Abstract
The significance of chlamydiosis as a cause of mortality in wild passerines (Order Passeriformes), and the role of these birds as a potential source of zoonotic Chlamydia psittaci infection, is unknown. We reviewed wild bird mortality incidents (2005–2011). Where species composition or post-mortem findings were indicative of chlamydiosis, we examined archived tissues for C. psittaci infection using PCR and ArrayTube Microarray assays. Twenty-one of 40 birds tested positive: 8 dunnocks (Prunella modularis), 7 great tits (Parus major), 3 blue tits (Cyanistes caeruleus), 2 collared doves (Streptopelia decaocto, Order Columbiformes), and 1 robin (Erithacus rubecula). Chlamydia psittaci genotype A was identified in all positive passerines and in a further three dunnocks and three robins diagnosed with chlamydiosis from a previous study. Two collared doves had genotype E. Ten of the 21 C. psittaci-positive birds identified in the current study had histological lesions consistent with chlamydiosis and co-localizing Chlamydia spp. antigens on immunohistochemistry. Our results indicate that chlamydiosis may be a more common disease of British passerines than was previously recognized. Wild passerines may be a source of C. psittaci zoonotic infection, and people should be advised to take appropriate hygiene precautions when handling bird feeders or wild birds.
Electronic supplementary material
The online version of this article (doi:10.1007/s10393-014-0951-x) contains supplementary material, which is available to authorized users.
Keywords: Chlamydia psittaci, chlamydiosis, collared dove Streptopelia decaocto, Order Passeriformes, passerine, wild bird
Introduction
Chlamydiosis is a disease of birds and mammals, including people, caused by infection with the Gram-negative, intracellular bacterium, Chlamydia (Chlamydophila) psittaci (Family Chlamydiaceae, Order Chlamydiales) (Vanrompay et al. 1995; Andersen and Franson 2007). Birds are the primary hosts of C. psittaci (Andersen and Franson 2007), and a wide range of avian species is susceptible to infection (Kaleta and Taday 2003). Avian infections are frequently asymptomatic (Kaleta and Taday 2003) but can also cause a broad spectrum of disease (“avian chlamydiosis”) including respiratory, enteric, and ocular disease (Vanrompay et al. 1995; Andersen and Franson 2007). Gross lesions typically include air sacculitis, serositis, hepatomegaly, and splenomegaly (Vanrompay et al. 1995), and there is often concurrent infectious disease (Pennycott et al. 2009). Microscopic lesions are variable: splenic, hepatic, renal, and/or myocardial necrosis may be evident in acute cases; other findings can include splenic and/or hepatic histiocytosis, hepatic periportal inflammatory cell infiltrates, and biliary hyperplasia (Vanrompay et al. 1995).
Avian chlamydiosis has been diagnosed in a variety of wild bird species in Europe, particularly columbiforms (Order Columbiformes) such as collared doves (Streptopelia decaocto), feral pigeons (Columbia livia), and wood pigeons (Columba palumbus) (Bracewell and Bevan 1986; Magnino et al. 2009). Also, chlamydiosis has been diagnosed occasionally in passerines (Order Passeriformes) (Simpson and Bevan 1989; Holzinger-Umlauf et al. 1997; Pennycott et al. 2009). The first reported occurrence of the disease in passerines in Britain was in 1988, when robins (Erithacus rubecula), dunnocks (Prunella modularis) and Paridae (tit species) were affected in a garden in south-west England (Simpson and Bevan 1989). Subsequently, Pennycott et al. (2009) reported mortality of Fringillidae (finches), Paridae, and robins in a Scottish garden in 2008, in which trichomonosis was considered the primary cause of disease and death, but in which concurrent chlamydiosis was diagnosed in some of the birds examined. Colvile et al. (2012) described a further six incidents which affected Paridae and/or dunnocks and/or robins in England in 2009 (1 incident) and 2011 (5 incidents).
Chlamydia psittaci is currently classified into seven ompA genotypes, each of which appears to have a certain host predilection: genotype A (parrots), B (pigeons), C (ducks and geese), D (turkeys), E (pigeons, ducks and other species), F (parakeets), and E/B (ducks, turkeys and pigeons) (Vanrompay et al. 1997; Geens et al. 2005; Sachse et al. 2009). These data are derived mainly from studies of captive or farmed birds and feral pigeons: C. psittaci genotypes infecting wild passerines have rarely been determined (Kaleta and Taday 2003; Kalmar et al. 2013). In a recently proposed extension of the ompA typing scheme, subgroups of genotypes A (A-VS1, A-6BC and A-8455), E/B (EB-E30, EB-859 and EB-KKCP), and D (D-NJ1 and D-9N) were described, and six further avian genotypes were identified [in corvids, parrots, an oriental stork (Ciconia boyciana), and a brown skua (Stercorarius antarcticus lonnbergi)] (Sachse et al. 2008).
While pigeons and doves appear to be the major wild bird reservoir of C. psittaci across Europe (Bracewell and Bevan 1986; Magnino et al. 2009), variable and potentially high prevalences of C. psittaci infection have been demonstrated in some wild passerine populations. For example, in Germany, 215 of 399 (54%) clinically healthy tits [including 30 of 43 (70%) blue tits (Cyanistes (Parus) caeruleus), 169 of 318 (53%) great tits (Parus major) and 12 of 32 (38%) marsh tits (Poecile (Parus) palustris)] were found to be Chlamydia sp. positive from cloacal and pharyngeal swabs using immunofluorescent antibody testing (Holzinger-Umlauf et al. 1997). Olsen et al. (1998) detected C. psittaci in 9 of 219 (3%) passerines sampled in Sweden (using PCR on fecal samples), including 2 of 29 (7%) robins and 1 of 21 (5%) great tits. Observation of sick birds was not reported; therefore, it seems likely that birds sampled in this study were apparently healthy. Others have failed to detect C. psittaci infection in passerines: Zweifel et al. (2009) from 527 passerines [including 211 chaffinches (Fringilla coelebs), 47 great tits and 12 robins] sampled in Switzerland (by PCR on cloacal swabs), and Prukner-Radovćic et al. (2005) from 53 passerines (including 15 robins) sampled in Croatia (by ELISA on cloacal swabs). The prevalence of C. psittaci infection in wild passerines in Britain is unknown.
Chlamydia psittaci infection causes a range of symptoms in human beings (in which the disease is termed “psittacosis”), ranging from asymptomatic infection or mild, flu-like illness to severe respiratory disease that, in rare cases, can be fatal (Smith et al. 2011; Rehn et al. 2013). Human cases have most often been attributed not only to direct or indirect contact with infected captive psittacine birds (Palmer 1982; Wreghitt and Taylor 1988; Smith et al. 2011), but also to contact with poultry (particularly ducks) (Palmer 1982; Gaede et al. 2008; Laroucau et al. 2009) and racing and feral pigeons (Haag-Wackernagel and Moch 2004; Harkinezhad et al. 2009; Magnino et al. 2009). The origins of human psittacosis cases, however, are often undetermined [e.g., Health Protection Agency (HPA), and Department for Environment, Food & Rural Affairs (Defra) 2012]. Other wild bird species have been implicated in some psittacosis outbreaks (Williams et al. 1998; Telfer et al. 2005; Herrmann et al. 2006; Rehn et al. 2013), including wild passerines, which were the suspected source of an outbreak that affected at least 25 people in southern Sweden in early 2013 (Rehn et al. 2013).
Wild bird carcasses tend not to be tested for C. psittaci infection routinely due to financial constraints (molecular tests are required to obtain a diagnosis) (Pennycott et al. 2009); therefore, the prevalence of chlamydiosis in British passerines has been under-investigated (Colvile et al. 2012). Here, we conducted a retrospective survey of selected garden bird carcasses submitted by members of the public across England and Wales in order to investigate the significance of chlamydiosis as a cause of disease in these species. We use the term “chlamydiosis” to describe cases in which C. psittaci infection was detected in birds which had gross, histological, and immunohistochemical findings consistent with the disease. We conducted C. psittaci genotyping of positive cases in order to further our understanding of the epidemiology of the infection in British garden birds.
Methods
Wild Bird Cases
Reports of sick and dead wild birds were received from the general public through a national disease surveillance network established as part of the Garden Bird Health initiative (GBHi) (Robinson et al. 2010). Morbidity and mortality incidents were reported either on an ad hoc basis or through a systematic volunteer scheme (Robinson et al. 2010). A detailed description of each incident was obtained, including the species and number of birds affected, date when sick and/or dead birds were first observed, location, and clinical signs. If available, carcasses suitable for post-mortem examination (PME) were submitted.
On receipt, carcasses were either refrigerated at 4°C and examined within 48 h, or frozen at −20°C and examined at a later date. PMEs followed a standardized protocol, as described by Lawson et al. (2011). Birds were assigned to the age classes “Nestling,” “Juvenile,” (fully fledged and independent from nest) or “Adult” (any individual beyond its post-juvenile molt), and sex was determined, where possible, on the basis of plumage characteristics or gonad inspection. Carcasses were weighed, and body condition was subjectively assessed (as “Emaciated,” “Thin,” “Normal,” or “Fat”) on the basis of subcutaneous fat deposits and pectoral muscle condition. Samples (liver, small-intestinal content, and tissues with macroscopic lesions) were routinely submitted for microbiological examination using a standardized protocol (Lawson et al. 2011). A saline mount preparation of small-intestinal contents was examined microscopically for parasites. A standard range of tissues from each case was frozen at −20°C pending further testing and, where the state of carcass preservation permitted, tissue samples were fixed in neutral-buffered 10% formalin pending histological examination. Tissues were submitted for further tests (in addition to those described below) as indicated by the macroscopic findings, including culture and PCR to detect Trichomonas sp. infection (Robinson et al. 2010), and histopathology and PCR to detect avipoxvirus infection (Lawson et al. 2012).
Cases were selected for C. psittaci testing from an archive of 1,578 passerine and columbiform carcasses received at the Institute of Zoology from across England and Wales, 2005–2011, on the basis of either (1) having gross lesions consistent with previously reported chlamydiosis incidents (hepatomegaly and/or splenomegaly and/or serositis), or (2) having been from a mortality incident in which the species assemblage of sick and dead birds was consistent with previously reported passerine chlamydiosis incidents (involvement of robins and/or Paridae and/or dunnocks). In addition, tissues from six passerine carcasses in which chlamydiosis had already been diagnosed (Colvile et al. 2012) were submitted for molecular C. psittaci testing.
Molecular Detection of C. psittaci Infection
DNA was extracted from frozen/thawed liver, or from pooled liver and spleen where both archived tissues were available, using a Biosprint 15 DNA Blood Kit (Qiagen Ltd., Manchester, M15 6SH, UK) according to the manufacturer’s instructions. The purified DNA was stored at 4°C, until the molecular analyses were performed.
All samples were examined by real-time PCR with primers specific for the 23S rRNA gene (Family Chlamydiaceae) using an ABI 7500 thermocycler (Applied Biosystems, Foster City, California, USA) following methods described by Ehricht et al. (2006) and Zweifel et al. (2009) which had a detection limit of 1 inclusion-forming unit (ifu) (Ehricht et al. 2006). A positive control (C. abortus DNA) and a negative control (reaction mix with molecular biology grade water) were included in each PCR run. Each sample was tested in duplicate. When both Ct-values were <38, a sample was considered as positive (Zweifel et al. 2009). Samples for which one or both duplicates gave Ct-values of >38 were considered as questionably positive. If amplification was absent in both duplicates, the sample was interpreted as negative, and no further molecular tests were performed. Positive and questionably positive samples were further examined using each of the following three tests:
A Chlamydia species-specific 23S ArrayTube (AT) Microarray assay (Alere Chip Technologies GmbH, Jena, Germany) as described by Borel et al. (2008), which had a detection limit of 1 ifu (Ehricht et al. 2006).
A C. psittaci ompA real-time PCR, which had a detection limit of 2 ifu, as described by Pantchev et al. (2009). Each sample was tested in duplicate with positive (C. psittaci DNA) and negative (molecular grade water) controls included. A sample was considered as positive when the average Ct-value was <36 (Pantchev et al. 2009), and as questionable positive when the average Ct-value was >36.
A C. psittaci genotyping assay, as described by Sachse et al. (2008). In the case of weak signals where the ompA genotype could not be accurately identified by the software, the assignment was done manually based on the closest match: these cases were termed “weak positive.” The lowest amount of DNA required for correct typing was equivalent to 2 ifu (Sachse et al. 2009).
Samples were considered positive for C. psittaci if they were positive (including—for the C. psittaci genotyping assay—“weak positive”) on at least one of these three further tests.
Histology and Immunohistochemistry
In C. psittaci-positive cases for which formalin-fixed tissues were available, the significance of the infection was investigated using histopathological examination and immunohistochemistry.
Formalin-fixed tissues were prepared for histopathological examination using routine methods (Bancroft 2008), and 5-µm-thick sections were examined using various stains including H&E, Ziehl-Neelsen, Giemsa, Periodic Acid-Schiff, and Gram-Twort.
Chlamydia spp.-specific immunohistochemistry, using anti-chlamydial lipopolysaccharide antibody (mouse IgG1, clone 13/4; Santa Cruz Biotechnology Inc., California, USA), was performed on paraffin-embedded, formalin-fixed tissue sections following the methodology described by Buxton et al. (1996).
A diagnosis of chlamydiosis was made for C. psittaci-positive cases which had co-localization of Chlamydia spp.-specific immunolabeling with histological lesions consistent with the disease [such as splenic, hepatic, renal and/or myocardial necrosis, splenic and/or hepatic histiocytosis, hepatic periportal inflammatory cell infiltrates, and biliary hyperplasia (Vanrompay et al. 1995)].
Results
Wild Bird Cases
Tissues from 40 birds (from 38 mortality incidents) in the case archive fulfilled our selection criteria and were tested for C. psittaci infection using molecular methods. These comprised 35 passerines (from 33 mortality incidents) and 5 columbiforms (from a further 5 mortality incidents) (Table 1).
Table 1.
Number of birds of each species submitted for molecular testing for C. psittaci infection and summary of results
| Taxonomic group | No. cases tested and results | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Order Passeriformes | |||
| Family Paridae | |||
| Great tit (Parus major) | 7 | 5 | 12 |
| Blue tit (Cyanistes caeruleus) | 3 | 1 | 4 |
| Family Prunellidae | |||
| Dunnock (Prunella modularis) | 8 | 0 | 8 |
| Family Turdidae | |||
| Robin (Erithacus rubecula) | 1 | 3 | 4 |
| Family Corvidae | |||
| Rook (Corvus frugilegus) | 0 | 2 | 2 |
| Jackdaw (Corvus monedula) | 0 | 2 | 2 |
| Family Fringillidae | |||
| Chaffinch (Fringilla coelebs) | 0 | 1 | 1 |
| Family Troglodytidae | |||
| Wren (Troglodytes troglodytes) | 0 | 1 | 1 |
| Family Motacillidae | |||
| Pied wagtail (Motacilla alba) | 0 | 1 | 1 |
| Order Columbiformes | |||
| Family Columbidae | |||
| Collared dove (Streptopelia decaocto) | 2 | 1 | 3 |
| Feral pigeon (Columba livia) | 0 | 2 | 2 |
| Total | 21 | 19 | 40 |
Molecular Detection of C. psittaci Infection
Tissues from 21 of the 40 selected cases tested positive for C. psittaci DNA: all of 8 dunnocks, 7 (of 12) great tits, 3 (of 4) blue tits, 2 (of 3) collared doves, and 1 (of 4) robins (Table 1 and Supplementary Table 1). For the positive cases, the results of each of the molecular tests are presented in Table 2. The 21 positive cases had been submitted from 20 separate mortality incidents, the details of which are presented in Table 3. Each of 4 corvids, 2 feral pigeons, 1 wren (Troglodytes troglodytes), 1 chaffinch, and 1 pied wagtail (Motacilla alba) tested were negative.
Table 2.
Results of PCR, ArrayTube Microarray, and genotyping assays in C. psittaci-positive birds
| Case no. | Species | 23S rtPCR for Chlamydiaceae b | 23S ArrayTube Microarrayd | C. psittaci ompA rtPCRe | C. psittaci genotyping assayf | ||
|---|---|---|---|---|---|---|---|
| Ct-value | Result | Ct-value | Result | ||||
| 1 | Blue tit | 39.6 | Ques | Neg | 38.6 | Ques | Genotype A-6BC |
| 2 | Dunnock | 17.2 | Pos | C. psittaci | 18.0 | Pos | Genotype A-VS1 |
| 3 | Dunnock | 41.3 | Ques | Neg | 38.6 | Ques | Weak positive |
| 4 | Dunnock | 18.8 | Pos | C. psittaci | 20.4 | Pos | Genotype A-VS1 |
| 5 | Great tit | 26.3 | Pos | C. psittaci | 27.5 | Pos | Genotype A-VS1 |
| 6 | Great tit | 38.9c | Ques | C. psittaci | 38.8 | Ques | Weak positive |
| 7 | Great tit | 37.1 | Pos | C. psittaci | 38.2 | Ques | Genotype A-VS1 |
| 8 | Great tit | 26.0 | Pos | C. psittaci | 27.0 | Pos | Genotype A-VS1 |
| 9 | Dunnock | 21.9 | Pos | C. psittaci | 22.7 | Pos | Genotype A-VS1 |
| 10 | Collared dove | 15.2 | Pos | C. psittaci | 20.1 | Pos | Genotype E |
| 11 | Dunnock | 19.8 | Pos | C. psittaci | 23.9 | Pos | Genotype A-VS1 |
| 12 | Dunnock | 15.9 | Pos | C. psittaci | 16.9 | Pos | Genotype A-VS1 |
| 13 | Robin | 40.5c | Ques | Neg | 29.6 | Pos | Neg |
| 14 | Blue tit | 43.7c | Ques | C. psittaci | 40.0 | Ques | Neg |
| 15 | Collared dove | 13.9 | Pos | C. psittaci | 17.7 | Pos | Genotype E |
| 16 | Great tit | 26.5c | Ques | C. psittaci | 30.6 | Pos | Genotype A-6BC |
| 17 | Dunnock | 14.6 | Pos | C. psittaci | 19.2 | Pos | Genotype A-6BC |
| 18 | Great tit | 25.9c | Ques | C. psittaci | 30.0 | Pos | Genotype A-6BC |
| 19 | Dunnock | 15.9c | Ques | C. psittaci | 20.9 | Pos | Genotype A-VS1 |
| 20 | Great tit | 19.0 | Pos | C. psittaci | 23.4 | Pos | Genotype A-VS1 |
| 21 | Blue tit | 34.3 | Pos | Neg | 39.5 | Ques | Genotype A-VS1 |
| Six further cases described in a previous studya | |||||||
| Robin | 16.7 | Pos | C. psittaci | 18.1 | Pos | Genotype A-VS1 | |
| Robin | 15.6 | Pos | C. psittaci | 19.9 | Pos | Genotype A-VS1 | |
| Dunnock | 19.1 | Pos | C. psittaci | 23.2 | Pos | Genotype A-VS1 | |
| Robin | 13.0 | Pos | C. psittaci | 17.7 | Pos | Genotype A-VS1 | |
| Dunnock | 23.8c | Ques | C. psittaci | 27.9 | Pos | Genotype A-VS1 | |
| Dunnock | 11.8 | Pos | C. psittaci | 16.5 | Pos | Genotype A-VS1 | |
Samples were considered positive for C. psittaci if they were (1) positive or questionably positive on 23S PCR, and (2) positive (including, for the C. psittaci genotyping assay, “weak positive”) on at least one of the subsequent molecular tests.
Pos positive, Ques questionable positive, Neg negative.
aSix additional chlamydiosis cases reported by Colvile et al. (2012) also submitted for molecular testing.
b23S rtPCR as described by Ehricht et al. (2006) and Zweifel et al. (2009). Ct-value averaged from two duplicate samples, cut-off value 38.0.
cOnly one Ct-value was determined.
d23S ArrayTube Microarray assay as described by Borel et al. (2008).
eOmpA rtPCR as described by Pantchev et al. (2009). Ct-value averaged from at least two duplicate samples, cut-off value 36.0.
f C. psittaci genotyping assay as described by Sachse et al. (2008). In the case of weak signals where the ompA genotype could not be accurately identified by the software, the assignment was done manually based on the closest match: these cases were termed “weak positive.”
Table 3.
Details of mortality incidents and gross post-mortem examination findings in C. psittaci-positive birds
| Case no. | Species and signalment | Details of mortality incident | Body condition, [bodyweight (g)] and gross findings on post-mortem examination | ||
|---|---|---|---|---|---|
| Date and location | Species affected: no. birds found dead (no. seen sick) (and total no. affected individuals) | Clinical signs (if sick birds were observed) and/or perceived cause of death (reported by members of the public) | |||
| 1 |
Blue tit Adult |
Oct 2005 Wiltshire, England |
Blue tit 1 (0) | None reported |
Normal (11.1) Suspected hepatomegaly |
| 2 |
Dunnock Adult male |
Jan–Feb 2006 East Sussex, England |
Dunnock 2 (1) (2 individuals) | One individual was fluffed up prior to death |
Emaciated (17.4) Suspected splenomegaly |
| 3 |
Dunnock Adult |
Sep 2006–Jan 2007 Staffordshire, England |
Dunnock 1 (1) (1 individual) | Dunnock was fluffed up and lethargic prior to death |
Thin (17.0) Hepatomegaly. Necrotic ingluvitis |
| Greenfinch 6 (some) | Some greenfinches were fluffed up and unable to fly | ||||
| Chaffinch 14 (some) | None reported | ||||
| House sparrow 2 (0) | None reported | ||||
| 4 |
Dunnock Adult female |
Feb 2007 Northamptonshire, England |
Dunnock 1 (0) | Suspected window strike |
Emaciated (13.4) Suspected splenomegaly |
| 5 |
Great tit Adult female |
Apr 2007 Wrexham, Wales |
Great tit 1 (0) | None reported |
Thin (14.1) Penetrating wound, rib fractures and fibrinous serositis |
| 6 |
Great tit Adult |
Sep–Oct 2007 East Sussex, England |
Great tit 1 (0) Greenfinch 0 (1) |
Great tit was predated by a cat Greenfinch was fluffed up and lethargic |
Normal (20.5) Splenomegaly. Pedunculated skin lesions on wing. Puncture wound |
| 7 |
Great tit Adult |
Jul–Sep 2007 Surrey, England |
Great tit 3 (3) (≥4 individuals) Blue tit 5 (0) |
Multiple individuals had skin growths, particularly on face and wing. Two of the dead great tits were euthanized |
Normal (17.6) Splenomegaly. Facial skin lesions. Hemorrhage (euthanasia) |
| 8 |
Great tit Adult male |
Jul–Oct 2007 East Sussex, England |
Great tit 2 (3) (3 individuals) | Two great tits were lethargic and one other was observed to have a skin growth on wing |
Normal (16.9) Splenomegaly. Suspected hepatomegaly. Fibrinous serositis. Hemorrhagic, inflamed neck lesion |
| Dunnock 1 (1) (1 individual) | Dunnock was fluffed up before death | ||||
| 9 |
Dunnock Adult |
From the same mortality incident as Case 8 (see above) |
Emaciated (15.9) Hepatomegaly and splenomegaly |
||
| 10 |
Collared dove Adult female |
Sep 2008 Essex, England |
Collared dove 1 (1) (1 individual) | Found sick following cat predation and later died |
Emaciated (108.5) Serositis, air sacculitis and pericarditis. Ingluvitis. Hepatomegaly |
| 11 |
Dunnock Adult male |
Nov 2008–Jan 2009 Powys, Wales |
Dunnock 2 (2) (2 individuals) Robin 1 (1) (1 individual) |
Dunnocks and robin were fluffed up and lethargic before death |
Emaciated (15.7) Anorexia |
| Greenfinch 0 (1) | None reported | ||||
| 12 |
Dunnock Adult male |
Feb 2009 West Sussex, England |
Blue tit 1 (0) | Blue tit was a possible window strike |
Thin (19.2) Fractures with no associated hemorrhage |
| Dunnock 3 (0) | One dunnock was a possible window strike | ||||
| Great tit 3 (0) | One great tit had avian pox (confirmed post-mortem) | ||||
| Robin 2 (0) | None reported | ||||
| Pheasant 1 (0) | None reported | ||||
| 13 |
Robin Nestling |
Apr 2009 Surrey, England |
Robin 3 (0) | All of a clutch of 3 nestlings found dead |
Thin (11.0) Hepatic congestion |
| 14 |
Blue tit Nestling |
May 2009 Staffordshire, England |
Blue tit 6 (0) | Six of a clutch of 7 nestlings died |
Thin (5.3) Suspected hepatomegaly. Anorexia |
| 15 |
Collared dove Juvenile |
Jun 2009 Tyne and Wear, England |
Collared dove 1 (1) (1 individual) | Fledgling, seen lethargic before death | Emaciated (104) |
| Hepatomegaly, splenomegaly and serositis | |||||
| 16 |
Great tit Adult female |
Feb 2010 Wiltshire, England |
Great tit 1 (1) (1 individual) | Lethargic prior to death, with skin lesion on head |
Thin (15.5) Large skin lesion on head. Suspected splenomegaly |
| 17 |
Dunnock Adult male |
Mar 2010 Kent, England |
Dunnock 1 (1) (1 individual) | Dunnock was fluffed up and lethargic then predated by a cat |
Thin (17.0) Wound, fracture and hemorrhage. Splenomegaly and suspected hepatomegaly. Numerous intestinal helminths |
| Blue tit 0 (1) | Blue tit was observed to be “sick” | ||||
| 18 |
Great tit Adult female |
Feb–Apr 2010 Surrey, England |
Great tit 1 (5) (5 individuals) | Multiple great tits had fleshy skin growths, one died |
Thin (13.7) Multiple skin lesions. Suspected splenomegaly. Numerous lice |
| 19 |
Dunnock Adult male |
Apr 2010 Hampshire, England |
Dunnock 1 (1) (1 individual) | Fluffed up and lethargic, euthanized |
Thin (17.0) Wounds, fractures and hemorrhage (euthanasia). Hepatomegaly and suspected splenomegaly |
| 20 |
Great tit Adult female |
Oct 2010 Surrey, England |
Great tit 1 (0) | Skin lumps, cat predation |
Normal (19.0) Multiple skin lesions. Fracture and hemorrhage. Splenomegaly |
| 21 |
Blue tit Adult male |
Mar–Apr 2011 Worcestershire, England |
Blue tit 6 (≥9) Great tit 0 (≥2) |
Sick blue tits and great tits were lethargic and some appeared to have dyspnea. Some appeared to have epiphora and/or blepharitis and/or blepharospasm. At least two sick blue tits were euthanized and some died |
Thin (8.16) Pulmonary congestion. Anorexia |
Nine C. psittaci-positive birds were from eight incidents of multi-species passerine mortality; eight positive birds were from incidents in which only a single bird had been observed to be sick or found dead; and four positive birds were from sites of multiple mortality where a single species had been affected [including two nestlings—a robin (Case 13) and a blue tit (Case 14)—from failed nests] (Table 3). Positive cases had either been observed with non-specific clinical signs (fluffed up plumage and/or lethargy) prior to death (11 cases), had been found dead (4 cases), had suffered trauma (including predation) (at least 6 cases), or had been euthanized for welfare reasons (2 cases). One positive blue tit (Case 21) had been submitted from an incident in which it, and other blue tits and great tits, had been observed with apparent dyspnea and ocular disease. Two positive collared doves were from separate incidents where no other sick or dead birds were observed; there was no report of columbiform morbidity or mortality at any of the positive passerine incidents (Table 3).
Eighteen of the 21 C. psittaci-positive birds were adults, comprising 7 males (4 great tits, 2 dunnocks, and 1 blue tit), 6 females (4 great tits, 1 dunnock, and 1 collared dove), and 5 birds of undetermined sex (2 great tits, 2 dunnocks and 1 blue tit) (Table 3). The remaining positive birds were nestlings (see above) and a juvenile collared dove (Table 3). Positive cases had been submitted in each year of the study: 1 (of 1 tested) was from 2005, 1 (2) from 2006, 7 (12) from 2007, 1 (4) from 2008, 5 (9) from 2009, 5 (8) from 2010, and 1 (4) was from 2011. The positive birds had been found dead in all seasonal quarters of the year: 7 had been found in January-March; 7 in April–June; 2 in July–September; and 5 in October–December. Figure 1 shows the locations of positive and negative cases.
Figure 1.
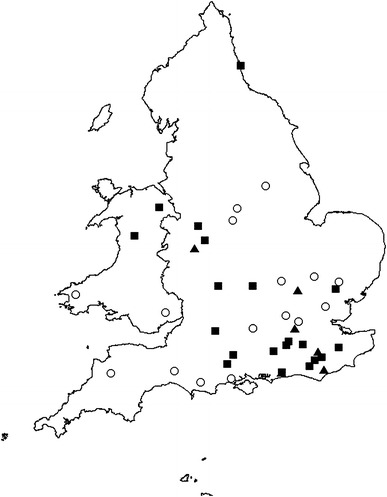
Geographical distribution of garden birds tested for C. psittaci (2005–2011). Closed squares represent sites from which C. psittaci-positive birds were submitted; closed triangles represent sites from which six additional positive birds (described by Colvile et al. 2012) were submitted; and open circles represent sites from which birds negative for C. psittaci were submitted.
The C. psittaci genotype involved was determined for 17 of the 21 positive birds (Table 2). Genotype A was present in all 15 passerine cases for which the genotype was determined (7 dunnocks, 6 great tits and 2 blue tits) and was subtyped as genotype A-VS1 in 11 cases (6 dunnocks, 4 great tits and 1 blue tit) and as genotype A-6BC in 4 cases (2 great tits, 1 dunnock, and 1 blue tit). A further 3 dunnocks and 3 robins confirmed to have chlamydiosis in a previous study (Colvile et al. 2012) were also found to have been infected with genotype A-VS1. The two positive collared doves examined were infected with C. psittaci genotype E.
Pathological Examination
Of the 21 C. psittaci-positive birds, the state of carcass preservation in six birds precluded histopathological or immunohistochemical evaluation (Supplementary Table 2). Of the 15 birds for which tissues were examined microscopically, the significance of C. psittaci infection was unclear in five (Supplementary Table 2), but chlamydiosis was diagnosed by histological and immunohistochemical examination in 10 (Table 4 and Fig 2): 5 dunnocks, 3 great tits, and 2 collared doves, from 9 separate mortality incidents. Of the chlamydiosis cases, body condition was “emaciated” in 6 cases, “thin” in 3 cases, and “normal” in 1 case; splenomegaly was suspected/confirmed in 7 cases, hepatomegaly was suspected/confirmed in 5 cases, and serositis was present in 4 cases (Table 4).
Table 4.
Pathological findings from wild birds with chlamydiosis
| Case no. | Species and signalment | Results of microbiological examination and additional tests | Histopathological findings | Immunohistochemical labeling for Chlamydia sp. specific antigens | Diagnoses |
|---|---|---|---|---|---|
| 2 | Dunnock (Adult male) | Liver and small intestine (SI): Escherichia coli 1. Spleen: no growth | Fibrinous to histiocytic hepatitis with fibrinous thrombosis of the hepatic veins. Fibrinonecrotic, focally extensive splenitis. Fibrinous pneumonia. Histiocytic, focal, mild epicarditis. Proventriculus and gizzard: histiocytic serositis. Giemsa-positive granules in Kupffer cells of the liver, with similar material in some dissociated cells (macrophages or autolyzed hepatocytes), possibly representative of Chlamydial inclusions (or conventional bacteria, such as the E. coli 1 isolated from the tissue). Ziehl-Neelsen (ZN) stain negative for acid-fast agents or inclusions | Intense positive immunolabeling in the heart (endo- and epicardium plus interstitial cells) and serosal surface of the trachea. Foci of positive labeling in the meninges, proventriculus and gizzard. Within the lung, spleen and liver, positive labeling in the cytoplasm of macrophage-like cells (possibly Kupffer cells in the liver) | Chlamydiosis; possible additional bacterial infection |
| 4 | Dunnock (Adult female) | Liver: mixed growth, predominantly E. coli 1. SI: E. coli 1 and Enterococcus sp. | Fibrinous to histiocytic hepatitis, with fibrinous thrombosis of hepatic veins. Fibrinous pneumonia. Marked atrophy of epicardial adipose tissue and pectoral muscle. Giemsa-positive, granular to linear material in Kupffer cells of the liver, possibly representative of Chlamydial inclusions (or conventional bacteria, such as the E. coli 1 isolated from the tissue; interpretation hindered by autolysis). ZN stain negative for acid-fast agents or inclusions | Positive immunolabeling in the heart (interstitium of the left ventricular wall, right ventricular wall cardio-myocytes, and epicardium), liver (macrophages, hepatocytes and white blood cells) and lung | Chlamydiosis; possible coli-septicemia; possible window strike |
| 5 | Great tit (Adult female) | Liver: confluent mixed growth. E. coli 1, Moellerella wisconsensis & Enterococcus spp.. Lung: mixed growth predominance Serratia fonticola & M. wisconsensis. Coelomic cavity: few colonies E. coli 1, M. wisconsensis & Enterococcus spp. | Fibrinous perihepatitis and striking cellular infiltrate of portal tracts throughout the liver parenchyma (interpretation hindered by autolysis). Multifocal, acute pulmonary edema. Moderate to marked atrophy of epicardial adipose tissue. ZN and Giemsa stain reveal no Chlamydial inclusions | Positive labeling in the heart (mainly associated with blood vessels), liver (hepatocytes and possibly Kupffer cells), kidney (interstitial tissue) and keel | Chlamydiosis; trauma (possible predation); possible additional (bacterial/viral) infection |
| 8 | Great tit (Adult male) | Lung, skin lesion and coelom: E. coli 1 & Enterococcus spp. | Vascular endothelial hypertrophy within heart and spleen, with intralesional Gram-negative organisms. Fibrinogranulomatous to mixed cellular, locally extensive, epicarditis, with intralesional Gram-negative organisms. Granulomatous to hemorrhagic, extensive dermatitis, possibly associated with an unidentified mite. Fibrinonecrotizing, focal, acute hepatitis. Fibrinonecrotizing splenitis. Mild pectoral muscle atrophy. Gram-Twort stain shows intra-endothelial organisms as Gram-negative coccobacilli or short rods and shows similar organisms in some epicardial macrophages. ZN and Giemsa stains show no evidence of Chlamydial inclusions | Positive labeling in the heart (epicardium and heart base), spleen (white blood cells), lung (parenchyma and white blood cells), liver (cell-associated, probably macrophages), and skin (inflammatory cells) | Chlamydiosis; possible other bacterial infection |
| 9 | Dunnock (Adult) | Liver: Mixed growth. E. coli 1 and Providencia stuartii. SI and bursa of Fabricus: E. coli 1 | Fibrinonecrotic, marked hepatitis with multifocal probable fibrinous thrombosis and with intralesional bacterial rods. Fibrinonecrotic splenitis. Fibrinous pneumonia with intrahistiocytic bacterial rods. Focal epicarditis. Giemsa stain shows moderate numbers of bacterial rods in blood vessels in all tissues, within pulmonary macrophages and within some of the fibrinous lesions in the liver and spleen. ZN stain negative for acid-fast agents | Positive labeling in the liver (cell-associated and extracellular labeling in sinusoids and blood vessels), spleen (sub-capsule area), heart (interstitial tissue and myocardium), lung (white blood cells and pleura), and trachea (serosal surface) | Chlamydiosis; possible other bacterial infection |
| 10 | Collared dove (Adult female) | Liver, SI, crop, pericardium and lung: mixed, E. coli 1 and Enterococcus spp.. Crop: also Candida tropicalis & C. albicans 1. Crop tissue negative for Trichomonas sp. on culture and PCR | Severe candidiasis (crop mucosa markedly thickened, containing massive numbers of Candida sp. spores and pseudohyphae) and secondary bacterial infection (consistent with E. coli 1 infection isolated on culture). Marked, necrotic pericarditis. Liver, gizzard and small intestine: serocoelomitis. Scattered cells within the spleen appear to contain large, basophilic, cytoplasmic inclusions (autolysis hinders interpretation) | Positive specific labeling in the liver, spleen, serosal surface of the small intestine and individual cells (presumed macrophages) in the lung | Chlamydiosis; cat predation; candidiasis |
| 11 | Dunnock (Adult male) | SI content: Campylobacter sp.. Liver and lung: no growth | Granulocytic enteritis associated with luminal and encysted trematode life stages (consistent with schistosomes but autolysis hinders interpretation). Fibrinonecrotic hepatitis. Focal epicarditis. Mild pulmonary edema (probably agonal). Sarcocystosis of the pectoral muscle. Severe atrophy of epicardial adipose tissue. ZN stain shows no acid-fast agents or inclusions. Giemsa stain faintly highlights Sarcocysts in the pectoral muscle and highlights scanty punctate material in the foci of hepatic necrosis (nuclear dust, or less likely, bacteria or Chlamydial inclusions) | Positive labeling in the lung (diffuse, in cells resembling macrophages and within blood vessels), trachea (serosal surface and intramuscular), pectoral muscle, liver (diffuse, and some associated with bile duct epithelium), proventriculus and gizzard (interstitium and mucosa), heart (cell-associated in interstitium, and myocardium), spleen, and intestines (serosal surface and mucosa) | Chlamydiosis; possible other septicemia; parasitic enteritis; sarcocystosis (probably incidental); intestinal Campylobacter sp. infection (probably incidental) |
| 15 | Collared dove (Juvenile) | Liver: moderate pure growth E. coli 1. SI: confluent nearly pure E. coli 1. Crop: Trichomonas sp. isolated in Bushby’s medium (subclinical infection). Circovirus-specific PCR on necrotic coelomic tissue negativea | Fibrinogranulomatous, extensive serositis with intralesional Gram-negative bacteria and some plant material (possible artefactual transfer, or alimentary tract rupture). Fibrinonecrotic splenitis with intralesional Gram-negative bacteria. Diffuse, marked atrophy of adipose tissue. Giemsa stain highlights bacteria in the coelomic exudate but shows no inclusions. A Periodic Acid-Schiff (PAS) preparation highlights plant matter in the exudate on the stomach and intestine but shows no fungal agents. A Gram-Twort highlights the Gram-negative bacteria as coccobacilli to short rods. ZN stain shows no acid-fast agents or inclusions | Positive labeling in the heart (predominantly cell-associated but also extracellular, often perivascular), spleen (capsule and parenchyma), crop (serosal surface), proventriculus and gizzard (serosal surfaces and intramuscular), intestine (within inflammatory cells on the serosal surface), lung (within macrophages in alveoli and interstitium), and kidney (interstitium) | Chlamydiosis; possible other Gram-negative bacterial infection; possible alimentary tract rupture; subclinical Trichomonas sp. infection |
| 16 | Great tit (Adult female) | Liver: Light nearly pure growth of Serratia ficaria. Small intestine and lung: no growth. Skin lesion: avipox PCR positive | Generalized vascular endothelial hypertrophy in most tissues, with intralesional Gram-negative, PAS-positive organisms. Acute, fibrinous pneumonia with atelectasis. Fibrinonecrotic, extensive hepatitis. Fibrinonecrotic, disseminated, acute or subacute splenitis. Proliferative, multifocally necrotizing, extensive, severe dermatitis with numerous intracytoplasmic inclusion bodies (pathognomonic for avian poxvirus infection) and with minor surface infection by bacterial cocci and mixed fungi. Apparent mild hemoparasitism (compatible with leucocytozoonosis, but other hemoprotozoa could be indistinguishable on histology). ZN and Giemsa stains show no inclusions. Gram-Twort stain shows many of the endothelial bodies as Gram-negative coccoid or short bacillary structures; PAS stain highlights most of the same structures in intense magenta | Positive immunolabeling in the liver (cell-associated, primarily perivascular) and head lesions (inflammatory cells, primarily macrophages). (Brain, trachea, heart, pectoral muscle, lung, esophagus, spleen, proventriculus and gizzard and large intestine devoid of immunolabeling) | Chlamydiosis; avian pox disease with secondary mixed infection; hemoparasitism (significance unclear) |
| 17 | Dunnock (Adult male) | Liver, SI and peritoneum: pure isolate E.coli 1. | Fibrinonecrotic splenitis with intralesional, coccoid to coccobacillary, Gram-negative bacteria. Pulmonary congestion, edema and atelectasis. Generalized perivascular cellular infiltrates (interpretation hindered by autolysis). Sarcocystosis of the pectoral muscle with no evidence of myositis. ZN and Giemsa stains reveal no Chlamydial inclusions. A Gram-Twort stain shows the splenic bacteria to be Gram-negative, and apparently coccoid to coccobacillary | Positive labeling in the brain (cell-associated and possibly extracellular), trachea (muscle), lung (diffuse, cell-associated and extracellular), heart (diffuse, myocardium), crop (serosa), proventriculus (white blood cells within mucosa and lamina propria), gizzard (mucosa), liver (diffuse, cell-associated and extracellular), spleen (sub-capsular region, associated with white blood cells), large and small intestines, kidney (cell-associated), testis (cell-associated, interstitium), pectoral muscle (myofibrils) | Chlamydiosis; possible other bacterial sepsis; cat predation; heavy intestinal helminth burden; sarcocytosis (presumed incidental infection) |
aCircovirus PCR performed by Biobest Laboratories Ltd., Penicuik, Scotland, EH26 0PY, UK.
Figure 2.
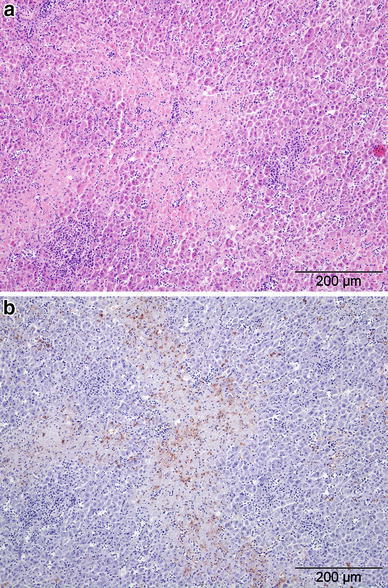
Liver of a dunnock (Prunella modularis) (Case 9), showing a multiple random foci of coagulative hepatocellular necrosis on H&E stain and b semi-serial section of liver subjected to immunohistochemistry (IHC) for Chlamydia spp. bacteria specific LPS: note positive labeling (red/brown pigment) in the cytoplasm of many of the necrotic hepatocytes (central area with pale blue poorly demarcated cells) and also some viable hepatocytes (IHC with haematoxylin counter-stain).
There was concurrent infectious disease in over half (8/15) of the C. psittaci-positive cases examined microscopically. Avian pox was confirmed (using PCR +/- histology +/- electron microscopy) in five C. psittaci-positive great tits from separate incidents, including one (Case 16) with confirmed chlamydiosis (Table 4; Supplementary Table 2). Trichomonosis was diagnosed (using PCR and histology) in one C. psittaci-positive dunnock (Case 3) from a mortality incident affecting predominantly finch species (Table 3). Concurrent trauma, most commonly cat predation, was either confirmed or suspected in nine C. psittaci-positive cases, including four cases of confirmed chlamydiosis (Table 4).
Discussion
When garden bird carcasses from 38 mortality incidents suggestive of chlamydiosis were examined retrospectively, chlamydiosis was diagnosed in at least one bird from each of nine incidents. Ten birds, submitted from 2006–2010, were positive for the disease: eight small passerines (5 dunnocks, 3 great tits) and two collared doves. The eight passerines were from seven separate mortality incidents, which add to eight previously confirmed incidents associated with chlamydiosis in small passerines in Britain (Simpson and Bevan 1989; Pennycott et al. 2009; Colvile et al. 2012). Colvile et al. (2012) described six small passerine chlamydiosis mortality incidents in England, five of which occurred in 2011 and questioned whether there had been a recent increase in the incidence of chlamydiosis in small passerines in Britain. Here, cases of passerine chlamydiosis were identified in each year of the study, indicating that any apparent increase in incidence is most likely to have been due to increased diagnostic effort. Furthermore, our results show that chlamydiosis is likely to have been a commoner cause of disease in small passerines than was previously recognized.
In addition to the 10 garden birds diagnosed with chlamydiosis, a further 11 birds found dead from 2005–2011 were positive for C. psittaci infection. Post-mortem tissue decomposition precluded histological or immunohistochemical examination in six of these cases, while in five cases there was equivocal evidence of chlamydiosis (tissues from four cases were negative on immunohistochemistry; one case had immunolabeling but no evidence of histological lesions consistent with chlamydiosis). Seven of the cases in which chlamydiosis was not confirmed (Cases 1, 3, 6, 13, 14, 18, and 19) had a high average Ct-value (38 or over) in the Chlamydiaceae-specific PCR assay, and in five of these cases at least one of the follow-up C. psittaci-specific assays was also negative, indicative of very low tissue concentrations of the bacterium. C. psittaci infection may not, therefore, have been a primary factor in the death of these birds and may have been incidental in some cases.
There was concurrent infectious disease in over half (8/15) of the C. psittaci-positive cases examined histologically: chlamydiosis was confirmed in 3 of these cases, while in 5 cases, histology was equivocal for chlamydiosis—indicating that another infectious disease may have been the primary cause of morbidity or death. Avian pox in great tits—an emerging infectious disease in Britain (Lawson et al. 2012)—was the most common concurrent infectious disease diagnosed (5 cases). In addition to chlamydiosis, a dunnock examined in the current study had trichomonosis. Concurrent chlamydiosis and trichomonosis were previously reported from a passerine mortality incident in Scotland in 2008 (Pennycott et al. 2009), and concurrent infectious disease is a common finding in other avian species affected by chlamydiosis (Vanrompay et al. 1995). At least four of the 21 C. psittaci-positive cases (including two cases with chlamydiosis) had evidence of cat predation. There have been rare reports of disease in cats and dogs associated with C. psittaci infection (Werth 1989), most commonly attributed to the animals having contact with pet parrots. The risk of pet cats or dogs acquiring the infection from wild birds is unknown but is likely to be low since there are few diagnosed cases of chlamydiosis in these companion animals.
Most (17 of 21) positive cases were selected for testing based on the presence of gross lesions typical of avian chlamydiosis (hepatomegaly, splenomegaly, and serositis), hence any C. psittaci-positive cases with different or no gross lesions would have been overlooked during case selection for this study. Also, only certain species were selected for diagnostic testing. It is therefore not possible to make inferences regarding the prevalence of chlamydiosis, or C. psittaci infection, in the general passerine population in Britain from this study. Further investigation, particularly of cases with no gross or macroscopic lesions (or clinical signs) typical of chlamydiosis, is warranted in order to explore the prevalence of C. psittaci infection in passerines.
Both the number and species of birds that had been observed sick or dead in each of the C. psittaci-positive mortality incidents we identified were highly variable. In the eight positive incidents in which there had been multi-species mortality, tits, dunnocks, robins, and finches were the most commonly affected species, as observed in previous studies (Simpson and Bevan 1989; Pennycott et al. 2009). Such a species complement, however, was one of the criteria used for the selection of cases for this study and was the sole basis for the selection of cases from three incidents, so this observation will be circular. No apparent sex predisposition to C. psittaci infection or seasonality of infection was evident, although the relatively small sample size may provide limited inferences regarding these factors. C. psittaci-positive incidents were widespread geographically (Fig. 1). Two C. psittaci-positive cases were from Wales, where (to the authors’ knowledge) infection with C. psittaci in free-living passerines has not been reported previously.
The use of four very sensitive assays—one family-specific screening assay, combined with three C. psittaci-specific assays—ensured that the overall molecular diagnostic method was highly sensitive and specific. C. psittaci was characterized as genotype A in all 15 passerines in which this could be determined. The sub-genotype was determined to be A-VS1 in 11 cases and A-6BC in 4 cases. A further six passerines diagnosed with chlamydiosis in a previous study (Colvile et al. 2012) also had genotype A-VS1. Genotype A has been identified most commonly in captive psittacines (Sachse et al. 2008, 2009), but our results suggest it is also a common genotype in wild passerines in Britain. Genotype A-VS1 is the most common subtype of genotype A, with the broadest host range of all C. psittaci genotypes, having previously been identified in psittacines, poultry, pigeons, canaries, and pheasants (Sachse and Rüttger 2014). Genotype A-6BC has been identified in a similar range of host species to A-VS1 but appears to be less prevalent (Sachse and Rüttger 2014). In two collared doves with chlamydiosis (Cases 10 and 15), C. psittaci was characterized as genotype E. Genotype E has been identified previously in feral pigeons (Magnino et al. 2009).
Although all C. psittaci genotypes are potentially zoonotic (Vanrompay et al. 2007), genotype A is the most commonly identified genotype in people, including in patients with severe psittacosis (Heddema et al. 2006; Vanrompay et al. 2007; Gaede et al. 2008). C. psittaci genotype A was identified in all four genotyped cases in a recent outbreak of human psittacosis in southern Sweden (that affected at least 25 people), in which wild passerines were implicated as the source of infection (Rehn et al. 2013). The identification of C. psittaci genotype A in passerines in the current study supports wild passerines as a potential source of human infection. Case-control investigations of human psittacosis outbreaks in Australia and Sweden have identified direct or indirect contact with live or dead wild birds (Telfer et al. 2005; Rehn et al. 2013), cleaning of wild bird feeders (Rehn et al. 2013), time spent in the garden, and lawn mowing (Williams et al. 1998; Telfer et al. 2005) as risk factors for disease. It is recommended that the public take sensible hygiene precautions when handling sick or dead wild birds and garden bird feeders, and that they wet areas contaminated with bird droppings prior to cleaning to minimize aerosolization, to reduce the risk of infection with C. psittaci and other zoonotic pathogens (Pennycott et al. 2009; Colvile et al. 2012; Rehn et al. 2013).
Although the overall risk of C. psittaci transmission from wild birds to humans is likely to be low (Haag-Wackernagel and Moch 2004; Rehn et al. 2013), considering that over 12 million households provide supplementary food for garden birds in Britain (Davies et al. 2009), it is important to determine the prevalence of subclinical C. psittaci carriage in wild passerines in order to understand the risks of zoonotic transmission (Colvile et al. 2012).
Conclusion
Through this retrospective study, we almost double (from 8 to 15) the number of small passerine mortality incidents in Britain in which chlamydiosis has been diagnosed, showing that chlamydiosis may be a more common cause of disease in British passerines than was previously recognized. We diagnosed further cases of C. psittaci infection in passerines, and showed that it was unlikely to have been a primary pathogen in some birds. C. psittaci was characterized as genotype A in all the passerines (dunnocks, great tits, robins, and blue tits) from which it was determined, indicating that this is likely to be a common genotype in these species. As this genotype is known to be capable of infecting people, our results support a potential role for wild passerines in the zoonotic transmission of C. psittaci. Further research is required to determine the prevalence of C. psittaci infection in wild birds in Britain; people should be advised to take appropriate hygiene precautions when cleaning wild bird feeders or when handling sick or dead wild birds.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Details of mortality incidents and gross post-mortem examination findings in birds negative for C. psittaci infection. (DOC 56 kb)
Chlamydia psittaci-positive birds in which histology and immunohistochemistry were either not performed, or in which the results were equivocal for chlamydiosis. (DOC 53 kb)
Acknowledgements
The authors would like to thank Gabriela Peniche, Chris Durrant, Shaheed MacGregor, Matthew Perkins, and Belinda Clark (Zoological Society of London); Carmen Kaiser (University of Zürich); Finn Pathologists; Clare Underwood (Moredun Research Institute); the Friedrich-Loeffler-Institute; Cynthia Dare (University of Liverpool); Kirsi Peck [Royal Society for the Protection of Birds (RSPB)], Mike Toms [British Trust for Ornithology (BTO)], James Kirkwood [Universities Federation for Animal Welfare (UFAW)], and Vic Simpson (Wildlife Veterinary Investigation Centre); Liz Poirier and the Open Access Funding Team, UCL Library Services (University College London); and MSc students from the Royal Veterinary College and Institute of Zoology for their assistance. We also thank the members of the public and the BTO’s Garden BirdWatch volunteers who reported disease incidents and submitted dead birds for post-mortem examination. This project received financial support from the UFAW, the RSPB, CJ Wildbird Foods, Gardman Ltd., Cranswick Pet Products, the Birdcare Standards Association, the Scottish Government, and the British Veterinary Association Animal Welfare Foundation. This work was also supported in part by the GB Wildlife Disease Surveillance Partnership which receives funding from Defra through the Surveillance Scanning Programme.
Contributor Information
K. M. Beckmann, Phone: 0044 (0)7790094150, FAX: 0044 (0)20 7483 2237, Email: katie.beckmann@ioz.ac.uk
N. Borel, Email: n.borel@access.uzh.ch
A. M. Pocknell, Email: ann.pocknell@finnpathologists.com
M. P. Dagleish, Email: mark.dagleish@moredun.ac.uk
K. Sachse, Email: konrad.sachse@fli.bund.de
S. K. John, Email: shinto.john@ioz.ac.uk
A. Pospischil, Email: apos@vetpath.uzh.ch
A. A. Cunningham, Email: a.cunningham@ioz.ac.uk
B. Lawson, Email: becki.lawson@ioz.ac.uk
References
- Andersen AA, Franson JC. Avian chlamydiosis. In: Thomas NJ, Hunter DB, Atkinson CT, editors. Infectious Diseases of Wild Birds. Oxford: Blackwell; 2007. pp. 303–316. [Google Scholar]
- Bancroft JD (2008) Theory and Practice of Histological Techniques. Bancroft JD, Stevens M (editors), Oxford: Elsevier Health Sciences, 725 pp
- Borel N, Kempf E, Hotzel H, Schubert E, Torgerson P, Slickers P, Ehricht R, Tasara T, Pospischil A, Sachse K. Direct identification of chlamydiae from clinical samples using a DNA microarray assay—a validation study. Molecular and Cellular Probes. 2008;22:55–64. doi: 10.1016/j.mcp.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Bracewell CD, Bevan BJ. Chlamydiosis in birds in Great Britain: 1. Serological reactions to Chlamydia in birds sampled between 1974 and 1983. The Journal of Hygiene. 1986;96:447–451. doi: 10.1017/S0022172400066225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D, Rae AG, Maley SW, Thomson KM, Livingstone M, Jones GE, Herring AJ. Pathogenesis of Chlamydia psittaci infection in sheep: detection of the organism in a serial study of the lymph node. Journal of Comparative Pathology. 1996;114:221–230. doi: 10.1016/S0021-9975(96)80044-2. [DOI] [PubMed] [Google Scholar]
- Colvile KM, Lawson B, Pocknell AM, Dagleish MP, John SK, Cunningham AA (2012) Chlamydiosis in British songbirds. Veterinary Record 171:177 (DOI: 10.1136/vr.100506, Online August 2, 2012) [DOI] [PubMed]
- Davies ZG, Fuller RA, Loram A, Irvine KN, Sims V, Gaston KJ. A national scale inventory of resource provision for biodiversity within domestic gardens. Biological Conservation. 2009;142:761–771. doi: 10.1016/j.biocon.2008.12.016. [DOI] [Google Scholar]
- Ehricht R, Slickers P, Goellner S, Hotzel H, Sachse K. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Molecular and Cellular Probes. 2006;20:60–63. doi: 10.1016/j.mcp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Gaede W, Reckling K-F, Dresenkamp B, Kenklies S, Schubert E, Noack U, Irmscher H-M, Ludwig C, Hotzel H, Sachse K. Chlamdyophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses and Public Health. 2008;55:184–188. doi: 10.1111/j.1863-2378.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- Geens T, Desplanques A, Van Loock M, Bönner BM, Kaleta EF, Magnino S, Andersen AA, Everett KDE, Vanrompay D. Sequencing of the Chlamydophila psittaci ompA gene reveals a new genotype, E/B, and the need for a rapid discriminatory genotyping method. Journal of Clinical Microbiology. 2005;43:2456–2461. doi: 10.1128/JCM.43.5.2456-2461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag-Wackernagel D, Moch H. Health hazards posed by feral pigeons. Journal of Infection. 2004;48:307–313. doi: 10.1016/j.jinf.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Harkinezhad T, Verminnen K, De Buyzere M, Rietzschel E, Bekaert S, Vanrompay D. Prevalence of Chlamydophila psittaci infections in a human population in contact with domestic and companion birds. Journal of Medical Microbiology. 2009;58:1207–1212. doi: 10.1099/jmm.0.011379-0. [DOI] [PubMed] [Google Scholar]
- Health Protection Agency (HPA), and Department for Environment, Food & Rural Affairs (Defra) (2012) Zoonoses Report UK 2011. Defra, London, UK. http://www.defra.gov.uk/animal-diseases/zoonotic/. Accessed 16 Nov 2013
- Heddema ER, Van Hannen EJ, Duim B, Vandenbroucke-Grauls CMJE, Pannekoek Y. Genotyping of Chlamydophila psittaci in human samples. Emerging Infectious Diseases. 2006;12:1989–1990. doi: 10.3201/eid1212.051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann B, Persson H, Jensen J-K, Joensen HD, Klint M, Olsen B. Chlamydophila psittaci in Fulmars, the Faroe Islands. Emerging Infectious Diseases. 2006;12:330–332. doi: 10.3201/eid1202.050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger-Umlauf HA-M, Marschang RE, Gravendyck M, Kaleta EF. Investigation on the frequency of Chlamydia sp. infections in tits (Paridae) Avian Pathology. 1997;26:779–789. doi: 10.1080/03079459708419252. [DOI] [PubMed] [Google Scholar]
- Kaleta EF, Taday EMA. Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Avian Pathology. 2003;32:435–462. doi: 10.1080/03079450310001593613. [DOI] [PubMed] [Google Scholar]
- Kalmar ID, Dicxk V, Dossche L, Vanrompay D (2013) Zoonotic infection with Chlamydia psittaci at an avian refuge centre. The Veterinary Journal (DOI:10.1016/j.tvjl.2013.10.034, Online November 12, 2013) [DOI] [PubMed]
- Laroucau K, De Barbeyrac B, Vorimore F, Clerc M, Bertin C, Harkinezhad T, Verminnen K, Obeniche F, Capek I, Bébéar C, Durand B, Zanella G, Vanrompay D, Garin-Bastuji B, Sachse K. Chlamydial infections in duck farms associated with human cases of psittacosis in France. Veterinary Microbiology. 2009;135:82–89. doi: 10.1016/j.vetmic.2008.09.048. [DOI] [PubMed] [Google Scholar]
- Lawson B, Lachish S, Colvile KM, Durrant C, Peck KM, Toms MP, Sheldon BC, Cunningham AC (2012) Emergence of a novel avian pox disease in British tit species. PLoS ONE 7(11):e40176 (DOI: 10.1371/journal.pone.0040176) [DOI] [PMC free article] [PubMed]
- Lawson B, Malnick H, Pennycott TW, Macgregor SK, John SK, Duncan G, Hughes LA, Chantrey J, Cunningham AA. Acute necrotising pneumonitis associated with Suttonella ornithocola infection in tits (Paridae) The Veterinary Journal. 2011;188:96–100. doi: 10.1016/j.tvjl.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Magnino S, Haag-Wackernagel D, Geigenfeind I, Helmecke S, Dovč A, Prukner-Radovčić E, Residbegović E, Ilieski V, Laroucau K, Donati M, Martinov S, Kaleta EF. Chlamydial infections in feral pigeons in Europe: review of data and focus on public health implications. Veterinary Microbiology. 2009;135:54–67. doi: 10.1016/j.vetmic.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Olsen B, Persson K, Broholm K-A. PCR detection of Chlamydia psittaci in faecal samples from passerine birds in Sweden. Epidemiology and Infection. 1998;121:481–483. doi: 10.1017/S0950268898001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SR. Psittacosis in man—recent developments in the UK: a review. Journal of the Royal Society of Medicine. 1982;75:262–267. doi: 10.1177/014107688207500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantchev A, Sting R, Bauerfeind R, Tyczka J, Sachse K. New real-time PCR tests for species-specific detection of Chlamydophila psittaci and Chlamydophila abortus from tissue samples. The Veterinary Journal. 2009;181:145–150. doi: 10.1016/j.tvjl.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Pennycott TW, Dagleish MP, Wood AM, Garcia C. Chlamydophila psittaci in wild birds in the UK. Veterinary Record. 2009;164:157–158. doi: 10.1136/vr.164.5.157-b. [DOI] [PubMed] [Google Scholar]
- Prukner-Radovćic E, Horvatek D, Gottstein Ź, Ciglar Grozdanić I, Mazija H. Epidemiological investigation of Chlamydophila psittaci in pigeons and free-living birds in Croatia. Veterinary Research Communications. 2005;29(Suppl. 1):17–21. doi: 10.1007/s11259-005-0083-4. [DOI] [PubMed] [Google Scholar]
- Rehn M, Ringberg H, Runehagen A, Herrmann B, Olsen B, Petersson AC, Hjertqvist M, Kühlmann-Berenzon S, Wallensten A (2013) Unusual increase of psittacosis in southern Sweden linked to wild bird exposure, January to April 2013. Euro Surveillance 18(19):20478. http://www.eurosurveillance.org/ViewArticle.aspx?Articleld=204/ (accessed November 13, 2013) [PubMed]
- Robinson RA, Lawson B, Toms MP, Peck KM, Kirkwood JK, Chantrey J, Clatworthy IR, Evans AD, Hughes LA, Hutchinson OC, John SK, Pennycott TW, Perkins MW, Rowley PS, Simpson VR, Tyler KM, Cunningham AA (2010) Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE 5(8):e12215 (DOI: 10.1371/journal.pone.0012215, Online August 18, 2010) [DOI] [PMC free article] [PubMed]
- Sachse K, Laroucau K, Hotzel H, Schubert E, Ehricht R, Slickers P. Genotyping of Chlamydophila psittaci using a new DNA micro-array assay based on sequence analysis of ompA genes. BMC Microbiology. 2008;8:63. doi: 10.1186/1471-2180-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse K, Laroucau K, Vorimore F, Magnino S, Feige J, Müller W, Kube S, Hotzel H, Schubert E, Slickers P, Ehricht R. DNA microarray-based genotyping of Chlamydophila psittaci strains from culture and clinical samples. Veterinary Microbiology. 2009;135:22–30. doi: 10.1016/j.vetmic.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Sachse K, Rüttger A (2014) Rapid microarray-based genotyping of Chlamydia spp. strains from clinical tissue samples. In: Methods in Molecular Biology, Vol. xxx:Molecular Diagnosis in Veterinary Laboratory Practice—Reviews and Protocols, Cunha MV, Inácio J (editors), New York, USA: Springer, pp xxx [DOI] [PubMed]
- Simpson VR, Bevan B. Chlamydia psittaci infection in robins. Veterinary Record. 1989;125:536. doi: 10.1136/vr.125.21.537-b. [DOI] [PubMed] [Google Scholar]
- Smith KA, Campbell CT, Murphy J, Stobierski MG, Tengelsen LA. Compendium of measures to control Chlamydophila psittaci infection among humans (Psittacosis) and pet birds (Avian Chlamydiosis), 2010 National Association of State Public Health Veterinarians (NASPHV) Journal of Exotic Pet Medicine. 2011;20:32–45. doi: 10.1053/j.jepm.2010.11.007. [DOI] [Google Scholar]
- Telfer BL, Moberley SA, Hort KP, Branley JM, Dwyer DE, Muscatello DJ, Correll PK, England J, McAnulty JM. Probable psittacosis outbreak linked to wild birds. Emerging Infectious Diseases. 2005;11:391–397. doi: 10.3201/eid1103.040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanrompay D, Butaye P, Sayada C, Ducatelle R, Haesebrouck F. Characterisation of avian Chlamydia psittaci strains using omp1 restriction mapping and serovar-specific monoclonal antibodies. Research in Microbiology. 1997;148:327–333. doi: 10.1016/S0923-2508(97)81588-4. [DOI] [PubMed] [Google Scholar]
- Vanrompay D, Ducatelle R, Haesebrouck F. Chlamydia psittaci infections: a review with emphasis on avian chlamydiosis. Veterinary Microbiology. 1995;45:93–119. doi: 10.1016/0378-1135(95)00033-7. [DOI] [PubMed] [Google Scholar]
- Vanrompay D, Harkinezhad T, Van De Walle M, Beeckman D, Van Droogenbroeck C, Verminnen K, Leten R, Martel A, Cauwerts K. Chlamydophila psittaci transmission from pet birds to humans. Emerging Infectious Diseases. 2007;13:1108–1110. doi: 10.3201/eid1307.070074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth D. The occurrence and significance of Chlamydia psittaci and Coxiella burnetii in dogs and cats. A study of the literature. Berliner und Münchener tierärztliche Wochenschrift. 1989;102:156–161. [PubMed] [Google Scholar]
- Williams J, Tallis G, Dalton C, Ng S, Beaton S, Catton M, Elliott J, Carnie J. Community outbreak of psittacosis in a rural Australian town. The Lancet. 1998;351:1697–1699. doi: 10.1016/S0140-6736(97)10444-5. [DOI] [PubMed] [Google Scholar]
- Wreghitt TG, Taylor CED. Incidence of respiratory tract chlamydial infections and importation of psittacine birds. The Lancet. 1988;331:582. doi: 10.1016/S0140-6736(88)91368-2. [DOI] [PubMed] [Google Scholar]
- Zweifel D, Hoop R, Sachse K, Pospischil A, Borel N (2009) Prevalence of Chlamydophila psittaci in wild birds—potential risk for domestic poultry, pet birds, and public health? European Journal of Wildlife Research 55:575–581 (DOI: 10.1007/s10344-009-0275-2, Online May 14, 2009)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of mortality incidents and gross post-mortem examination findings in birds negative for C. psittaci infection. (DOC 56 kb)
Chlamydia psittaci-positive birds in which histology and immunohistochemistry were either not performed, or in which the results were equivocal for chlamydiosis. (DOC 53 kb)


