Abstract
The short- and long-term morbidity and mortality in acute heart failure is still unacceptably high. There is an unmet need for new therapy options with new drugs with a new mode of action. One of the drugs currently in clinical testing in Phase III is ularitide, which is the chemically synthesized form of the human natriuretic peptide urodilatin. Urodilatin is produced in humans by differential processing of pro-atrial natriuretic peptide in distal renal tubule cells. Physiologically, urodilatin appears to be the natriuretic peptide involved in sodium homeostasis. Ularitide exerts its pharmacological actions such as vasodilation, diuresis, and natriuresis through the natriuretic peptide receptor/particulate guanylate cyclase/cyclic guanosine monophosphate pathway. In animal models of heart failure as well as Phase I and II clinical studies in heart failure patients, ularitide demonstrated beneficial effects such as symptom relief and vasodilation, while still preserving renal function. Subsequently, the pivotal acute decompensated heart failure (ADHF) Phase III study, called TRUE-AHF, was started with the objectives to evaluate the effects of ularitide infusion on the clinical status and cardiovascular mortality of patients with ADHF compared with placebo. This review summarizes preclinical and clinical data supporting the potential use of ularitide in the treatment of ADHF.
Keywords: Ularitide, Vasodilation, Diuresis, Natriuresis, Acute decompensated heart failure, Guanylate cyclase
Introduction
Ularitide is the chemically synthesized form of urodilatin, a human natriuretic peptide, produced by differential processing of pro-atrial natriuretic peptide in distal renal tubule cells.1,2 It is a 32-amino acid-containing peptide with the same structure as the 28-amino acid-containing atrial natriuretic peptide (ANP), except for the addition of four amino acids at the N-terminal extension.3 Urodilatin is secreted into urine and forms the basis for a paracrine system regulating water and sodium reabsorption at the level of the collecting duct (Figure 1).1,4 Urodilatin was first isolated from human urine by Wolf-Georg Forssmann's group in 1988.3
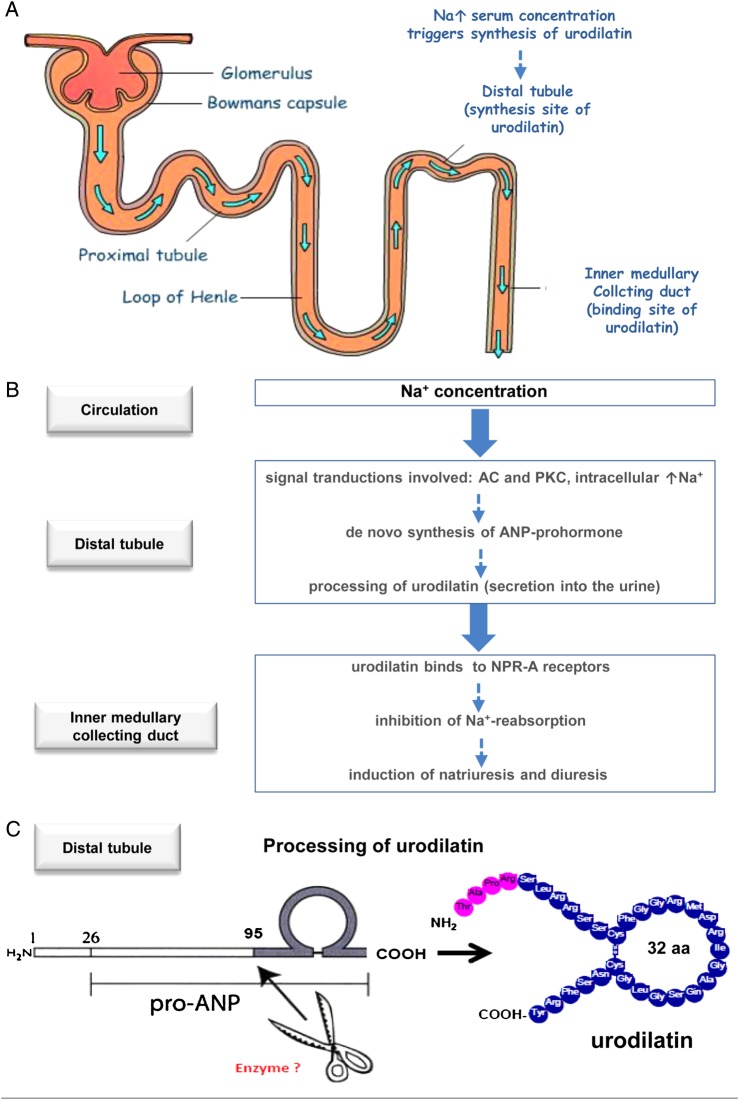
Figure 1.
Schematic drawing of the role of urodilatin under physiological and pathophysiological conditions. (A) Nephron highlighting distal tubule (synthesis site of urodilatin) and collecting duct (binding site of urodilatin). (B) sodium concentration as stimulus for synthesis of urodilatin in distal tubule cells and excretion into the urine, mediated by transduction mechanisms:19 AC, adenylyl cyclyse; PKC, protein kinase (C) and binding to NPR-A receptor in the inner medullary collecting duct. (C) Processing of urodilatin from pro-ANP. Enzyme responsible not known yet (aa, amino acids).
Molecular mode of action
Ularitide binds primarily to the extracellular domain of natriuretic peptide receptor-A (NPR-A) which is expressed in the heart, kidney, vascular smooth muscle tissue, and other organs5,6 binding to and activates the intracellular guanylate cyclase domain of the receptor, also called particulate (membrane-bound) guanylate cyclase (pGC).7 Guanylate cyclases (GC) are enzymes that catalyse the conversion of guanosine 5′-triphosphate to cyclic guanosine-3′,5′-monophosphate (cGMP). The GC family includes both membrane-bound and soluble isoforms that are expressed in nearly all cell types. Membrane-bound pGC serves as a receptor for natriuretic peptides, whereas soluble GC acts as a receptor for the biological messenger nitric oxide. Subsequently, cGMP effectors include cGMP-dependent protein kinases, cGMP-regulated phosphodiesterases, and cyclic nucleotide-gated ion channels (Figure 2).
Figure 2.

Schematic drawing of intracellular cyclic guanosine monophosphate signalling induced by nitric oxide and soluble guanylate cyclase and natriuretic peptides via natriuretic peptide receptor A and particulate guanylate cyclase. Cyclic guanosine monophosphate acts through a number of distinct pathways such as cyclic guanosine monophosphate-dependent protein kinases and the cyclic guanosine monophosphate-regulated phosphodiesterase-5 contributing to main effects such as vasodilation through vasorelaxation of smooth muscle cells and natriuresis and diuresis through inhibition of Na+-reabsorption in distal tubular cells. NO, nitric oxide; sGC, soluble guanylate cyclase; NP, natriuretic peptide; cGMP, cyclic guanosine monophosphate; NPR-A, natriuretic peptide receptor A; pGC, particulate guanylate cyclase; cGK, cyclic guanosine monophosphate-dependent protein kinases; PDE-5, phosphodiesterase-5.
Pharmacological effects of Ularitide include induction of natriuresis, diuresis, vasodilation, bronchodilation, and inhibition of the renin–angiotensin–aldosterone system (RAAS).8–10
Physiology
In a series of in vitro experiments, as well as physiological animal and human studies, it was shown that endogenous urodilatin seems to play an essential role in mediating urinary sodium excretion.
In cardiac denervated conscious dogs, sodium urodilatin excretion is correlated more closely with urodilatin in urine than with circulating ANP at baseline, and following intravenous (IV) saline infusion or left atrial distension in both cardiac innervated and denervated animals.11 In cephalic hypertonia dog models, hypertonic infusion to the brain-induced natriuresis, which was paralleled by increased urodilatin concentrations in the urine, rather than increased ANP concentrations in the blood.12
In healthy subjects, chronic adaptation to increasing oral sodium intake demonstrated a close correlation between an increase in sodium excretion and an increase in urinary urodilatin.13 Additionally, both the circadian rhythm of natriuresis and the natriuresis following acute isotonic saline infusion closely correlated with urinary urodilatin.14 Also, upon hypervolemic stimuli, such as head-out-water immersion or an acute posture, change from an upright to a supine posture led to an increased natriuresis which was paralleled by an increase of urodilatin.4
Thus, physiologically, urodilatin, in concert with other hormones such as the renin–aldosterone system, seems to play a paracrine role in renal function
Pathophysiology
The role of endogenous urinary urodilatin has been investigated in patients with fluid balance disturbances. After termination of tachycardia in patients with paroxysmal supraventricular tachycardia, natriuresis is paralleled by increased urinary urodilatin, rather than by increased circulating ANP.15 Urinary urodilatin is also increased in patients with heart failure New York Heart Association (NYHA) functional classes III and IV compared with classes I and II. Nevertheless, patients retained sodium.16,17 However, the lack of increase in natriuresis with increasing NYHA class may be due to known NPR-A down-regulation in CHF.18
Urodilatin: from stimuli to synthesis
Molecular mechanisms responsible for urodilatin synthesis and excretion were investigated by Lenz et al.19 Using a human kidney cell line (HEK-293) showing many similarities of distal tubule cells, they demonstrated that at basal as well as after stimulation by increasing sodium chloride, these cells release urodilatin. Signal transduction mechanisms involved in this process are adenylyl cyclase, protein kinase C, and increased intracellular calcium, leading to an increased urodilatin secretion. While corin is the responsible enzyme for cleavage of ANP from its prohormone,20 the differential processing of urodilatin from the same prohormone is unknown (Figure 1).
By the application of a urodilatin-specific antibody with no ANP cross-reactivity,21 the immunohistochemical localization of urodilatin was shown exclusively in the distal tubule cell of the human nephron.22 Urodilatin immunoreactivity is present in human urine and has only been found in low plasma concentrations in healthy or diseased humans.21 Hence, it is suggested that urodilatin is released to the luminal part of the distal nephron and binds further downstream in the inner medullary collecting duct to the NPR-A.1,22
Preclinical pharmacology
Rats
The effects of ularitide in models of heart failure were investigated in male Wistar rats with or without (sham-operated) surgically induced chronic aortocaval fistula.23 Following surgery, rats with an aortocaval fistula developed sodium retention and other signs of high-output HF. Ularitide was then infused at incremental doses of 0.75–12 µg/kg. Doses of 1.5 µg/kg and above produced significant (P≤ 0.05), dose-dependent increases in urinary flow (7 ± 1 µL/min at baseline to 25 ± 4 µL/min), glomerular filtration rate (GFR) (1.7 ± 0.1 µL/min at baseline to 2.6 ± 0.1 mL/min), sodium excretion (0.4 ± 0.1 µL/min at baseline to 1.9 ± 0.4 mL/min), and urinary cGMP excretion compared with baseline. Mean arterial pressure (MAP) decreased significantly from 114 ± 5 mmHg at baseline to 96 ± 7 mmHg and 94 ± 4 mmHg only at doses of 6 and 12 µg/kg, respectively.
Dogs
The effects of ularitide on heart failure were also investigated in conscious female dogs with or without surgically induced chronic aortocaval fistula.24 Dogs received 5 pmol/kg/min (∼17.5 ng/kg/min) IV infusions of ularitide or ANP for 75 min and thereafter at 10 pmol/kg/min (∼35 ng/kg/min) for another 75 min. Both the initial and subsequent ularitide infusions demonstrated increases in urine volume and sodium excretion in dogs with high-output CHF (all P < 0.05 vs. controls). Effects were more pronounced than after ANP in both normal dogs and dogs with chronic aortocaval fistula.24
Moreover, the effects of ularitide, ANP, and B-type natriuretic peptide (BNP) were investigated in anaesthetized male dogs with overt CHF that received 2 pmol/kg/min IV infusions of ularitide, canine ANP, or canine BNP.25 Infusion of 2 pmol/kg/min (∼7 ng/kg/min) ularitide resulted in significant decreases of cardiac filling pressures [right atrial pressure (RAP), pulmonary artery pressure (PAP), and pulmonary capillary wedge pressure (PCWP)] (all P < 0.05 vs. baseline) while cardiac output remained unchanged. Although haemodynamic parameter changes were similar between groups, BNP infusions increased urinary sodium excretion and GFR (<0.05) more than ularitide (P < 0.05) while ANP did not result in urinary sodium excretion and GFR change.
In conscious female dogs with low-output CHF, an incrementally increasing 30 min ularitide infusion of 3.2–195 pmol/kg/min (∼11–683 ng/kg/min), followed by 60-min infusions of 0.9% saline, resulted in no effects on MAP, CO, stroke volume, or peripheral vascular resistance, but reduced RAP and PAP.26 Moreover, ularitide led to a significant increase in urinary flow and sodium excretion (P < 0.05 vs. controls).
Thus, in animal models of HF, ularitide administration resulted in little vasodilation but significant increases in diuresis and natriuresis. When comparing with ANP, ularitide appears to have stronger renal effects.23,24 This is most likely due to ularitide’s higher resistance to neutral endopeptidase (NEP) based on its N-terminal extension of four additional amino acids.27,28 One of the main locations of NEP is in the brush border of the proximal tubule. Thus, in contrast to ANP, ularitide is more likely to reach the inner medullary collecting duct to bind to the NPR-A and inhibit sodium reabsorption (Figure 3).
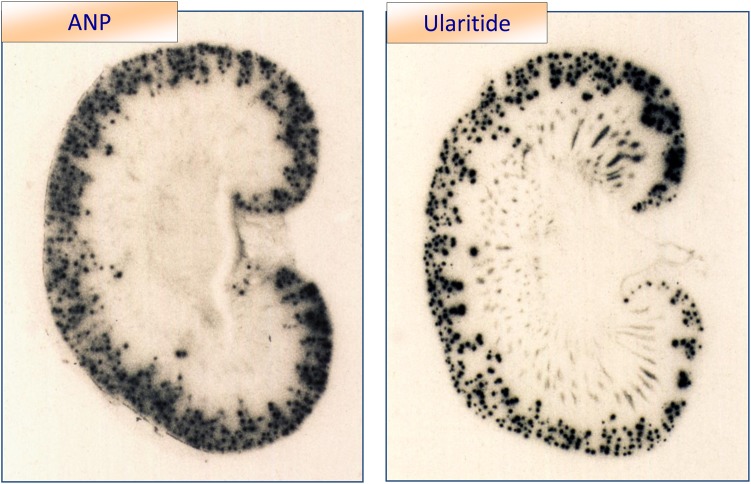
Figure 3.
Radioactive labeled atrial natriuretic peptide and ularitide were infused into the renal artery. Binding sites in the kidney are seen by autoradiography. Atrial natriuretic peptide is not bound to medullary structures while binding of ularitide in medullary stripes indicates that ularitide may not be degraded by neutral endopeptidase in the proximal tubule.1
Clinical studies
Phase I
In an initial open label study, 18 healthy men received an IV bolus injection of 1, 2, or 4 µg/kg ularitide (n = 6 per dose) 15 min after a placebo (0.9% saline) injection.29 Pulmonary capillary wedge pressure started to decrease 5 min after dosing and remained below baseline until 90 min post-administration in a dose-dependent manner. This effect was paralleled by increased plasma and urinary cGMP. Haemodynamic effects following an IV bolus injection of 2 µg/kg ANP (n = 6) were considerably less pronounced than that occurring after all doses of ularitide.
In a randomized, open-label study, 12 NYHA class IV AHF patients received two IV bolus injections of 4 µg/kg ularitide (n = 6) or ANP (n = 6) at 30 min interval.30 Five minutes after the first ularitide dose, PCWP decreased from 22.7 ± 2.3 to 13.0 ± 1.8 mmHg (all data as mean ± standard error of mean [SEM], P < 0.01 vs. baseline) and systemic vascular resistance (SVR) decreased from 1788 ± 210 to 1160 ± 116 dyn s cm−5. Measures of PCWP and SVR remained at those levels until the second injection, then tended to increase at the end of the 90-min study period. Decreases in PCWP and SVR after ANP were considerably less pronounced and shorter. Cardiac index (CI) and stroke volume index increased after ularitide injections, whereas ANP injections resulted in considerably less pronounced and shorter increases. The same was true for plasma cGMP concentrations. During the first 30-min urine collection period, the use of either ularitide or ANP was associated with an ∼4-fold increase in urine flow and sodium excretion (P < 0.05 vs. baseline). Urinary cGMP excretion increased by ∼3-fold (P < 0.05 vs. baseline) in both groups. Thus, ularitide was shown to have stronger haemodynamic effects when compared with ANP and decreases PCWP to a greater extent in patients with acute decompensated heart failure (ADHF).
In one of the first randomized, placebo-controlled, double-blind studies in humans, 12 patients with stable heart failure (NYHA functional class II or III) received 15 ng/kg/min IV ularitide (n = 6) or placebo (n = 6) over 10 h.31 Systolic blood pressure (SBP), MAP, and central venous pressure decreased and heart rate increased during ularitide infusion, whereas these parameters showed only minimal changes in the placebo group. The total urine volume and sodium excretion (all data mean ± SEM) were 795 ± 305 and 77.3 ± 37.0 mmol during ularitide infusion compared with 475 ± 35 mL and 41.8 ± 9.6 mmol with placebo. Owing to the high variability of renal effects in the ularitide group, and the limited number of patients, that difference was not statistically significant.
In these same patients, neurohormone levels (plasma renin activity, plasma concentrations of aldosterone, norepinephrine, vasopressin, and ANP) did not change during ularitide or placebo infusion. However, this may be due to the large variation of respective values in the groups and the small number of patients. Plasma and urine cGMP concentrations rose in parallel to plasma ularitide concentrations. They increased rapidly and reached a plateau after ∼30 min. The half-life of ularitide, determined after end of infusion, was ∼5 min. The authors concluded that ularitide decreases preload and increases diuresis and natriuresis without activation of neurohormonal systems, thus favouring the drug for heart failure patients.
Phase II
The SIRIUS (Safety and efficacy of an Intravenous placebo-controlled Randomized Infusion of Ularitide in a prospective double-blind Study in patients with symptomatic, decompensated chronic heart failure) studies represent the phase II programme to explore the potential clinical value of ularitide in ADHF.
SIRIUS I (Phase IIa)
SIRIUS I was a randomized, double-blind, ascending-dose safety study of 24 ADHF patients requiring hospitalization and monitoring via right heart catheterization, was NYHA functional class III or IV, and had a CI ≤ 2.5 L/min/m2 and PCWP ≥ 18 mmHg).32 Main exclusion criteria were SBP ≤ 90 mmHg, myocardial infarction within the previous 4 weeks, severe valvular stenosis, or cardiogenic shock.
Demographic, medical history, and haemodynamic data at baseline were similar in all four groups.
Compared with baseline, ularitide decreased mean PCWP by 10 mmHg in the 15 ng/kg/min group (P < 0.05; n = 6) and by 15 mmHg in the 30 ng/kg/min group (P < 0.05; n = 6) at 6 h. In both groups, RAP decreased, self-assessed dyspnoea tended to improve, but urine output did not significantly differ. However, loop diuretics were given more frequently in the placebo and 7.5 ng/kg/min groups compared with the higher doses of ularitide (n = 6 vs. n = 3).
At 24 h, 15 and 30 ng/kg/min ularitide infusions decreased mean plasma N-terminal prohormone B-type natriuretic peptide (NT-proBNP) by 40 and 45%, respectively, compared with baseline. Plasma cGMP concentrations did not change in the placebo group, whereas 15 and 30 ng/kg/min ularitide infusions were associated with increased plasma cGMP (P ≤ 0.05 vs. baseline and vs. placebo between 1 and 12 h after start of infusion).
Overall, there was no obvious difference in the safety profiles of the four treatments. During infusion, three subjects experienced transient asymptomatic hypotension with SBP ≤ 90 mmHg at an ularitide dose of 7.5 (n = 1) and 30 ng/kg/min (n = 2).
SIRIUS II (Phase IIb)
In this randomized, double-blind, placebo-controlled study, 221 patients with ADHF (NYHA functional class III or IV; CI ≤ 2.5 L/min/m2, PCWP ≥ 18 mmHg) received either placebo (n = 53) or ularitide at 7.5 ng/kg/min (n = 60), 15 ng/kg/min (n = 53), or 30 ng/kg/min (n = 55) as a 24-h continuous infusion, in addition to standard therapy.33 Haemodynamic parameters (PCWP, RAP, CI, and SVR) were determined at 30 min, 1, 2, 4, 6, 8, 24, and 26 h. Self-assessed changes in dyspnoea were documented for all patients at 6 and 24 h after the start of the infusion using a 7-point Likert scale (prior to haemodynamic assessments). Main exclusion criteria were de novo ADHF, SBP ≤ 90 mmHg, myocardial infarction in the previous 4 weeks, serum creatinine >2.5 mg/dL, or cardiogenic shock.
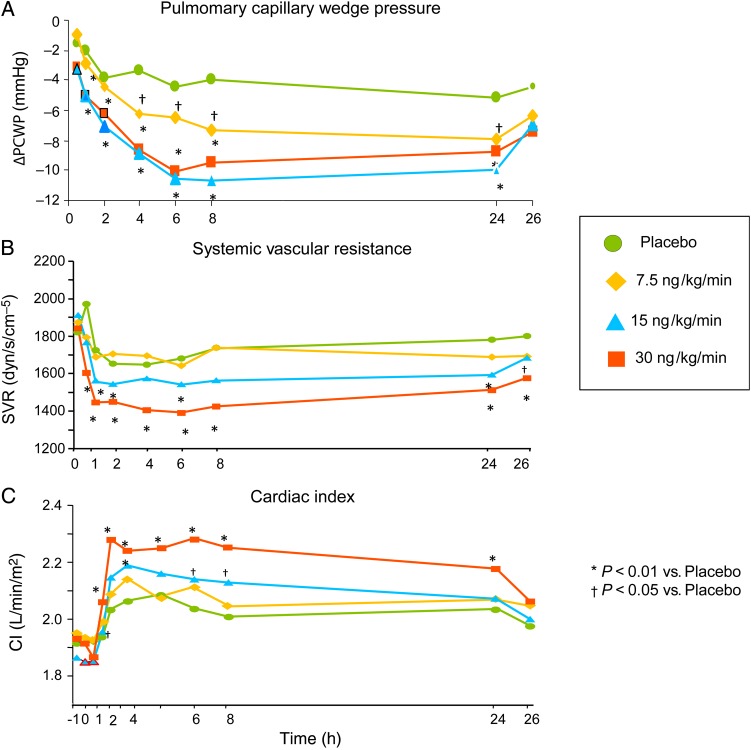
At 6 h, patients receiving ularitide demonstrated a significant decrease in PCWP in the 7.5, 15, and 30 ng/kg/min groups (Figure 4A). Ularitide use was also associated with reduced SVR (Figure 4B) and increased CI (Figure 4C) for the 15 and 30 ng/kg/min groups, beginning 1 h after start of infusion until end of infusion. The time course of the SVR reduction mirrored that of CI increase.
Figure 4.
Haemodynamic parameters for placebo (filled circles) and ularitide 7.5 ng/kg/min (diamonds), 15 ng/kg/min (squares), 30 ng/kg/min (triangles) dose groups. (A) Changes from baseline in pulmonary capillary wedge pressure, (B) systemic vascular resistance (SVR), and (C) cardiac index. P values are given (repeated-measures mixed effects analysis of covariance, including baseline as covariate, treatment, and time of assessment as factors as well as treatment-by-assessment interaction). Adapted from Mitrovic et al.33
In the ularitide groups, more patients assessed their dyspnoea as moderately or markedly improved (Figure 5A), whereas in the placebo group, patients most frequently reported no change. The gradual decrease of PCWP at 6 h correlated well with pooled ularitide- and placebo-treated patients' dyspnoea assessments (Figure 5B).
Figure 5.
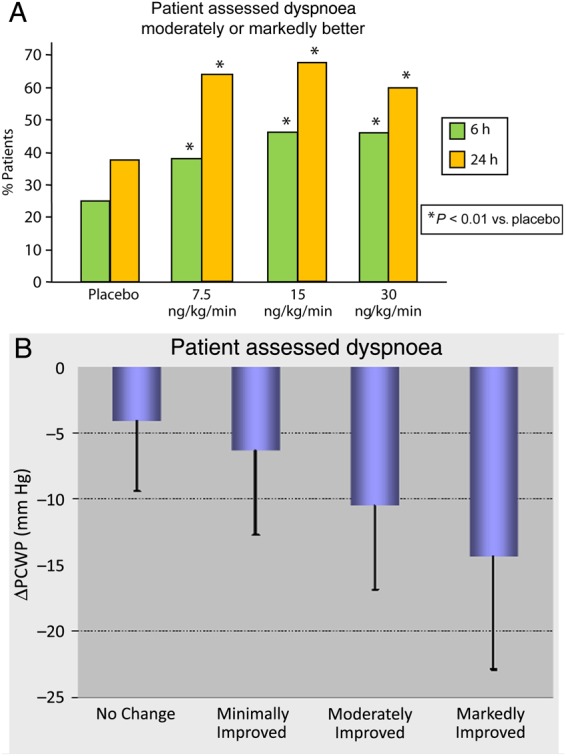
(A) Summarized patients' dyspnoea assessments of ‘moderately improved’ or ‘markedly improved’ at 6 and 24 h. P values of tests vs. placebo, considering all scores (Uleman test). (B) Mean (standard deviation) change from baseline in pulmonary capillary wedge pressure (ΔPCWP) at 6 h in pooled ularitide- and placebo-treated patients who assessed dyspnoea as ‘no change’, ‘minimally improved’, ‘moderately improved’, or ‘markedly improved’.
At 24 h, 15 and 30 ng/kg/min ularitide infusions were associated with decreased NT-proBNP in plasma, compared with placebo.
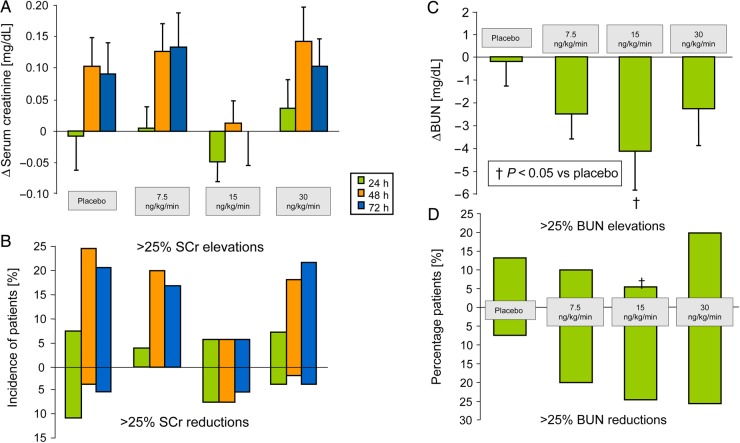
The effects of ularitide on renal function were investigated in further detail.34 Serum creatinine was determined at baseline as well as 24, 48, and 72 h after start of infusion. Blood urea nitrogen (BUN) was only determined at baseline and 24 h (also used for calculation of GFR at 48 and 72 h. The overall, renal function was not impaired by ularitide throughout the 24-h infusion and during a 2-day follow-up period, interestingly, the most favourable renal function was found with the 15 ng/kg/min ularitide dose, although this may be a result of the small numbers of patients studied (Figure 6A and B).
Figure 6.
(A and C) Serum creatinine (SCr) and blood urea nitrogen (BUN) over 72 and 24 h respectively. (B) Percentage patients with BUN elevations and reductions >25% from baseline at 24 h post treatment. (D) Percentage patients with SCr elevations and reductions >25% from baseline at 24, 48 and 72 h post treatment. †P< 0.05 vs. placebo (repeated-measures mixed effects analysis of covariance, including baseline as covariate, treatment, and time of assessment as factors as well as treatment-by-assessment interaction). Adapted from Mitrovic et al.33 and Luss et al.34
Biomarkers for myocardial (troponin I) or renal damage (cystatin C) did not change throughout the 72-h study period.10 At 26 h, ularitide was associated with a significantly decreased plasma endothelin-1 of ∼15% across all doses compared with placebo. Decreased endothelin-1 strongly correlated with decreased PCWP at 24 h (r = 0.509, P < 0.0001). At 6 h, 15 ng/kg/min ularitide infusion was associated with a decreased plasma aldosterone by 40% (P = 0.05).
Systolic blood pressure decreased dose dependently. In two, four, and seven patients of the 7.5, 15, and 30 ng/kg/min groups, respectively, the infusion was temporarily interrupted due to an SBP decrease to ≤80 mmHg. In one patient of each ularitide group, the study drug was discontinued permanently due to that SBP decrease. All patients spontaneously recovered from hypotension. The most frequently reported drug-related adverse events were hypotension (5.4%) and decreased BP (5.4%) in the ularitide groups. Decreases in BP usually occurred 4–12 h after start of infusion; about half of them were asymptomatic. If a patient was symptomatic, symptoms developed over several hours and were usually mild. Compared with other vasodilators, including nesiritide, or nitroglycerin administered in ADHF patients,35–38 hypotension occurred at similar or lower incidence rates.
When compared with other haemodynamic studies in ADHF, the SIRIUS II study allowed inclusion of patients with a low SBP starting from 90 mmHg, which is in contrast to the majority of trials in AHF with other vasodilators.35,39,40
Overall, 12 patients died through Day 30: 7 (13.2%) in the placebo group and 2, 2, and 1 in the 7.5, 15, and 30 ng/kg/min ularitide groups, respectively (3.4, 3.8, and 1.8%, respectively).
Phase III (TRUE-AHF)
The randomized, double-blind, placebo-controlled Phase III TRial of Ularitide's Efficacy and safety in patients with Acute Heart Failure (TRUE-AHF) is ongoing in more than 200 centres in North America, Europe, and Latin America (NCT01661634). TRUE-AHF is an event driven trial, which can enrol up to 4304 patients ADHF with the goal to evaluate the effects of ularitide on clinical status and 180-day mortality outcomes in AHF. TRUE-HF requires early enrolment, a maximum of 12 h and ideally within 6 h after emergency department presentation or hospitalization, followed by a 48-h blinded IV infusion of either 15 ng/kg/min ularitide or placebo, added to standard-of-care therapy.
The objectives of this trial are to evaluate the effects of this ularitide infusion on the clinical status and CV mortality of patients with ADHF compared with placebo. There are two co-primary endpoints: (i) a composite score of assessing ‘worsening heart failure’ that includes a patient global assessment compared with baseline using a 7-point Likert scale of symptoms, persistent or worsening HF, and all-cause mortality at 6, 24, and 48 h after start of study drug infusion, and (ii) CV mortality following randomization for the entire duration of the trial.
TRUE-AHF and ASCEND-HF
In 2001, FDA approved nesiritide to reduce PCWP and improve dyspnoea. ASCEND-HF was initiated as a response to questions raised regarding renal toxicity and mortality associated with nesiritide (BNP). In total, 7141 patients who were hospitalized with ADHF were randomly assigned to receive either nesiritide or placebo for 24–168 h in addition to the standard care. There was no significant difference in rates of death at 30 days or in rates of worsening renal failure. Additionally, there was no improvement in dyspnoea except in the subset of patients who received nesiritide earlier than 15 h after presentation.38
Ularitide (urodilatin) and nesiritide (BNP) belong to the natriuretic peptide family, bind to NPR-A receptor, and share pharmacological properties.41 When comparing study designs of TRUE-AHF (NCT01661634) and ASCEND-HF, there are relevant differences. In short, in TRUE-AHF, patients have to be randomized within 12 h after their initial clinical presentation. This is based on the idea that elevated ventricular filling pressures and distention may lead to stretch-related myocardial injury, the most vulnerable time being the very early hours following the onset of ADHF. TRUE-AHF also enrols a population of ostensibly sicker patients than did ASCEND (more dyspnoea required at enrollment and higher natriuretic peptide level entry criteria) with the objective to maximize the potential ularitide's vasorelaxant effect. Further, TRUE-AHF selects for patients with a higher initial blood pressure so as to minimize the risk associated with iatrogenic hypotension, which is known to be associated with increased risk of death.
In summary, TRUE-AHF is characterized by more stringent inclusion criteria when compared with ASCEND-HF. Main idea is to maximize the effect of ularitide and to minimize adverse events such as hypotensions. However, whether this rationale will translate into better clinical outcome when compared with those in ASCEND-HF needs to be awaited.
Summary of clinical results
In Phase I–II clinical heart failure studies, ularitide demonstrated beneficial effects such as vasodilation and preserved renal function for up to 3 days. In a randomized, double-blind, placebo-controlled Phase IIa and IIb study of 245 patients with ADHF, 24-h infusions of ularitide lowered cardiac filling pressures, improved dyspnoea, and preserved short-term renal function. In addition, troponin assessments did not indicate short-term myocardial damage, while the potent vasoconstrictor endothelin was significantly decreased. Because of the beneficial clinical effects and promising surrogate results seen in these early Phase II studies, the Phase III TRUE-AHF study was begun and currently has enrolled more than 1800 patients.
Funding
Funding to pay the Open Access publication charges for this article was provided by the University of Göttingen Faculty of Medicine.
Conflict of interest: S.D.A., P.P., V.M., W.F.P., and G.F. are reporting honoraria for participating in the executive committee of clinical trials and for speaking from Cardiorentis and Novartis.
References
- 1.Forssmann WG, Richter R, Meyer M. The endocrine heart and natriuretic peptides: histochemistry, cell biology, and functional aspects of the renal urodilatin system. Histochem Cell Biol. 1998;110:335–357. doi: 10.1007/s004180050295. [DOI] [PubMed] [Google Scholar]
- 2.Saba SR, Ramirez G, Vesely DL. Immunocytochemical localization of ProANF 1-30, ProANF 31-67, atrial natriuretic factor and urodilatin in the human kidney. Am J Nephrol. 1993;13:85–93. doi: 10.1159/000168596. [DOI] [PubMed] [Google Scholar]
- 3.Schulz-Knappe P, Forssmann K, Herbst F, Hock D, Pipkorn R, Forssmann WG. Isolation and structural analysis of “urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin Wochenschr. 1988;66:752–759. doi: 10.1007/BF01726570. [DOI] [PubMed] [Google Scholar]
- 4.Goetz KL. Renal natriuretic peptide (urodilatin?) and atriopeptin: evolving concepts. Am J Physiol. 1991;261(6 Pt 2):F921–F932. doi: 10.1152/ajprenal.1991.261.6.F921. [DOI] [PubMed] [Google Scholar]
- 5.Nagase M, Katafuchi T, Hirose S, Fujita T. Tissue distribution and localization of natriuretic peptide receptor subtypes in stroke-prone spontaneously hypertensive rats. J Hypertens. 1997;15:1235–1243. doi: 10.1097/00004872-199715110-00007. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox JN, Augustine A, Goeddel DV, Lowe DG. Differential regional expression of three natriuretic peptide receptor genes within primate tissues. Mol Cell Biol. 1991;11:3454–3462. doi: 10.1128/mcb.11.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitrovic V, Hernandez AF, Meyer M, Gheorghiade M. Role of guanylate cyclase modulators in decompensated heart failure. Heart Fail Rev. 2009;14:309–319. doi: 10.1007/s10741-009-9149-7. [DOI] [PubMed] [Google Scholar]
- 8.Carstens J, Jensen KT, Pedersen EB. Effect of urodilatin infusion on renal haemodynamics, tubular function and vasoactive hormones. Clin Sci (Lond) 1997;92:397–407. doi: 10.1042/cs0920397. [DOI] [PubMed] [Google Scholar]
- 9.Fluge T, Fabel H, Wagner TO, Schneider B, Forssmann WG. Urodilatin (ularitide, INN): a potent bronchodilator in asthmatic subjects. Eur J Clin Invest. 1995;25:728–736. doi: 10.1111/j.1365-2362.1995.tb01951.x. [DOI] [PubMed] [Google Scholar]
- 10.Mitrovic V, Luss H, Seferovic PM, Simeunovic D, Ristic AD, Felix S, et al. Effects of ularitide on the endothelin and aldosterone system, cardiac troponins, and renal function in acute decompensated heart failure. Eur J Heart Fail. 2013;15(Suppl. 1):S10. [Google Scholar]
- 11.Goetz K, Drummer C, Zhu JL, Leadley R, Fiedler F, Gerzer R. Evidence that urodilatin, rather than ANP, regulates renal sodium excretion. J Am Soc Nephrol. 1990;1:867–874. doi: 10.1681/ASN.V16867. [DOI] [PubMed] [Google Scholar]
- 12.Emmeluth C, Drummer C, Gerzer R, Bie P. Roles of cephalic Na+ concentration and urodilatin in control of renal Na+ excretion. Am J Physiol. 1992;262(3 Pt 2):F513–F516. doi: 10.1152/ajprenal.1992.262.3.F513. [DOI] [PubMed] [Google Scholar]
- 13.Meyer M, Richter R, Brunkhorst R, Wrenger E, Schulz-Knappe P, Kist A, et al. Urodilatin is involved in sodium homeostasis and exerts sodium-state-dependent natriuretic and diuretic effects. Am J Physiol. 1996;271(3 Pt 2):F489–F497. doi: 10.1152/ajprenal.1996.271.3.F489. [DOI] [PubMed] [Google Scholar]
- 14.Drummer C, Fiedler F, Konig A, Gerzer R. Urodilatin, a kidney-derived natriuretic factor, is excreted with a circadian rhythm and is stimulated by saline infusion in man. J Am Soc Nephrol. 1991;1:1109–1113. doi: 10.1681/ASN.V191109. [DOI] [PubMed] [Google Scholar]
- 15.Kentsch M, Kuhrmann T, Drummer C, Rodemerk U, Gerzer R, Muller-Esch G. [Renal urodilatin secretion is associated with diuresis and natriuresis after spontaneous, supraventricular tachycardia] Z Kardiol. 1998;87:134–138. doi: 10.1007/s003920050165. [DOI] [PubMed] [Google Scholar]
- 16.Drummer C, Kentsch M, Otter W, Heer M, Herten M, Gerzer R. Increased renal natriuretic peptide (urodilatin) excretion in heart failure patients. Eur J Med Res. 1997;2:347–354. [PubMed] [Google Scholar]
- 17.Jovanovic A, Lehinant S, Forssmann WG, Hamm C, Mitrovic V. Plasma and urinary cGMP in heart failure in relation to natriuretic peptides (Abstract 949) Eur J Heart Fail Suppl. 2010;9(Suppl 1):S168. [Google Scholar]
- 18.Charloux A, Piquard F, Doutreleau S, Brandenberger G, Geny B. Mechanisms of renal hyporesponsiveness to ANP in heart failure. Eur J Clin Invest. 2003;33:769–778. doi: 10.1046/j.1365-2362.2003.01222.x. [DOI] [PubMed] [Google Scholar]
- 19.Lenz W, Herten M, Gerzer R, Drummer C. Regulation of natriuretic peptide (urodilatin) release in a human kidney cell line. Kidney Int. 1999;55:91–99. doi: 10.1046/j.1523-1755.1999.00242.x. [DOI] [PubMed] [Google Scholar]
- 20.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummer C, Fiedler F, Bub A, Kleefeld D, Dimitriades E, Gerzer R, et al. Development and application of a urodilatin (CDD/ANP-95–126)-specific radioimmunoassay. Pflugers Arch. 1993;423:372–377. doi: 10.1007/BF00374930. [DOI] [PubMed] [Google Scholar]
- 22.Herten M, Lenz W, Gerzer R, Drummer C. The renal natriuretic peptide urodilatin is present in human kidney. Nephrol Dial Transplant. 1998;13:2529–2535. doi: 10.1093/ndt/13.10.2529. [DOI] [PubMed] [Google Scholar]
- 23.Abassi ZA, Powell JR, Golomb E, Keiser HR. Renal and systemic effects of urodilatin in rats with high-output heart failure. Am J Physiol. 1992;262(Pt 2):F615–F621. doi: 10.1152/ajprenal.1992.262.4.F615. [DOI] [PubMed] [Google Scholar]
- 24.Villarreal D, Freeman RH, Johnson RA. Renal effects of ANF (95–126), a new atrial peptide analogue, in dogs with experimental heart failure. Am J Hypertens. 1991;4:508–515. doi: 10.1093/ajh/4.6.508. [DOI] [PubMed] [Google Scholar]
- 25.Chen HH, Cataliotti A, Schirger JA, Martin FL, Burnett JC., Jr Equimolar doses of atrial and brain natriuretic peptides and urodilatin have differential renal actions in overt experimental heart failure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1093–R1097. doi: 10.1152/ajpregu.00682.2004. [DOI] [PubMed] [Google Scholar]
- 26.Riegger GA, Elsner D, Forssmann WG, Kromer EP. Effects of ANP-(95-126) in dogs before and after induction of heart failure. Am J Physiol. 1990;259(Pt 2):H1643–H1648. doi: 10.1152/ajpheart.1990.259.6.H1643. [DOI] [PubMed] [Google Scholar]
- 27.Gagelmann M, Hock D, Forssmann WG. Urodilatin (CDD/ANP-95-126) is not biologically inactivated by a peptidase from dog kidney cortex membranes in contrast to atrial natriuretic peptide/cardiodilatin (alpha-hANP/CDD-99-126) FEBS Lett. 1988;233:249–254. doi: 10.1016/0014-5793(88)80436-8. [DOI] [PubMed] [Google Scholar]
- 28.Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993;291(Pt 1):83–88. doi: 10.1042/bj2910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kentsch M, Ludwig D, Drummer C, Gerzer R, Muller-Esch G. Haemodynamic and renal effects of urodilatin in healthy volunteers. Eur J Clin Invest. 1992;22:319–325. doi: 10.1111/j.1365-2362.1992.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 30.Kentsch M, Ludwig D, Drummer C, Gerzer R, Muller-Esch G. Haemodynamic and renal effects of urodilatin bolus injections in patients with congestive heart failure. Eur J Clin Invest. 1992;22:662–669. doi: 10.1111/j.1365-2362.1992.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 31.Elsner D, Muders F, Muntze A, Kromer EP, Forssmann WG, Riegger GA. Efficacy of prolonged infusion of urodilatin [ANP-(95–126)] in patients with congestive heart failure. Am Heart J. 1995;129:766–773. doi: 10.1016/0002-8703(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 32.Mitrovic V, Luss H, Nitsche K, Forssmann K, Maronde E, Fricke K, et al. Effects of the renal natriuretic peptide urodilatin (ularitide) in patients with decompensated chronic heart failure: a double-blind, placebo-controlled, ascending-dose trial. Am Heart J. 2005;150:1239. doi: 10.1016/j.ahj.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Miric M, Moiseyev VS, et al. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur Heart J. 2006;27:2823–2832. doi: 10.1093/eurheartj/ehl337. [DOI] [PubMed] [Google Scholar]
- 34.Luss H, Mitrovic V, Seferovic PM, Simeunovic D, Ristic AD, Moiseyev VS, et al. Renal effects of ularitide in patients with decompensated heart failure. Am Heart J. 2008;155:1012–1018. doi: 10.1016/j.ahj.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Publication Committee for the VMAC Investigators (Vasodilatation in theManagement of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 36.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 37.Hobbs RE, Miller LW, Bott-Silverman C, James KB, Rincon G, Grossbard EB. Hemodynamic effects of a single intravenous injection of synthetic human brain natriuretic peptide in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;78:896–901. doi: 10.1016/s0002-9149(96)00464-x. [DOI] [PubMed] [Google Scholar]
- 38.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 39.McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA. 2007;298:2009–2019. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 40.Ponikowski P, Mitrovic V, Ruda M, Fernandez A, Voors AA, Vishnevsky A, et al. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J. 2014;35:431–441. doi: 10.1093/eurheartj/eht459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CY, Burnett JC., Jr Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–142. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]