Abstract
Background:
Drug addiction is a chronic brain disease characterized by recurrent episodes of relapse to drug-seeking/-taking behaviors. The ventral subiculum, the primary output of the hippocampus, plays a critical role in mediating drug-seeking behavior.
Methods:
A d-amphetamine intravenous self-administration rat model was employed along with focal electrical stimulation of the ventral subiculum (20 Hz/200 pulses) to examine its role in reinstatement of drug-seeking behavior. Dopamine efflux in the nucleus accumbens was measured by in vivo microdialysis and subsequent HPLC-ED analyses. Pharmacological antagonism of dopamine and ionotropic glutamate receptors locally within the nucleus accumbens was employed to assess the role of glutamate and dopamine in reinstatement of drug-seeking behavior induced by stimulation of the ventral subiculum.
Results:
Here, we demonstrate that reinstatement of drug-seeking behavior following extinction of d-amphetamine self-administration by rats was induced by electrical stimulation in the ventral subiculum but not the cortex. This reinstatement was accompanied by a significant increase in dopamine efflux in the nucleus accumbens and was disrupted by microinfusion of a dopamine D1 or D2 antagonist into the nucleus accumbens. Inhibition of N-methyl-D-aspartate or non- N-methyl-D-aspartate receptors had no effect on the reinstatement induced by ventral subiculum stimulation, whereas co-infusion of D1 and N-methyl-D-aspartate antagonists at formerly ineffective doses prevented drug-seeking behavior.
Conclusions:
These data support the hypothesis that dopamine/glutamate interactions within the ventral striatum related to memory processes are involved in relapse to addictive behavior.
Keywords: deep brain-stimulation, ventral subiculum, nucleus accumbens, dopamine amphetamine-reinstatement
Introduction
Drug addiction is a chronic brain disease characterized by recurrent episodes of relapse to drug-seeking/-taking behaviors (Leshner, 1999; White, 2002; Kalivas and Volkow, 2005), and research on the neurobiology of learning and memory may hold the key to effective therapies (Berke and Hyman, 2000; Hyman et al., 2006). Presentation of stimuli that trigger memory of prior use of addictive drugs, such as those associated with previous episodes of drug intake or administration of a priming dose of drug itself, can often reinstate drug-seeking behaviors in humans and experimental animals (O’Brien et al., 1992; Stewart, 2000; Shalev et al., 2002). Brain-imaging studies with human drug addicts report that craving induced by drug-associated stimuli can activate corticolimbic structures, including the prefrontal cortex, hippocampus, amygdala, anterior cingulate, and ventral striatum (Breiter et al., 1997; Childress et al., 1999; Volkow et al., 2002; Leyton and Vezina, 2013), regions involved in different aspects of cognition.
The ventral subiculum (vSub), a primary output of the hippocampus (Groenewegen et al., 1987), plays a critical role in memory formation and contextual information processing (Squire et al., 2004). This brain region projects to the mesocorticolimbic dopamine (DA) system, which subserves the primary rewarding effects of many drugs of abuse, including psychostimulants and opiates (Wise and Bozarth, 1987), and is also involved in synaptic plasticity (Nicola et al., 2000) and memory function (Seamans et al., 1998). Importantly, glutamatergic afferents from the vSub modulate DA efflux in the nucleus accubmens (NAc) (Blaha et al., 1997; Taepavarapruk et al., 2000) and are implicated in approach behavior guided by memory for the location of reward stimuli (Floresco et al., 1997) as well as behavioral sensitization to repeated amphetamine administration (Lodge and Grace, 2008). Thus, activation of the vSub may provide a means by which aspects of drug-related memories could trigger renewed drug-seeking behavior. Indeed, previous studies have demonstrated a critical role of the vSub in different psychostimulant- and opiate-induced reinstatement models (Vorel et al., 2001; Sun and Rebec, 2003; Taepavarapruk and Phillips, 2003; Bossert and Stern, 2014).
Drug-related cues associated with amphetamine, cocaine, and heroin increase DA transmission in the ventral striatum of humans (Volkow et al., 2006; Boileau et al., 2007; Zijlstra et al., 2008). Furthermore, DA D1 receptors (Anderson et al., 2003; Bachtell et al., 2005; Bossert et al., 2007), DA D2 receptors (Bachtell et al., 2005; Anderson et al., 2006), and ionotropic glutamate receptors (Cornish et al., 1999; Cornish and Kalivas, 2000; Shen et al., 2011) within the NAc are implicated in cue- or drug-induced reinstatement of cocaine- and heroin-seeking behavior. In this context, it is of interest that vSub stimulation significantly increases DA efflux in the NAc, which in turn leads to the reinstatement of d-amphetamine (d-AMPH) self-administration during voluntary abstinence following prolonged drug use (Taepavarapruk and Phillips, 2003). Thus, the present study sought to examine the role of DA and glutamate receptors in the NAc in mediating the vSub-induced reinstatement of d-AMPH.
Methods
Subjects
Subjects were 89 male Long-Evans rats (Charles River) weighing between 320 and 350g at the time of surgery. Rats were individually housed in plastic cages in a colony room with an ambient temperature of 25oC and a 12-hour light-dark cycle (7:00 am-7:00 pm; lights on at 7:00 am). Rat chow and water were available ad libitum. Testing was conducted during the light phase. All experiments conformed to the standards of the Canadian Council on Animal Care and were approved by the Committee on Animal Care, University of British Columbia.
Intravenous Self-Administration
To facilitate acquisition of drug self-administration, all rats were trained to press a lever for food pellets (Bioserv) on a fixed-ratio 2 (FR-2) schedule until a criterion of 150 food pellets in a 1-hour session for 3 of 4 days was reached. Depression of second inactive lever had no consequences. After training, all rats were given approximately 15g of rat chow per day in their home cages. Upon completion of FR-2 schedule training, they were returned to free-feeding conditions.
Prior to surgery, rats were anesthetized with ketamine hydrochloride (100mg/kg, intraperitoneal; MTC Pharmaceuticals) and xylazine (10mg/kg, intraperitoneal; Rompun). First, a chronic Silastic jugular catheter (made from custom-made 22-gauge cannula with elongated ends, 5mm, Plastic Products Inc.) was implanted into the jugular vein to the left vena cava. The catheter was tied and glued to the vein, and the free end of the cannula was passed subcutaneously to the top of the head. Immediately following the catheter surgery, a bipolar stimulating electrode was implanted stereotaxically with the tip centered in the vSub/CA1 region of the hippocampus (anteroposterior [AP]=−5.8mm from bregma; mediolateral (ML)=+5.5mm from midline; and dorsoventral (DV)=−6.0mm from dura or in the area V1B of the primary visual cortex (AP=−5.8mm, ML=±5.5mm, and DV=−1.0mm (Paxinos and Watson, 1997). For dialysis experiments, 2 microdialysis probe guide cannulae (19 gauge, 15mm) were implanted bilaterally dorsal to the NAc (AP=+1.7mm, ML=±1.1mm, and DV=−1.0mm). For pharmacological studies, 2 microinfusion guide cannulae (23 G, 15mm) were implanted bilaterally dorsal to the NAc. The guide cannulae, stimulating electrode, and catheter were secured to the skull with 4 jeweler’s screws and dental acrylic. After the surgery, all cannulae were regularly flushed with 0.1mL heparinized saline (10 IU/mL) to maintain patency. Animals recovered from surgery for at least 5 days before testing.
Following recovery from surgery, rats were trained to self-administer d-AMPH solution. The start of the drug session was signaled by illumination of the house light (2W) 2 seconds prior to a single experimenter-administered infusion of d-AMPH (“prime”) at the same dose as all other drug infusions (0.1mg/0.1mL/infusion/5 s). Following the prime, the house light remained illuminated for the remainder of the drug session to indicate the availability of drug. Rats were allowed to self-administer 12 additional infusions of d-AMPH on a FR-2 schedule on 6 daily sessions. Extinction of drug-seeking behavior was initiated on day 7 by substituting a 0.9% saline solution after rats had self-administered 6 infusions of d-AMPH. On subsequent 5-hour extinction trials, only saline solution was available when rats pressed the drug-paired lever, and extinction training continued for at least 5 days until responding on the drug-paired lever was ≤2 presses/h.
Microdialysis
A detailed description of the microdialysis probe construction and perfusion procedure and the analytical method for quantifying DA in dialysate is provided elsewhere (Taepavarapruk et al., 2000). Briefly, probes were constructed with a semipermeable hollow-fiber membrane (2mm of exposed membrane, 340 µm outer diameter, 65,000 Dalton molecular weight cutoff, Filtral 12, Hospal-gambro, Germany). The microdialysis probe was implanted into the NAc and continuously perfused with artificial cerebrospinal fluid at a constant flow rate of 1 µL/min approximately 8 to 12h prior to the start of the drug self-administration session. The dialysate samples were collected every 10 minutes and immediately analyzed by high pressure liquid chromatography with electrochemical detection. DA and its metabolites, dihydroxyphenylacetic acid and homovanillic acid, were quantified from each sample by comparing sample peak heights to peak heights from a calibration curve of the standard solution containing DA and metabolites at 3 different concentrations.
Experimental Protocol
The first experiment tested the effectiveness of brain stimulation in the reinstatement of drug-seeking behavior. Following extinction of responding on the drug-paired lever, constant current cathodal pulses were delivered to the vSub/CA1 or the control placements in the primary visual cortex through an isolator (Iso-flex, A.M.P.I) via a Master-8 stimulator (A.M.P.I., Israel). Parameters for all electrical stimulation were a total of 200 square-wave pulses, 300 µA, at 20 Hz delivered for 10 seconds. In the second experiment, microdialysis was employed to monitor changes of DA in the NAc of rats during the initial extinction session. After extinction was established, DA efflux was measured in the contralateral NAc of rats displaying drug-seeking behavior following electrical stimulation of the vSub. A third experiment examined the effects of intra-NAc infusion of different ionotropic glutamate and DA receptor antagonists on the reinstatement of drug-seeking behavior induced by vSub stimulation. The specific NMDA antagonist, 2-amino-5-phosphonovaleric acid (AP-V); the specific α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate antagonist, antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX); the DA D1 receptor antagonist SCH 23390; or the DA D2 antagonist sulpiride were microinjected into the NAc 15 minutes prior to the vSub stimulation. Bilateral infusions of drug or saline were made via stainless-steel needles (30 gauge) inserted through guide cannulae implanted over the NAc. Saline, AP-V (200 or 400ng in 1 µL/side), or DNQX (200 and 400ng in 1 µL/side) and SCH 23390 (300 and 600ng in 1 µL/side) or sulpiride (300ng in 1 µL/side) were delivered at a rate of 0.5 µL/1min by an infusion pump (Sage Instruments, Model 341). Injection needles were left in place for an additional 1 minute after injection to allow for diffusion. Intra-NAc infusions of saline or drug were counterbalanced across the 2 test sessions.
Drug Preparation
d-AMPH sulphate (Sigma-Aldrich) was dissolved in sterile 0.9% NaCl daily and filtered through a 0.22-µm sterile nylon filter unit (Millipore) prior to use. The dose of d-AMPH used in all experiments was 0.1mg/0.1mL/infusion. AP-V and DNQX were purchased from Precision Biochemical Inc. (Vancouver, Canada). Sulpiride and SCH 23390 were purchased from Research Biochemical Inc. (Natick, MA). A 10-mM AP-V or DNQX stock solution was prepared by dissolving with 50 to 100 µL of 0.1M NaOH solution and was adjusted to pH 7.0. The solution was then topped up to 1mL with normal perfusion medium and kept frozen as aliquots at −20 oC. Drug was diluted from frozen aliquots with sterile saline solution immediately prior to use. Sulpiride was prepared with 1 drop of NaOH solution and was adjusted to pH 7 with HCl solution. SCH 23390 was dissolved in sterile 0.9% NaCl prior to use.
Histology
After completion of each experiment, DC current (100 µA for 10 seconds) was passed through the bipolar stimulating electrode. Animals were euthanized with an overdose of chloral hydrate and transcardially perfused with 0.9% saline followed by 10% formaldehyde solution. The brains were then removed and placed in 10% sucrose in 10% formaldehyde for a few days. Serial 50-µm coronal sections were cut on a freezing microtome and stained for Nissl substance with cresyl violet. Placements of the dialysis probe, infusion needles, and the bipolar stimulating electrode were determined under a light microscope.
Data Analysis
Behavioral data were recorded via a computer interface system (MED Associates Inc.), which also controlled delivery of drug and food. The number of presses on both active (drug-paired lever) and inactive levers was summed in 10-minute or 1-hour bins and continuously recorded throughout the testing period. Group means of lever presses on drug-paired and inactive levers were compared for statistical significance using Student’s paired t test. Concentration of DA and its metabolites in 10-minute dialysate samples were expressed as percentage of values in 4 baseline samples immediately preceding the drug alone or brain stimulation session. Levels of DA and metabolites were uncorrected for probe recovery. All values were presented as the mean±SEM. Statistical analyses of the neurochemical data employed SigmaPlot software for Windows (version 12; Systat Inc). One-way or 2-way ANOVA with repeated measures followed by the Tukey’s posthoc test or Dunnett’s test were employed where appropriate. All P values<0.05 were considered statistically significant.
Results
Effect of vSub Stimulation on d-AMPH–Seeking Behavior during Extinction
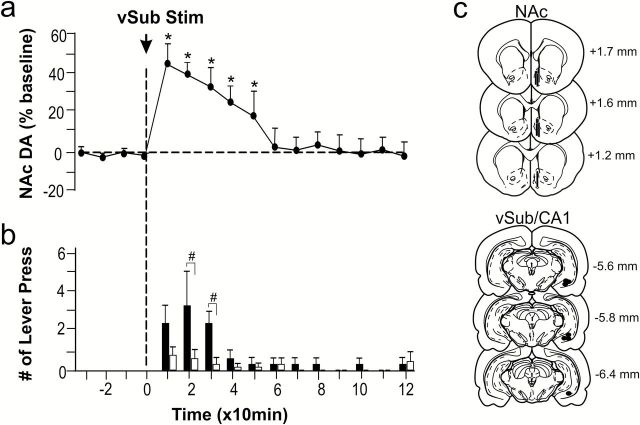
To determine the role of the vSub in d-AMPH–seeking behaviour following extinction, rats that met the extinction criterion (ie, ≤2 responses on the drug-paired lever/hour after 5 consecutive 5-h extinction sessions in which saline was substituted for d-AMPH) were exposed to electrical stimulation of the vSub or the visual cortex. The visual cortex was chosen for the control stimulation site, because this cortical area is situated above the target site of the hippocampus and the electrode targeting the hippocampus passed through this region. As illustrated in Figure 1a, stimulation of the vSub induced reinstatement of drug-seeking behavior as reflected in a significant difference in mean responses on the drug-paired lever as compared with the final day of extinction (F (1,14)=26.66, P<.05 and Tukey’s P<.05, n=8). A significant difference in mean responses on drug-paired active lever vs inactive lever (P<.05, Tukey’s) was also observed. In contrast, stimulation at the control placements in the cortex had no significant effect on mean responses on either lever during extinction (F (1,14) = 0.00, P>.05, n=8). Placements of stimulating electrodes from this experiment are presented in Figure 1b. Electrode tips were all located in the vSub/CA1 regions of the hippocampus or in the area V1B of the primary visual cortex.
Figure 1.

Effect of brain stimulation on reinstatement of d-amphetamine (d-AMPH)– seeking behavior during extinction. a, Stimulation at the ventral subiculum (vSub) or the visual cortex occurred on the day after rats met extinction criterion. Filled bars with black (vSub) or grey (visual cortex) represent mean responses (±SEM) on drug-paired lever in the first 1 hour of an extinction session with brain-stimulation. Unfilled bars represent mean responses (±SEM) on inactive lever. * denotes significant differences in mean responses vs pretest day value, P<.05. # denotes significant differences in mean responses on drug-paired lever versus inactive lever, P<.05. b, Histological placement of stimulating electrodes located with the vSub and visual cortex. Black circles indicate tip of electrodes. Serial coronal brain sections are computer-generated drawings taken from Paxinos and Watson (1997). The numbers beside each plate correspond to millimeters from bregma.
Effect of vSub Stimulation on Extracellular DA Efflux in the NAc during Extinction
To examine the neurochemical correlates of relapse induced by vSub stimulation during extinction, changes in DA efflux were measured in the NAc. In response to the initial 6 infusions of d-AMPH prior to the start of extinction training, DA efflux in the NAc was significantly increased by 549.44±124.42% (F (30,150)=16.20, P<.05 and Dunnett’s, P <.05, n=7) relative to predrug baseline and remained elevated for another 60 minutes before gradually returning to baseline values after approximately 180 minutes (Figure 2a). The marked decrease in DA metabolites was significantly different from predrug baseline values (F (30,150)=12.19, P<.05 and Dunnett’s, P<.05) for dihydroxyphenylacetic acid and (F (30,150)=4.90, P<.05 and Dunnett’s, P<.05) for homovanillic acid (Figure 2b). During the first 5-hour extinction session, all rats responded significantly more on the drug-paired lever in the first hour when d-AMPH was available (ie, 14.0±3.23 vs 2.71±0.96; P<.05, paired Student’s t test). Following saline substitution, higher responding was observed on the drug-paired lever for the next 2 hours (Figure 2c). In the final hour of the session, rats no longer discriminated between active and inactive levers (Figure 2c). Responses on both levers were markedly reduced in the 4 subsequent sessions of saline replacement (data not shown), and extinction was confirmed by the absence of responding on the active or inactive lever in a 40-minute period prior to the application of vSub stimulation (Figure 3b). During the reinstatement test, following a stable baseline of DA efflux in the NAc, the brief train of vSub stimulation caused a significant increase in DA efflux that remained elevated for 50 minutes before returning to baseline values (F (12,72)=14.08, P<.05 and Dunnett’s, P<.05) (Figure 3a) and evoked a significant increase in mean responses on the drug-paired lever at 20 minutes (3.14±1.69 vs 0.57±0.39, inactive lever, P<.05, paired Student’s t test) and 30 minutes poststimulation (2.28±0.56 vs 0.28±0.30, P<.05, paired Student’s t test) (Figure 3b). Placements of microdialysis probes and stimulating electrodes are presented in Figure 3c. Microdialysis probes were located on the border between shell and core regions within the NAcm and electrode tips were all located in the ipsilateral vSub/CA1 region of the hippocampus.
Figure 2.
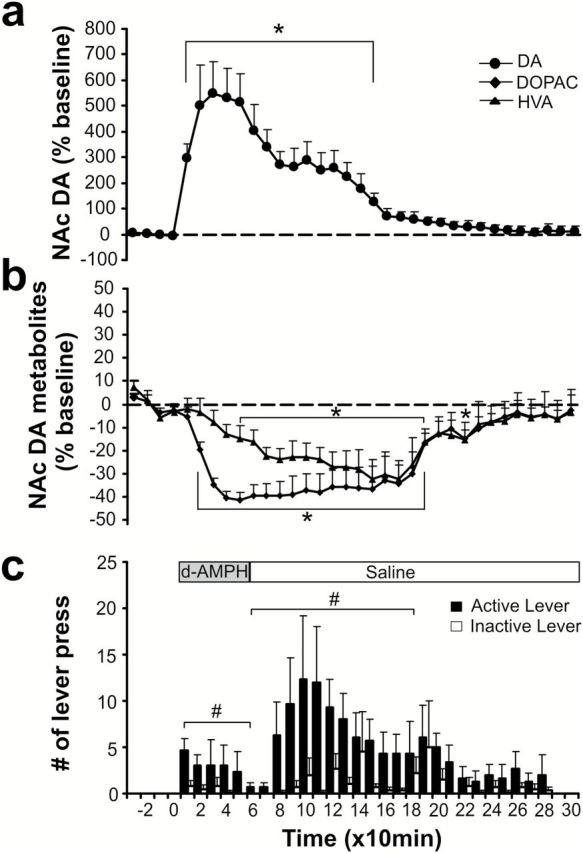
Changes in dopamine (DA), dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) efflux during the first 5-hour extinction session. Rats received 6 infusions of d-amphetamine (d-AMPH) before saline substitution. a, Black circles represent percent change (±SEM) in DA efflux relative to baseline. b, Diamonds and triangles represent mean percent change (±SEM) in DOPAC and HVA efflux relative to baseline, respectively. * denotes significant differences in DA efflux vs prestimulation value (last baseline sample), P<.05. c, Filled and unfilled bars represent mean responses (±SEM) on drug-paired and inactive levers in 10-minute bins, respectively. # denotes significant differences in mean responses on drug-paired lever vs inactive lever, P<.05.
Figure 3.
Effect of stimulation of the ventral subiculum (vSub) on extracellular dopamine (DA) efflux in the nucleus accumbens (NAc) and on lever pressing during extinction. a, Circles represent mean percent change (±SEM) in DA efflux relative to baseline. * denotes significant differences in DA efflux vs prestimulation value (last baseline sample) at P<.05. b, Filled and unfilled bars represent mean responses (±SEM) on drug-paired and inactive levers in 10-minute bins, respectively. # denotes significant differences in mean responses on drug-paired lever vs inactive lever at P<.05. c, Locations of microdialysis probes implanted in the NAc (black bars) and stimulating electrode tips in the ipsilateral vSub (black circles) from all rats in the second experiment. Serial coronal brain sections are computer-generated drawings taken from Paxinos and Watson (1997). The numbers beside each plate correspond to millimeters from bregma.
The Effects of Microinfusion of Glutamate and Dopamine Receptor Antagonists into the NAc on Reinstatement Of Drug-Seeking Induced by vSub Stimulation
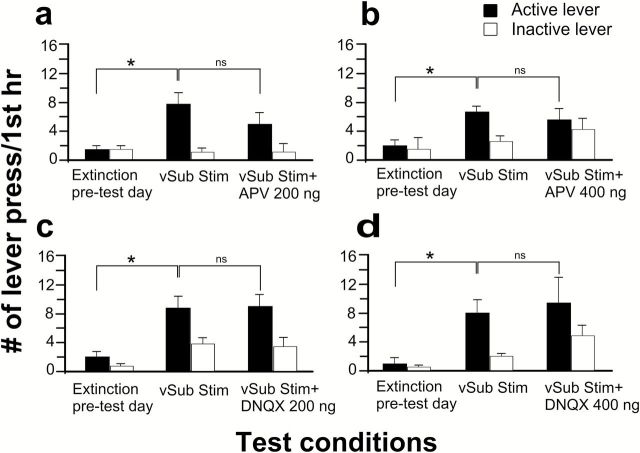
To determine the role of glutamate and DA receptors in mediating the vSub stimulation-induced relapse, different groups of rats received microinjections of glutamate and/or DA antagonists into the NAc prior to vSub stimulation. Replicating the main effect shown in Figure 1a, vSub stimulation induced a significant increase in mean responses on the drug-paired lever relative to extinction data in all groups tested (F values not shown, P<.05) (Figures 4a-d and 5a-d).
Figure 4.
Effects of ionotropic glutamate receptor antagonists on reinstatement induced by stimulation of the ventral subiculum (vSub). The NMDA antagonist 2-amino-5-phosphonopentanoic acid (AP-V) (200 [a] or 400ng [b]) or the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX) (200 [c] or 400ng [d]) was infused bilaterally into the nucleus accumbens (NAc) 15 minutes before stimulation of the vSub. Filled and unfilled bars represent mean responses (±SEM) on drug-paired and inactive levers in first 1 hour of the extinction session, respectively. * denotes significant differences in mean responses on drug-paired lever vs pretest day value at P<.05. # denotes significant differences in mean responses on drug-paired lever between vSub stimulation and treatment groups at P<.05.
Figure 5.
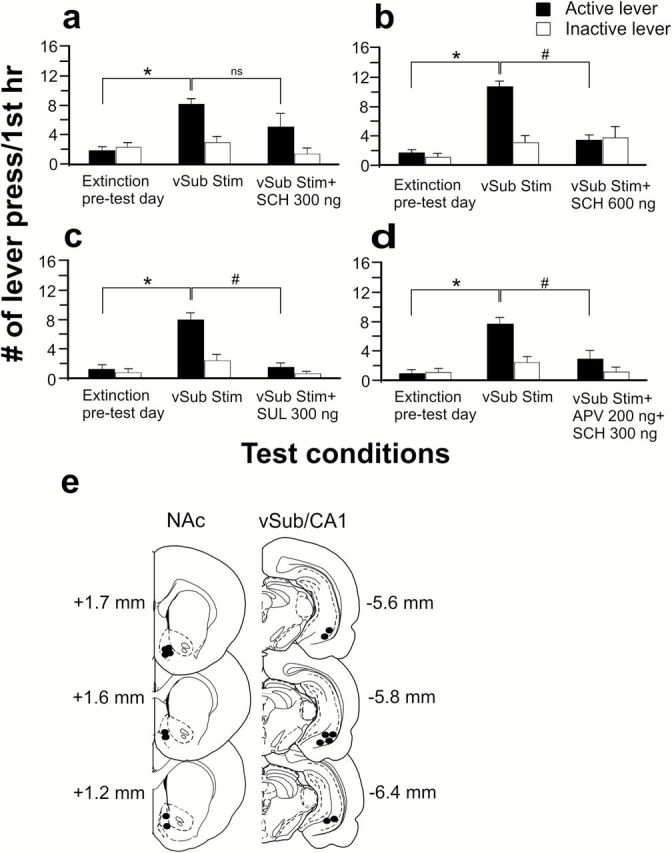
Effects of dopamine (DA) receptor antagonists on reinstatement induced by
stimulation of the ventral subiculum (vSub). The DA D1 antagonist SCH 23390 (300 [a] or 600ng [b]) or the DA D2 antagonist sulpiride 300ng (c) or a combination of AP-V (200ng) and SCH 23390 (300ng) (d) were infused bilaterally into the NAc 15 minutes before stimulation of the vSub. Filled and unfilled bars represent mean responses (±SEM) on the drug-paired lever and inactive lever in first 1 hourr of the extinction session, respectively. * denotes significant differences in mean responses on the drug-paired lever vs pretest day value at P<.05. # denotes significant differences in mean responses on drug-paired lever between vSub stimulation and treatment groups at P<.05. e, Location of microinfusion tips in the NAc (black circles) and stimulation electrode tips in the ipsilateral vSub (black circles) from all rats in the third experiment. Serial coronal brain sections are computer-generated drawings taken from Paxinos and Watson (1997). The numbers beside each plate correspond to millimeters from bregma.
Glutamate Antagonists
Analysis of the mean responses on the drug-paired and inactive lever following a 200-ng dose of AP-V (n = 8) showed no significant differences compared with vSub stimulation (F (1,14)=2.37, p>.05) (Figure 4a). Similarly, when tested with a 400-ng dose of AP-V (n=9), mean responses on the levers were again not significantly different compared with vSub stimulation alone (F (1,16)=1.98, p>.05) (Figure 4b). Infusion of DNQX into the NAc at doses of either 200ng (F (1,14)=1.24, p>.05, n=8) or 400ng (F 1,10)=0.21, p>0.05, n=6) also failed to block vSub-induced reinstatement of drug-seeking behavior (Figure 4c-d).
Dopamine Antagonists
Intra-NAc infusions of the DA D1 antagonist SCH 23390 (300ng, n = 14) did not block the increase in mean responses on the drug-paired lever following vSub stimulation (F (1,26)=1.95, P>.05) (Figure 5a). In contrast, the effects of vSub stimulation were blocked by intra-NAc infusion of SCH 23390 at a dose of 600ng (n=6), as mean responses on the drug-paired lever were significantly different from vSub stimulation scores (F (1,10)=30.86, P<.05 and Tukey’s, P<.05) (Figure 5b). There was no significant difference in mean responses between the inactive levers (P>.05, Tukey’s). Infusion of the DA D2 antagonist sulpiride (300ng, n=8) into the NAc prior to vSub stimulation also blocked the increased responding on the drug-paired lever induced by vSub stimulation (F (1,14)=18.38, P<.05 and Tukey’s, P<.05) (Figure 5c). Mean responses on the inactive levers did not significantly differ (P>.05, Tukey’s). Previous experiments have shown that coincident activation of NMDA and DA D1 receptors within the NAc is required for appetitive instrumental learning (Smith-Roe and Kelley, 2000); therefore, it was of interest to determine if a similar interaction may block reinstatement of drug-seeking behavior induced by vSub stimulation. As shown in Figure 5d, APV (200ng) and SCH 23390 (300ng) infused in combination into the NAc prior to vSub stimulation blocked the increase in responding on the drug-paired lever normally induced by vSub stimulation (F (1,18)=8.12, P<.05 and Tukey’s, P<.05, n=10). There was no significant difference in mean responses between the inactive levers (P>.05, Tukey’s). It is important to note that at the doses employed, neither drug by itself blocked reinstatement induced by vSub stimulation (Figures 4a and 5a). Placements of microinfusion cannulae and stimulating electrodes are presented in Figure 5e. Infusion tracks were located on the border between shell and core regions within the NAc, and electrode tips were all located in the ipsilateral vSub/CA1 region of the hippocampus.
Discussion
The present study confirms that electrical stimulation of glutamatergic afferents to the NAc originating in the vSub of the hippocampal formation can reinstate drug-seeking behavior in rats following extinction of intravenous self-administration of d-AMPH. Stimulation at cortical sites in this study and in the cerebellum or lateral hypothalamus as reported by Vorel et al. (2001) were ineffective; therefore, this form of reinstatement of drug-seeking behavior does not appear to be a general property of brain stimulation per se. The present data also demonstrate that vSub stimulation-induced reinstatement is accompanied by a significant increase in DA efflux in the NAc that is attenuated during the process of extinction despite the continuation of responding on a drug-paired lever. The close relationship between the vSub-evoked increase in DA efflux in the NAc and reinstatement of drug-seeking behavior parallels the effects reported following cocaine- and d-AMPH–induced reinstatement (Neisewander et al., 1996; Di Ciano et al., 2001; Taepavarapruk and Phillips, 2003). Microinjection into the NAc of either the D1 antagonist SCH 23390 (600ng) or the D2 antagonist sulpiride (300ng), or co-infusion of SCH-23390 (300ng) and AP-V (200ng) into the NAc prevented increased responding on the drug-paired lever following vSub stimulation. The ability of intra-NAc DA D1 and D2 antagonists to inhibit vSub-induced d-AMPH reinstatement is consistent with their effects in cue- or drug-induced reinstatement of cocaine- and heroin-seeking behavior (Anderson et al., 2003, 2006; Bachtell et al., 2005; Bossert et al., 2007). However, the role of accumbal NMDA receptors in the reinstatement of drug-seeking behavior has yielded contradictory findings. Administration of either AP-V (3 and 30 μg) (Famous et al., 2007) into the NAc shell or cis-ACDA, an NMDA agonist, into the NAc (Cornish et al., 1999) has been shown to reinstate cocaine-seeking behavior. The present data revealed that co-infusion of SCH-23390 and AP-V into the NAc at doses that were ineffective alone inhibited vSub stimulation-induced reinstatement of psychostimulant self-administration, suggesting that DA/glutamate interactions within the NAc play an important role in relapse through activation of synapses that encode learned associations between drug reward and environmental stimuli. Although the low dose of D2 receptor antagonist blocked vSub stimulation-induced relapse, the possibility still remains that had a subthreshold dose of sulpiride been used in combination with APV, it would also be effective in blocking relapse.
The involvement of AMPA receptors in the NAc in vSub stimulation-induced reinstatement of d-AMPH–seeking behavior appears to differ from reinstatement induced by systemic cocaine or intracerebral infusion of cocaine into the prefrontal cortex, which can be blocked by injection of the AMPA receptor antagonist CNQX into the NAc (Cornish et al., 1999; Cornish and Kalivas, 2000; Park et al., 2002). The absence of DNQX (200–400ng) effects on vSub stimulation on drug-seeking behavior cannot be attributed to the dose of drug, as they were comparable in all 3 studies. However, differences in drug potency between these 2 AMPA antagonists may have been a factor (Honore et al., 1988). Importantly, the method of relapse used in these previous studies differs from that used in the present study in which relapse is induced by administration of cocaine rather than electrical stimulation of the hippocampus. As reinstatement of amphetamine-induced conditioning place preference is associated with decreased expression of AMPA receptors in the NAc (Cruz et al., 2008), future studies need to understand the cellular differences between the different reinstatement methods. We previously reported that vSub stimulation-evoked increases in DA efflux in the NAc could be blocked by reverse dialysis of DNQX (10 µM) for 30 minutes (Taepavarapruk et al., 2000). It remains to be determined if an acute injection of DNQX at the doses employed here would be equally effective in blocking the facilitatory effect of vSub stimulation on DA efflux, and negative results may explain the present failure of DNQX to block reinstatement of drug-seeking behavior.
The neural circuitry underlying vSub stimulation-induced relapse is complex. The vSub sends strong glutamatergic projections to both the NAc and medial prefrontal cortex (mPFC) (Groenewegen et al., 1987; Sesack et al., 1989; Jay and Witter, 1991), and thus, glutamatergic projections from mPFC to NAc (Sesack et al., 1989; Gorelova and Yang, 1997) may be a factor in the effects of vSub stimulation on NAc DA efflux. However, previous studies in our laboratory found no effect of mPFC inactivation on vSub-induced DA efflux in the NAc in control animals (Taepavarapruk et al., 2008). Similarly, Floresco et al. (2001) demonstrated that inactivating the mPFC had no effect on the increase in VTA neuron population activity induced by chemical stimulation of the vSub. Instead, a multisynaptic pathway involving vSub stimulation-induced activation of NAc neurons that inhibit tonically active GABAergic ventral pallidal neurons leading to increased neuron population activity in the VTA has been proposed (Floresco et al., 2001, 2003). This is in agreement with a previous study conducted by Vorel and colleagues (2001) that demonstrated vSub stimulation-induced cocaine reinstatement could be blocked by administration of kynurenic acid and elicited by NMDA into the VTA. As the mPFC plays a critical role in cue- or context-induced reinstatement of opiates and psychostimulants (Rocha and Kalivas, 2010; Shen et al., 2011; Bossert et al., 2014), future studies are needed to determine if inactivation of the mPFC eliminates vSub stimulation-induced reinstatement to d-AMPH.
Exposure to specific sensory cues, including environmental context, previously associated with drug reward can induce relapse to drug use in humans (O’Brien et al., 1992; Stewart, 2000; Shalev et al., 2002) and is associated with hyperactivation of the ventral striatum (Leyton and Vezina, 2013). Taken together, our data suggest that a brief train of focal brain stimulation of a major glutamatergic afferent pathway from the ventral hippocampus to the ventral striatum can reactivate a neural circuit that has been modified by prior association of environmental stimuli with drug reward and thereby reinitiate drug-seeking behavior. Furthermore, we provide evidence that DA/glutamate interactions within the NAc are necessary for context-induced reinstatement of drug seeking. As attenuation of DA transmission has been demonstrated to be effective in reducing cocaine cue-induced craving (Berger et al., 1996; Leyton et al., 2005), our data also suggest that a combination therapy based on coadministration of low doses of DA D1 and NMDA antagonists may prove effective in blocking relapse to psychostimulant self-administration. The ability of vSub stimulation to trigger relapse to both opiates and psychostimulants holds promise as a model system for the further study of adaptive neural processes that encode different facets of addiction. These effects of vSub stimulation also provide a note of caution with regards to the possible use of deep brain stimulation (DBS) at sites within neural circuits implicated in the induction and maintenance of drug-seeking behaviors (Luigjes et al., 2012). Although the stimulus parameters employed in our studies differ from those shown to be effective in attenuating drug-seeking behavior in recent preclinical studies (Luigjes et al., 2012), the pronounced and sustained elevation in DA efflux in the NAc induced by relatively brief trains of electrical brain-stimulation in major afferent projections to the NAc (Taepavarapruk and Phillips, 2003; Taepavarapuk et al., 2008) raises the possibility that DBS may exacerbate addictive behavior depending on the locus of stimulation. As such, we would endorse the recommendation by Carter and Hall (2011) that it is premature on both empirical and ethical grounds to employ DBS for the clinical treatment of drug addiction.
Statement of Interest
A.G.P. was a Director of Allon Therapeutics. P.T. and K.A.B. declare no conflict of interest.
Acknowledgments
We gratefully acknowledge Fred Lepiane, Christina Cheng, and Niwat Taepavarapruk for their assistance. This research was supported by a grant from the Canadian Institutes of Health Research to A.G.P.
References
- Anderson SM, Bari AA, Pierce RC. (2003). Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl) 168:132–138. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. (2006). Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology 31:1452–1461. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. (2005). Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 183:41–53. [DOI] [PubMed] [Google Scholar]
- Berger SP, Hall S, Mickalian JD, Reid MS, Crawford CA, Delucchi K, Carr K. (1996). Haloperidol antagonism of cue-elicited cocaine craving. Lancet 347:504–508. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. (2000). Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25:515–532. [DOI] [PubMed] [Google Scholar]
- Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. (1997). Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci 9:902–911. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. (2007). Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci 27:3998–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. (2007). Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27:12655–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. (2012). Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J Neurosci 32:4982–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL. (2014). Role of ventral subiculum in context-induced reinstatement of heroin seeking in rats. Addict Biol19:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. (1997). Acute effects of cocaine on human brain activity and emotion. Neuron 19:591–611. [DOI] [PubMed] [Google Scholar]
- Carter A, Hall W. (2011). Proposals to trial deep brain stimulation to treat addiction are premature. Addiction 106:235–237. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. (1999). A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience 93:1359–1367. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. (2000). Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Marin MT, Planeta CS. (2008). The reinstatement of amphetamine-induced place preference is long-lasting and related to decreased expression of AMPA receptors in the nucleus accumbens. Neuroscience 151:313–319. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. (2001). Changes in dopamine efflux associated with extinction, CS-induced and d-amphetamine-induced reinstatement of drug-seeking behavior by rats. Behav Brain Res 120:147–158. [DOI] [PubMed] [Google Scholar]
- Famous KR, Schmidt HD, Pierce RC. (2007). When administered into the nucleus accumbens core or shell, the NMDA receptor antagonist AP-5 reinstates cocaine-seeking behavior in the rat. Neurosci Lett 420:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. (1997). Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci 17:1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. (2001). Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci 21:4915–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. (2003). Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Yang CR. (1997). The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience 76:689–706. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. (1987). Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience 23:103–120. [DOI] [PubMed] [Google Scholar]
- Honore T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE. (1988). Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science 241:701–703. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. (1991). Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol 313:574–586. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Leshner AI. (1999). Science-based views of drug addiction and its treatment. JAMA 282:1314–1316. [DOI] [PubMed] [Google Scholar]
- Leyton M, Casey KF, Delaney JS, Kolivakis T, Benkelfat C. (2005). Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci 119:1619–1627. [DOI] [PubMed] [Google Scholar]
- Leyton M, Vezina P. (2013). Striatal ups and downs: Their roles in vulnerability to addictions in humans. Neurosci Biobehav Rev 37:1999–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. (2008). Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci 28:7876–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R, Mazaheri A, De Vries TJ, Denys D. (2012). Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry 17:572–583. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, O’Dell LE, Tran-Nguyen LT, Castaneda E, Fuchs RA. (1996). Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology 15:506–514. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. (2000). Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23:185–215. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. (1992). Classical conditioning in drug-dependent humans. Ann N Y Acad Sci 654:400–415. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. (2002). Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci 22:2916–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1997). The rat brain in stereotaxic coordinates. 3rd ed. San Diego: Academic. [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. (2010). Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci 31:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. (1998). D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci 18:1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 290:213–242. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. (2002). Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42. [DOI] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. (2011). Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A 108:19407–19412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. (2000). Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci 20:7737–7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. (2004). The medial temporal lobe. Annu Rev Neurosci 27:279–306. [DOI] [PubMed] [Google Scholar]
- Stewart J. (2000). Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci 25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. (2003). Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci 23:10258–10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taepavarapruk P, Floresco SB, Phillips AG. (2000). Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology (Berl) 151:242–251. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Howland JG, Ahn S, Phillips AG. (2008). Neural circuits engaged in ventral hippocampal modulation of dopamine function in medial prefrontal cortex and ventral striatum. Brain Struct Funct 213:183–195. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Phillips AG. (2003). Neurochemical correlates of relapse to d-amphetamine self-administration by rats induced by stimulation of the ventral subiculum. Psychopharmacology (Berl) 168:99–108. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. (2002). Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem 78:610–624. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. (2006). Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. (2001). Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292:1175–1178. [DOI] [PubMed] [Google Scholar]
- White FJ. (2002). A behavioral/systems approach to the neuroscience of drug addiction. J Neurosci 22:3303–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. (1987). A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492. [PubMed] [Google Scholar]
- Zijlstra F, Booij J, van den Brink W, Franken IH. (2008). Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol 18:262–270. [DOI] [PubMed] [Google Scholar]