Abstract
Carmustine wafers (CW; Gliadel® wafers) are approved to treat newly-diagnosed high-grade glioma (HGG) and recurrent glioblastoma. Widespread use has been limited for several reasons, including concern that their use may preclude enrollment in subsequent clinical trials due to uncertainty about confounding of results and potential toxicities. This meta-analysis estimated survival following treatment with CW for HGG. A literature search identified relevant studies. Overall survival (OS), median survival, and adverse events (AEs) were summarized. Analysis of variance evaluated effects of treatment (CW vs non-CW) and diagnosis (new vs recurrent) on median survival. The analysis included 62 publications, which reported data for 60 studies (CW: n = 3,162; non-CW: n = 1,736). For newly-diagnosed HGG, 1-year OS was 67 % with CW and 48 % without; 2-year OS was 26 and 15 %, respectively; median survival was 16.4 ± 21.6 months and 13.1 ± 29.9 months, respectively. For recurrent HGG, 1-year OS was 37 % with CW and 34 % without; 2-year OS was 15 and 12 %, respectively; median survival was 9.7 ± 20.9 months and 8.6 ± 22.6 months, respectively. Effects of treatment (longer median survival with CW than without; P = 0.043) and diagnosis (longer median survival for newly-diagnosed HGG than recurrent; P < 0.001) on median survival were significant, with no significant treatment-by-diagnosis interaction (P = 0.620). The most common AE associated with wafer removal was surgical site infection (SSI); the most common AEs for repeat surgery were mass effect, SSI, hydrocephalus, cysts in resection cavity, acute hematoma, wound healing complications, and brain necrosis. These data may be useful in the context of utilizing CW in HGG management, and in designing future clinical trials to allow CW-treated patients to participate in experimental protocols.
Keywords: BCNU, Carmustine, Gliadel, Glioblastoma, Glioma, High-grade, Meta-analysis, Wafer
Introduction
High-grade gliomas (HGG; WHO grade 3 or 4) account for the majority of newly-diagnosed malignant brain tumors, with glioblastoma multiforme (GBM) representing the most common subtype [1]. These highly infiltrative and aggressive tumors generally have a poor prognosis, as they are difficult to treat and recurrence is common [2]. Treatment for HGG generally includes surgical resection followed by radiotherapy and chemotherapy [2]. In particular, the addition of the alkylating agent temozolomide (TMZ) to post-surgical radiotherapy and as adjuvant therapy has become standard treatment for many patients with HGG [2, 3]. Factors associated with prolonged survival include complete resection (≥98 % of tumor volume) [4], younger age [5], better performance status [5], MGMT promoter status [6, 7], oligodendroglial phenotype [5], p53 mutation [8], and IDH1 mutation [9].
Carmustine wafer (CW) implant (Gliadel® Wafer, Arbor Pharmaceuticals, LLC, Atlanta, GA) is approved for treatment of newly-diagnosed HGG as an adjunct to surgery and radiation and for treatment of recurrent GBM as an adjunct to surgery [10]. Local chemotherapy with CW was shown to significantly increase survival compared with placebo in newly-diagnosed HGG [11, 12] and in recurrent GBM [13]. Risks associated with CW include cerebral edema, healing abnormalities, intracranial infections, seizures, intracranial hypertension, and cerebrospinal fluid leaks [14].
Treatment guidelines recommend CW as appropriate for some patients (e.g., patients in whom near total resection is feasible [Category 2B recommendation] [2] or in whom craniotomy is indicated [Level II recommendation] [15]); however, questions remain as to its optimal use. For example, randomized controlled trials (RCT) comparing CW and TMZ as single treatments have not been conducted, and while several reports on the use of TMZ following CW implantation have been published (see review by Dixit et al. [16]), there remain concerns about the safety of this approach [2]. Currently, many clinical trials of new chemotherapies exclude patients treated with CW [2, 17] because of concerns about potential toxicities, confounding of results (e.g., due to wafer-induced imaging changes), and a paucity of reliable survival statistics. More reliable data regarding expected survival times with CW might be helpful in the context of designing future clinical trials, so that new protocols might accommodate the use of CW as part of a comprehensive approach utilizing multiple treatment modalities maximizing benefit to patients.
This meta-analysis was designed to estimate survival times for patients treated with CW for newly-diagnosed or recurrent HGG, using data from published studies.
Methods
Search strategy and study selection
A literature search was conducted in January, 2014 using Medline (includes PubMed), Embase, and BIOSIS, with the following search criteria: gliadel OR [(“BCNU” OR carmustine) AND (polymer OR polymers OR wafer* OR polifeprosan OR interstitial)] AND (glioma OR glioblastoma); no restrictions on publication date were used. The abstract of each publication was screened to determine relevance. Much of the published evidence on CW is derived from retrospective studies of heterogeneous populations and varying treatment regimens, which generally precludes inclusion of these publications in meta-analyses. However, in an effort to utilize as much of the available data as possible and increase the generalizability of our results, we chose to exclude only preclinical or phase 1 studies, individual case reports, or small case series (n < 10); also excluded were review articles, editorials, and studies of carmustine administered in a formulation other than wafers. Each remaining publication was reviewed to determine if overall survival or selected safety/toxicity outcomes (seizures, wound healing complications, infection, or mass effect) were reported for patients treated with CW. Relevant congress abstracts published in 2009 or later were identified (via the Northern Light database in addition to sources listed above) and screened using the same process and criteria described above. Abstracts of studies with full results published were also excluded.
Data collection
Data were extracted and reviewed. The following were collected: (1) characteristics of study participants, including age, sex, diagnosis (new or recurrent), tumor grade (grade 3 or 4 vs grade 4 only); (2) study treatment (specific treatment regimens, general categories of CW alone, CW + other treatment(s), no CW, radiotherapy use); (3) survival outcomes (1-, 2-, and 3-year survival rate; median survival time); (4) safety outcomes (adverse events [AEs], deaths due to AEs, wafer removal, repeat surgery).
Statistical analyses
Overall survival (OS) rates at 1, 2, and 3 years, and median survival time were summarized by tumor grade (grade 3 or 4 vs grade 4 only), by new or recurrent diagnosis, and by use of CW with or without TMZ. A factorial analysis of variance was performed to evaluate the effects of treatment (CW vs no CW), diagnosis (new vs recurrent), and use of TMZ (among CW-treated patients) on median survival. All statistical analyses including tests of hypotheses and P-values are based on weighted statistics, where the weights were derived from the total safety or efficacy sample size. Statistical significance for all weighted statistical tests were set at P ≤ 0.05. No Bonferroni correction for multiple inferences was applied. All analyses were performed using SAS (version 9.2). The incidence of safety outcomes in patients treated with CW was summarized.
Results
Search results
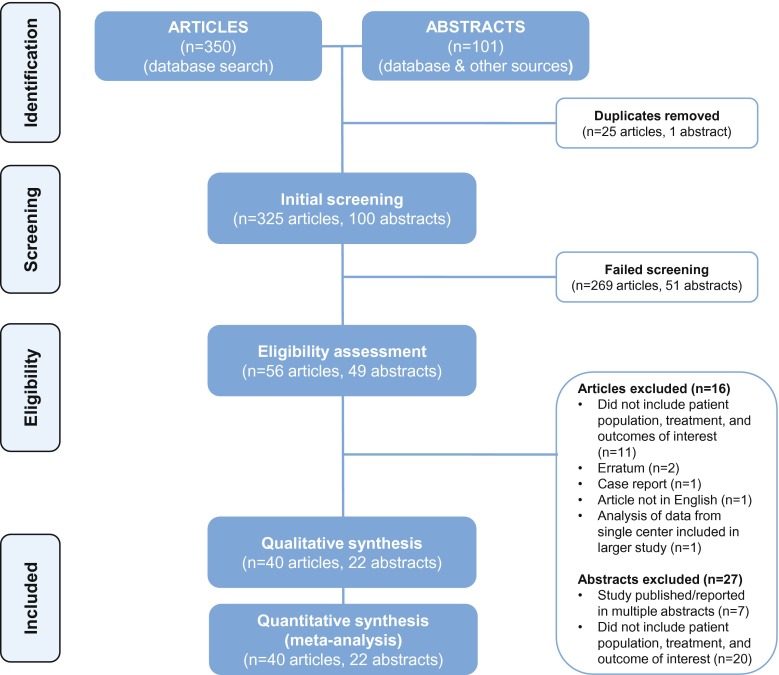
The initial PubMed search retrieved 350 possible references. Twenty-five duplicate articles were excluded. Based on initial screening of the article abstracts, 269 were excluded (100 were reviews or editorials; 83 did not evaluate CW; 56 were preclinical or phase 1 studies; 22 were individual case reports or small case series; 3 did not evaluate patients with HGG; 3 were pediatric studies; and 2 were secondary or subgroup analyses of published studies). The full text of the remaining 56 references were obtained and reviewed. Sixteen of these references were excluded from the analyses, leaving 40 published reports that were included in the survival or safety analyses. The search of the abstract database identified 22 abstracts for analysis (Fig. 1). Thus, a total of 62 publications were included (Table 1) [6, 11–13, 18–75].
Fig. 1.
Flow diagram
Table 1.
Characteristics of published studies included in analysis
| Study | Design | Diagnosis: new/or recurrent | Grade 3, 4, or both | CW treatment regimen | Other treatment | CW no. of patients | 1-year OS (%) | 2-year OS (%) |
|---|---|---|---|---|---|---|---|---|
| Affronti et al. [18] | RCS, cohort, SC | New | 4 | Surgery + CW + RT + TMZ + multiagent rotational chemo | Surgery + RT + TMZ + multiagent rotational chemo | 36 | 81 | 47 |
| Anderson and Thomson (abstr) [19] | RCS, SC | Both | Both | Surgery + CW | n/a | 11 | Not reported | Not reported |
| Aoki et al. [20] | Phase 1/2 | Both | Both | Surgery + CW + RT + TMZ ± INF-B | n/a | New diagnosis, grade 3 or 4: 16; new diagnosis: grade 4 only: 9; recurrent, grade 3 or 4: 8; recurrent, grade 4 only: 4 | New diagnosis, grade 3 or 4: 100; new diagnosis, grade 4 only: not reported; recurrent, grade 3 or 4: 62.5; recurrent, grade 4 only: 50 | New diagnosis, grade 3 or 4: 68.8; new diagnosis, grade 4 only: 44.4; recurrent, grade 3 or 4: 25; recurrent, grade 4 only: not reported |
| Attenello et al. [21] | RCS, cohort, SC | Both | Both | Surgery + CW | Surgery | Primary resection: 166; revision resection: 122; grade 3: 250; grade 4: 38 | Primary, grade 3: 78; primary, grade 4: 57; revision, grade 3: 68; revision, grade 4: 47 | Primary, grade 3: 66; primary, grade 4: 20; revision, grade 3: 47; revision, grade 4: 13 |
| Barr and Grundy [22] | RCS, SC | Both | Both | Surgery + CW ± RT ± TMZ | n/a | Primary resection: 59; revision resection: 5 | Primary: 61; revision: not reported | Primary: 20; revision: not reported |
| Bock et al. [23] | RCS, MC | New | 4 | Surgery + CW + RT + TMZ | n/a | 44 | 58 | 13 |
| Brem et al. [24] | Phase 1/2 | Recurrent | Both | Surgery + CW | n/a | 21 | 38 | n/a |
| Brem et al. [13] | RCT, MC | Recurrent | Both | Surgery + CW ± chemo | Surgery + Placebo ± chemo | 110 | 24 | 11 |
| Catalán-Uribarrena et al. [25] | Prospective cohort | New | Both | Surgery + CW + RT | Surgery + RT ± TMZ | 55 | 52 | 11 |
| Chaichana et al. [26] | RCCS | New | 4 | Surgery + CW ± RT ± TMZ | Surgery ± RT ± TMZ | 45 | 33 | 9 |
| Damilakis 2011 (abstr) [27] | RCS, SC | New | 4 | Surgery + CW + RT | n/a | 22 | Not reported | Not reported |
| Darakchiev et al. [28] | Phase 1/2 | Recurrent | 4 | Surgery + CW + I-125 (± RT ± chemo) | n/a | 34 | 66 | 23 |
| De Bonis et al. [29] | RCS, SC | Both | 4 | Surgery + CW ± RT ± TMZ | Surgery ± RT ± TMZ | New: 19 Recurrent: 28 | Not reported | Not reported |
| Della Puppa et al. [30] | RCS, SC | Both | Both | Surgery + CW + chemo ± RT | n/a | 36 | Not reported | Not reported |
| Della Puppa et al. [31] | RCS, SC | Both | Both | Surgery + CW + chemo ± RT | n/a | 55 | Not reported | Not reported |
| De’Santi et al. [32] | Case series, SC | Both | 4 | Surgery + 5-ALA + CW + RT + TMZ | n/a | 10 | Not reported | Not reported |
| Desjardins et al. (abstr) [33] | Phase 2 | New | 4 | Surgery + CW + RT + TMZ + Bev | n/a | 33 | Not reported | Not reported |
| Dörner et al. [34] | RCS, SC | Both | Both | Surgery + CW | n/a | 88 | Not reported | Not reported |
| Duntze et al. [35] | Prospective cohort | New | Both | Surgery + CW + RT + TMZ | n/a | 92 | 70.3 | 37 |
| Giese et al. [36] | Phase 3 RCT (subgroup analysis) | New | 4 | Surgery + CW + RT | Surgery + placebo + RT | 11 | Not reported | Not reported |
| Gutenberg et al. [37] | RCS | Both | 4 | Surgery + CW ± RT ± TMZ | n/a | New diagnosis, without TMZ: 13; new diagnosis, with TMZ: 17; recurrent, unmethylated: 11; recurrent, methylated: 6 | Without TMZ: 78; with TMZ: 100; recurrent: Not reported | Without TMZ: 0; with TMZ: 38; recurrent: not reported |
| Ho et al. (abstr) [38] | RCS, SC | Not specified | Not stated | Surgery + CW ± RT ± TMZ | Surgery ± RT ± TMZ | 26 | Not reported | Not reported |
| Hoffmann (abstr) [39] | RCS, SC | Both | Both | Surgery + CW ± RT ± TMZ | Not specified | 34 | Not reported | Not reported |
| Kleinberg 2004 [40] | RCS, SC | New | Both | Surgery + CW +RT | n/a | 39 | Not reported | Not reported |
| Ko et al. [41] | RCS | Recurrent | 4 | Surgery + CW ± I-125 | n/a | CW +I-125: 17; CW alone: 7 | CW + I-125: 41; CW alone: 16 | CW + I-125: 22; CW alone: 0 |
| Krex et al. (abstr) [42] | RCS, SC | Recurrent | 4 | Surgery + CW + RT + TMZ | n/a | 60 | Not reported | Not reported |
| Kunwar et al. [43] | Phase 3 RCT | Recurrent | 4 | Surgery + CW | Surgery + CB | 93 | 38 | 13 |
| Lechapt-Zalcman et al. [44] | Prospective observational MC | New | 4 | Surgery + CW + RT + TMZ | n/a | 111 | 71.7 | 34.2 |
| Lemcke et al. [45] | RCS | Not specified | Grade 4 | Surgery + CW | Surgery alone | 30 | Not reported | Not reported |
| Lopez et al. (abstr) [46] | RCS, SC | Recurrent | 4 | Surgery + CW ± TMZ | n/a | 14 | 64.3 | 50 |
| McGirt et al. [47] | RCS, cohort, SC | New | 4 | Surgery + CW + RT ± TMZ | Surgery + RT + TMZ | With TMZ: 30; no TMZ: 78 | With TMZ: 92; no TMZ: 57 | With TMZ: 39; no TMZ: 18 |
| McGovern et al. [48] | RCS, SC | Recurrent | Both | Surgery + CW | n/a | 33 | Not reported | Not reported |
| Menei et al. [49] | RCS, MC | Both | Both | Surgery + CW ± RT ± TMZ/chemo | n/a | New: 83; recurrent: 80 | Not reported | Not reported |
| Metellus et al. [6] | Prospective cohort | Recurrent | 4 | Surgery + CW | n/a | 22 | 36.4 | Not reported |
| Metellus et al. (abstr) [50] | Prospective cohort | New | 4 | Surgery + CW + RT | n/a | 29 | 51 | Not reported |
| Miglierini et al. [51] | RCS, SC | New | 4 | Surgery + CW + RT + TMZ | n/a | 24 | 78 | 24 |
| Noël et al. [52] | RCS, SC | New | Both | Surgery + CW + RT + TMZ | Surgery + RT + TMZ | Grade 3 or 4: 28; grade 4 only: 20 | Grade 3 or 4: 78.6; grade 4 only: 75 | Grade 3 or 4: 40.9; grade 4 only: 38.9 |
| Pan et al. [53] | RCS, SC | New | 4 | Surgery + CW + RT + TMZ | n/a | 21 | 75 | 39 |
| Perez Gomez et al. [54] | RCS | New | Both | Surgery + CW + RT + TMZ | n/a | 49 | 60.5 | 21.3 |
| Qadri et al. (abstr) [55] | RCS, SC | Recurrent | Both | Surgery + CW | n/a | Grade 3 o4 4: 20; grade 4 only: 15 | Grade 3 or 4: not stated; grade 4 only: 41 | Not reported |
| Qadri et al. (abstr) [56] | RCS, SC | Recurrent | 4 | Surgery + CW | n/a | 14 | Not reported | Not reported |
| Quinn 2009 [57] | Phase 2, OL, SC | Recurrent | 4 | Surgery + CW + O-6-BG | n/a | 52 | 47 | 10 |
| Quiros [58] | Retrospective cohort | New | Both | Surgery + CW + RT + TMZ | Surgery + RT + TMZ | 35 | Not reported | Not reported |
| Ranjan et al. [59] | Phase 2 | New | 4 | Surgery + CW + RT + TMZ + Bev | n/a | 41 | Not reported | Not reported |
| Rezazadeh et al. [abstr] [60] | Phase 2 | New | 4 | Surgery + CW + RT + TMZ + Bev | n/a | 10 | Not reported | Not reported |
| Ryken (abstr) [61] | Prospective cohort | New | 4 | Surgery + CW + RT + TMZ | n/a | 21 | Not reported | Not reported |
| Salmaggi et al. [62] | Phase 2 | New | 4 | Surgery + CW + RT + TMZ | n/a | 35 | 85 | 30 |
| Salvati et al. [63] | RCS, SC | New | 4 | Surgery + CW + RT + TMZ | n/a | 32 | 100 | Not reported |
| Samis Zella et al. [64] | RCS | Recurrent | 4 | Surgery + CW ± TMZ ± other chemo | Surgery ± TMZ ± other chemo | 63 | Not reported | Not reported |
| Satilmis et al. (abstr) [65] | RCS, SC | Recurrent | 4 | Surgery + CW | n/a | 71 | Not reported | Not reported |
| Shah et al. [66] | RCS | Both | Both | Surgery + CW ± RT ± TMZ | n/a | 177 patients (181 surgeries) | Not reported | Not reported |
| Silvani et al. (abstr) [67] | Phase 2 | New | 4 | Surgery + CW + RT + TMZ | n/a | 35 | Not reported | Not reported |
| Smith et al. [68] | Phase 1/2 prospective, SC | New | 4 | Surgery + CW + GKS + RT | n/a | 27 | 51 | 22 |
| Subach et al. [69] | RCS matched cohort, SC | Recurrent | 4 | Surgery + CW | Surgery | 17 | 0 | Not reported |
| Sumrall et al. (abstr) [70] | Phase 1/2 | New | Both | Surgery + CW + RT + TMZ | n/a | Grade 4 only: 43 | 74 | Not reported |
| Uff et al. (abstr) [71] | RCS, SC | Recurrent | Not stated | Surgery + CW | n/a | 30 | 37 | Not reported |
| Ulmer et al. [72] | RCS | Both | 4 | Surgery + CW ± RT ± TMZ | n/a | 44 | 32 | 5 |
| Valtonen et al. [11] | Phase 3 RCT | New | Both | Surgery + CW + RT | Surgery + placebo + RT | Grade 3 or 4: 16; grade 4 only: 11 | Grade 3 or 4: 64; grade 4 only: 55 | Grade 3 or 4: 32; grade 4 only: 19 |
| Watts et al. [73] | Prospective single-arm | New | 4 | Surgery + 5-ALA + CW + RT + TMZ | n/a | 59 | Not reported | Not reported |
| Westphal et al. [12] | Phase 3 RCT | New | Both | Surgery + CW + RT | Surgery + Placebo + RT | Grade 3 or 4: 120; grade 4 only: 101 | Grade 3 or 4: 59.2; grade 4 only: 58 | See Westphal 2006 |
| Westphal et al. [74] | Long-term follow-up | New | Both | Surgery + CW + RT | Surgery + placebo + RT | Grade 3 or 4: 120; Grade 4 only: 101 | See Westphal 2003 | Grade 3 or 4: 15.8; grade 4 only: 10 |
| Zhu et al. (abstr) [75] | RCS, SC | New | 4 | Surgery + CW | n/a | 57 | Not reported | 29.63 |
| Study | 3-year OS (%) | Median survival (months) | Other treatment (N) | 1-year OS (%) | 2-year OS (%) | 3-year OS (%) | Median survival (months) |
|---|---|---|---|---|---|---|---|
| Affronti et al. [18] | 21 | 22.35 | 49 | 69 | 29 | 20 | 18.175 |
| Anderson and Thomson (abstr) [19] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Aoki et al. [20] | Not reported | New diagnosis, grade 3 or 4: not calculable; new diagnosis, grade 4 only: 20.2; recurrent, grade 3 or 4: 12; recurrent, grade 4 only: 8.6 | n/a | n/a | n/a | n/a | n/a |
| Attenello et al. [21] | Primary, grade 3: 58; primary, grade 4: 20; revision, grade 3: 29; revision, grade 4: 8 | Primary, grade 3: 57; primary: grade 4: 13.5; revision, grade 3: 23.6; revision, grade 4: 11.3 | n/a | n/a | n/a | n/a | n/a |
| Barr and Grundy [22] | Primary: 10; revision: not reported | Primary: 15.3; revision: 7.5 | n/a | n/a | n/a | n/a | n/a |
| Bock et al. [23] | 13 | 12.7 | n/a | n/a | n/a | n/a | n/a |
| Brem et al. [24] | n/a | 11.5 | n/a | n/a | n/a | n/a | n/a |
| Brem et al. [13] | 9 | 7.75 | 112 | 20 | 11 | 8 | 5.75 |
| Catalán-Uribarrena et al. [25] | 11 | 13.4 | 55 | 43 | 18 | 11 | 11.0 |
| Chaichana et al. [26] | 1 | 8.7 | 45 | 9 | 0 | 0 | 5.5 |
| Damilakis 2011 (abstr) [27] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Darakchiev et al. [28] | 20 | 17.25 | n/a | n/a | n/a | n/a | n/a |
| De Bonis et al. [29] | Not reported | New: 14; recurrent: 6 | New: 58 Recurrent: 60 | Not reported | Not reported | Not reported | New: 11; recurrent:9 |
| Della Puppa et al. [30] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Della Puppa et al. [31] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| De’Santi et al. [32] | Not reported | 21 | n/a | n/a | n/a | n/a | n/a |
| Desjardins et al. (abstr) [33] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Dörner et al. [34] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Duntze et al. [35] | Not reported | 18.8 | n/a | n/a | n/a | n/a | n/a |
| Giese et al. [36] | Not reported | 14.7 | 13 | Not reported | Not reported | Not reported | 9.5 |
| Gutenberg et al. [37] | Not reported | New diagnosis, without TMZ: 14.7; new diagnosis, with TMZ: 18.9; recurrent, unmethylated: 11.2; recurrent, methylated: 10 | n/a | n/a | n/a | n/a | n/a |
| Ho et al. (abstr) [38] | Not reported | Not reported | 42 | Not reported | Not reported | Not reported | Not reported |
| Hoffmann (abstr) [39] | Not reported | 19.5 | n/a | n/a | n/a | n/a | n/a |
| Kleinberg 2004 [40] | Not reported | 12.8 | n/a | n/a | n/a | n/a | n/a |
| Ko et al. [41] | Not reported | CW + I-125: 11.67; CW alone: 7; CW ± I-125: 11.2 | n/a | n/a | n/a | n/a | n/a |
| Krex et al. (abstr) [42] | Not reported | 8.9 | n/a | n/a | n/a | n/a | n/a |
| Kunwar et al. [43] | Not reported | 8.8 | 183 | 38 | 13 | Not reported | 9.1 |
| Lechapt-Zalcman et al. [44] | 18.1 | 17.5 | n/a | n/a | n/a | n/a | n/a |
| Lemcke et al. [45] | Not reported | 12.8 | 58 | Not reported | Not reported | Not reported | 11.4 |
| Lopez et al. (abstr) [46] | Not reported | 26 | n/a | n/a | n/a | n/a | n/a |
| McGirt et al. [47] | With TMZ: 32; no TMZ: 12 | With TMZ: 21.3; no TMZ: 12.4 | 45 | Not reported | Not reported | Not reported | 14.7 |
| McGovern et al. [48] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Menei et al. [49] | Not reported | New: 17; recurrent: 7 | n/a | n/a | n/a | n/a | n/a |
| Metellus et al. [6] | Not reported | 9.9 | n/a | n/a | n/a | n/a | n/a |
| Metellus et al. (abstr) [50] | Not reported | 12.6 | n/a | n/a | n/a | n/a | n/a |
| Miglierini et al. [51] | 15 | 19.2 | n/a | n/a | n/a | n/a | n/a |
| Noël et al. [52] | Not reported | Grade 3 or 4: 20.6; grade 4 only: 20.8 | Grade 3 or 4: 37; grade 4 only: 16 | Grade 3 or 4: 78.4; grade 4 only: 62.5 | Grade 3 or 4: 33.3; grade 4 only: 0 | Not reported | Grade 3 or 4: 20.8; grade 4 only: 13.8 |
| Pan et al. [53] | 24 | 17 | n/a | n/a | n/a | n/a | n/a |
| Perez Gomez et al. [54] | 13.3 | 15 | n/a | n/a | n/a | n/a | n/a |
| Qadri et al. (abstr) [55] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Qadri et al. (abstr) [56] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Quinn 2009 [57] | 5 | 12.575 | n/a | n/a | n/a | n/a | n/a |
| Quiros [58] | Not reported | 20 | 35 | Not reported | Not reported | Not reported | 20 |
| Ranjan et al. [59] | Not reported | 16.1 | n/a | n/a | n/a | n/a | n/a |
| Rezazadeh et al. [abstr] [60] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Ryken (abstr) [61] | Not reported | 18.2 | n/a | n/a | n/a | n/a | n/a |
| Salmaggi et al. [62] | Not reported | 17.8 | n/a | n/a | n/a | n/a | n/a |
| Salvati et al. [63] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Samis Zella et al. [64] | Not reported | Not reported | 32 | Not reported | Not reported | Not reported | Not reported |
| Satilmis et al. (abstr) [65] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Shah et al. [66] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Silvani et al. (abstr) [67] | Not reported | 23 | n/a | n/a | n/a | n/a | n/a |
| Smith et al. [68] | 9 | 12.5 | n/a | n/a | n/a | n/a | n/a |
| Subach et al. [69] | Not reported | 3.5 | 45 | 50 | Not reported | Not reported | 13.5 |
| Sumrall et al. (abstr) [70] | 12 | 19.35 | n/a | n/a | n/a | n/a | n/a |
| Uff et al. (abstr) [71] | Not reported | 15.25 | n/a | n/a | n/a | n/a | n/a |
| Ulmer et al. [72] | Not reported | 10.4 (mean) | n/a | n/a | n/a | n/a | n/a |
| Valtonen et al. [11] | Grade 3 or 4: 27; grade 4 only: 19 | Grade 3 or 4: 14.52; grade 4 only: 13.33 | Grade 3 or 4: 16; grade 4 only: 16 | Grade 3 or 4: 20; grade 4 only: 20 | Grade 3 or 4: 7; grade 4 only: 7 | Grade 3 or 4: 7; grade 4 only: 7 | Grade 3 or 4: 9.98; grade 4 only: 9.98 |
| Watts et al. [73] | Not reported | Not reported | n/a | n/a | n/a | n/a | n/a |
| Westphal et al. [12] | See Westphal 2006 | Grade 3 or 4: 13.8; grade 4 only: 13.1 | Grade 3 or 4: 120; grade 4 only: 106 | Grade 3 or 4: 49.6; grade 4 only: 48 | See Westphal 2006 | See Westphal 2006 | Grade 3 or 4: 11.6; grade 4 only: 11.4 |
| Westphal et al. [74] | Grade 3 or 4: 9.2; grade 4 only: 1 | See Westphal 2003 | Grade 3 or 4: 120; grade 4 only: 106 | See Westphal 2003 | Grade 3 or 4: 8.3; grade 4 only: 5 | Grade 3 or 4: 1.7; Grade 4 only: 0 | See Westphal 2003 |
| Zhu et al. (abstr) [75] | Not reported | 19.38 (mean) | n/a | n/a | n/a | n/a | n/a |
AA anaplastic astrocytoma, AO anaplastic oligodendroglioma, CW carmustine wafer, GBM glioblastoma multiforme, GKS Gamma Knife surgery, HGG high-grade glioma, MC multicenter, O 6 -BG O6-benzylguanine, OL open label, RCCS retrospective case control study, RCS retrospective case series, RCT randomized controlled trial, RT radiotherapy, SC single center, TMZ temozolomide
Publication and population characteristics
The 62 publications in this analysis reported data for 60 separate studies; one study (Westphal 2003 [12]) was reported in 3 publications: the primary study report and 2 follow-up analyses [36, 74]. Thus, in all, 60 different study populations were included. In all analyses, sample sizes were calculated for each variable. The total number of patients treated with CW in 60 studies was 3,162 (mean ± SD sample size = 53 ± 47, range 10–288). The total number of patients treated without CW in 17 studies was 1,736 (mean ± SD sample size = 102 ± 167, range 10–725). A total of 3,071 patients treated with CW and 1,663 patients without CW were evaluated for safety; efficacy populations were 2,637 and 1,685, respectively (all analyses were based on the number of patients with data for a specific outcome). The mean ± SD age of patients in the 25 studies reporting mean age was 55 ± 37 years (range from 33 studies reporting range = 17–83 years).
Thirty-eight studies were retrospective studies, seven studies were prospective observational studies, and fifteen were phase 1/2 through 3 clinical trials and/or randomized controlled trials.
Twenty-eight studies included only newly-diagnosed patients, 16 studies included only recurrent patients, and 14 studies included both newly-diagnosed and recurrent patients, while 2 studies [38, 45]) did not specify. Thirty-three studies included patients with grade 4 tumors only; 25 studies included patients with grade 3 or grade 4 HGG; tumor grade was not stated for 2 studies.
Study treatments (for patients treated with CW) were listed as: Surgery + CW-only in 19 studies, Surgery + CW + other treatment(s) in 28 studies, both Surgery + CW-only, and Surgery + CW + other treatments in 10 studies, and not stated in 2 studies. Radiotherapy was used with CW in 38 (63 %) studies, chemotherapy with TMZ was used with CW in 32 (53 %) studies, and other chemotherapy was used in 9 (15 %) studies.
Efficacy
Overall survival
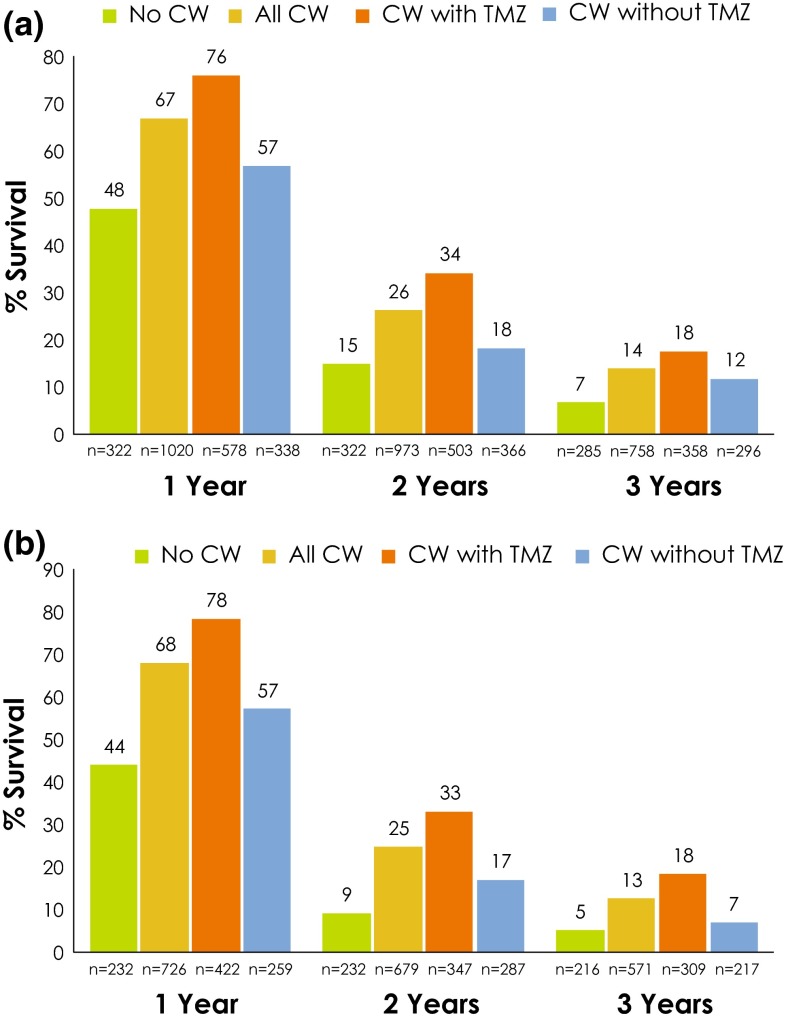
OS was summarized separately for patients with newly-diagnosed HGG and for those with recurrent HGG. Among patients with newly-diagnosed HGG, OS at 1, 2, and 3 years was numerically greater for patients who received treatment with CW compared with those who did not; among those treated with CW, OS was numerically higher for patients who also received TMZ compared with those who did not (Fig. 2a). The same general pattern was observed when data from only patients with grade 4 tumors were analyzed (Fig. 2b).
Fig. 2.
a, b Overall survival rates in patients with newly-diagnosed HGG a grade 3 or 4 and b grade 4 only. CW carmustine wafer, HGG high-grade glioma, TMZ temozolomide. If the sum of the n values for CW + TMZ and CW without TMZ subgroups does not equal the n for the All CW subgroup, this is due to the fact that a few studies in the meta-analysis included some patients who received TMZ and some who did not, but results were reported for the entire study group (i.e., not reported separately based on use of TMZ); data from these studies were included in the analysis for All CW, but were excluded from the with/without TMZ analyses
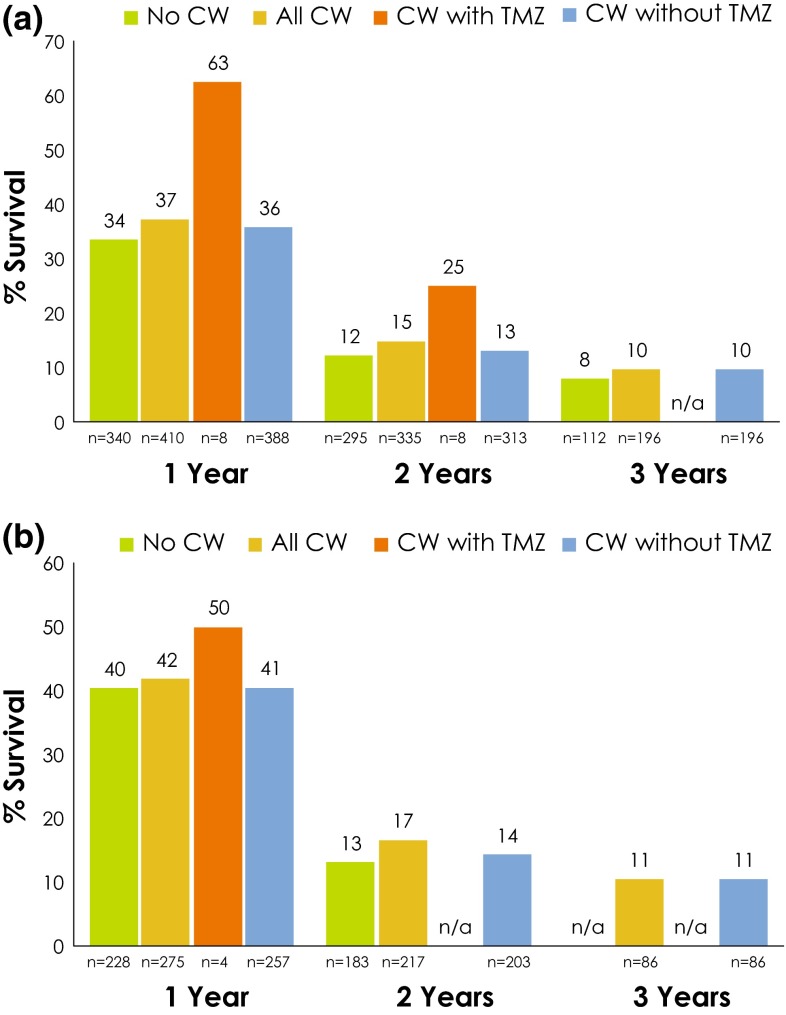
Survival among patients with recurrence was based on time from diagnosis of surgery for recurrence. Among patients with recurrent HGG, OS at 1, 2, and 3 years was numerically greater for patients treated with CW compared with those who were not; among those treated with CW, OS was numerically higher for patients who also received TMZ compared with those who did not (Fig. 3a). Results were similar in the analysis of data from only patients with grade 4 tumors (Fig. 3b). In both cases, results for patients treated with CW + TMZ should be interpreted with caution, as they are based on a very limited sample of patients.
Fig. 3.
a, b Overall survival rates in patients with recurrent HGG a grade 3 or 4 and b grade 4 only. CW carmustine wafer, n/a not available, HGG high-grade glioma, TMZ temozolomide. Limited sample size for CW + TMZ. Survival based on time after diagnosis of, or surgery for, recurrent disease. If the sum of the n values for CW + TMZ and CW without TMZ subgroups does not equal the n for the All CW subgroup, this is due to the fact that a few studies in the meta-analysis included some patients who received TMZ and some who did not, but results were reported for the entire study group (i.e., not reported separately based on use of TMZ); data from these studies were included in the analysis for All CW, but were excluded from the with/without TMZ analyses
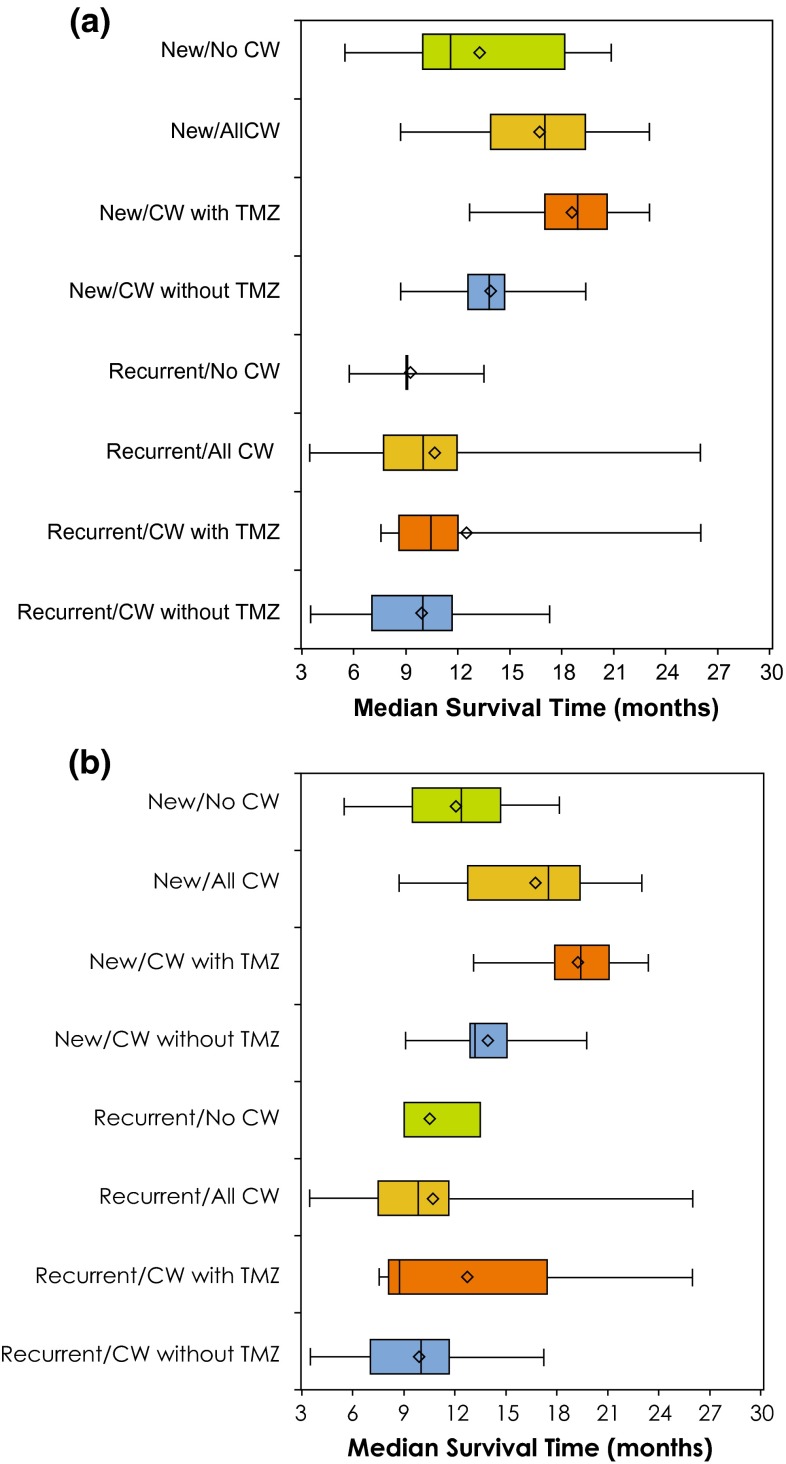
Median survival
Median survival for patients with newly-diagnosed and recurrent HGG is shown in Fig. 4a (grade 3 or 4) and Fig. 4b (grade 4 only). Analysis of median survival data showed a significant effect of treatment (median survival was longer with CW than without; P = 0.043) and diagnosis (median survival was longer for newly diagnosed HGG than recurrent HGG; P < 0.001), with no treatment-by-diagnosis interaction (P = 0.620); the effect of TMZ was also significant (P < 0.001).
Fig. 4.
a, b Median survival among patients with newly-diagnosed or recurrent HGG a grade 3 or 4 and b grade 4 only; box plots with average (diamond), 25th, 50th and 75th percentiles (lines of the box) and range (minimum and maximum hash marks) are also provided for the median survival times (months). Significant effects of treatment (CW vs no CW; P = 0.043) and diagnosis (new diagnosis vs recurrence; P < 0.001) were detected, with no treatment-by-diagnosis interaction (P = 0.620); the effect of TMZ was also significant (P < 0.001). CW carmustine wafer, HGG high-grade glioma, TMZ temozolomide. Limited sample size for Recurrent/CW + TMZ. Survival for recurrent diagnosis based on time after diagnosis of, or surgery for, recurrent disease
Safety
There were 28 deaths (28/3,071; 0.91 %) reported as adverse events (AEs) among patients receiving CW, and 34 deaths (34/1,663; 2.0 %) among patients not receiving CW. The single large RCT of only recurrent diagnosed patients of CW vs cintredekin besudotox [43] had all 34 deaths in patients who did not receive CW (34/177 = 19.2 %), and 13 deaths among CW patients (13/92 = 14.1 %, P > 0.05). The remaining 15 deaths were reported in 11 studies; most (n = 10) were among newly-diagnosed patients. Not all studies indicated specific AEs resulting in death; among the specific AEs that were cited (for 16 patients treated with CW), pulmonary embolism (n = 3) and stroke (n = 2) were the most common (all others were 1 patient each).
CW removal was performed on 12 patients (12/3,071; 0.39 %) in 5 studies, where 5 patients were recurrent diagnosis patients. In 8 of the 12 patients, the AE term associated with wafer removal was infection at the surgical site.
Repeat surgeries were performed in 83 patients treated with CW (83/3,071; 2.7 %) in 13 studies. The most common AE terms associated with repeat surgeries were surgical site infection (n = 11), hydrocephalus (n = 9), hematoma (n = 8), cysts in resection cavity (n = 7), and wound healing complications (n = 6).
Discussion
In this meta-analysis of data from patients with newly-diagnosed HGG treated with CW (±other adjuvant treatments), median survival time was 16 months, with 1- and 2-year OS of 67 and 26 %, respectively. Among patients from the same studies who were treated with other modalities, median survival time was 13 months, with 1-year OS of 48 % and 2-year OS of 15 %.
As expected, OS rates were lower (1-year: 37 %; 2-year: 15 % and median survival (approximately 10 months) was shorter among patients treated with CW with recurrent disease relative to those with newly-diagnosed disease. The median survival times reported here are slightly longer than those reported in the prescribing information for CW (13.8 and 7.4 months for new and recurrent glioma, respectively) [10], which are based on 2 phase 3 RCTs [12, 13]. This difference may be due in part to the inclusion of TMZ and other adjuvant treatments or advances in surgical resection techniques, among other factors.
The majority studies included in this analysis enrolled patients with grade 4 gliomas; several studies also included patients with grade 3 gliomas, although outcomes in those studies were not always reported separately by tumor grade. In our analysis, survival outcomes among the subset of patients treated with CW with grade 4 HGG were generally similar to those among all patients (i.e., patients with grade 3 or 4 HGG).
In this analysis, survival was generally improved among patients treated with CW who also received TMZ than among those who received CW without TMZ. This is not unexpected, considering the complementary mechanisms of action of CW and TMZ, as has previously been reviewed [76]. However, the sample sizes for these subgroups were limited. In addition, only a few of the studies that evaluated CW with TMZ accounted for MGMT promoter status [37, 44, 52, 62, 70]. In light of evidence that MGMT promoter status may be a significant predictor of survival in patients treated with CW or TMZ [6, 7], this further limits the ability to draw definitive conclusions from these data with regard to potential treatment-related differences in survival benefit. An extensive exploration of molecular mechanisms and optimization of HGG treatment in an era of personalized medicine is beyond the scope of this discussion. However, we believe that studies will need to be conducted further evaluating the role of CW for use as part of a multi-modal approach with the current “standard of care” of newly-diagnosed GBM, i.e., radiotherapy plus TMZ, followed by monthly adjuvant TMZ, as well as with the many new emerging targeted therapies, including drugs such as bevacizumab. Also in light of the newly-presented data this past year regarding the upfront glioblastoma multiforme bevacizumab studies (RTOG 0825 [77], AVAglio [78]), the utility of CW in HGG may need to be revisited: given the heterogeneity of HGG at a tumor biology level, it may seem prudent to treat these tumors in a multimodality approach fashion and utilize CW with TMZ and radiotherapy in the appropriate patients in the upfront setting.
Among patients treated with CW, the incidence of death reported as an AE was 0.9, <1 % of patients required wafer removal, and approximately 3 % required repeat surgery. The AEs/complications associated with these events were generally consistent with the known safety profile of CW (e.g., surgical site infection, hydrocephalus, wound healing abnormalities, etc.) [10, 14]. Good surgical practice should include proper and careful technique toward ensuring a water-tight dural closure, thus lowering the risk of known AEs.
Two meta-analyses and several reviews have been published that summarized efficacy and safety data from studies of CW [16, 17, 79–83]. In the most recent Cochrane review of CW, Hart and colleagues reported significantly increased survival with CW relative to placebo in primary disease (HR 0.65, 95 % CI 0.48–0.86, P = 0.003), and a non-significant difference in recurrence (HR 0.83, 95 % CI 0.62–1.10, P = 0.2). Consistent with the aims of that meta-analysis, estimates of survival times were not calculated and data were largely limited to those from RCTs. A recent meta-analysis based on 19 studies that included newly-diagnosed patients with glioblastoma who were treated with CW found a median survival time of 16.2 months [84]. This is consistent with our finding of 16.4 months for the same type of patients. A number of systematic reviews have been published that included data from multiple studies of various designs [16, 17, 79–82]; however, again, estimates of survival times were not calculated, as these reviews summarized individual study data without further analysis. The lack of similarly designed analyses in the literature therefore limits our ability to compare our results with many published reviews.
In contrast to previous reports, with the current meta-analysis we sought to better characterize outcomes with CW using data from as many studies as possible, to aid clinicians in making treatment recommendations and to assist researchers in developing more inclusive clinical trial designs by providing the most comprehensive and reliable survival-related dataset. As such, the inclusion criteria we used in selecting studies were less restrictive than those of more traditional meta-analyses. Thus, the heterogeneity of the included studies in terms of study design, patient characteristics, and study treatments (variability was present not only between studies but within individual studies), must be noted as a limitation. Because our analysis did not control for the potential effects of these variables, which can have an impact on survival outcomes, these factors should be kept in mind when considering the results. In addition, we did not systematically assess each study for potential bias. The nature of the majority of studies (retrospective, single-arm) largely eliminates bias in terms of favoring one treatment over another. However, there is a degree of selection bias inherent in the patient populations studied; that is, patients who are candidates for CW treatment generally have better performance status and have accessible tumors that can be almost completely resected, and therefore a better prognosis than patients who are not candidates for CW treatment.
In this comprehensive review of the literature on CW and meta-analysis of published survival data, we attempted to summarize the cumulative data of numerous studies that have been reported over the past 18 years. Our results highlight benefits in survival of patients in the CW arms versus patients who did not receive CW. There was significant effect of CW treatment on median survival (P = 0.043), with higher OS rates for patients with new or recurrent HGG within the cohort treated with CW.
CW is an FDA-approved treatment modality for all newly-diagnosed HGGs, including GBM, anaplastic astrocytoma, anaplastic oligodendroglioma, and anaplastic oligoastrocytoma, as well as for recurrent GBM. It is also now an accepted form of therapy for newly-diagnosed and recurrent HGG in the most recent NCCN guidelines for CNS tumors [2]. Traditionally, the use of CW has precluded patients from accrual to many clinical trials. One frequently cited reason for exclusion is the potential ambiguity that the presence of the wafers have on the assessment of treatment response (or lack thereof) on follow-up MRI scans. However, it should be considered that following the phase of the treatment involving chemotherapy (in the time between resection and radiotherapy), an inflammatory response occurs, which may also contribute to the effect. In the current era of new biologicals entering clinical trials, the combination of an active inflammatory milieu together with an empowered immune system (e.g., dendritic cells, immune checkpoint modulators) may have positive anti-tumor interactions that will have to be determined in trials. Considering the recent trend toward a greater emphasis on OS (rather than PFS), which is largely independent of imaging measures, it may be helpful to reconsider the notion that CW precludes any trial participation. Another important issue contributing to reluctance to use CW involves the lack of reliable survival data for patients treated with CW, which might lead to confusion during the statistical analysis of the survival data of patients in a given trial. With the publication of this new, comprehensive dataset regarding the survival of more than 3,000 patients treated with CW, it should be easier to design clinical trials that can include patients who had received CW. In addition, statisticians will now have more reliable median survival times, 1-year survival rates, and 2-year survival rates to use for the analysis of protocols that will allow accrual of these patients.
Acknowledgments
We thank Harvey Kushner, PhD, of BioMedical Computer Research Institute for statistical analysis/support. We also thank Sherri D. Jones, PharmD, of MedVal Scientific Information Services for medical writing and editorial assistance. This manuscript was prepared according to ISMPP’s GPP2 Guidelines. Funding to support this study and the preparation of this manuscript was provided by Arbor Pharmaceuticals and Eisai, Inc.
Ethical standards
This meta-analysis does not report original data from human or animal subjects. Please consult the original publications for information about ethical procedures.
Abbreviations
- AA
Anaplastic astrocytoma
- AE
Adverse event
- AO
Anaplastic oligodendroglioma
- CW
Carmustine wafers
- GBM
Glioblastoma multiforme
- GKS
Gamma Knife surgery
- HGG
High-grade glioma
- MC
Multicenter
- O6-BG
O6-benzylguanine
- OS
Overall survival
- RCCS
Retrospective case control study
- RCS
Retrospective case series
- RCT
Randomized controlled trial
- RT
Radiotherapy
- SC
Single center
- SSI
Surgical site infection
- TMZ
Temozolomide
References
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network (2013) NCCN clinical practice guidelines in oncology (NCCN Guidelines®): central nervous system cancers, version 2.2013. National Comprehensive Cancer Network, Fort Washington, PA [DOI] [PubMed]
- 3.Stupp R, Tonn JC, Brada M, Pentheroudakis G. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v190–v193. doi: 10.1093/annonc/mdq187. [DOI] [PubMed] [Google Scholar]
- 4.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 5.Dehais C, Laigle-Donadey F, Marie Y, Kujas M, Lejeune J, Benouaich-Amiel A, Pedretti M, Polivka M, Xuan KH, Thillet J, Delattre JY, Sanson M. Prognostic stratification of patients with anaplastic gliomas according to genetic profile. Cancer. 2006;107(8):1891–1897. doi: 10.1002/cncr.22211. [DOI] [PubMed] [Google Scholar]
- 6.Metellus P, Coulibaly B, Nanni I, Fina F, Eudes N, Giorgi R, Barrie M, Chinot O, Fuentes S, Dufour H, Ouafik L, Figarella-Branger D. Prognostic impact of O6-methylguanine-DNA methyltransferase silencing in patients with recurrent glioblastoma multiforme who undergo surgery and carmustine wafer implantation: a prospective patient cohort. Cancer. 2009;115(20):4783–4794. doi: 10.1002/cncr.24546. [DOI] [PubMed] [Google Scholar]
- 7.Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, Bacci A, Agati R, Calbucci F, Ermani M. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115(15):3512–3518. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 8.Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, O’Fallon JR, Schaefer PL, Scheithauer BW, James CD, Buckner JC, Jenkins RB. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93(16):1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 9.Cheng HB, Yue W, Xie C, Zhang RY, Hu SS, Wang Z. IDH1 mutation is associated with improved overall survival in patients with glioblastoma: a meta-analysis. Tumour Biol. 2013;34(6):3555–3559. doi: 10.1007/s13277-013-0934-5. [DOI] [PubMed] [Google Scholar]
- 10.Inc Eisai. Gliadel® wafer (polifeprosan 20 with carmustine implant) [prescribing information] Woodcliff Lake: Eisai Inc.; 2010. [Google Scholar]
- 11.Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41(1):44–48. doi: 10.1097/00006123-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/S0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 14.Sabel M, Giese A. Safety profile of carmustine wafers in malignant glioma: a review of controlled trials and a decade of clinical experience. Curr Med Res Opin. 2008;24(11):3239–3257. doi: 10.1185/03007990802508180. [DOI] [PubMed] [Google Scholar]
- 15.Fadul CE, Wen PY, Kim L, Olson JJ. Cytotoxic chemotherapeutic management of newly diagnosed glioblastoma multiforme. J Neurooncol. 2008;89(3):339–357. doi: 10.1007/s11060-008-9615-4. [DOI] [PubMed] [Google Scholar]
- 16.Dixit S, Hingorani M, Achawal S, Scott I. The sequential use of carmustine wafers (Gliadel®) and post-operative radiotherapy with concomitant temozolomide followed by adjuvant temozolomide: a clinical review. Br J Neurosurg. 2011;25(4):459–469. doi: 10.3109/02688697.2010.550342. [DOI] [PubMed] [Google Scholar]
- 17.Kleinberg L. Polifeprosan 20, 3.85% carmustine slow-release wafer in malignant glioma: evidence for role in era of standard adjuvant temozolomide. Core Evid. 2012;7:115–130. doi: 10.2147/CE.S23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Affronti ML, Heery CR, Herndon JE, Rich JN, Reardon DA, Desjardins A, Vredenburgh JJ, Friedman AH, Bigner DD, Friedman HS. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009;115(15):3501–3511. doi: 10.1002/cncr.24398. [DOI] [PubMed] [Google Scholar]
- 19.Anderson IA, Thomson S. Complications following Gliadel wafer insertion during surgery for glioblastoma multiforme; a single centre-experience. Neuro Oncol. 2011;13(Suppl 2):ii1. [Google Scholar]
- 20.Aoki T, Nishikawa R, Sugiyama K, Nonoguchi N, Kawabata N, Mishima K, Adachi JI, Kurisu K, Yamasaki F, Tominaga T, Kumabe T, Ueki K, Higuchi F, Yamamoto T, Ishikawa E, Takeshima H, Yamashita S, Arita K, Hirano H, Yamada S, Matsutani M. A multicenter phase I/II study of the BCNU implant (Gliadel wafer) for Japanese patients with malignant gliomas. Neurol Med Chir (Tokyo) 2014;54(4):290–301. doi: 10.2176/nmc.oa2013-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 22.Barr JG, Grundy PL. The effects of the NICE Technology Appraisal 121 (Gliadel and temozolomide) on survival in high-grade glioma. Br J Neurosurg. 2012;26(6):818–822. doi: 10.3109/02688697.2012.697221. [DOI] [PubMed] [Google Scholar]
- 23.Bock HC, Puchner MJ, Lohmann F, Schutze M, Koll S, Ketter R, Buchalla R, Rainov N, Kantelhardt SR, Rohde V, Giese A. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev. 2010;33(4):441–449. doi: 10.1007/s10143-010-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brem H, Mahaley MS, Jr, Vick NA, Black KL, Schold SC, Jr, Burger PC, Friedman AH, Ciric IS, Eller TW, Cozzens JW. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74(3):441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 25.Catalan-Uribarrena G, Bilbao-Barandica G, Pomposo-Gaztelu I, Undabeitia-Huertas J, Ruiz de Gopegui-Ruiz E, Galbarriatu-Gutierrez L, Canales-Llantada M, Aurrecoechea-Obieta J, Igartua-Azkune A, Carbayo-Lozano G. Prognostic factors and survival in a prospective cohort of patients with high-grade glioma treated with carmustine wafers or temozolomide on an intention-to-treat basis. Acta Neurochir. 2012;154(2):211–222. doi: 10.1007/s00701-011-1199-7. [DOI] [PubMed] [Google Scholar]
- 26.Chaichana KL, Zaidi H, Pendleton C, McGirt MJ, Grossman R, Weingart JD, Olivi A, Quinones-Hinojosa A, Brem H. The efficacy of carmustine wafers for older patients with glioblastoma multiforme: prolonging survival. Neurol Res. 2011;33(7):759–764. doi: 10.1179/1743132811Y.0000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damilakis K, Paleologos TS, Papanikolaou PG, Venetikidis A, Mallios G, Stamatopoulos G, Chatzidakis E, Fratzoglou M, Kyriakou T (2011) Carmustine wafers treatment for firstly diagnosed malignant gliomas: a study of 22 cases. Presented at: 14th European Congress of Neurosurgery, October 9–14, Rome, Italy
- 28.Darakchiev BJ, Albright RE, Breneman JC, Warnick RE. Safety and efficacy of permanent iodine-125 seed implants and carmustine wafers in patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108(2):236–242. doi: 10.3171/JNS/2008/108/2/0236. [DOI] [PubMed] [Google Scholar]
- 29.De Bonis P, Anile C, Pompucci A, Fiorentino A, Balducci M, Chiesa S, Maira G, Mangiola A. Safety and efficacy of Gliadel wafers for newly diagnosed and recurrent glioblastoma. Acta Neurochir. 2012;154(8):1371–1378. doi: 10.1007/s00701-012-1413-2. [DOI] [PubMed] [Google Scholar]
- 30.Della Puppa A, Rossetto M, Ciccarino P, Del MG, Rotilio A, Manara R, Paola Gardiman M, Denaro L, d’Avella D, Scienza R. The first 3 months after BCNU wafers implantation in high-grade glioma patients: clinical and radiological considerations on a clinical series. Acta Neurochir. 2010;152(11):1923–1931. doi: 10.1007/s00701-010-0759-6. [DOI] [PubMed] [Google Scholar]
- 31.Della Puppa A, Denaro L, Rossetto M, Ciccarino P, Manara R, Lombardi G, Del Moro G, Rotilio A, d’Avella D, Scienza R. Postoperative seizure in high grade glioma patients treated with BCNU wafers: a mono-institutional experience. J Neurooncol. 2011;105(2):275–280. doi: 10.1007/s11060-011-0577-6. [DOI] [PubMed] [Google Scholar]
- 32.De’Santi MS, Genovese M, Amoroso E, Manto A, Piucci B. 5-ALA guided surgery plus carmustine wafer implant and the Stupp regimen (radiotherapy and temozolomide) for the treatment of glioblastoma multiforme. Chirurgia. 2011;24(6):353–356. [Google Scholar]
- 33.Desjardins A, Peters KB, Herndon JE, II, Bailey LA, Alderson LM, Ranjan T, Sampson JH, Friedman AH, Bigner DD, Friedman HS, Vredenburgh JJ. Newly diagnosed glioblastoma treated with Gliadel® followed by radiation therapy (RT), temozolomide and bevacizumab, and post-RT bevacizumab and temozolomide: a phase II study. Neuro Oncol. 2012;14(Suppl 6):104. [Google Scholar]
- 34.Dorner L, Ulmer S, Rohr A, Mehdorn HM, Nabavi A. Space-occupying cyst development in the resection cavity of malignant gliomas following Gliadel® implantation—incidence, therapeutic strategies, and outcome. J Clin Neurosci. 2011;18(3):347–351. doi: 10.1016/j.jocn.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 35.Duntze J, Litre CF, Eap C, Theret E, Debreuve A, Jovenin N, Lechapt-Zalcman E, Metellus P, Colin P, Guillamo JS, Emery E, Menei P, Rousseaux P, Peruzzi P. Implanted carmustine wafers followed by concomitant radiochemotherapy to treat newly diagnosed malignant gliomas: prospective, observational, multicenter study on 92 cases. Ann Surg Oncol. 2013;20(6):2065–2072. doi: 10.1245/s10434-012-2764-x. [DOI] [PubMed] [Google Scholar]
- 36.Giese A, Kucinski T, Knopp U, Goldbrunner R, Hamel W, Mehdorn HM, Tonn JC, Hilt D, Westphal M. Pattern of recurrence following local chemotherapy with biodegradable carmustine (BCNU) implants in patients with glioblastoma. J Neurooncol. 2004;66(3):351–360. doi: 10.1023/B:NEON.0000014539.90077.db. [DOI] [PubMed] [Google Scholar]
- 37.Gutenberg A, Bock HC, Bruck W, Doerner L, Mehdorn HM, Roggendorf W, Westphal M, Felsberg J, Reifenberger G, Giese A. MGMT promoter methylation status and prognosis of patients with primary or recurrent glioblastoma treated with carmustine wafers. Br J Neurosurg. 2013;27(6):772–778. doi: 10.3109/02688697.2013.791664. [DOI] [PubMed] [Google Scholar]
- 38.Ho J, Bruch J, Watts C, Price SJ. The use of intra-operative carmustine chemotherapy wafers in the management of high grade gliomas: an assessment of complications and access to further therapy. Br J Neurosurg. 2011;25(2):163. [Google Scholar]
- 39.Hoffmann D (2009) Should Gliadel be mandatory in first intention operative treatment of high grade glioma? Presented at the Marseille Neurosurgery EANS-SFNC 2009 Joint Annual Meeting, March 27–31, Marseille
- 40.Kleinberg LR, Weingart J, Burger P, Carson K, Grossman SA, Li K, Olivi A, Wharam MD, Brem H. Clinical course and pathologic findings after Gliadel and radiotherapy for newly diagnosed malignant glioma: implications for patient management. Cancer Invest. 2004;22(1):1–9. doi: 10.1081/CNV-120027575. [DOI] [PubMed] [Google Scholar]
- 41.Ko AL, Fink KR, Stelzer KM, Silbergeld DL. Safety and efficacy of concomitant chemotherapeutic wafers and iodine-125 seeds for recurrent glioblastoma. Surg Neurol Int. 2012;3:137. doi: 10.4103/2152-7806.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krex D, Juratli T, Lindner C, Raue C, Schackert G. Local therapy in recurrent glioblastoma. Neuro-Oncology. 2011;13(Suppl 3):iii158. [Google Scholar]
- 43.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, Shaffrey M, Ram Z, Piepmeier J, Prados M, Croteau D, Pedain C, Leland P, Husain SR, Joshi BH, Puri RK. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lechapt-Zalcman E, Levallet G, Dugue AE, Vital A, Diebold MD, Menei P, Colin P, Peruzzy P, Emery E, Bernaudin M, Chapon F, Guillamo JS. O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation and low MGMT-encoded protein expression as prognostic markers in glioblastoma patients treated with biodegradable carmustine wafer implants after initial surgery followed by radiotherapy with concomitant and adjuvant temozolomide. Cancer. 2012;118(18):4545–4554. doi: 10.1002/cncr.27441. [DOI] [PubMed] [Google Scholar]
- 45.Lemcke J, Grawe A, Golz L, Meier U (2013) Benefits and risks of camustin wavers in the therapy of malign glioblastoma. Presented at the European Association of Neurosurgical Societies, November 11–14, Tel Aviv
- 46.Lopez MJ, Garcia del Busto N, Cuenca M, Quintana B, Antonino G, Agustin S. Use of carmustine implant in glioblastoma multiforme. Int J Clin Pharm. 2012;34(1):240. [Google Scholar]
- 47.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quinones-Hinojosa A. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGovern PC, Lautenbach E, Brennan PJ, Lustig RA, Fishman NO. Risk factors for postcraniotomy surgical site infection after 1,3-bis (2-chloroethyl)-1-nitrosourea (Gliadel) wafer placement. Clin Infect Dis. 2003;36(6):759–765. doi: 10.1086/368082. [DOI] [PubMed] [Google Scholar]
- 49.Menei P, Metellus P, Parot-Schinkel E, Loiseau H, Capelle L, Jacquet G, Guyotat J. Biodegradable carmustine wafers (Gliadel) alone or in combination with chemoradiotherapy: the French experience. Ann Surg Oncol. 2010;17(7):1740–1746. doi: 10.1245/s10434-010-1081-5. [DOI] [PubMed] [Google Scholar]
- 50.Metellus P, Nanni-Metellus I, Chinot O, Fina F, Fuentes S, Dufour H, Barrie M, L’Houcine O, Figarella-Branger D. Prognostic significance of MGMT promoter methylation status in elderly patients with newly diagnosed glioblastoma treated with BCNU water implantation: a prospective patient cohort. Neuro Oncol. 2011;13(Suppl 3):56. [Google Scholar]
- 51.Miglierini P, Bouchekoua M, Rousseau B, Dam HP, Malhaire JP, Pradier O. Impact of the per-operatory application of Gliadel wafers (BCNU, carmustine) in combination with temozolomide and radiotherapy in patients with glioblastoma multiforme: efficacy and toxicity. Clin Neurol Neurosurg. 2012;114(9):1222–1225. doi: 10.1016/j.clineuro.2012.02.056. [DOI] [PubMed] [Google Scholar]
- 52.Noel G, Schott R, Froelich S, Gaub MP, Boyer P, Fischer-Lokou D, Dufour P, Kehrli P, Maitrot D. Retrospective comparison of chemoradiotherapy followed by adjuvant chemotherapy, with or without prior Gliadel implantation (carmustine) after initial surgery in patients with newly diagnosed high-grade gliomas. Int J Radiat Oncol Biol Phys. 2012;82(2):749–755. doi: 10.1016/j.ijrobp.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 53.Pan E, Mitchell SB, Tsai JS. A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol. 2008;88(3):353–357. doi: 10.1007/s11060-008-9576-7. [DOI] [PubMed] [Google Scholar]
- 54.Pérez Gómez R, Villanueva Álvarez A, Herruzo Cabrera I, Fortes de La Torre I, García Sánchez L, Ramos Trujillo A, Márquez Márquez B. First-line treatment of malignant glioma with carmustine implants. Seven years-results. Rep Pract Oncol Radiother. 2013;18:S190. doi: 10.1016/j.rpor.2013.03.129. [DOI] [Google Scholar]
- 55.Qadri SRM, Nicholae L, Jenkinson MD, Brodbelt A (2011) Treatment of recurrent high grade gliomas with Gliadel wafers after completion of adjuvant chemo-radiotherapy—the Walton Centre experience. Presented at the EORTC-EANO conference 2011, March 25–26, Bucharest
- 56.Qadri SRM, Jenkinson MD, Brodbelt A. Recurrent high grade glioma after local carmustine wafer implant into the tumour bed. Does residual tumour affect progression? Br J Neurosurg. 2011;25(5):555. [Google Scholar]
- 57.Quinn JA, Jiang SX, Carter J, Reardon DA, Desjardins A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, Bigner DD, Sampson JH, McLendon RE, Herndon JE, Threatt S, Friedman HS. Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res. 2009;15(3):1064–1068. doi: 10.1158/1078-0432.CCR-08-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quiros J. Radiotherapy with concomitant and adyuvant temozolomide with or without carmustune wafers in high grade glioma. Radiother Oncol. 2010;96:S269. [Google Scholar]
- 59.Ranjan T, Peters KB, Vlahovic G, Alderson LM, Herndon JE, McShery F, Threatt S, Sampson JH, Friedman AH, Bigner DD, Friedman HS, Vredenburgh JJ, Desjardins A. Phase II trial for patients with newly diagnosed glioblastoma (GBM) treated with carmustine wafers followed by concurrent radiation therapy (RT), temozolomide (TMZ), and bevacizumab (BV), then followed by TMZ and BV post-RT. J Clin Oncol. 2013;31(15 suppl):e13015. [Google Scholar]
- 60.Rezazadeh A, LaRocca RV, Vitaz TW, Villanueva WG, Hodes J, Haysley L. A phase II study of multimodal therapy employing surgery, carmustine (BCNU) wafer, concomitant temozolomide and radiation, followed by dose dense therapy with temozolomide plus bevacizumab for newly diagnosed glioblastoma (GBM) Neuro Oncol. 2011;13(Suppl 3):iii90. [Google Scholar]
- 61.Ryken T. Prospective multimodality therapy for newly diagnosed glioblastoma with surgical resection, implantable BCNU chemotherapy and combination radiotherapy and temozolomide: long-term follow-up. Neuro Oncol. 2011;13(Suppl 3):iii85. [Google Scholar]
- 62.Salmaggi A, Milanesi I, Silvani A, Gaviani P, Marchetti M, Fariselli L, Solero CL, Maccagnano C, Casali C, Guzzetti S, Pollo B, Ciusani E, DiMeco F. Prospective study of carmustine wafers in combination with 6-month metronomic temozolomide and radiation therapy in newly diagnosed glioblastoma: preliminary results. J Neurosurg. 2013;118(4):821–829. doi: 10.3171/2012.12.JNS111893. [DOI] [PubMed] [Google Scholar]
- 63.Salvati M, D’elia A, Frati A, Brogna C, Santoro A, Delfini R. Safety and feasibility of the adjunct of local chemotherapy with biodegradable carmustine (BCNU) wafers to the standard multimodal approach to high grade gliomas at first diagnosis. J Neurosurg Sci. 2011;55(1):1–6. [PubMed] [Google Scholar]
- 64.Samis Zella MA, Wallocha M, Slotty PJ, Isik G, Hanggi D, Schroeteler J, Ewelt C, Steiger HJ, Sabel M. Evaluation of post-operative complications associated with repeat resection and BCNU wafer implantation in recurrent glioblastoma. Acta Neurochir. 2014;156(2):313–323. doi: 10.1007/s00701-013-1931-6. [DOI] [PubMed] [Google Scholar]
- 65.Satilmis G, Samis Zella MA, Wallocha M, Schroeteler J, Steiger HJ, Sabel MC (2012) Evaluation of complications of carmustine wafer implantation in recurrent GBM. Presented at: 63rd annual meeting of the German Society of Neurosurgery Joint Meeting with the Japanese Neurosurgical Society, June 13–16, Leipzig
- 66.Shah RS, Homapour B, Casselden E, Barr JG, Grundy PL, Brydon HL. Delayed post-operative haemorrhage after carmustine wafer implantation: a case series from two UK centres. Br J Neurosurg. 2014;28(4):488–494. doi: 10.3109/02688697.2013.861387. [DOI] [PubMed] [Google Scholar]
- 67.Silvani A, Gaviani P, Lamperti E, Botturi A, DiMeco F, Broggi G, Fariselli L, Solero CL, Salmaggi A. Phase II study: carmustine implant (Gliadel wafer) plus adjuvant and concomitant temozolomide in combination with radiotherapy in primary glioblastoma patients. Neuro Oncol. 2011;13(Suppl 3):iii46. [Google Scholar]
- 68.Smith KA, Ashby LS, Gonzalez LF, Brachman DG, Thomas T, Coons SW, Battaglia M, Scheck A. Prospective trial of gross-total resection with Gliadel wafers followed by early postoperative Gamma Knife radiosurgery and conformal fractionated radiotherapy as the initial treatment for patients with radiographically suspected, newly diagnosed glioblastoma multiforme. J Neurosurg. 2008;109(Suppl 6):106–117. doi: 10.3171/JNS/2008/109/12/S17. [DOI] [PubMed] [Google Scholar]
- 69.Subach BR, Witham TF, Kondziolka D, Lunsford LD, Bozik M, Schiff D. Morbidity and survival after 1,3-bis(2-chloroethyl)-1-nitrosourea wafer implantation for recurrent glioblastoma: a retrospective case-matched cohort series. Neurosurgery. 1999;45(1):17–22. doi: 10.1097/00006123-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Sumrall AL, Burri S, Brick W, Asher A. Temozolomide (TMZ) can be safely administered in the immediate postoperative period after tumor resection and Gliadel® wafer placement in patients with newly diagnosed high-grade gliomas: final results of a prospective, multi-institutional, phase I/II trial. Neuro Oncol. 2012;14(Suppl 6):vi66. doi: 10.1007/s11060-015-1793-2. [DOI] [PubMed] [Google Scholar]
- 71.Uff CE, McGregor DI, Levy S, Bradford R, Thorne LW. The use of Gliadel® wafers in recurrent high grade glioma. Br J Neurosurg. 2009;23(5):482. [Google Scholar]
- 72.Ulmer S, Spalek K, Nabavi A, Schultka S, Mehdorn HM, Kesari S, Dorner L. Temporal changes in magnetic resonance imaging characteristics of Gliadel wafers and of the adjacent brain parenchyma. Neuro Oncol. 2012;14(4):482–490. doi: 10.1093/neuonc/nos003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watts C, Wanek K, Counsell N, Smith P (2013) Preliminary results of the GALA-5 study: an evaluation of the tolerability and feasibility of combining 5-amino-levulinic acid (5-ALA) with carmustine wafers (Gliadel) in the surgical management of primary glioblastoma. Presented at the NCRI cancer conference, November 3–6, Liverpool
- 74.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir. 2006;148(3):269–275. doi: 10.1007/s00701-005-0707-z. [DOI] [PubMed] [Google Scholar]
- 75.Zhu JJ, Esquenazi-Levy Y, Friedman ER, Tandon N. Retrospective review of 57 cases of glioblastoma treated with surgery and implantable BCNU wafer placement. Neuro Oncol. 2012;14(Suppl 6):vi60. [Google Scholar]
- 76.Gutenberg A, Lumenta CB, Braunsdorf WE, Sabel M, Mehdorn HM, Westphal M, Giese A. The combination of carmustine wafers and temozolomide for the treatment of malignant gliomas: a comprehensive review of the rationale and clinical experience. J Neurooncol. 2013;113(2):163–174. doi: 10.1007/s11060-013-1110-x. [DOI] [PubMed] [Google Scholar]
- 77.Gilbert MR, Dignam J, Won M, Blumenthal DT, Vogelbaum MA, Aldape KD, Colman H, Chakravarti A, Jeraj R, Armstrong TS, Wefel JS, Brown PD, Jaeckle KA, Schiff D, Atkins JN, Brachman DG, Werner-Wasik M, Komaki R, Sulman EP, Mehta MP. Phase III double-blind placebo-controlled trial evaluating bevacizumab (bev) in patients (pts) with newly diagnosed glioblastoma (GBM) J Clin Oncol. 2013;31(18 Suppl):1. [Google Scholar]
- 78.Wick W, Cloughsey TF, Nishikawa R, Mason W, Saran F, Henriksson R, Hilton M, Kerloeguen Y, Chinot OL. Tumor response based on adapted Macdonald criteria and assessment of pseudoprogression (PsPD) in the phase III AVAglio trial of bevacizumab (Bv) plus temozolomide (T) plus radiotherapy (RT) in newly diagnosed glioblastoma. J Clin Oncol. 2013;31(18 Suppl):2002. [Google Scholar]
- 79.La Rocca RV, Rezazadeh A. Carmustine-impregnated wafers and their impact in the management of high-grade glioma. Expert Opin Pharmacother. 2011;12(8):1325–1332. doi: 10.1517/14656566.2011.580737. [DOI] [PubMed] [Google Scholar]
- 80.Nagpal S. The role of BCNU polymer wafers (Gliadel) in the treatment of malignant glioma. Neurosurg Clin N Am. 2012;23(2):289–295. doi: 10.1016/j.nec.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 81.Perry J, Chambers A, Spithoff K, Laperriere N. Gliadel wafers in the treatment of malignant glioma: a systematic review. Curr Oncol. 2007;14(5):189–194. doi: 10.3747/co.2007.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, Somerville M, Price A, Stein K. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess. 2007;11(45):iii–iv. doi: 10.3310/hta11450. [DOI] [PubMed] [Google Scholar]
- 83.Hart MG, Grant R, Garside R, Rogers G, Somerville M, Stein K. Chemotherapy wafers for high grade glioma. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD007294.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bregy A, Shah AH, Diaz MV, Pierce HE, Ames PL, Diaz D, Komotar RJ. The role of Gliadel wafers in the treatment of high-grade gliomas. Exp Rev Anticancer Ther. 2013;13(12):1453–1461. doi: 10.1586/14737140.2013.840090. [DOI] [PubMed] [Google Scholar]