Abstract
Background:
Oxytocin, a neurohypophyseal neuropeptide, is a potential mediator and regulator of drug addiction. However, the cellular mechanisms of oxytocin in drug seeking remain unknown.
Methods:
In the present study, we used a self-administration/reinstatement model to study the effects of oxytocin on cocaine seeking and its potential interaction with glutamate function at the receptor level.
Results:
Systemic oxytocin dose-dependently reduced cocaine self-administration during various schedules of reinforcement, including fixed ratio 1, fixed ratio 5, and progressive ratio. Oxytocin also attenuated reinstatement to cocaine seeking induced by cocaine prime or conditioned cues. Western-blot analysis indicated that oxytocin increased phosphorylation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor GluA1 subunit at the Ser 845 site with or without accompanying increases in phosphorylation of extracellular signal-regulated kinase, in several brain regions, including the prefrontal cortex, bed nucleus of the stria terminalis, amygdala, and dorsal hippocampus. Immunoprecipitation of oxytocin receptor and GluA1 subunit receptors further demonstrated a physical interaction between these 2 receptors, although the interaction was not influenced by chronic cocaine or oxytocin treatment. Oxytocin also attenuated sucrose seeking in a GluA1- or extracellular-signal-regulated kinase-independent manner.
Conclusions:
These findings suggest that oxytocin mediates cocaine seeking through interacting with glutamate receptor systems via second messenger cascades in mesocorticolimbic regions.
Keywords: cocaine, glutamate receptor, oxytocin, reinstatement, self-administration.
Introduction
Oxytocin (OT) is an endogenous peptide that is primarily synthesized in magnocellular neurons of the supraoptic and paraventricular nuclei of the hypothalamus and is secreted by axon terminals in the posterior pituitary into systemic blood circulation (Choy and Watkins, 1977). In addition, OT is produced in parvocellular and magnocellular neurons in the paraventricular nuclei that project to various brain regions and release OT in the brain (Sofroniew, 1983; Knobloch et al., 2012). OT receptors (OTRs) are found in both the peripheral and central nervous systems, including localization in multiple forebrain structures (Gimpl and Fahrenholz, 2001). OTR promiscuously couples to several G proteins (Gq, Gi, Gs) under different conditions and in different brain regions, thereby mediating various functions in neuronal and peripheral tissues via multiple signaling pathways (Stoop, 2012). OT is a potent modulator of a variety of brain functions, including emotions, social interactions, sexual behavior, learning, and memory (Gimpl and Fahrenholz, 2001).
A critical role of OT in drug addiction was proposed many years ago. For instance, OT inhibited the development of tolerance to morphine, morphine withdrawal, and heroin self-administration (Kovacs et al., 1985). Exogenous OT also attenuated hyperlocomotion and stereotyped behavior after acute cocaine, tolerance to chronic cocaine, and cocaine self-administration (Sarnyai and Kovacs, 1994). More recently, intracerebroventricular OT infusion inhibited methamphetamine-induced conditioned place preference (CPP) and its reinstatement induced by stress (Qi et al., 2009). Similarly, intraperitoneal (IP) OT treatment inhibited methamphetamine self-administration and methamphetamine prime-induced reinstatement of drug seeking (Carson et al., 2010; Cox et al., 2013). Based on this promising preclinical data, OT has been proposed as a therapeutic target for drug addiction (McGregor and Bowen, 2012). Although preliminary data from clinical studies are still limited, acute OT blocked alcohol withdrawal symptoms in humans (Pedersen et al., 2013) and ameliorated stress-induced reactivity and craving in marijuana-dependent individuals (McRae-Clark et al., 2013).
Although OT can decrease drug reward-related behavior, the potential cellular mechanisms of OT’s actions on motivated drug seeking remain unknown. Since OT interacts with central dopamine (DA) and glutamate (Glu) pathways, it is possible that OT attenuates reward-related behavior via altered DA activity in mesolimbic regions as well as Glu neurotransmission in cortical regions (Yang et al., 2010). For example, OT inhibited the cocaine-induced enhancement of DA utilization in the nucleus accumbens (NAc) (Kovacs et al., 1990). A recent study indicated that OT antagonized methamphetamine-induced increases in DA turnover in the dorsal striatum and NAc and inhibited the increase of extracellular Glu levels in the medial prefrontal cortex (PFC) after stress-induced reinstatement of CPP in methamphetamine-treated mice (Qi et al., 2009). Atosiban, an OTR antagonist, attenuated both of these effects, indicating that OT exerts its action through an OTR-dependent process (Qi et al., 2008, 2009). However, the molecular cascades that mediate the effect of OT on cocaine-motivated behaviors have not been examined.
Herein, we systematically assessed whether OT would attenuate various measures of cocaine taking and cocaine seeking using the self-administration/reinstatement model of cocaine addiction and relapse. To this end, we first evaluated the effects of OT on cocaine self-administration in rats on various schedules of reinforcement. Second, we determined whether OT would decrease reinstatement of cocaine seeking induced by a cocaine-priming injection or conditioned cues. Finally, we studied the impact of OT on intracellular molecular changes in multiple brain regions involved in cocaine addiction. Because OT increased protein kinase A (PKA) activity and phospho-ERK-1/2 (p-ERK) in cell lysates (Makarevich et al., 2004), we focused on the phosphorylation of GluA1 at Ser845 (p-GluA1, an indicator of PKA activity) and p-ERK expression. Further, since OT altered drug-induced GluN1 protein expression and modulated the neuronal activity in the PFC via AMPA receptors (Qi et al., 2012), we examined a possible interaction between cortical OTR and Glu receptors.
Methods
Subjects
Adult male (initial weight 250–300g) Sprague Dawley rats (Charles River Laboratories, Wilmington, NC) were single-housed in a temperature- and humidity-controlled vivarium on a reversed 12-h–light/–dark cycle (lights off at 6:00 am). All experimental procedures occurred during the dark cycle. Rats were given ad libitum access to water and were maintained on standard chow (20–25g/d, Harlan, Indianapolis, IN) for the duration of each experiment. The experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and complied with federal guidelines in the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources on Life Sciences, 1996).
Surgery
For cocaine self-administration and yoked saline controls, rats underwent catheter implantation 5 to 7 days before the start of self-administration as previously described (Zhou et al., 2012). Briefly, rats were anesthetized (IP) using a mixture of ketamine hydrochloride (66mg/kg; Vedco Inc., St. Joseph, MO) and xylazine (1.33mg/kg; Lloyd Laboratories, Shenandoah, IA), followed by Equithesin (0.5mL/kg) and Ketorolac (2mg/kg; Sigma Aldrich) as a preoperative analgesic. One end of a silastic catheter was implanted into the right jugular vein, and the other end was attached to an infusion harness (Instech Solomon, Plymouth Meeting, PA) for IV drug delivery.
Cocaine Self-Administration
Daily 2-hour self-administration sessions were carried out in standard operant conditioning chambers with 2 retractable levers, 2 stimulus lights, a tone generator, and a house light, and controlled by a computerized data collection program (MED-PC, Med-Associates, St Albans, VT). The house light signaled the initiation of the session and remained on throughout the session. Presses on the active (ie, cocaine-paired) lever resulted in a 2-second activation of the infusion pump (0.2mg/50 μL infusion) and a 5-second presentation of a stimulus light and tone (4.5kHz, 78 dB) complex. After each infusion, a 20-second time-out occurred, during which responses on the active lever were recorded but resulted in no programmed consequences. Presses on the inactive lever had no consequences. Yoked controls received a 50-μL saline infusion and light and tone stimulus whenever the matched cocaine self-administering subject received a cocaine infusion, with concurrent recording of their inconsequential responses. Cocaine hydrochloride (National Institute on Drug Abuse, Research Triangle Park, NC) was dissolved in 0.9% sterile saline.
Extinction and Reinstatement of Cocaine Seeking
Following self-administration and between reinstatement tests, rats underwent daily 2-hour extinction sessions, during which responses on either lever had no consequences. Extinction sessions continued until animals met the criteria of 2 consecutive sessions with <20 active lever presses for a minimum of 7 sessions prior to the first reinstatement test and a minimum of 2 sessions between reinstatement tests. Reinstatement tests consisted of 2-hour cue-induced reinstatement, whereby responses on the previously cocaine-paired lever resulted in a 5-second presentation of the light and tone cues, and 2-hour (1 hour in experiment 3) cocaine-primed reinstatement, whereby rats received cocaine (10mg/kg, IP) immediately prior to the test and active lever presses had no programmed consequences.
Sucrose Self-Administration, Extinction, and Reinstatement
For comparison with OT effects on cocaine-induced cellular changes, experiment 3 (described below) also involved sucrose reinforcement using the same procedure as cocaine self-administration experiments, with the following exceptions. First, sucrose rats did not undergo surgery. Second, instead of cocaine, rats received sucrose pellets (45mg, Noyes pellets, Fisher Scientific, Pittsburg, PA) as the primary reinforcer. Third, during sucrose-primed reinstatement tests, rats received 1 noncontingent pellet every 2 minutes for the first 10 minutes of the session and 1 pellet every 30 minutes thereafter, modified from previous procedures (Kumaresan et al., 2009).
Western Blotting
The procedure of Western blotting has been previously described (Sun et al., 2009). Immediately after cocaine prime-induced restatement, rats were rapidly decapitated and brains were removed, flash frozen, and stored in −80°C until used. Brain regions were dissected, including PFC, NAc, caudate-putamen (CPu), bed nucleus of the stria terminalis (BNST), hypothalamus, amygdala (Amy), dorsal hippocampus (dHip), ventral hippocampus, and ventral tegmental area. Protein was extracted by sonication and loaded on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels then transferred to polyvinylidene difluoride membranes. Membranes were then incubated with primary antibody against p-ERK (1:5000), ERK (1:2000) (both from Cell Signaling, Beverly, MA), p-GluA1 Ser845 (1:1000; Millipore, Billerica, MA), GluA1 (1:10000; Abcam, Cambridge, MA), OTR (1:500; Abbiotec, San Diego, CA), and calnexin (1:10000; Enzo Life Sciences, Farmingdale, NY), followed by their appropriate secondary antibodies. Antibody binding was detected by using an enhanced chemiluminescence kit (ECL Plus; GE Healthcare Bio-Sciences, Piscataway, NJ). The integrated density of protein band was measured using ImageJ software (National Institute of Health, Bethesda, MD).
Co-Immunoprecipitation
The procedure of co-immunoprecipitation has been previously described (Sun et al., 2009). Briefly, equal amounts of protein extracts (250 µg) were incubated with GluA1 antibody (9 µg; Millipore, Billerica, MA) overnight, followed by the addition of TrueBlot anti-rabbit Ig immunoprecipitation beads (60 µL; eBioscience, San Diego, CA) for 4 hours at 4°C. Beads were washed 3 times, centrifuged, and then boiled in Lammeli buffer for 5 minutes for protein detachment. Proteins were loaded on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and analyzed for GluA1 and OTR by immunoblotting as described above. The primary antibody against OTR (1:500) was used to detect the association between GluA1 and OTR.
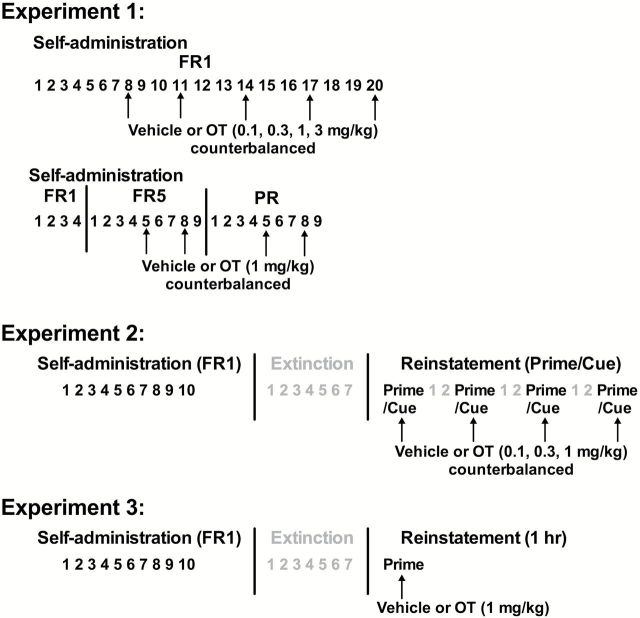
Experiment 1: OT Treatment during Self-Administration on Different Schedules of Reinforcement
In this experiment, we tested the effects of OT administration on established cocaine self-administration using an FR1 schedule of reinforcement, which is used in the majority of reinstatement models of relapse. Once cocaine intake was stabilized (>10 infusions/session) for 7 sessions, all rats received vehicle or OT (0.1, 0.3, 1, 3mg/kg, IP) in a counterbalanced manner 30 minutes prior to the self-administration sessions, with at least 2 sessions to verify stable responding between tests.
We further explored the impact of OT on responding for cocaine using higher demand schedules of reinforcement. We used a 1-mg/kg dose of OT, as it decreased cocaine seeking on the FR1 schedule of reinforcement (results from experiment 1) without affecting locomotor activity (L. Zhou, PhD, unpublished data, January, 2013). Another set of rats experienced 4 cocaine self-administration sessions on an FR1 schedule of reinforcement followed by 9 sessions using an FR5 schedule. Thirty minutes prior to the fifth and eighth sessions, rats received either vehicle or OT in a counterbalanced order. Rats then underwent 9 daily self-administration sessions on a PR schedule, during which reinforcement was contingent upon an increasing number of responses that incrementally increased through the following equation: Response ratio=(5e [injection number×0.2])−5 (Richardson and Roberts, 1996). Rats were pretreated with vehicle or OT in a counterbalanced order 30 minutes prior to the fifth and eighth sessions. The PR sessions were 5 hours long but were terminated earlier if the rat failed to receive an infusion within 1 hour. A time line for experiment 1 is shown in Figure 1.
Figure 1.
Timeline of experiments 1, 2, and 3. Phases of self-administration, extinction, and reinstatement are shown. Arrows indicate the administration of vehicle or oxytocin (OT).
Experiment 2: OT Treatment during Reinstatement
A separate cohort of subjects first experienced 10 sessions of cocaine self-administration (>10 infusions/session) on an FR1 schedule of reinforcement and at least 7 sessions of extinction until extinction criteria were met. They then received vehicle or OT (0.1, 0.3, 1mg/kg) in a counterbalanced order 30 minutes prior to discrete trials for conditioned cue-induced or cocaine-primed reinstatement of cocaine seeking (2 separate groups), with a minimum of 2 extinction sessions between reinstatement tests. A time line for experiment 2 is shown in Figure 1.
Experiment 3: OT Effects on Cocaine-Induced Cellular Changes
Since the GluA1 subunit has been implicated in cocaine seeking induced by a cocaine prime (Bachtell et al., 2008; Ping et al., 2008), we examined the possible effects of OT and cocaine on the p-GluA1 expression in cocaine prime-induced reinstatement. After 10 sessions of cocaine self-administration and 7 sessions of extinction, rats received vehicle or OT (1mg/kg) treatment 30 minutes before a 1-hour cocaine-primed reinstatement test, as the most pronounced OT effect was observed during the first hour (experiment 2). Yoked saline subjects also experienced a cocaine priming injection and were used as controls for the effects of chronic cocaine self-administration on the selected proteins. To determine whether OT effects on molecular changes were cocaine specific, another set of rats served as controls for no cocaine experience using sucrose self-administration and a 1-hourr sucrose-primed reinstatement. For all subjects, rats underwent rapid decapitation, and brains were removed immediately after the reinstatement test. Nine separate brain regions were then dissected out and homogenized for Western blot and/or immunoprecipitation analysis. A time line for experiment 3 is shown in Figure 1.
Data Analysis
To evaluate dose-dependent OT effects on cocaine self-administration on the FR1 schedule of reinforcement and subsequent reinstatement, 1-way repeated-measures analysis of variance (ANOVA) was used to analyze active lever responses or cocaine intake/sucrose pellets. Paired sample t tests were used to compare active lever pressing or cocaine intake on the FR5 schedule of reinforcement as well as lever responding or breakpoint on the PR schedule of reinforcement. Cocaine intake was calculated as cocaine received during a session adjusted to an individual rat’s body weight (0.2mg/infusion×infusion number/body weight). Two-way ANOVAs (cocaine) or t tests (sucrose) were used to analyze active lever responses or protein levels in Western blotting and immunoprecipitation. Pairwise multiple comparisons were conducted using Bonferroni correction. Pearson correlations were used to determine correlations between active lever presses and protein levels measured. All data are presented as the mean±SEM and α was set at P<.05.
Results
OT Dose-Dependently Reduced Cocaine Seeking during Self-Administration
Results from experiment 1 consistently indicated the attenuating effect of OT on cocaine self-administration during the maintenance phase under different schedules of reinforcement. In rats trained on an FR1 schedule of reinforcement, OT reduced both active lever responding (F(4,49)=14.69, P<.001) (Figure 2a) and cocaine intake (F(4,49)=13.34, P<.001) (Figure 2b). Pairwise comparisons indicated that OT reduced active lever presses and cocaine intake at the doses of 0.3, 1, and 3mg/kg. When the demand increased to FR5, OT (1mg/kg) significantly reduced active lever presses (t10=7.66, P<.01) (Figure 2c) and cocaine intake (t10=6.99, P<.01) (Figure 2d). Further, when the schedule of reinforcement changed to a PR, OT also significantly attenuated active lever presses (t8=2.52, P<.05) (Figure 2e), with a marginal effect on the break point (t8=2.11, P=.068) (Figure 2f). OT did not affect responding on the inactive lever during FR1 (F(4,49)=0.72, P>.05), FR5 (t10=0.84, P>.05), or PR (t8=0.59, P>.05) schedules of reinforcement (data not shown).
Figure 2.
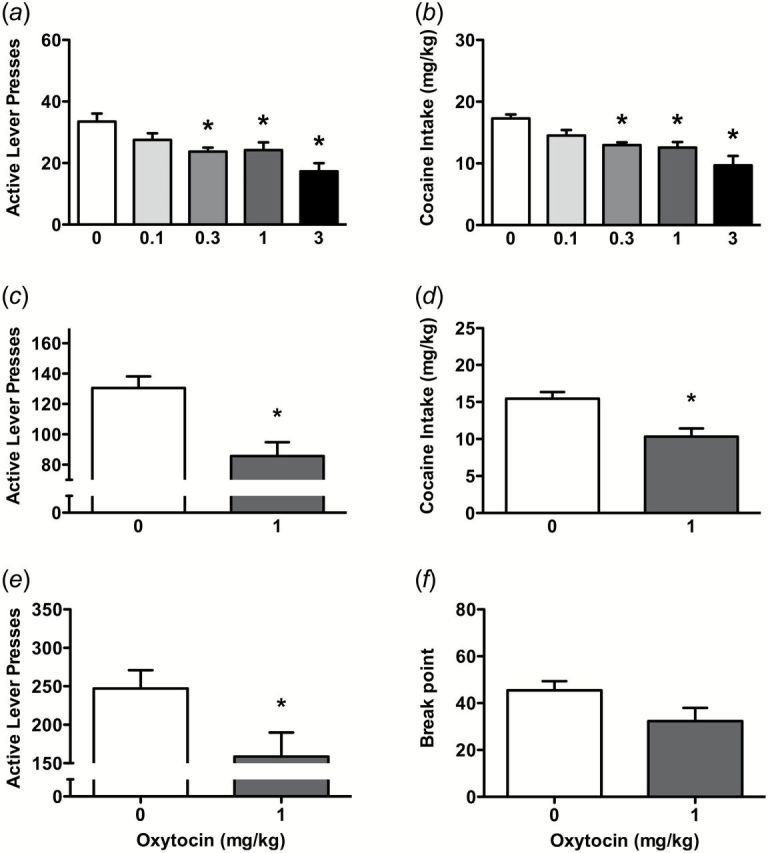
Oxytocin (OT) effects on cocaine self-administration and reinstatement. Data are shown for active lever presses and cocaine intake during cocaine self-administration on fixed ratio 1 (a-b) and fixed ratio 5 (c-d) schedules of reinforcement, and lever presses and breakpoint on progressive ratio schedule of reinforcement (e-f). Significant differences are indicated for OT treatment compared with vehicle controls (*P<.05) (n=9–11/group).
OT Dose-Dependently Reduced Cocaine Seeking during Reinstatement
Similar to its attenuating effect on cocaine seeking during self-administration, OT also dose-dependently decreased reinstatement to cocaine seeking induced by a cocaine-prime (F(3,35)=6.36, P<.01) (Figure 3a) or conditioned cues (F(3,39)=4.73, P<.01) (Figure 3b). Pairwise comparisons revealed significant inhibitory effects of OT at 0.3 and 1mg/kg for cocaine-primed reinstatement and 1mg/kg in cue-induced reinstatement. OT had no effect on inactive lever presses during cocaine-primed (F(3,35)=0.91, P>.05) or cue-induced reinstatement (F(3,39)=0.47, P>.05) (data not shown).
Figure 3.
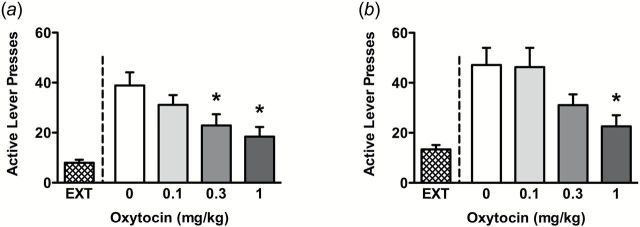
Oxytocin (OT) effects on cocaine seeking during reinstatement. Active lever presses in cocaine-primed (a) or cue-induced (b) reinstatement to cocaine seeking are shown, respectively. Significant differences are indicated for OT treatment compared with vehicle controls (*P<.05) (n=9–10/group).
OT Effects on Cocaine-Induced Intracellular Changes
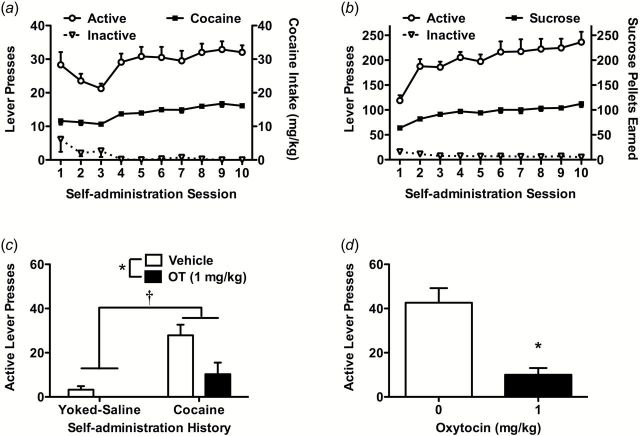
A comparison of responding showed that rats readily acquired self-administration of either cocaine (Figure 4a) or sucrose (Figure 4b) with robust and stable active lever responding and intake as well as similar lever discrimination between active and inactive levers for both the cocaine and sucrose groups.
Figure 4.
Acquisition of self-administration and oxytocin (OT) effects on primed reinstatement in rats used for Western-blot analysis. Data (n=6–8/group) are shown for active and inactive lever presses and number of reinforcers received during cocaine (a) or sucrose (b) self-administration. Active lever presses during cocaine- (c) or sucrose-primed (d) reinstatement are shown. Significant differences are indicated for OT treatment (filled bar) compared with vehicle controls (open bar) (*P<.05) and cocaine compared with yoked-saline (†P<.05).
In cocaine-primed reinstatement, the behavioral results in rats used for Western blotting and immunoprecipitation analyses confirmed our earlier findings. Two-way ANOVA indicated a significant main effect of treatment (F(1,25)=6.65, P<.05) and training history (F(1,25)=18.46, P<.01), but not a significant interaction (Figure 4c). Specifically, rats that received OT (1mg/kg) showed attenuated active lever presses compared with rats that received vehicle. During cocaine-primed reinstatement, rats that self-administered cocaine exhibited higher active lever presses compared with controls that received yoked-saline during self-administration. In sucrose-primed reinstatement, OT (1mg/kg) reduced active lever responding compared with vehicle (t14=4.48, P<.01) (Figure 4d). Consistent with results from experiment 2, OT (1mg/kg) did not attenuate inactive lever presses during cocaine-primed reinstatement (P>.05 for both main effects and interaction; data not shown). However, OT did attenuate inactive lever presses during sucrose-primed reinstatement (vehicle: 4.00±0.91; OT 1mg/kg: 0.88±0.35; t14=3.22, P<.01).
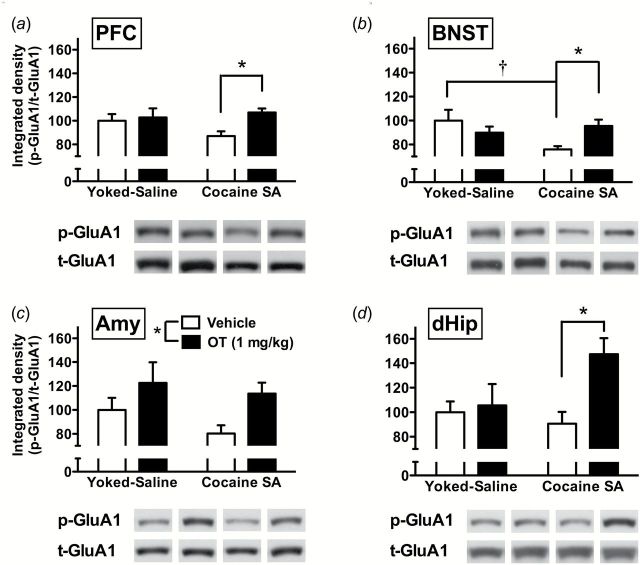
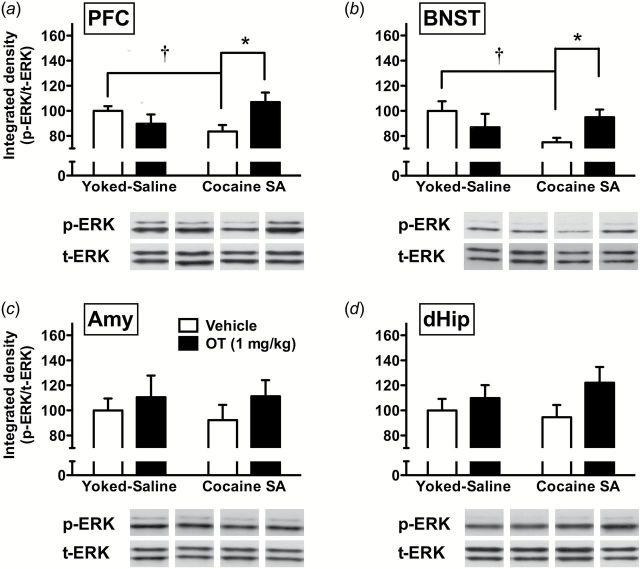
At the molecular level, in general, OT increased p-GluA1 and p-ERK in multiple brain regions (Figure 5 and Figure 6). In the PFC, 2-way ANOVA revealed a significant interaction (F(1,25)=5.22, P<.05) (Figure 5a). Pairwise comparisons showed that OT increased p-GluA1 in cocaine but not yoked saline rats. Similarly, a significant interaction was obtained in the BNST (F(1,25)=6.38, P<.05) (Figure 5b). Pairwise comparisons indicated that compared with vehicle treatment, OT selectively increased p-GluA1 in rats with a cocaine self-administration history, but failed to affect p-GluA1 levels in yoked saline rats. Additionally, p-GluA1 was significantly lower in rats with cocaine vs yoked-saline history, but not after OT treatment. In the Amy, 2-way ANOVA revealed a significant main effect of treatment (F(1,25)=6.80, P<.05) (Figure 5c), whereby OT increased p-GluA1 compared with vehicle controls, regardless of cocaine history. In the dHip, a significant main effect of treatment (F(1,25)=6.44, P<.05) and interaction (F(1,25)=4.35, P<.05) were observed (Figure 5d). Pairwise comparisons showed that OT increased p-GluA1 in cocaine, but not yoked-saline, rats. Compared with p-GluA1, p-ERK showed more limited changes that were restricted to the PFC and BNST (Figure 6). Although no significant main effects were observed, significant interactions were seen in both PFC (F(1,25)=7.38, P<.05) (Figure 6a) and BNST (F(1,25)=5.60, P<.05) (Figure 6b). Pairwise comparisons further revealed similar patterns in the 2 areas, whereby OT increased p-ERK in cocaine, but not yoked-saline, rats compared with vehicle controls. In vehicle-treated animals, rats that received cocaine during self-administration had decreased p-ERK compared with controls with yoked-saline.
Figure 5.
Oxytocin (OT) effects on phosphorylation of GluA1 at Ser845 (p-GluA1) protein levels after cocaine prime in multiple brain regions. Data (n=6–8/group) are shown for integrated density of p-GluA1 normalized with total GluA1 (t-GluA1) in prefrontal cortex (PFC) (a), bed nucleus of the stria terminalis (BNST) (b), amygdala (Amy) (c), and dorsal hippocampus (dHip) (d). Significant differences are indicated for OT treatment (filled bar) compared with vehicle controls (open bar) (*P<.05) and cocaine self-administration compared with yoked-saline (†P<.05).
Figure 6.
Oxytocin (OT) effects on phospho-ERK-1/2 (p-ERK) protein levels after cocaine prime in multiple brain regions. Data (n=6–8/group) are shown for integrated density of p-ERK normalized with total ERK (t-ERK) in prefrontal cortex (PFC) (a), bed nucleus of the stria terminalis (BNST) (b), amygdala (Amy) (c), and dorsal hippocampus (dHip) (d). Significant differences are indicated for OT treatment (filled bar) compared with vehicle controls (open bar) (*P<.05) and cocaine self-administration compared with yoked-saline (†P<.05).
No changes of p-GluA1 or p-ERK were observed in any other brain regions of interest (Table 1), thus indicating a good degree of regional specificity of OT effects. Furthermore, OT failed to affect the levels of p-GluA1 or p-ERK in the PFC, BNST, Amy, or dHip of sucrose-experienced rats (Table 2), indicating that different mechanisms are likely involved in sucrose-induced reinstatement. In addition, OT or chronic cocaine did not alter OTR levels in any of the measured regions of cocaine or sucrose rats (Table 3), suggesting a possible functional rather than expressional regulation of OTR. Lastly, although the immunoprecipitation assay revealed binding between OTR and GluA1 in the PFC and dHip, neither cocaine nor OT treatment altered the binding levels (Figure 7). This evidence weighs against a direct protein-protein interaction that mediates OT effects on GluA1 signaling. No significant correlations between active lever presses and measured protein levels were found in any of the analyzed brain regions (P>.05; data not shown).
Table 1.
Protein Levels of p-GluA1 and p-ERK Normalized with Correspondent Total Proteins in Brain Areas without OT Effects in Cocaine-Trained Rats.
| p-GluA1 | p-ERK | |||||||
|---|---|---|---|---|---|---|---|---|
| Sal-Veh | Sal-OT | Coc-Veh | Coc-OT | Sal-Veh | Sal-OT | Coc-Veh | Coc-OT | |
| NAc | 100.0±6.3 | 101.8±10.8 | 98.6±6.4 | 107.2±5.3 | 100.0±4.9 | 100.2±13.0 | 87.8±10.0 | 102.0±5.0 |
| CPu | 100.0±6.3 | 98.1±13.0 | 77.3±5.2 | 94.6±11.6 | 100.0±12.0 | 91.2±13.4 | 71.6±10.9 | 87.4±7.6 |
| HT | 100.0±16.2 | 88.5±13.2 | 76.4±8.6 | 95.2±12.6 | 100.0±9.7 | 85.7±6.1 | 117.4±18.3 | 116.0±15.0 |
| vHip | 100.0±7.1 | 104.1±9.4 | 90.3±7.4 | 96.7±8.6 | 100.0±5.3 | 94.8±1.5 | 96.1±8.5 | 99.9±7.0 |
| VTA | 100.0±12.0 | 137.5±30.7 | 92.4±8.8 | 103.0±7.7 | 100.0±5.8 | 88.9±8.7 | 99.8±11.4 | 108.2±10.6 |
Abbreviations: Coc, cocaine; CPu, caudate-putamen; HT, hypothalamus; NAc, nucleus accumbens; OT, oxytocin; p-ERK, phospho-ERK-1/2; p-GluA1, phosphorylation of GluA1 at Ser845; Sal, saline; Veh, vehicle; vHip, ventral hippocampus; VTA, ventral tegmental area.
Data are presented as the mean±SEM (n=6–8/group).
Table 2.
Protein Levels of p-GluA1 and p-ERK Normalized with Correspondent Total Proteins in Sucrose-Trained Rats.
| p-GluA1 | p-ERK | |||
|---|---|---|---|---|
| Veh | OT | Veh | OT | |
| PFC | 100.0±4.4 | 109.0±7.5 | 100.0±8.2 | 106.6±8.9 |
| BNST | 100.0±10.1 | 107.5±9.2 | 100.0±5.6 | 106.3±7.1 |
| Amy | 100.0±10.2 | 116.7±11.6 | 100.0±10.9 | 107.4±10.3 |
| dHip | 100.0±5.8 | 113.3±3.7 | 100.0±9.5 | 113.4±9.5 |
Abbreviations: Amy, amygdala; BNST, bed nucleus of the stria terminalis; dHip, dorsal hippocampus; OT, oxytocin; p-ERK, phospho-ERK-1/2; PFC, prefrontal cortex; p-GluA1, phosphorylation of GluA1 at Ser845; Veh, vehcile.
Data are presented as the mean±SEM (n=8/group).
Table 3.
Unchanged OTR Protein Levels Normalized with Calnexin Protein Levels in Cocaine and Sucrose-Trained Rats.
| Cocaine Self-Administration | Sucrose Self-Administration | |||||
|---|---|---|---|---|---|---|
| Sal-Veh | Sal-OT | Coc-Veh | Coc-OT | Veh | OT | |
| PFC | 100.0±16.4 | 108.7±14.5 | 105.3±3.9 | 97.6±5.1 | 100.0±11.8 | 93.9±16.4 |
| NAc | 100.0±10.3 | 105.0±10.6 | 91.5±8.1 | 98.2±4.2 | – | – |
| CPu | 100.0±10.3 | 118.9±6.2 | 115.1±6.8 | 106.3±7.9 | – | – |
| BNST | 100.0±8.0 | 116.7±8.9 | 95.7±6.6 | 114.4±10.9 | 100.0±9.7 | 93.5±11.6 |
| HT | 100.0±3.9 | 105.8±7.0 | 107.2±5.1 | 103.9±6.4 | – | – |
| Amy | 100.0±10.7 | 98.8±6.4 | 92.5±8.1 | 99.8±12.3 | 100.0±8.8 | 100.0±10.1 |
| dHip | 100.0±20.8 | 133.1±12.8 | 125.6±13.4 | 139.9±14.3 | 100.0±3.3 | 98.4±7.6 |
| vHip | 100.0±8.9 | 107.4±8.9 | 97.8±7.9 | 102.2±9.2 | – | – |
| VTA | 100.0±11.8 | 114.4±9.9 | 102.9±6.8 | 92.3±12.6 | – | – |
Abbreviations: Amy, amygdala; BNST, bed nucleus of the stria terminalis; Coc, cocaine; CPu, caudate-putamen; dHip, dorsal hippocampus; HT, hypothalamus; NAc, nucleus accumbens; OT, oxytocin; PFC, prefrontal cortex; Sal, saline; Veh, vehicle; vHip, ventral hippocampus; VTA, ventral tegmental area.
Data are presented as mean±SEM (n=6–8/group).
Figure 7.
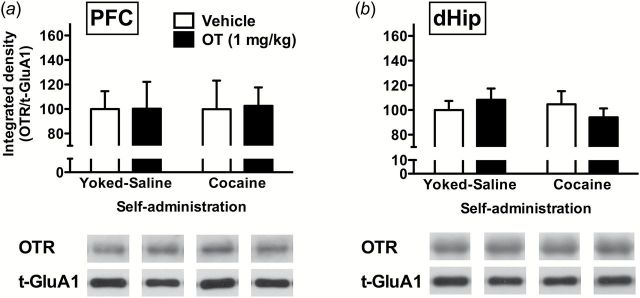
Oxytocin (OT) effects on physical interaction between OT receptors (OTRs) and GluA1 subunit in prefrontal cortex (PFC) (a) and dorsal hippocampus (dHip) (b). Data (n=4/group) are shown for integrated density of OTR normalized with total GluA1 (t-GluA1).
Discussion
In this study, acute OT reduced cocaine self-administration under various schedules of reinforcement and dose-dependently attenuated cocaine-primed and cue-induced reinstatement of cocaine seeking. A potential mechanism for the effects of OT on cocaine-induced drug seeking may reside in the increased p-GluA1 and p-ERK in specific brain regions found in cocaine-experienced rats, whereby the reductions seen after chronic cocaine self-administration are rescued by acute OT. In that such changes were not seen in rats with a history of sucrose reinforcement, they suggest a potentially unique role for OT on p-GluA1 and p-ERK activity in cocaine addiction.
Although previous studies have provided some evidence that acute OT attenuated drug reward (Sarnyai and Kovacs, 1994), these studies primarily investigated abused drugs other than cocaine and used animal models with limited degrees of face validity. In contrast, the current results comprehensively showed that OT reduces cocaine self-administration and nonreinforced seeking under low (FR1) and high (FR5) motivational demand as well as on a PR schedule that determined the influence of OT on motivational limit. We also demonstrated the suppressive effect of OT on reinstatement induced by a cocaine prime or conditioned cues, 2 major triggers for reinstatement of extinguished drug seeking. Our results strongly support the OT system as a promising therapeutic target for treating cocaine addiction.
Several possible mechanisms could account for OT’s suppressive effects on cocaine seeking. First, OT has been shown to decrease DA release and receptor binding in mesolimbic brain structures (Sarnyai and Kovacs, 1994), resulting in reduced rewarding effects of cocaine. Second, OT itself could be rewarding, as a high dose of systemic OT can induce CPP (Liberzon et al., 1997). Thus, in the presence of OT, less cocaine intake may be needed to reach the same level of reinforcement. Third, OT has an inhibitory effect on learning and memory processes (Engelmann et al., 1996). It is possible that OT reduces operant performance by impairment of information processing during cocaine self-administration and reinstatement after extinction, which requires the restoration of a conditioned operant response. The finding that OT has no effect on nonspecific responding on the inactive lever further supports the contention that OT reduced cocaine self-administration and reinstatement via effects on motivated cocaine seeking.
Acute OT had notable and selective effects on p-ERK and p-GluA1 in multiple brain regions important for cocaine-primed cocaine seeking. First, in PFC and BNST, reinstatement of cocaine seeking resulted in a reduction of p-ERK that was normalized by OT pretreatment. ERK-mediated signaling plays a critical role in the neuronal and behavioral adaptations in response to cocaine (Ren et al., 2010; Besnard et al., 2011). In vitro, the elevation of p-ERK and enhancement of long-term potentiation has been documented after OT application (Tomizawa et al., 2003). Further, a similar change in p-GluA1 expression was observed after OT treatment. The increase of p-GluA1 results in enhanced AMPA receptor-mediated excitatory current by promoting GluA1 plasma membrane insertion (Roche et al., 1996; Banke et al., 2000; Oh et al., 2006). In cellular and neuronal culture, OT application recruited cAMP/PKA signaling (Cassoni et al., 2000; Ayar et al., 2014), the activation of which is necessary for the p-GluA1 induction (Sun et al., 2014). The current results and data from a previous study (Sun et al., 2013) show that cocaine self-administration decreased p-ERK and p-GluA1 in PFC and BNST. It is plausible that OT attenuates cocaine-induced drug seeking via rescuing the reduction in protein phosphorylation. However, to determine whether these effects play a causal role or reflect compensatory phenomena, future studies will require direct manipulation of p-GluA1 (eg, PKA inhibitor) or p-ERK (eg, MEK inhibitor) after OT treatment.
Second, systemic OT increased p-GluA1 in Amy and dHip. A recent study has shown that peripheral OT treatment elevated extracellular OT levels in the Amy and dHip (Neumann et al., 2013). Elevated OT successively increases p-GluA1, possibly through PKA activation, which is necessary for tonically maintained OTR binding (Bale et al., 2001). Acute cocaine injection has been shown to elevate OT levels in the Hip, whereas chronic cocaine decreased OT levels (Sarnyai et al., 1992). The fact that OT failed to further potentiate p-GluA1 expression in the dHip of the yoked-saline controls may have been due to a ceiling effect of OT levels after the acute cocaine prime. Finally, cocaine and/or OT had no effect on either p-ERK or p-GluA1 in the remaining brain areas examined. This finding is consistent with a previous study demonstrating that a cocaine injection did not alter p-GluA1 on Ser845 in the NAc (Anderson et al., 2008). However, cocaine prime increased phosphorylation of GluA1 at the Ser831 site (a CaM-KII and PKC site) in NAc (Anderson et al., 2008) and CPu (White et al., 2013), suggesting different Glu mechanisms may be involved in these striatal regions.
Similar to cocaine, sucrose-induced reinstatement was also reduced by OT (1mg/kg), which concurs with our earlier findings of OT modulation of sucrose seeking (Cox et al., 2013). It is not surprising that OT should have some impact on natural reward given its innervation of central appetitive pathways. Indeed, previous findings indicated that OT infusion into the ventral tegmental area suppressed sucrose intake (Mullis et al., 2013), and OT knockout mice displayed enhanced intake of sucrose solution (Sclafani et al., 2007). Our findings further suggested broader actions of OT on learned reward seeking, regardless of a history of drug or a natural reinforcer. Although OT had similar effects on sucrose and cocaine seeking, the effects on p-ERK and p-GluA1 were cocaine specific, as OT did not influence p-ERK or p-GluA1 in any of the brain areas examined in sucrose rats. This difference indicates that the observed OT-induced increases in p-ERK and p-GluA1 after reinstatement of cocaine seeking were not solely due to the act of lever pressing. Furthermore, it suggests the involvement of different mechanisms and/or sites of action. One possibility is that p-ERK and p-GluA1 signaling may not be critically involved in OT-regulated reinstatement, whereby the observed phosphorylation changes in cocaine-experienced rats reflect compensatory effects and OT-regulated reinstatement of cocaine and sucrose seeking engage as-yet-to-be-determined common mechanisms. Alternatively, different sites of action may mediate OT effects on sucrose seeking. OT has been shown to act on neurons in the nucleus of the solitary tract (NTS) to modulate their response to peripheral satiety signals (Blevins et al., 2004). In addition, lesions of OTR-containing neurons in the NTS profoundly blocked the orexigenic effect of intracerebroventricular administration of an OTR antagonist (Baskin et al., 2010), suggesting that endogenous OT acts primarily at the NTS to inhibit food intake. Future studies focusing on molecular changes in other brain regions, including the NTS, will be necessary to determine this possibility.
After methamphetamine administration, OT altered drug-induced GluN1 protein expression, along with AMPA receptor-dependent neuronal activity in the PFC (Qi et al., 2012), indicating a possible interaction between OTR and Glu receptors. Either a direct protein-protein interaction or an indirect second messenger-signaling mechanism mediates the interaction between receptors. Our current data demonstrate for the first time that a protein-protein complex was formed between the OTR and GluA1 subunit in both PFC and dHip. However, none of the experimental manipulations altered this receptor interaction, suggesting that OT-mediated second messenger cascades are likely responsible for the activating effect of p-GluA1 in an ERK-dependent (PFC) or ERK-independent (dHip) manner.
Some limitations of the current study should be noted. First, as vasopressin (VP) is very closely related to OT, it will be important to extend such studies to more selectively examine VP activity. Although OT exhibits more specificity at the OTR, it does bind to VP receptors with a 100-fold lower affinity (Mouillac et al., 1995). Similar to OT, systemic VP administration decreased cocaine self-administration (van Ree et al., 1988) and food intake (Langhans et al., 1991). More selective OTR compounds, both agonists and antagonists, will be useful to address OT selectivity in cocaine addiction. Second, the use of whole tissue dissection limits the ability to assess discrete subregions that may mediate differential effects in cocaine seeking (eg, NAc subregions of the core and shell). Additional techniques, including local microinjection of OTR agents into precise brain regions, will be required. Third, the underlying mechanisms of OT actions that alter p-GluA1 and p-ERK levels need to be explored. Finally, we used a widely applied self-administration model of 2-hour daily cocaine access. Longer daily access regimens of cocaine self-administration lead to escalation of cocaine intake over time, a feature more akin to human addiction (Ahmed and Koob, 1998; Orio et al., 2009; Gipson et al., 2011; Zlebnik et al., 2012). Although it remains unclear as to whether extended periods of daily cocaine self-administration contribute to fundamentally different alterations in neuronal functions (Purgianto et al., 2013; Sun et al., 2013), it will be important to assess the effects of OT after varied durations of cocaine self-administration in future studies.
In conclusion, the current results further support the development of OT and OT analogues as potential drug therapies for addiction treatment. We found that OT decreased cocaine taking and seeking across different stages of addiction for cocaine under different motivational demands. Further, our data suggest a potential Glu-mediated regulation of the inhibitory effects of OT on cocaine reward-related behaviors. Additional studies should follow up on OT regulation of GluA1 function to determine whether these effects are immediately critical for cocaine seeking or represent compensatory phenomena. In addition, elucidation of the OT signaling pathways and their interaction with other neurotransmission systems in vivo is necessary to better understand the role of OT in drug addiction for possible translational studies and development of OTR targets. Finally, future assessment of the safety, effectiveness (eg, possible development of tolerance), and potential side effects (eg, food intake) of OT usage, especially after long-term treatment (a potentially more relevant paradigm to the clinical treatment of addiction), is warranted.
Statement of Interest
None.
Acknowledgments
This work was supported by NIH grants P50 DA016511, P50 DA015369, RO1 DA033479, and C06 RR015455 and NCMHD grant P20 MD003942. We thank Dr. Carmela Reichel for her helpful comments on this manuscript. We also thank Shannon Ghee, Andrew Novak, Phong Do, Clifford Chan, Jennifer Hergatt, Kelsey Brown, and Kyle Brown for technical assistance.
References
- Ahmed SH, Koob GF. (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. (2008). CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci 11:344–353. [DOI] [PubMed] [Google Scholar]
- Ayar A, Ozcan M, Alcin E, Serhatlioglu I, Ozcan S, Kutlu S, Kelestimur H. (2014). Oxytocin activates calcium signaling in rat sensory neurons through a protein kinase C-dependent mechanism. J Physiol Biochem 70:43–48. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. (2008). Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci 27:2229–2240. [DOI] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. (2001). CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci 21:2546–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. (2000). Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, Simhan HN, Moralejo DH, Blevins JE. (2010). A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology 151:4207–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard A, Bouveyron N, Kappes V, Pascoli V, Pages C, Heck N, Vanhoutte P, Caboche J. (2011). Alterations of molecular and behavioral responses to cocaine by selective inhibition of Elk-1 phosphorylation. J Neurosci 31:14296–14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG. (2004). Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–96. [DOI] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. (2010). Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology 58:38–43. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Fulcheri E, Carcangiu ML, Stella A, Deaglio S, Bussolati G. (2000). Oxytocin receptors in human adenocarcinomas of the endometrium: presence and biological significance. J Pathol 190:470–477. [DOI] [PubMed] [Google Scholar]
- Choy VJ, Watkins WB. (1977). Immunocytochemical study of the hypothalamo-neurohypophysial system. II. Distribution of neurophysin, vasopressin and oxytocin in the normal and osmotically stimulated rat. Cell Tissue Res 180:467–490. [DOI] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM. (2013). Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology 38:2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. (1996). Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev 20:341–358. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. (2001). The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. (2011). Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 214:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73:553–566. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Horvath Z, Sarnyai Z, Faludi M, Telegdy G. (1985). Oxytocin and a C-terminal derivative (Z-prolyl-D-leucine) attenuate tolerance to and dependence on morphine and interact with dopaminergic neurotransmission in the mouse brain. Neuropharmacology 24:413–419. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Barbarczi E, Szabo G, Telegdy G. (1990). The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology 29:365–368. [DOI] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. (2009). Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res 202:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans W, Delprete E, Scharrer E. (1991). Mechanisms of vasopressin’s anorectic effect. Physiol Behav 49:169–176. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Trujillo KA, Akil H, Young EA. (1997). Motivational properties of oxytocin in the conditioned place preference paradigm. Neuropsychopharmacology 17:353–359. [DOI] [PubMed] [Google Scholar]
- Makarevich AV, Sirotkin AV, Franek J, Kwon HB, Bulla J. (2004). The role of oxytocin, protein kinase A, and ERK-related MAP-kinase in the control of porcine ovarian follicle functions. Exp Clin Endocrinol Diabetes 112:108–114. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. (2012). Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav 61:331–339. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MM, Brady KT. (2013). Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology (Berl) 228:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillac B, Chini B, Balestre MN, Jard S, Barberis C, Manning M, Tribollet E, Trumpp-Kallmeyer S, Hoflack J, Elands J, et al. (1995). Identification of agonist binding sites of vasopressin and oxytocin receptors. Adv Exp Med Biol 395:301–310. [PubMed] [Google Scholar]
- Mullis K, Kay K, Williams DL. (2013). Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res 1513:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38:1985–1993. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. (2006). Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem 281:752–758. [DOI] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. (2009). A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci 29:4846–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. (2013). Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res 37:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping A, Xi J, Prasad BM, Wang MH, Kruzich PJ. (2008). Contributions of nucleus accumbens core and shell GluR1 containing AMPA receptors in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. Brain Res 1215:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME. (2013). Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology 38:1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Yang JY, Song M, Li Y, Wang F, Wu CF. (2008). Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn Schmiedebergs Arch Pharmacol 376:441–448. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. (2009). Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology 56:856–865. [DOI] [PubMed] [Google Scholar]
- Qi J, Han WY, Yang JY, Wang LH, Dong YX, Wang F, Song M, Wu CF. (2012). Oxytocin regulates changes of extracellular glutamate and GABA levels induced by methamphetamine in the mouse brain. Addict Biol 17:758–769. [DOI] [PubMed] [Google Scholar]
- Ren Z, Sun WL, Jiao H, Zhang D, Kong H, Wang X, Xu M. (2010). Dopamine D1 and N-methyl-D-aspartate receptors and extracellular signal-regulated kinase mediate neuronal morphological changes induced by repeated cocaine administration. Neuroscience 168:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. [DOI] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. (1996). Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16:1179–1188. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL. (1994). Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology 19:85–117. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Vecsernyes M, Laczi F, Biro E, Szabo G, Kovacs GL. (1992). Effects of cocaine on the contents of neurohypophyseal hormones in the plasma and in different brain structures in rats. Neuropeptides 23:27–31. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Rinaman L, Vollmer RR, Amico JA. (2007). Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol 292:R1828–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. (1983). Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res 60:101–114. [DOI] [PubMed] [Google Scholar]
- Stoop R. (2012). Neuromodulation by oxytocin and vasopressin. Neuron 76:142–159. [DOI] [PubMed] [Google Scholar]
- Sun WL, Zhou L, Quinones-Jenab V, Jenab S. (2009). Cocaine effects on dopamine and NMDA receptors interactions in the striatum of Fischer rats. Brain Res Bull 80:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Zelek-Molik A, McGinty JF. (2013). Short and long access to cocaine self-administration activates tyrosine phosphatase STEP and attenuates GluN expression but differentially regulates GluA expression in the prefrontal cortex. Psychopharmacology (Berl) 229:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WL, Coleman NT, Zelek-Molik A, Barry SM, Whitfield TW, Jr., McGinty JF. (2014). Relapse to cocaine-seeking after abstinence is regulated by cAMP-dependent protein kinase A in the prefrontal cortex. Addict Biol 19:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. (2003). Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci 6:384–390. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Burbach-Bloemarts EM, Wallace M. (1988). Vasopressin neuropeptides and acquisition of heroin and cocaine self-administration in rats. Life Sci 42:1091–1099. [DOI] [PubMed] [Google Scholar]
- White SL, Schmidt HD, Vassoler FM, Pierce RC. (2013). Acute cocaine increases phosphorylation of CaMKII and GluA1 in the dorsolateral striatum of drug naive rats, but not cocaine-experienced rats. Neurosci Lett 537:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JY, Qi J, Han WY, Wang F, Wu CF. (2010). Inhibitory role of oxytocin in psychostimulant-induced psychological dependence and its effects on dopaminergic and glutaminergic transmission. Acta Pharmacol Sin 31:1071–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE. (2012). Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther 340:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. (2012). Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology (Berl) 224:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]