, the precise relationship between the high glucose-induced inflammation and SIRT1 expression has not been extensively investigated. Therefore, further studies are needed to thoroughly understand the mechanism on high glucose-induced inflammatory response in sepsis.
Acknowledgments
We thank Chaowu Tang (Department of Burns and Cutaneous Surgery, Xijing Hospital) for his kind technique support in this study.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Natural Science Foundation of China (NSFC) to Dahai Hu (81372069), Xiongxiang Zhu (81272084) and Maolong Dong (81372055). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hotchkiss RS, Opal S. Immunotherapy for sepsis—a new approach against an ancient foe. N Engl J Med. 2010; 363: 87–89. 10.1056/NEJMcibr1004371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoo CH, Yeom JH, Heo JJ, Song EK, Lee SI, Han MK. Interferon beta protects against lethal endotoxic and septic shock through SIRT1 upregulation. Sci Rep. 2014; 4: 4220 10.1038/srep04220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu KP, Li Y, Ljubimov AV, Yu FS. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009; 58: 1077–1085. 10.2337/db08-0997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013; 8: e54514 10.1371/journal.pone.0054514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luan R, Liu S, Yin T, Lau WB, Wang Q, Guo W, et al. High glucose sensitizes adult cardiomyocytes to ischaemia/reperfusion injury through nitrative thioredoxin inactivation. Cardiovasc Res. 2009; 83: 294–302. 10.1093/cvr/cvp085 [DOI] [PubMed] [Google Scholar]
- 6. Dong J, Jimi E, Zeiss C, Hayden MS, Ghosh S. Constitutively active NF-kappaB triggers systemic TNFalpha-dependent inflammation and localized TNFalpha-independent inflammatory disease. Genes Dev. 2010; 24: 1709–1717. 10.1101/gad.1958410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004; 23: 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013; 25: 1939–1948. 10.1016/j.cellsig.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 9. Rai E, Sharma S, Kaul S, Jain K, Matharoo K, Bhanwer AS, et al. The interactive effect of SIRT1 promoter region polymorphism on type 2 diabetes susceptibility in the North Indian population. PLoS One. 2012; 7: e48621 10.1371/journal.pone.0048621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takeda-Watanabe A, Kitada M, Kanasaki K, Koya D. SIRT1 inactivation induces inflammation through the dysregulation of autophagy in human THP-1 cells. Biochem Biophys Res Commun. 2012; 427: 191–196. 10.1016/j.bbrc.2012.09.042 [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Zhao X, Shi D, Chen P, Yu Y, Yang L, et al. Overexpression of SIRT1 promotes high glucose-attenuated corneal epithelial wound healing via p53 regulation of the IGFBP3/IGF-1R/AKT pathway. Invest Ophthalmol Vis Sci. 2013; 54: 3806–3814. 10.1167/iovs.13-12091 [DOI] [PubMed] [Google Scholar]
- 12. Winnik S, Stein S, Matter CM. SIRT1—an anti-inflammatory pathway at the crossroads between metabolic disease and atherosclerosis. Curr Vasc Pharmacol. 2012; 10: 693–696. [DOI] [PubMed] [Google Scholar]
- 13. Yang H, Zhang W, Pan H, Feldser HG, Lainez E, Miller C, et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-kappaB activity. PLoS One. 2012; 7: e46364 10.1371/journal.pone.0046364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010; 285: 19051–19059. 10.1074/jbc.M110.123620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008; 177: 861–870. 10.1164/rccm.200708-1269OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009; 9: 327–338. 10.1016/j.cmet.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jungo F, Dayer JM, Modoux C, Hyka N, Burger D. IFN-beta inhibits the ability of T lymphocytes to induce TNF-alpha and IL-1beta production in monocytes upon direct cell-cell contact. Cytokine. 2001; 14: 272–282. [DOI] [PubMed] [Google Scholar]
- 18. Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, et al. Bacterial lipopolysaccharide activates nuclear factor-kappaB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999; 274: 7611–7614. [DOI] [PubMed] [Google Scholar]
- 19. Chung EY, Kim BH, Lee MK, Yun YP, Lee SH, Min KR, et al. Anti-inflammatory effect of the oligomeric stilbene alpha-Viniferin and its mode of the action through inhibition of cyclooxygenase-2 and inducible nitric oxide synthase. Planta Med. 2003; 69: 710–714. [DOI] [PubMed] [Google Scholar]
- 20. De Simone R, Andres RM, Aquino M, Bruno I, Guerrero MD, Terencio MC, et al. Toward the discovery of new agents able to inhibit the expression of microsomal prostaglandin E synthase-1 enzyme as promising tools in drug development. Chem Biol Drug Des. 2010; 76: 17–24. 10.1111/j.1747-0285.2010.00984.x [DOI] [PubMed] [Google Scholar]
- 21. Murakami A, Matsumoto K, Koshimizu K, Ohigashi H. Effects of selected food factors with chemopreventive properties on combined lipopolysaccharide- and interferon-gamma-induced IkappaB degradation in RAW264.7 macrophages. Cancer Lett. 2003; 195: 17–25. [DOI] [PubMed] [Google Scholar]
- 22. Peng C, Ma J, Gao X, Tian P, Li W, Zhang L. High glucose induced oxidative stress and apoptosis in cardiac microvascular endothelial cells are regulated by FoxO3a. PLoS One. 2013; 8: e79739 10.1371/journal.pone.0079739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma J, Chadban SJ, Zhao CY, Chen X, Kwan T, Panchapakesan U, et al. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS One. 2014; 9: e97985 10.1371/journal.pone.0097985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yi CO, Jeon BT, Shin HJ, Jeong EA, Chang KC, Lee JE, et al. Resveratrol activates AMPK and suppresses LPS-induced NF-kappaB-dependent COX-2 activation in RAW 264.7 macrophage cells. Anat Cell Biol. 2011; 44: 194–203. 10.5115/acb.2011.44.3.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centeno-Baez C, Dallaire P, Marette A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am J Physiol Endocrinol Metab. 2011; 301: E922–930. 10.1152/ajpendo.00530.2010 [DOI] [PubMed] [Google Scholar]
- 26. Shen Z, Ajmo JM, Rogers CQ, Liang X, Le L, Murr MM, et al. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-alpha production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol. 2009; 296: G1047–1053. 10.1152/ajpgi.00016.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vachharajani VT, Liu T, Brown CM, Wang X, Buechler NL, Wells JD, et al. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J Leukoc Biol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ichikawa T, Hayashi R, Suzuki K, Imanishi S, Kambara K, Okazawa S, et al. Sirtuin 1 activator SRT1720 suppresses inflammation in an ovalbumin-induced mouse model of asthma. Respirology. 2013; 18: 332–339. 10.1111/j.1440-1843.2012.02284.x [DOI] [PubMed] [Google Scholar]
- 29. Vachharajani VT, Liu T, Brown CM, Wang X, Buechler NL, Wells JD, et al. SIRT1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J Leukoc Biol. 2014; 96: 785–796. 10.1189/jlb.3MA0114-034RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu TF, Yoza BK, El Gazzar M, Vachharajani VT, McCall CE. NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance. J Biol Chem. 2011; 286: 9856–9864. 10.1074/jbc.M110.196790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang H, Lee SM, Gao B, Zhang J, Fang D. Histone deacetylase sirtuin 1 deacetylates IRF1 protein and programs dendritic cells to control Th17 protein differentiation during autoimmune inflammation. J Biol Chem. 2013; 288: 37256–37266. 10.1074/jbc.M113.527531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, Bae EJ, et al. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab. 2010; 298: E419–428. 10.1152/ajpendo.00417.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, et al. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009; 29: 1363–1374. 10.1128/MCB.00705-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005; 434: 113–118. [DOI] [PubMed] [Google Scholar]
- 35. Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem. 2012; 287: 25758–25769. 10.1074/jbc.M112.362343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen J. The immunopathogenesis of sepsis. Nature. 2002; 420: 885–891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.