Abstract
Background:
Use of synthetic cathinones, which are designer stimulants found in “bath salts,” has increased dramatically in recent years. Following governmental bans of methylenedioxypyrovalerone, mephedrone, and methylone, a second generation of synthetic cathinones with unknown abuse liability has emerged as replacements.
Methods:
Using a discrete trials current intensity threshold intracranial self-stimulation procedure, the present study assessed the effects of 2 common second-generation synthetic cathinones, α‐pyrrolidinopentiophenone (0.1–5mg/kg) and 4-methyl-N-ethcathinone (1–100mg/kg) on brain reward function. Methamphetamine (0.1–3mg/kg) was also tested for comparison purposes.
Results:
Results revealed both α‐pyrrolidinopentiophenone and 4-methyl-N-ethcathinone produced significant intracranial self-stimulation threshold reductions similar to that of methamphetamine. α‐Pyrrolidinopentiophenone (1mg/kg) produced a significant maximal reduction in intracranial self-stimulation thresholds (~19%) most similar to maximal reductions produced by methamphetamine (1mg/kg, ~20%). Maximal reductions in intracranial self-stimulation thresholds produced by 4-methyl-N-ethcathinone were observed at 30mg/kg (~15%) and were comparable with those observed with methamphetamine and α‐pyrrolidinopentiophenone tested at the 0.3-mg/kg dose (~14%). Additional analysis of the ED50 values from log-transformed data revealed the rank order potency of these drugs as methamphetamine ≈ α‐pyrrolidinopentiophenone>4-methyl-N-ethcathinone.
Conclusions:
These data suggest that the newer second-generation synthetic cathinones activate the brain reward circuitry and thus may possess a similar degree of abuse potential as prototypical illicit psychostimulants such as methamphetamine as well as the first generation synthetic cathinone methylenedioxypyrovalerone, as previously reported.
Keywords: bath salts, synthetic cathinones, ICSS, abuse liability, psychostimulants
Introduction
For the first time in history, the number of unregulated novel psychoactive substances on international drug markets now exceeds those under international control (United Nations, 2013). One of the most problematic classes of novel psychoactive substances to emerge are synthetic cathinones, comprising approximately 18% of all unregulated substances in international markets (United Nations, 2013). Synthetic cathinones first appeared in Europe in the mid 2000s and in the United States around 2009, and their use led to numerous reports of abuse, bizarre behavior, toxicity, and death (Prosser and Nelson, 2012; Rosenbaum et al., 2012). The rise in popularity of synthetic cathinones is linked to their ease of procurement over the internet and in gas stations, smoke shops, and novelty stores (Spiller et al., 2011). Synthetic cathinones have been falsely marketed as numerous products, the most recognizable being “bath salts,” and are typically labeled “not for human consumption” or “for research purposes only” to evade drug laws (United States Department of Justice, 2011c). Although many synthetic cathinone derivatives exist, 3,4-methylenedioxypyrovalerone (MDPV), mephedrone, or methylone, initially comprised approximately 98% of all synthetic cathinones encountered in US drug seizures (United States Department of Justice, 2011a). Citing imminent threats to public health and safety, the US Drug Enforcement Administration (DEA) used their emergency scheduling authority to temporarily classify these 3 drugs (now often referred to as first-generation bath salts) as Schedule I substances in October of 2011 (United States Department of Justice, 2011b). As of 2013, these first-generation synthetic cathinones are now permanently classified as Schedule I substances in the United States (United States of America, 2012; United States Department of Justice, 2013a).
On January 22, 2013, the US DEA published a request for information specifically regarding 8 additional synthetic cathinones, 2 of the most prominent being 4-methyl-N-ethcathinone (4-MEC) and α‐pyrrolidinopentiophenone (α-PVP) (DEA, 2013). Their similarity in chemical structure suggests that α-PVP and 4-MEC likely emerged as replacements for MDPV and mephedrone, respectively (Figure 1).
Figure 1.
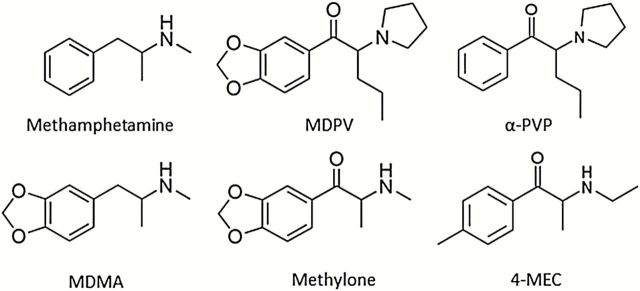
Chemical structures of the traditional psychostimulants methamphetamine (METH) and methylenedioxymethamphetamine (MDMA), first-generation synthetic cathinones methylenedioxypyrovalerone (MDPV) and methylone, and second-generation synthetic cathinones α‐pyrrolidinopentiophenone (α-PVP) and 4-methyl-N-ethcathinone (4-MEC).
Although literature regarding the neurochemistry, toxicology, and abuse liability of first-generation synthetic cathinones has emerged in recent years (Spiller et al., 2011; Coppola and Mondola, 2012; Baumann et al., 2013; Simmler et al., 2013; Watterson et al., 2013; Watterson and Olive, 2014), relatively little information exists regarding second-generation analogues such as α-PVP and 4-MEC (United States Department of Justice, 2013b). With regards to abuse liability, the potential for compulsive use (ie, addiction) of stimulant drugs generally increases as dopamine (DA) to serotonin transporter (DAT/SERT) reuptake (IC50 values) and/or release (EC50 values) ratios increase (ie, synaptic levels of DA are greater than 5-hydroxytrypamine, 5-HT). On the other hand, higher SERT to DAT ratios are generally associated with more entactogenic effects and episodic abuse patterns (Rothman and Baumann, 2003, 2006; Bauer et al., 2013a). Studies have revealed that α-PVP is a potent DA and norepinephrine transporter (DAT and NET, respectively) inhibitor, has relatively little affinity for the serotonin transporter (DAT/SERT IC50>781) (Meltzer et al., 2006; Marusich et al., 2014), increases extracellular DA release in the striatum (Kaizaki et al., 2014), and has locomotor enhancing properties similar to MDPV (DAT/SERT IC50≈806–816) and methamphetamine (DAT/SERT IC50≈10–25; DAT/SERT EC50≈152) (Rothman and Baumann, 2003; Baumann et al., 2012b; Kaizaki et al., 2014; Marusich et al., 2014). On the other hand, in vitro assays have shown that 4-MEC inhibits the reuptake of DAT, NET, and SERT with approximately equal affinity but also acts as a 5-HT releaser with a similar DAT to SERT ratio (DAT/SERT IC50≈1.85) (Iversen et al., 2013; Simmler et al., 2014) to methylone (DAT/SERT IC50≈2; DAT/SERT EC50≈1.82; Baumann et al., 2012a) and 3,4-methylenedioxymethamphetamine (MDMA; DAT/SERT EC50≈0.97; Baumann et al., 2012a). To our knowledge, the effects of 4-MEC on locomotor activity have not been reported. It is also important to note here that DAT to SERT ratios differ slightly between laboratories and/or as a result of cell types used (eg, rat brain synaptosomes, HEK 293 cells expressing human transporters, etc.). Thus, despite their somewhat unique in vitro profiles, these newer synthetic cathinones appear to exert effects on monoaminergic signaling and suggest that α-PVP will have stimulant effects and high compulsive abuse potential similar to methamphetamine (METH) and the first-generation synthetic cathinone MDPV (Aarde et al., 2013; Watterson et al., 2014). In contrast, 4-MEC is predicted to have entactogenic effects and relatively lower compulsive abuse potential (ie, episodic use) similar to MDMA and the first-generation synthetic cathinone methylone (Watterson et al., 2012).
However, there are currently no published behavioral studies that have directly assessed the potential abuse liability of α-PVP and 4-MEC. Thus, the current study sought to determine the effects of α-PVP and 4-MEC, along with methamphetamine for comparison, on thresholds for intracranial self-stimulation (ICSS) using a discrete trials current threshold determination procedure (Markou and Koob, 1992). The discrete trials current threshold ICSS task is commonly employed to assess abuse liability, with reductions in ICSS thresholds representing facilitation of brain reward functioning and increases in ICSS threshold representing anhedonic/depression-like effects and inhibition of brain reward function (Markou and Koob, 1992; Vlachou and Markou, 2011).
Methods
Subjects
All procedures were conducted with the approval of the Institutional Animal Care and Use Committee at Arizona State University in accordance with the Guide for Care and Use of Laboratory Animals as adopted by the National Institutes of Health. Male Sprague-Dawley rats (n=5 for α-PVP, n=5 for 4-MEC, and n=4 for METH) were obtained from Harlan Laboratories (Livermore, CA) and weighed approximately 250g on arrival. Rats were individually housed according to National Institutes of Health standards on a reversed 12-hour light-dark cycle (lights off at 6:00 am) and given ad libitum access to food and water during all experimental procedures, except during behavioral testing. All behavioral testing occurred during the dark phase between 8:00 am and 6:00 pm.
Drugs
α-PVP and 4-MEC were both obtained through Internet websites (NicePriceResearchChems.biz and www.researchchemz.com, respectively). Ten mg samples of both drugs were analyzed by liquid chromatography-mass spectrometry for purity and chemical composition at the Research Triangle Institute (Durham, NC). Samples were dissolved in methanol and analyzed using a Waters Synapt HDMS (Milford, MA) quadrupole time-of-flight mass spectrometer interfaced to a Waters Acquity UPLC system. Data were acquired using a capillary voltage of 3kV, source temperature of 120°C, desolvation temperature of 450°C, sampling cone at 30V, and extraction cone at 3V. The mass spectrometer was externally calibrated from 50 to 700Da using sodium formate solution, and mass shifts during acquisition were corrected using leucine enkephalin as a lockmass. Liquid chromatography was performed using a BEH C18 column (2.1×50mm, 1.7-µm particles) held at 30°C. Sample identity was confirmed based on exact mass, retention time, and fragmentation match to a certified reference standard from Cerilliant (Round Rock, TX). Both samples were determined to have an apparent purity of >95%. For all behavioral studies, α-PVP, 4-MEC, and METH (Sigma Aldrich, St. Louis, MO) were dissolved in 0.9% sterile saline and administered via the intraperitoneal route in a volume of 1mL/kg.
ICSS Surgical Procedures
Rats were anesthetized with isoflurane (2% vol/vol) vaporized oxygen and unilaterally implanted (right and left hemispheres counterbalanced across rats) with a stainless steel bipolar electrode (PlasticsOne, Roanoke, VA; 2-mm diameter, insulated except at the ventral tip) into the medial forebrain bundle (anterior-posterior −0.05mm; medial-lateral±1.7mm, dorsal-ventral −8.3mm from dura) and secured to the skull with skull screws and dental cement. Rats were given 7 days to recover from surgery before commencement of ICSS procedures, during which they received daily injections of 2.5mg/mL meloxicam (0.15mL volume) to minimize postsurgical discomfort.
ICSS Apparatus
All ICSS testing was conducted in operant conditioning chambers (ENV-007CT; Med Associates) housed in sound-attenuating cubicles equipped with an exhaust fan to mask external noise. Chambers were equipped with a house light and a nose-poke aperture containing a light-emitting diode (LED) stimulus light (ENV-114M; Med Associates). The nose-poke aperture was 2.5cm in diameter, located 5cm above a stainless steel grid floor, and contained an infrared detector placed 0.64cm from the front edge of the panel for recording responses. Located outside the chambers was a dual programmable ICSS stimulator (PHM-150B/2; Med Associates) interfaced to a computer to deliver electrical current to the electrode. Chambers were interfaced to a PC using Med-PC IV software that controlled all stimulation parameters, test functions, and data collection.
ICSS Testing Procedures
The discrete-trials current threshold ICSS procedures used in the present study were identical to those described in previous publications from our laboratory (Watterson et al., 2012, 2013, 2014; also see Markou and Koob, 1992; Vlachou and Markou, 2011). During all ICSS testing procedures, stimulation availability was signaled by illumination of the LED stimulus light located within the nose-poke aperture. Training began by allowing rats to spontaneously acquire nose-poke responses on an FR1 schedule of reinforcement, which delivered a 200-millisecond square-wave cathodal pulse of 120 µA at 100 Hz. Rats were required to exert a minimum of 600 nose pokes in a 30-minute session for 2 sessions to progress to discrete trials training. During discrete trials training, each trial began with a free stimulation of 120 µA followed by a 7.5-second period during which the LED light remained on until the rat emitted a response that would yield an identical stimulation. Following the initial trial, the LED light was turned off and an inter-trial interval (ITI) was initiated, during which responses were recorded but yielded no stimulation. Progression through discrete trial training required rats to meet criterion (>60% of total [trial + ITI] responses were correct trial responses) at 4 ITI lengths (2, 5, 10, and 15 seconds). Once rats completed discrete trials training, discrete trial current threshold determination procedures began. All discrete trials current threshold sessions began with a stimulus intensity of 120 µA and progressed through 4 cycles of ascending and descending blocks of trials. At a given current intensity, 5-trial blocks began with a free stimulation, followed by a 7.5-second interval during which rats could emit a nose-poke response to receive an identical stimulation. Following a single trial response, the LED stimulus light was turned off, initiating an ITI period between 7.5 and 15 seconds (mean 10 seconds) that separated trials. Responses during the ITI further lengthened the ITI by 12.5 seconds. When rats emitted an appropriate response on ≥3 of 5 trials, electrical stimulation was decreased by 5 µA for the next 5-trial block. Stimulation intensities continued to descend until the rats responded ≤2 of 5 trials during a given trial block, at which point the current intensities reversed into ascending mode, with 5-µA increases in current intensity for the subsequent blocks. Therefore, the discrete trial current threshold procedure determined the lowest amount of current intensity (threshold) for which a rat was willing to emit responses. For each session, raw threshold scores were calculated by averaging the midpoint of current intensities between positive (responses to ≥3 of 5 trials) or negative (responses to ≤2 of 5) trial blocks.
Prior to all drug and vehicle testing, rats received a minimum of 10 days of baseline threshold assessment and were required to meet stable baseline criteria. These criteria were determined by threshold means for the most recent 8 sessions as well as submeans for the first and last 4 of these sessions. The difference in submeans was divided by the overall mean, and threshold stability was considered to be met if the resulting percentage was <5 (Sidman, 1960). Baseline testing continued throughout experimentation to monitor stability, and drug testing was stopped if animals no longer displayed stability across baseline scores. In all cases, loss of stability occurred as a result of either loosening or complete detachment of the electrode implant from the skull.
Drugs were administered 20 minutes prior to placement into ICSS procedures. Drug doses were given in a randomized block design such that rats received each dose once before beginning another block of testing. At the beginning of the experiment, all subjects were to receive 5 determinations of each dose; however, loss of electrode implant or baseline stability meant some subjects received less. Rats that were administered 4-MEC received 2 to 5 determinations at each dose, and all rats that were administered α-PVP received 1 to 4 determinations at each dose. A 100-mg/kg dose for 4-MEC and 5-mg/kg dose for α-PVP were also administered, but only once and only for a subset of rats (N=4 for α-PVP; N=3 for 4-MEC) because of apparent aversive effects as indicated by robust ICSS threshold elevations. Because only a subset of rats received these higher doses, ICSS threshold determinations were not included in the statistical analysis. Rats receiving METH received 2 to 3 determinations at each dose with the exception of the 3-mg/kg dose, which was only assessed once in rats, also because of ICSS threshold increases. However, because all rats received a 3-mg/kg determination, ICSS thresholds at this dose for METH were included in statistical analyses.
Statistical Analyses
All statistical analyses were conducted using SigmaPlot (Systat Software, Inc. San Jose, CA). A significance criterion of P<.05 was used for all analyses. For each rat, raw ICSS current intensity thresholds (in µA) for all vehicle and drug sessions conducted once drug administration began were converted to scores reflecting the percent change from the mean of baseline thresholds obtained after reaching stabilization. For each dose, including vehicle, scores reflect the average percent change from the baseline score, which immediately preceded its determination for each individual animal. In addition to dose means, corresponding 95% confidence intervals (Figure 2) were calculated, and significance (between individual doses and vehicle) occurred when the 95% confidence intervals between individual doses and vehicle did not overlap (Cardinal and Aitken, 2006).
Figure 2.
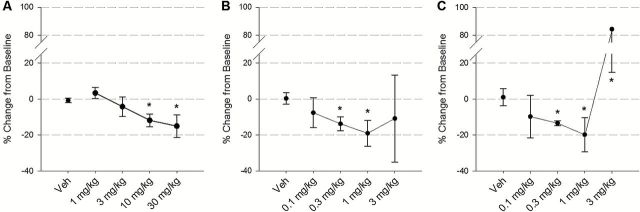
Effects of the second-generation synthetic cathinone (A) 4-methyl-N-ethcathinone (4-MEC) (1, 3, 10, 30, mg/kg), (B) α‐pyrrolidinopentiophenone (α-PVP) (0.1, 0.3, 1, and 3mg/kg), and the traditional psychostimulant (C) methamphetamine (METH) (0.1, 0.3, 1, and 3mg/kg) on intracranial self-stimulation (ICSS) thresholds. Data represent mean±95% confidence interval and are expressed as a percent change in ICSS thresholds relative to the previous baseline session. N=5, 5, and 4 in A, B, and C, respectively. *Symbols represent P<.05 vs. saline. In C, the confidence interval upper limit (no shown) for the 3-mg/kg dose=153.87.
To perform dose-effect comparisons across the different drugs tested, doses were log transformed for each animal and a linear slope (line of best fit) was calculated on group means for the descending portion of dose-effect curves starting with the lowest dose tested and ending with the dose producing the largest observed mean maximal reduction in ICSS thresholds. Linear slopes were then used to calculate ED50 values for each drug and animal. Maximal reductions for each animal, regardless of dose, were also calculated. Next, slopes for the log-transformed drug doses on the linear portion of the descending slopes (ED50 values in mg/kg) and maximal ICSS threshold decreases for METH, α-PVP, and 4-MEC were compared by a 1-way between-subjects analysis of variance with Bonferroni posthoc tests. Slope and ED50 values from previously published data (Watterson et al., 2012, 2014) were also calculated (Table 1) but were not compared statistically because of the possibility of cohort effects.
Table 1.
Slope Values Represent the Means for the Descending Linear Slope of Log-Transformed Doses.
| Drug | Slope | ED50mg/kg | Maximal % Reduction |
|---|---|---|---|
| METH | −50.23 | 0.20: UL = .45, LL = 0.004 | −21.11 |
| α-PVP | −46.70 | 0.35: UL = 0.82, LL = 0.023 | −25.76 |
| 4-MEC | −21.12 | 6.41: UL = 9.23, LL = 4.37 | −17.40 |
| MDPV | −96.07 | 0.35: UL = 0.55, LL = 0.17 | −42.03 |
| Methylone | −17.59 | 1.00: UL = 1.51, LL = 0.58 | −21.50 |
ED50 values represent the mean dose leading to 50% maximal response with upper 95% confidence limits (UL) and lower 95% confidence limits (LL). Maximal response values represent the mean maximum intracranial self-stimulation (ICSS) threshold reduction (independent of dose)±95% confidence intervals. Results reported for methylenedioxypyrovalerone (MDPV) and methylone were obtained from previous publications (Watterson et al., 2012, 2014).
Results
For 4-MEC (Figure 2A), significant reductions in ICSS thresholds vs vehicle were seen in the 10- (M=−11.86) and 30- (M=−15.09) mg/kg dose groups. For α-PVP (Figure 2B), significant reductions vs vehicle were seen in the 0.3- (M=−13.79) and 1- (M=−19.03) mg/kg doses. For METH (Figure 2C), significant reductions vs vehicle were seen in the 0.3- (M=−13.40) and 1- (M=−19.82) mg/kg doses.
For α-PVP, 4-MEC, and METH, higher doses (5, 100, and 3mg/kg, respectively) produced elevations in ICSS threshold values, with only METH producing significant elevations (mean±95% CI; α-PVP, 19.83±38.64; 4-MEC, 28.00±31.72; METH, 84.39±69.48%).
The slope, ED50, and maximal effect values are shown in Table 1. There were no significant differences in slopes of the linear portions of the dose-response curves between the 3 drugs (F[2,11]=1.63, P>.05). For ED50 values, a significant effect of drug was observed (F[2,11]=46.05, P<.001), and posthoc analyses revealed significant differences between METH and 4-MEC, METH and α-PVP, and α-PVP and 4-MEC (all Ps<.001). For comparison purposes, slope and ED50 values for MDPV and methylone were also calculated from previously published data (Watterson et al., 2012, 2014) but were not compared statistically. Finally, there were no significant differences observed in maximal reductions in ICSS thresholds (F[2,11]=1.64, P>.05).
Discussion
To our knowledge, there are currently no published studies directly assessing the potential abuse liability of the second-generation synthetic cathinones α-PVP or 4-MEC. The present study revealed that, similar to methamphetamine in the present study and to MDPV and methylone in previous studies (Watterson et al., 2012, 2014; Bonano et al., 2013), both α-PVP and 4-MEC dose-dependently decreased ICSS thresholds in a discrete trials current threshold procedure. ICSS threshold reductions are commonly accepted as indicative of facilitated brain reward function and the interoceptive rewarding effects of drugs of abuse and thus provide evidence of abuse potential in humans (Vlachou and Markou, 2011). At the highest doses tested, α-PVP (5mg/kg), 4-MEC (100mg/kg), and METH (3mg/kg) no longer decreased but instead produced increased ICSS thresholds (although these increases were not significant for α-PVP or 4-MEC). Increases in ICSS thresholds have been postulated to indicate decreases in brain reward function, aversive effects, and/or depression-like anhedonia (Vlachou and Markou, 2011). These higher doses were assessed only once in the current study and only in a subset of animals receiving 4-MEC and α-PVP because of the appearance of apparent aversive effects and thus were not included in statistical analyses. However, these observations suggest that high doses of all 3 of these psychostimulants may result in the emergence of deficits in brain reward function and/or aversion and anhedonia.
For α-PVP, the observed decreases in ICSS thresholds were very similar to those reported here for METH as well as those previously reported for MDPV (Watterson et al., 2014). The most robust threshold decrease observed for α-PVP was ~19% at the 1-mg/kg dose, which was similar to the 1mg/kg dose of METH presented here (~20%) and the 0.5-mg/kg dose of MDPV (~18%) (Watterson et al., 2012). Both METH and α-PVP resulted in significant ICSS threshold reductions at the 0.3- and 1-mg/kg doses. However, at the 3-mg/kg dose, METH led to an increase in ICSS thresholds (84.39±69.48, mean±95% CI), an effect not seen until a dose of 5mg/kg α-PVP was administered (19.83±38.64; mean±95% CI). Although these effects were not previously observed with MDPV doses tested up to 2mg/kg (Watterson et al., 2014), it is likely that higher doses of MDPV would also produce elevations in ICSS threshold effects similar to those observed in the current study with high doses of α-PVP and METH. In addition, the slope of the descending portion of the dose-effect curves for α-PVP was most similar to those observed after METH (−46.70 vs −50.23), along with the maximal ICSS threshold reductions (25.76% vs 21.11%) and ED50 values (0.35 vs 0.20mg/kg; see Table 1). Thus, α-PVP and METH are approximately equipotent in reducing ICSS thresholds; however, when compared with our previously published data on MDPV, maximal ICSS threshold reductions and slopes for both α-PVP and METH were approximately one-half those produced by MDPV (maximal reduction = 42.03%, slope = −96.07) under identical experimental procedures (Watterson et al., 2014). The ED50 dose for MDPV was the same α-PVP at 0.35mg/kg. Again, however, this dose was determined by maximal ICSS reductions that were approximately twice as robust for MDPV (42.03%) as α-PVP (25.76%). Thus, the ability of α-PVP to reduce ICSS thresholds is most similar to that of METH but approximately one-half that of MDPV.
For 4-MEC, the changes in ICSS thresholds observed in the present study closely resemble those previously observed for methylone (Watterson et al., 2012), albeit with a rightward shift in the dose response curve. The most robust decrease produced by 4-MEC was at the 30-mg/kg dose (~15%), similar to the 0.3-mg/kg doses of α-PVP (~14%) and METH (~13%) in the present study, the lowest dose of MDPV (0.1mg/kg, ~17%) previously tested (Watterson et al., 2014), and most robust, but nonsignificant, methylone dose (10mg/kg, ~13%) previously reported (Watterson et al., 2012). This maximal ICSS threshold decrease is also similar to that produced by MDMA (Hubner et al., 1988), but significantly less than maximal decreases produced by MDPV and METH (Watterson et al., 2014). At the 100-mg/kg dose (only assessed in a subset of animals, see Methods), 4-MEC increased ICSS thresholds (28.00%±31.73; mean±95% CI) indicative of a biphasic dose-response pattern that is typical of other illicit stimulants (Vlachou and Markou, 2011). In addition, neither the slope of the descending portion of the dose-effect curve for 4-MEC (−21.12) nor the maximal ICSS threshold decrease (17.40%) was different from α-PVP or METH. However, the ED50 value for 4-MEC was significantly different from α-PVP and METH, indicating that 4-MEC is less potent than these drugs. When compared with our previously published ICSS data for methylone (Watterson et al., 2012), 4-MEC led to similar maximal ICSS threshold reductions (−17.40% vs −21.50%) but much higher ED50 values (6.41 vs 1.00) and a slightly steeper slopes (−21.12 vs −17.59). However, our previously published study on methylone did not assess doses >10mg/kg; thus, it is possible that methylone may have led to greater maximal ICSS threshold reductions at higher doses. Although the results from the present study suggest that 4-MEC appears to be less potent than methylone, yet more effective in reducing ICSS thresholds, further experimentation is needed before definitive conclusions can be made.
The present study is, to our knowledge, the first to directly assess the potential abuse liability of second-generation synthetic cathinones. These results reveal that, like the first-generation synthetic cathinones now classified as Schedule I controlled substances for their high abuse potential, replacement synthetic cathinones possess similar rewarding effects as measured in ICSS procedures and thus likely possess similar degrees of abuse liability in humans. Furthermore, as with first-generation synthetic cathinones, these newer replacement cathinones appear to produce rewarding effects similar to the illicit stimulants methamphetamine and MDMA (Hubner et al., 1988; Vlachou and Markou, 2011). When considering the ICSS data from the present study, along with data previously published for MDPV (Watterson et al., 2014) and methylone (Watterson et al., 2012) under identical ICSS experimental conditions, the rank order potency of these drugs is MDPV>METH≈α-PVP>methylone≈4-MEC.
Synthetic cathinones, like prototypical psychostimulants, primarily exert their effects through substrate releasing or plasma membrane transporter blocking effects at monoaminergic terminals (Meltzer et al., 2006; Iversen et al., 2013; Lehner and Baumann, 2013; Marusich et al., 2014; Simmler et al., 2014). As previously mentioned, numerous studies suggest that there is a large degree of correspondence between ICSS threshold reductions, propensity for drug self-administration, and the balance between effects on DA vs 5-HT transmission in the mesolimbic reward pathway (Rothman and Baumann, 2003, 2006; Wee et al., 2005; Bauer et al., 2013a, 2013b). Specifically, higher DA/5-HT transporter affinity to release ratios correlate with increased self-administration propensity, more robust decreases in ICSS thresholds, and greater abuse liability as indicated by a progressively increased risk for compulsive use. Alternatively, lower DA to 5-HT ratios correlate with reduced propensity for self-administration, less robust decreases in ICSS thresholds, and a lower potential for compulsive drug intake. When considering all of the ICSS data from the present study along with previously published data on first-generation synthetic cathinones (Watterson et al., 2012, 2014) and prototypical psychostimulants (Hubner et al., 1988; Vlachou and Markou, 2011), these data generally support a strong positive relationship between differential effects on DA/5-HT signaling and rewarding effects, as revealed by maximal ICSS thresholds and steepness of slope of the descending portion of dose response curve. However, individual comparisons of the in vitro DAT/SERT affinities of all of these compounds is not appropriate, since some act primarily as presynaptic plasma membrane transporter blockers (MDPV and α-PVP) and others are transporter substrates and monoamine releasers (METH, methylone, and MDMA) or a combination of both (4-MEC). Thus, direct comparison of DAT to SERT data (ie, IC50 values) derived from inhibition of transporter function as assessed in competitive binding assays and data from monoamine release assays (ie, EC50 values) is problematic. However, when considering the aforementioned rank order potency (MDPV>α-PVP>4-MEC) based on ICSS threshold decreases and slopes of dose response curves within the class of monoamine transporter blockers, this rank order of potency is in close agreement with DAT to SERT ratios derived from IC50 values obtained in previous in vitro studies (MDPV [806–816]>α-PVP [806]>4-MEC [1.85]) (Baumann et al., 2012b, 2013; Marusich et al., 2014; Simmler et al., 2014). As previously mentioned, 4-MEC is also a weak 5-HT releaser (Simmler et al., 2014), which likely further decreases its abuse liability. When considering maximal ICSS threshold decreases and slopes of the dose response curve for monoamine releasers, their rank order potency is METH>methylone, which corresponds with their DAT to SERT ratios derived from EC50 values obtain in vitro (METH [152]>methylone [1.82–2]) (Baumann et al., 2012a, 2013).
Taken together, we predict that α-PVP possesses a potential for compulsive abuse (ie, addiction) that is roughly similar to that of METH and MDPV but much greater than that of 4-MEC, methylone, and MDMA. Accordingly, we also predict that 4-MEC will have a relatively lower potential for compulsive use than that of MDPV and METH, would be most similar to methylone and MDMA (ie, episodic use), and may exert primarily entactogenic effects.
Finally, it is also important to mention that both first- and second-generation synthetic cathinones are often sold as mixtures. Specifically, synthetic cathinone products have been shown to often contain more than one cathinone, as well as other adulterants, including illicit amphetamines, piperazines, cutting/binding agents, caffeine, and topical anesthetics (Brandt et al., 2010; German et al., 2014). Thus, although abuse liability assessment of these individual drugs is now emerging, assessment of the effects and abuse potential of combinations of these drugs will be more difficult yet should be a central focus of future research. Together, the results of the present study suggest that second-generation synthetic cathinones likely possess a similar potential for abuse as their first-generation predecessors as well as the illicit amphetamines they are designed to mimic. Furthermore, these findings have important implications for future research on synthetic cathinone abuse and dependence and legislative efforts to classify these drugs according to the proper controlled substance schedule.
Statement of Interest
None.
Acknowledgments
The authors wish to acknowledge the support of grant DA025606 from the National Institute on Drug Abuse. The authors would also like to thank Craig Trevor Johnson and Scott Wegner for their assistance in experimental procedures.
References
- Bauer C, Banks M, Blough B, Negus S. (2013). Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol 168:850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. (2012). The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37:192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KS. (2013). Psychoactive “bath salts”: not so soothing. Eur J Pharmacol 698:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. (2013). Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 231:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Aitken MR. (2006). ANOVA for the behavioural sciences researcher. L Erlbaum Association: Mahway, NJ, pp 94–103. [Google Scholar]
- Coppola M, Mondola R. (2012). 3,4-Methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol Lett 208:12–15. [DOI] [PubMed] [Google Scholar]
- Hubner C, Bird M, Rassnick S. (1988). The threshold lowering effects of MDMA (ecstasy) on brain-stimulation reward. Psychopharmacology 95:49–51. [DOI] [PubMed] [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang X-P, Roth BL. (2013). Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol 700:147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizaki A, Tanaka S, Numazawa S. (2014). New recreational drug 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (alpha-PVP) activates central nervous system via dopaminergic neuron. J Toxicol Sci 39:1–6. [DOI] [PubMed] [Google Scholar]
- Lehner KR, Baumann MH. (2013). Psychoactive “bath salts”: compounds, mechanisms, and toxicities. Neuropsychopharmacol 38:243–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. (1992). Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav 51:111–119. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzi KR, Wiley JL, Blough BE, Partialla JS, Baumann MH. (2014). Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. (2006). 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem 49:1420–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. (2011). The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol 8:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum C, Carreiro S, Babu K. (2012). Here today, gone tomorrow and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine. J Med Toxicol 8:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. (2003). Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479:23–40. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. (2006). Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann NY Acad Sci 1074:245–260. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gittings D, Johnstone M, Evangline D. (2003). Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology 169:21–27. [DOI] [PubMed] [Google Scholar]
- Schenk S, Colussi-Mas J, Do J, Bird J. (2012). Profile of MDMA self-administration from a large cohort of rats: MDMA develops a profile of dependence with extended testing. J Drug Alcohol Res 1:1–6. [Google Scholar]
- Sidman M. (1960). Tactics of scientific research. New York: Basic Books, Inc. [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu L-H, Huwyler J, Chaboz S, Hoener M, Liechti M. (2013). Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler L, Rickli A, Hoener MC, Liechti ME. (2014). Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 79:152–160. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. (2011). Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol 49:499–505. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (2013). World Drug Report. Vienna: United Nations.
- United States Department of Justice Drug Enforcement Administration (2011a). background, data and analysis of synthetic cathinones: mephedrone (4-MMC), methylone (MDMC) and 3,4-methylenedioxypyrovalerone (mdpv). Washington, DC: U.S. Government Printing Office.
- United States Department of Justice Drug Enforcement Administration (2011b). Chemicals used in “bath salts” Now under federal control and regulation Washington, DC: U.S. Government Printing Office.
- United States Department of Justice Drug Enforcement Administration (2013a). Federal Register, Vol. 78, No. 71, Friday, April 12, 2013/Rules and regulations.
- United States Department of Justice Drug Enforcement Administration (2013b). Synthetic cannabinoids and cathinones - DEA request for information.
- United States Department of Justice NDIC (2011c). Synthetic cathinones (bath salts): an emerging domestic threat.
- United States of America, One Hundred Twelfth Congress (2012). S. 3187: Food and Drug Administration Safety and Innovation Act.
- Vlachou S, Markou A. (2011). Intracranial self-stimulation. In: Animal models of drug addiction (Olmstead MC, ed), pp 3–56. New York: Springer. [Google Scholar]
- Watterson LR, Hood LE, Sewalia K, Tomek SE, Yahn S, Johnson CT, Wegner S, Blough BE, Marusich JA, Olive MF. (2012). The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts.” J Addict Res Ther s9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Watterson E, Olive MF. (2013). Abuse liability of novel “legal high” designer stimulants: evidence from animal models. Behav Pharmacol 24:341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. (2014). Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol 19:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Olive MF. (2014). Synthetic cathinones and their rewarding and reinforcing effects in rodents. Adv Neurosci 2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. (2005). Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther 313:848–854. [DOI] [PubMed] [Google Scholar]


