Abstract
Background:
Extensive research efforts have generated genomic, transcriptomic, proteomic, and functional data hoping to elucidate psychiatric pathophysiology. Selected reaction monitoring, a recently developed targeted proteomic mass spectrometric approach, has made it possible to evaluate previous findings and hypotheses with high sensitivity, reproducibility, and quantitative accuracy.
Methods:
Here, we have developed a labelled multiplexed selected reaction monitoring assay, comprising 56 proteins previously implicated in the aetiology of major psychiatric disorders, including cell type markers or targets and effectors of known psychopharmacological interventions. We analyzed postmortem anterior prefrontal cortex (Brodmann area 10) tissue of patients diagnosed with schizophrenia (n=22), bipolar disorder (n=23), and major depressive disorder with (n=11) and without (n=11) psychotic features compared with healthy controls (n=22).
Results:
Results agreed with several previous studies, with the finding of alterations of Wnt-signalling and glutamate receptor abundance predominately in bipolar disorder and abnormalities in energy metabolism across the neuropsychiatric disease spectrum. Calcium signalling was predominantly affected in schizophrenia and affective psychosis. Interestingly, we were able to show a decrease of all 4 tested oligodendrocyte specific proteins (MOG, MBP, MYPR, CNPase) in bipolar disorder and to a lesser extent in schizophrenia and affective psychosis. Finally, we provide new evidence linking ankyrin 3 specifically to affective psychosis and the 22q11.2 deletion syndrome-associated protein septin 5 to schizophrenia.
Conclusions:
Our study highlights the potential of selected reaction monitoring to evaluate the protein abundance levels of candidate markers of neuropsychiatric spectrum disorders, providing a high throughput multiplex platform for validation of putative disease markers and drug targets.
Keywords: SRMstats, myelination, GSK3b, CamKII, microglia.
Introduction
Psychiatric disorders represent a considerable burden for healthcare providers around the world, affecting >20% of the global population. Despite extensive research efforts during the past decades, the aetiologies of the major psychiatric disorders schizophrenia (SZ), bipolar disorder (BD), and major depressive disorder (MDD) are largely unknown. These disorders are heterogeneous in nature, with genetic and environmental factors contributing to pathogenesis and aetiology (Levinson, 2006; van Os and Kapur, 2009; Kim et al., 2011; Craddock and Sklar, 2013; Klengel and Binder, 2013). Symptom profiles greatly overlap with regards to clinical psychopathology as well as putative pathophysiology (Smoller et al., 2013). So far, no single gene, mRNA transcript, or protein has been found that can account for the pathology of any of these disorders. However, the existence of a variety of risk genes and risk-associated protein alterations is now widely accepted. Genome-wide association studies, identifying a large number of single nucleotide polymorphisms in candidate genes and structural genetic differences such as copy number variations, as well as micro-array and proteomic analyses have greatly contributed to this knowledge (Pickard, 2011). However, at present, there is a lack of information regarding which of the genetic risk polymorphisms are associated with changes at the mRNA and protein levels.
Recent technological developments in proteomic methods have now made it possible to validate high-throughput findings with great accuracy and sensitivity (Wesseling et al., 2014). SRM is currently the most advanced targeted mass spectrometry-based technology. In contrast to shot-gun proteomics strategies, in which the goal is to simultaneously investigate abundance changes of hundreds to thousands of proteins at the expense of sensitivity, SRM mass spectrometry platforms allow monitoring of a predetermined selection of proteins/peptides with high sensitivity, reproducibility, and quantitative accuracy (Picotti et al., 2010; Picotti and Aebersold, 2012). In addition, the SRM approach has emerged as an alternative to affinity-based assays such as enzyme-linked immune-sorbent assays with the advantage of faster and more cost-effective assay development (Whiteaker et al., 2011). Protein quantification by SRM in complex samples using predefined assay coordinates is reproducible across different laboratories and instrument platforms, facilitating reproducibility of assays in follow-up studies. Developed assays are universally applicable to test hypotheses across a variety of samples of different origins (eg, brain tissue, blood) and easily adjustable to different matrices. Therefore, SRM is ideal to investigate whether previously reported candidate risk genes for psychiatric disorders translate to changes at the protein level, providing evidence of functional effects and clinical relevance.
We designed a targeted multiple isotope labelled SRM-assay, comprising 56 proteins that have been implicated in the major psychiatric disorders and screened a total of 89 postmortem brain tissue samples of 22 SZ patients, 23 BD patients, 11 MDD patients, 11 MDD patients with psychotic features (MDD-P), and 22 healthy controls (CTs). The proteins tested have previously been associated with psychiatric pathophysiology at the gene, mRNA transcript, or protein level. Our main objective was to investigate the potential of SRM to systematically elucidate the pathophysiology of neuropsychiatric disorders and to further evaluate putative risk markers as novel molecular targets for the next generation of drugs.
Materials and methods
Clinical Samples
A total of 89 postmortem anterior prefrontal cortex (Broadmann Area 10) brain samples were provided by the Stanley Medical Research Institute (Bethesda, MD) (Torrey et al., 2000) and consisted of 22 SZ patients, 23 BD patients, 11 MDD patients without psychotic symptoms, 11 MDD-P patients with psychotic symptoms and 22 CTs. Tissue was collected postmortem from patients and controls with full informed consent obtained from a first-degree relative in compliance with the Declaration of Helsinki, and consent was obtained by questionnaires conducted over the phone and signed by 2 witnesses. The sample groups were matched for age of death, gender, and brain pH (t test). There were no significant differences in the brain side from which samples were obtained, secondary axis diagnosis of alcohol abuse/dependency, and drug abuse/dependency between patients and CTs (Fisher’s exact test). Tissue was sectioned using a Leica Cryostat (Milton Keynes, UK) and stored at −80°C until use. All tissue samples used contained equal amounts of white and grey matter. A summary of the demographic details and statistical values is shown in supplementary Table S1. Additional information is provided in supplementary Table S2.
Sample Preparation
Approximately 50mg of tissue per sample was used. Samples were added to fractionation buffer containing 7M urea, 2M thiourea, 4% CHAPS, 2% ASB14, and 70mM dithiothreitol at a 5:1 (vol/wt) ratio (Ernst et al., 2012). After sonication and vortexing for 30 minutes, protein concentrations of the lysates were determined using a Bradford assay (Bio-Rad, Hemel Hempstead, UK). Protein (approximately 100 μg) was precipitated using acetone. After dissolving the precipitate in 50mM ammonium bicarbonate, protein concentrations were determined in quadruplets. Reduction of sulfhydryl groups on proteins was performed with 5mM dithiothreitol at 60°C for 30 minutes and alkylation was carried out using 10mM iodacetamide and incubating in the dark at 37°C for 30 minutes. Proteins were digested using trypsin at a 1:50 (wt/wt) ratio for 17 hours at 37°C, and reactions were stopped by the addition of 8.8M HCl in a 1:60 (vol/vol) ratio. Sample aliquots were stored at −80°C until analysis.
Label-Based Selected Reaction Monitoring Mass Spectrometry
Abundance alterations of a panel of 56 candidate proteins implicated in the pathology of the major psychiatric disorders or associated with drug treatments were measured using targeted SRM mass spectrometry on a Xevo TQ-S mass spectrometer (Waters Corporation) coupled online through a New Objective nanoESI emitter (7cm long, 10-mm tip; New Objective) to a nanoAcquity UPLC system (Waters Corporation). The system was comprised of a C18 trapping column (180 μmx20mm, 5-μm particle size) and a C18 BEH nano-column (75 μmx200mm, 1.7-mm particle size). The separation buffers were (A) 0.1% formic acid and (B) 0.1% formic acid in acetonitrile. For separation of peptides, the following 48-minute gradient was applied: 97/3% (A/B) to 60/40% in 30 minutes; 60/40% to 15/85% in 2 minutes; 5 minutes at 15/85%; and returning to the initial condition in 1 minute. The flow rate was 0.3 µL/min and the column temperature was 35°C.
SRM assays were developed following a high-throughput strategy (Picotti et al., 2010) (Figure 1a). We initially started with more than 200 selected proteins. Up to 12 unique peptides ranging from 6 to 20 amino acids in length containing tryptic ends and no missed cleavages were chosen for each of the selected proteins. All peptides containing amino acids prone to undergo modifications (eg, Met, Trp, Asn, and Gln), potential ragged ends, lysine/arginine followed by proline, or bearing NXT/NXS glycosylation motifs were avoided and selected only when no other options were available (Lange et al., 2008). Peptides were checked by Protein BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) searches to ensure uniqueness. For method refinement, up to 12 transitions per peptide were tested in SRM mode. Transitions were calculated using Skyline version 1.2.0.3425 (MacLean et al., 2010) and corresponded to singly charged y-ions from doubly or triply charged precursors in the range of 350 to 1250Da. Transitions were selected based on software internal predictions, discovery proteomics data, and spectral data available through the Human NIST spectral libraries (Farrah et al., 2011). Method refinement was performed on quality control samples. For the final SRM assay, the 2 to 3 peptides with the maximal intensities and highest spectral library similarity (dotp) were selected. We also analyzed heavy-label spiked quality control samples (Figure 1b) in scheduled SRM mode to confirm identity via coelution, extracted the optimal fragment ions for SRM analysis, obtained accurate peptide retention times, and optimized collision energy and cone voltage for the quantification run applying skyline software (MacCoss Lab Software; Seattle, WA) (MacLean et al., 2010). Heavy labelled forms of the selected peptides (spiketides L) were chemically synthesized via SPOT synthesis (JPT Peptide Technologies GmBH, Berlin, Germany). The final transitions, collision energy, and retention time windows used for each peptide can be found in the supplementary information (supplementary Table S3).
Figure 1.
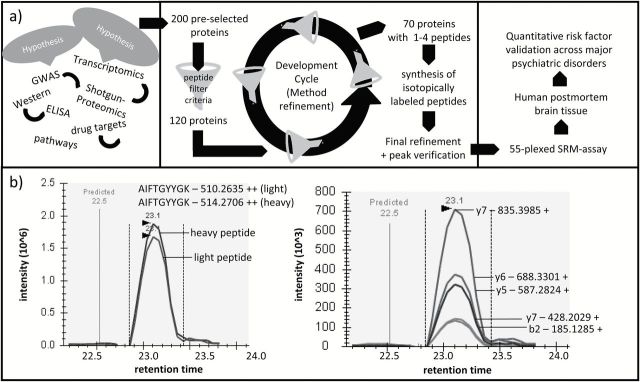
a, Schematic overview of the study design. b, left, Chromatographic SRM profile of the sample’s endogenic tryptic peptide AIFTGYYGK and of the spiked heavy labelled reference peptide. Right, Chromatographic SRM profile of the transitions of the light peptide.
Quantitative SRM measurements comparing patients and controls were performed in scheduled SRM acquisition mode using the optimized parameters defined during the assay refinement. For each target peptide, a heavy isotope labelled internal standard (JPT Peptide Technologies GmbH) was spiked in the peptide mixture for accurate quantification and identification. All SRM functions had a 2-minute window of the predicted retention time and scan times were 20 milliseconds. For each peptide, at least 3 transitions were monitored for the heavy and light version. Samples were run randomized and blocked (Oberg and Vitek, 2009) in triplicates, and blanks and quality control peptide injections (yeast alcohol dehydrogenase; supplementary Table S3) were performed alternating after every biological replicate. Resulting SRM data were analyzed using Skyline and explanatory data analysis (quality assessment), and model-based statistical analysis was conducted using SRMstats (Chang et al., 2011). The data preprocessing consisted of a log2 transformation of the data to stabilise the variance. A constant normalization was performed based on reference transitions for all proteins to equalize the median peak intensities of reference transitions from all proteins across all mass spectrometry runs and adjusted the bias to both reference and endogenous signals. Protein level quantification and testing for differential abundance among patient and control groups were carried out using the linear mixed-effects model implemented in SRMstats. The linear mixed model function supported by SRMstats employs a “restricted” or “expanded” scope of conclusions (Chang et al., 2011; Surinova et al., 2013). In the restricted scope model, the individual samples being modelled are the population of interest, whereas in the expanded scope model, the samples being modelled are treated as a random sample from the population of interest. Consequently, the expanded scope model allowed us to draw conclusions about the population from which the samples were drawn, and the restricted scope allowed conclusions within the data itself. Initially, we assumed the restricted scope, taking into account the measurement error of transitions across runs (technical variation), to quantify protein abundance changes in our sample cohorts. Subsequently, we continued with the expanded scope, accounting for technical variation and considering the individual biological replicates (biological variation) of our sample cohorts as random selection of their respective populations of origin to test which protein changes could be considered as representative of their underlying populations. The P-values were adjusted to control the false discovery rate at a cut-off of 0.05 according to Benjamini and Hochberg (Chang et al., 2011).
Results
Applying label-based SRM mass spectrometry, we measured differential protein abundance levels of 56 predefined proteins (Table 1) in postmortem brain samples from 22 SZ, 23 BD, 11 MDD, and 11 MDD-P patients compared with 22 CTs. Additionally, MDD-P was compared directly with MDD to identify proteins associated with affective psychosis in MDD. We detected common and unique alterations in individual protein levels across the disorders using a statistical modelling framework for protein significance analysis based on the SRM spectral data. The model required the scope of conclusion validity to be specified as either “restricted” or “expanded” corresponding to drawing conclusions about the data itself or about the population from which the individual samples were drawn.
Table 1.
Overview of the Multiplex SRM Assay
| Protein Name | UP-ID | Function Summary | CL | G | T | P | Link to | ||
|---|---|---|---|---|---|---|---|---|---|
| SZ | BD | MDD | |||||||
| 22q11.2 Deletion Syndrome | |||||||||
| Catechol O-methyltransferase | COMT | Catalyzes inactivation, of catecholamine neurotransmitters and catechol hormones | 22q11.21 | √ | [1–4] | [5, 6] | [7] | ||
| Proline dehydrogenase 1, mito | PROD | Converts proline to delta-1-pyrroline-5- carboxylate | 22q11.21 | √ | [1, 8, 9] | ||||
| Ran-binding protein 1 | RANG | Inhibits GTP exchange on Ran. Increases GTP hydrolysis induced by RANGAP1 | 22q11.21 | √ | [1] | ||||
| Septin 5 | SEPT5 | Filament-forming cytoskeletal GTPase, may play a role in cytokinesis | 22q11.21 | √ | [1] | ||||
| TCA transport protein, mito | TXTP | Involved in citrate-H+/malate exchange | 22q11.21 | √ | [1] | ||||
| Receptors | |||||||||
| Glutamate receptor 1 | GRIA1 | Ionotropic glutamate receptor (AMPA1) | 5q31.1 | √ | [2, 10] | ||||
| Glutamate receptor 2 | GRIA2 | Ionotropic glutamate receptor (AMPA2) | 4q32.1 | √ | [10–12] | [12] | [12] | ||
| Glutamate receptor 3 | GRIA3 | Ionotropic glutamate receptor (AMPA3) | 7q21.1- q21.2 | √ | [10] | [12] | |||
| NMDA receptor 1 | NMDZ1 | NMDA receptor subtype, high calcium permeability, voltage-dependent sensitivity to Mg2+ | 9q34.3 | √ | √ | √ | [10, 13, 14] | [15] | [16–18] |
| Scaffolding proteins | |||||||||
| Ankyrin 3 | ANK3 | Neuronal scaffolding protein in nodes of Ranvier and axon initial segments | 10q21 | √ | [19, 20] | [19, 21, 22] | [19] | ||
| Disks large homolog 4 (PSD95) | DLG4 | Interacts with the NMDA receptor subunits and shaker-type potassium channels | 17p13.1 | √ | √ | √ | [13, 14, 23–31] | [13, 23] | |
| Shank3 | SHAN3 | Post-synaptic density protein, interconnects NMDA and metabotropic glutamate receptors | 22q13.3 | √ | [32–34] | [35, 36] | |||
| Downstream signalling | |||||||||
| Calcium signalling | |||||||||
| Calmodulin (CaM) | CaM | Calmodulin mediates the control of a large number of enzymes, ion channels by Ca2+ | 14q32.11 | √ | [37, 38] | ||||
| CamK2α | KCC2A | Major protein kinase in the CNS, functions in LTP and neurotransmitter release | 5q32 | √ | √ | [39, 40] | |||
| CamK2β | KCC2B | Function autonomously after Ca2+/calmodulin-binding and autophosphorylation, involved in dendritic spine and synapse formation, neuronal plasticity | 7p14.3- p14.1 | √ | [41, 42] | [41] | |||
| CamK2γ | KCC2G | 10q22 | |||||||
| Calcineurin subunit B type 1 | CANB1 | Regulatory subunit of calcineurin, Ca2+/ calmodulin protein phosphatase | 2p15 | √ | [43] | ||||
| IP3 receptor isoform 1 | ITPR1 | Intracellular channel, mediates calcium release from the endoplasmic reticulum | 3p26.1 | [44] | |||||
| Neurochondrin | NCDN | Involved in signal transduction, increases cell surface localization of GRM5 | 1p34.3 | [45] | |||||
| Calmodulin-dep. calcineurin A subunit beta isoform | PP2BB | Ca2+-dependent, calmodulin-stimulated protein phosphatase, may have a role in the calmodulin activation of calcineurin. | 10q22 | √ | √ | [46, 47] | |||
| Protein Kinase | |||||||||
| Protein kinase C α type | KPCA | Ca2+-act. Ser/Thr -protein kinase, involved in cell proliferation, apoptosis, differentiation, migration | 17q22- q23.2 | √ | √ | [48, 49] | [50] | ||
| Protein kinase C β type | KPCB | Ca2+-act. Ser/Thr-protein kinase involved in oxidative stress-induced apoptosis & insulin signaling | 16p11.2 | √ | [48, 49] | [50, 51] | |||
| Protein kinase C γ type | KPCG | Ca2+-act. Ser/Thr-protein kinase, regulates neuronal receptor functions, mediates synaptic function | 19q13.4 | √ | [48, 49] | ||||
| MARCKS | MARCS | MARCKS is the most prominent cellular substrate for protein kinase C | 6q22.2 | √ | [52–54] | [55] | |||
| ERK-signalling | |||||||||
| ERK2 | MK01 | Serine/threonine kinase, essential component of the MAPK signal transduction pathway | 22q11.21 | √ | [56–58] | [59] | |||
| ERK1 | MK03 | Serine/threonine kinase, essential component of the MAPK signal transduction pathway | 16p11.2 | √ | [56–58] | ||||
| PED | PEA15 | Blocks Ras-mediated inhibition of integrin activation, modulates the ERK MAP kinase cascade | 1q21.1 | √ | [60–62] | ||||
| mTOR | |||||||||
| mTOR | MTOR | Central regulator of cellular metabolism, growth and survival | 1p36.2 | √ | [63] | ||||
| 40S ribosomal protein RS3A | RS3A | Ribosomal subunit | 8q24.3 | ||||||
| 40S ribosomal protein S4, X iso. | RS4X | Ribosomal subunit | Xq13.1 | ||||||
| Wnt signalling | |||||||||
| Catenin β-1 | CTNB1 | Key downstream component of the canonical Wnt signaling pathway | 3p21 | √ | [64] | ||||
| Glycogen synthase kinase-3 β | GSK3B | Active protein kinase, neg. regulator in the hormonal control of glucose homeostasis, | 3q13.3 | √ | √ | [65–67] | [68–72] | [73, 74] | |
| Phosphoprotein F1-20 | AP180 | Component of the adapter complexes which link clathrin to receptors in coated vesicles | 6q14.2 | √ | [75] | ||||
| Cell-type specific proteins | |||||||||
| Oligodendrocytes | |||||||||
| CNPase | CN37 | Involved in RNA metabolism in the myelinating cell, third most abundant protein in CNS myelin | 17q21 | √ | √ | [76–79] | |||
| Myelin basic protein | MBP | Most abundant component of myelin membrane, plays role in myelin formation and stabilization | 18q23 | √ | √ | [2, 80–84] | [83] | [84] | |
| Myelin proteolipid protein | MYPR | Major myelin protein from CNS, plays role in multi-laminar myelin formation or maintenance | Xq22 | √ | [85, 86] | [87] | |||
| Myelin-oligodendrocyte glycoprotein | MOG | Minor component of the myelin sheath, myelin sheath completion/maintenance, cell-cell communication | 6p22.1 | √ | [88–90] | [88] | [87] | ||
| Astrocytes | |||||||||
| Glial fibrillary acidic protein | GFAP | GFAP, a class-III intermediate filament, is a cell-specific marker for astrocytes | 17q21 | √ | √ | [91–94] | [95, 96] | ||
| Microglia | |||||||||
| Coronin-1A | COR1A | Crucial component of the cytoskeleton of highly motile cells | 16p11.2 | √ | √ | [94, 97–100] | |||
| Energy metabolism | |||||||||
| ATP synthase subunit b | ATP5F | Mitochondrial membrane ATP synthase | 18q21 | √ | √ | √ | [101] | [102] | [103] |
| Citrate synthase, mito | CISY | Pace-making enzyme in the first step of the tricarboxylic acid cycle (TCA) | 12q13.2 | √ | [104] | ||||
| Complex I-75 kDa | NDUS1 | Core subunit of the mitochondrial membrane respiratory chain, required for catalysis | 2q33-q34 | √ | √ | [105–107] | [107, 108] | [107] | |
| Malate dehydrogenase | MDHC | Catalyzes the oxidation of malate to oxalacetate, involved in TCA, gluconeogenesis | 2p13.3 | √ | √ | √ | [11, 78, 105, 109–112] | ||
| Phosphoglycerate kinase 1 | PGK1 | Major ATP-generating enzyme in glycolysis, reversed reaction in gluconeogenesis | Xq13.3 | √ | [113] | [114] | |||
| Triosephosphate isomerase | TPIS | Glycolytic enzyme, seems to act as a polymerase alpha cofactor protein | 12p13 | √ | [94, 113] | ||||
| Oxidative Stress | |||||||||
| Catalase | CATA | Protects cells from the toxic effects of hydrogen peroxide | 11p13 | √ | √ | [115, 116] | [117–120] | ||
| Glutathione peroxidase 1 | GPX1 | Protects the hemoglobin in erythrocytes from oxidative breakdown | 3p21.3 | √ | √ | [121–123] | [124] | ||
| Protein DJ-1 | PARK7 | Protects cells against oxidative stress and cell death | 1p36.23 | √ | [104, 125] | ||||
| Peroxiredoxin-3 | PRDX3 | Redox regulation of the cell. Protects radical- sensitive enzymes from oxidative damage | 10q25-q26 | ||||||
| Neuronal structure/plasticity | |||||||||
| Amyloid beta A4 protein | A4 | cell surface receptor, plays role in neurite growth, neuronal adhesion and axonogenesis | 21q21 | √ | [126] | [127] | |||
| BDNF/NT-3 growth factors receptor | NTRK2 | Receptor tyrosine kinase, involved in the nervous system development via regulation of neuron survival, proliferation, migration, differentiation, synapse formation and plasticity | 9q22.1 | √ | √ | √ | [128–130] | [128, 131–133] | [128, 134–138] |
| Neural cell adhesion molecule 1 | NCAM1 | Cell adhesion molecule involved in neuron- neuron adhesion, neurite fasciculation & outgrowth | 11q23.1 | √ | √ | [2, 139] | [140] | ||
| Neuromodulin | NEUM | Major component of the motile “growth cones”, role in axonal and dendritic filopodia induction | 3q13.31 | √ | [141, 142] | [142] | |||
| Neurofilament light polypeptide | NFL | Maintenance of neuronal caliber | 8p21 | √ | √ | [28] | [143] | ||
| Transcription Factors | |||||||||
| Nuclear factor NF- kappa-B p105 | NFKB1 | Pleiotropic transcription factor, endpoint of a series of signal transduction events | 4q24 | √ | [144] | [145, 146] | |||
| Transcriptional activator protein Purα | PURA | Transcription activator, plays a role in the initiation of DNA replication and in recombination | 5q31 | √ | [147] | ||||
Abbreviations: CL, chromosome location; G, implicated at genetic level; P, implicated at protein level; T, implicated at transcript level; UP-ID, UniProt identification code.
Assays were selected based on literature findings. For references, see supplementary Table S4.
Statistical analysis using the restricted model resulted in the identification of 12 differentially expressed proteins in the SZ/CT, 27 in BD/CT, 11 in MDD/CT, 6 in MDD-P/CT, and 15 in the MDD-P/MDD group comparisons (Table 2).
Table 2.
Significantly Changed Proteins Identified By Label-Based LC-SRM in the SZ/CT, BD/CT, MDD/CT, MDD-P/CT, and MDD-P/MDD Comparisons
| Protein | SZ/CT | BD/CT | MDD/CT | MDD-P/CT | MDD-P/MDD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratio | p | p* | Ratio | p | p* | Ratio | p | p* | Ratio | p | p* | Ratio | p | p* | |
| 22q11.2 Deletion Syndrome | |||||||||||||||
| Catechol O-methyltransferase | not significant | 1.25 | 0.0038 | 0.0102 | not significant | not significant | not significant | ||||||||
| Ran-binding protein 1 | not significant | not significant | 1.16 | 0.009 | 0.048 | not significant | not significant | ||||||||
| Septin 5 | 0.66 | 7.0E-06 | 7E-05 | not significant | not significant | not significant | not significant | ||||||||
| not significant in any comparison: | TCA transport protein, mito; Proline dehydrogenase 1, mito | ||||||||||||||
| Receptors | |||||||||||||||
| Glutamate receptor 2 | not significant | 1.16 | 0.0039 | 0.0102 | not significant | not significant | not significant | ||||||||
| NMDA receptor 1 | not significant | 1.27 | 0.019 | 0.0418 | not significant | not significant | not significant | ||||||||
| not significant in any comparison: | Glutamate receptor 1, Glutamate receptor 3 | ||||||||||||||
| Scaffolding proteins | |||||||||||||||
| Ankyrin-3 | not significant | not significant | 1.17 | 0.003 | 0.019 | 0.80 | 4.9E-05 | 0.0006 | 0.68 | 1.1E-09 | 6E-08 | ||||
| Disks large homolog 4 (PSD95) | 0.88 | 1.0E-03 | 0.006 | 1.13 | 0.002 | 0.006 | not significant | not significant | not significant | ||||||
| not significant in any comparison: | Shank 3 | ||||||||||||||
| Downstream signalling | |||||||||||||||
| Calcium signalling | |||||||||||||||
| CamK2α | not significant | 1.12 | 0.0002 | 0.0007 | not significant | not significant | 0.88 | 0.0035 | 0.026 | ||||||
| CamK2β | not significant | not significant | 1.15 | 8E-04 | 0.008 | not significant | 0.87 | 0.00515 | 0.031 | ||||||
| CamK2γ | not significant | 1.22 | 2E-07 | 1E-06 | 1.21 | 6E-05 | 0.001 | not significant | 0.87 | 0.01239 | 0.05 | ||||
| PP2BB | not significant | not significant | not significant | not significant | 0.83 | 0.00447 | 0.03 | ||||||||
| Calcineurin subunit B type 1 | not significant | 1.19 | 0.0052 | 0.012 | not significant | not significant | not significant | ||||||||
| Neurochondrin | not significant | 1.19 | 0.0051 | 0.012 | not significant | not significant | not significant | ||||||||
| not significant in any comparison: | Calmodulin, IP3 receptor isoform 1 | ||||||||||||||
| Protein Kinase | |||||||||||||||
| Protein kinase C α type | not significant | 1.17 | 0.0015 | 0.0048 | not significant | not significant | not significant | ||||||||
| Protein kinase C γ type | not significant | not significant | not significant | not significant | 0.85 | 0.01158 | 0.05 | ||||||||
| MARCKS | not significant | 1.10 | 0.0086 | 0.02 | not significant | not significant | not significant | ||||||||
| not significant in any comparison: | Protein kinase C, β type | ||||||||||||||
| ERK-signalling | |||||||||||||||
| ERK1 | not significant | 1.11 | 5E-05 | 0.0002 | 1.10 | 0.002 | 0.015 | not significant | 0.88 | 0.00046 | 0.005 | ||||
| not significant in any comparison: | ERK2, PED | ||||||||||||||
| mTOR | |||||||||||||||
| 40S ribosomal protein S4, X isoform | not significant | 1.16 | 0.0004 | 0.0014 | not significant | not significant | 0.85 | 0.00853 | 0.043 | ||||||
| not significant in any comparison: | mTOR, 40S ribosomal protein RS3A | ||||||||||||||
| Wnt signalling | |||||||||||||||
| Catenin β-1 | not significant | 1.21 | 1E-06 | 7E-06 | not significant | not significant | not significant | ||||||||
| Glycogen synthase kinase-3 β | 0.90 | 7.5E-03 | 0.041 | 1.13 | 0.0037 | 0.0102 | not significant | not significant | not significant | ||||||
| ‘not significant in any comparison | Phosphoprotein F1-20 | ||||||||||||||
| Cell-type specific proteins | |||||||||||||||
| Oligodendrocytes | |||||||||||||||
| CNPase | 0.79 | 1.1E-07 | 2.2E-06 | 0.53 | <1E-16 | <1E-16 | not significant | 0.79 | 1.8E-05 | 0.0003 | not significant | ||||
| Myelin basic protein | 0.79 | 2.3E-06 | 2.7E-05 | 0.64 | <1E-16 | <1E-16 | not significant | 0.79 | 1.4E-04 | 0.0014 | not significant | ||||
| Myelin proteolipid protein | 0.79 | 2.1E-11 | 6.2E-10 | 0.61 | <1E-16 | <1E-16 | not significant | 0.82 | 2.1E-06 | 4E-05 | not significant | ||||
| Myelin-oligodendrocyte glycoprotein | 0.79 | 2.4E-12 | 1.5E-10 | 0.56 | <1E-16 | <1E-16 | not significant | 0.82 | 1.5E-06 | 4E-05 | 0.86 | 0.00131 | 0.011 | ||
| Astrocytes | |||||||||||||||
| Glial fibrillary acidic protein | 0.89 | 1.7E-04 | 0.001 | not significant | 0.83 | 5E-07 | 3E-05 | 0.79 | 8.4E-11 | 5E-09 | not significant | ||||
| Microglia | |||||||||||||||
| Coronin-1A | not significant | not significant | 0.77 | 4E-04 | 0.005 | not significant | not significant | ||||||||
| Energy metabolism | |||||||||||||||
| Citrate synthase, mito | not significant | 1.17 | 0.0001 | 0.0004 | not significant | not significant | not significant | ||||||||
| Complex I-75 kDa | not significant | 1.25 | 0.0002 | 0.0006 | not significant | not significant | not significant | ||||||||
| Malate dehydrogenase, mito | 0.93 | 8.5E-03 | 0.042 | 1.19 | 1E-10 | 1E-09 | 1.11 | 0.002 | 0.015 | not significant | 0.91 | 0.01183 | 0.05 | ||
| not significant in any comparison | ATP synthase subunit b, Phosphoglycerate kinase 1, Triose phosphate isomerase | ||||||||||||||
| Oxidative Stress | |||||||||||||||
| Peroxiredoxin-3 | not significant | 1.19 | 1E-06 | 7E-06 | not significant | not significant | not significant | ||||||||
| not significant in any comparison | Glutathione peroxidase 1, Protein DJ-1, Catalase | ||||||||||||||
| Neuronal structure/plasticity | |||||||||||||||
| Neuromodulin | 0.89 | 3.6E-04 | 0.003 | not significant | not significant | not significant | 1.17 | 0.0007 | 0.007 | ||||||
| Neurofilament light polypeptide | 0.91 | 6.8E-04 | 0.005 | 0.84 | 5E-11 | 6E-10 | 1.10 | 0.002 | 0.015 | not significant | 0.86 | 6.3E-05 | 0.002 | ||
| not significant in any comparison: | APP A4; Neural cell adhesion molecule 1; BDNF/NT-3 growth factors receptor | ||||||||||||||
| Transcription factors | |||||||||||||||
| Transcriptional activator protein Pur-α | not significant | 1.13 | 2E-05 | 1E-04 | 1.18 | 1E-05 | 2E-04 | not significant | 0.85 | 0.00016 | 0.002 | ||||
| not significant in any comparison: | Nuclear factor NF-kappa-B p105 | ||||||||||||||
Abbreviations: BD, bipolar disorder; CT, controls; MDD, major depressive disorder; SZ, schizophrenia.P-values were determined using SRMstats (fixed-subject effects) and corrected to control for multiple hypothesis testing after Benjamini-Hochberg (Chang et al., 2011). Significant findings using the mixed subject effect model of the SRMstats framework are indicated by grey shading. For reasons of clarity, only ratios and significance levels of significantly changing proteins are shown. For full information, see supplementary Table S5.
Most alterations in protein abundance levels were found in the BD/CT comparison in which the majority of proteins were increased, with the exception of oligodendrocyte-specific proteins (CN37, MBP, MOG, MYPR) and neurofilament light polypeptide, which were decreased. A less pronounced decrease in oligodendrocyte-specific proteins was found in the SZ/CT and MDD-P/CT comparisons. In MDD/CT, lower levels of the astrocyte marker glial fibrillary acidic protein and the microglia marker coronin 1a were identified. In the BD/CT comparison, the Wnt signalling pathway appeared to be upregulated. The MDD/CT comparison showed increases in calmodulin-dependent protein kinase 2 subunits β and γ as well as ERK signalling. All proteins found to be significantly different in MDD-P/MDD were decreased similar to the finding in the SZ/CT comparison, although this involved different proteins. In the MDD-P/MDD comparison, all 3 calmodulin-dependent protein kinase 2 subunits (CAMK2α, CAMK2β, CAMK2y) were decreased. To test for potential medication effects, we correlated the SRM intensity estimates and fluphenazine milligram equivalents and found no significant effect on any measured protein (Spearman correlation P>.1). However, effects of other psychotropic medication such as antidepressants and mood-stabilizing agents cannot be ruled out. To exclude further confounding effects, we correlated the SRM intensity estimates and additional demographic characteristics (brain pH, postmortem interval, age of death, age of disease onset, disease duration). We were unable to find any correlation (Spearman correlation P>.1). For further information, see supplementary Table S6.
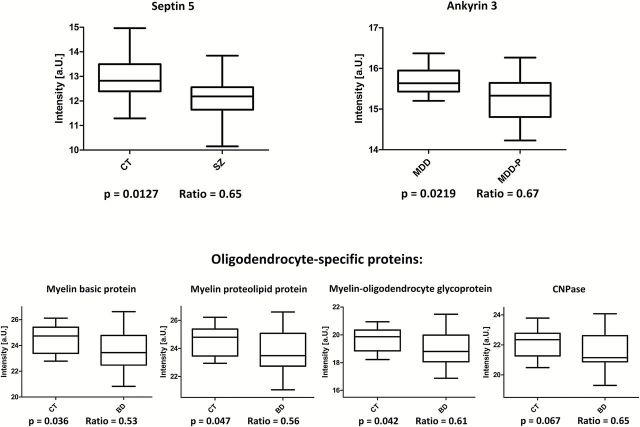
Since these proteomic findings resulted from the analysis of our sample cohort, we subsequently applied an expanded model to determine the likelihood of finding similar results in a wider population. These results supported the decrease in oligodendrocytic proteins (MBP, MOG, MYP, CN37) in BD/CT and the decrease of Septin 5 (SEPT5) in SZ/CT and Ankyrin 3 (ANK3) in MDD-P/MDD (Figure 2).
Figure 2.
Box plots of the normalized SRM estimates illustrating the proteins detected as significantly changed in the expanded model. Ratios represent disease/CT or MDD-P/MDD, respectively.
Discussion
This study represents the first and largest label-based quantitative targeted proteomics investigation in human brain tissue to date, evaluating expression changes of high-risk genes and risk-associated proteins in 4 different psychiatric disorders.
Applying linear mixed effect models, we were able to detect a range of significantly altered proteins that have been implicated at the genetic, transcriptomic, and proteomic levels in psychiatric research. We provide evidence for microglial dysfunction in MDD (Frick et al., 2013; Kreisel et al., 2013) compared with controls as shown by a decrease in coronin 1A levels. The astrocyte marker glial fibrillary acidic protein was decreased in SZ and MDD, indicating a potential disturbance of neuronal maintenance and glutamate clearance, and all CamK2 isoforms were changed in the MDD-P/MDD comparison. In addition, we identified a decrease of proteins associated with energy metabolism in SZ and an increase in BD and MDD, in line with previous findings (Prabakaran et al., 2004; Marazziti et al., 2012). It is also of note that we were able to validate the widely reported expression changes of malate dehydrogenase in psychiatric disorders.
One of the most striking results in this study was the systematic decrease of all investigated oligodendrocyte-specific proteins in SZ, BD, and MDD-P. Myelin-related abnormalities have previously been observed in postmortem brain and imaging studies of SZ and BD (Davis et al., 2003; Dwork et al., 2007; Brambilla et al., 2009; Mcintosh et al., 2009; Andreasen et al., 2011; Bartzokis et al., 2011). In accordance with previous findings of mRNA changes (Tkachev et al., 2003), this is the first study to show a more prominent decrease in oligodendrocyte-specific proteins in BD compared with SZ. Myelination in the prefrontal cortex occurs predominantly in adolescence and early adulthood (Benes, 1989), consistent with the typical age of onset of SZ and BD. Interestingly, myelination deficits in SZ, BD, and MDD-P corresponded with changes in the expression of the GSK3β and Wnt signalling regulatory protein catenin β in SZ and BD. GSK3β is a negative regulator of oligodendrocyte differentiation and myelination and has been implicated in BD and SZ. GSK3β inhibitors such as lithium are widely prescribed as mood stabilizers in the treatment of BD and are known to increase oligodendrocyte differentiation and promote myelination (Azim and Butt, 2011). Furthermore, the NMDA receptor subunit NR1, which we found to be upregulated in BD, is a functional regulator of oligodendrocyte precursor cell differentiation and remyelination (Li et al., 2013). Consequently, the development and re-profiling of drugs that promote remyelination may represent novel pharmacological targets for psychiatric disorders, especially BD. The compound XAV939 has been shown to enhance oligodendrocyte differentiation and remyelination by stabilizing Axin2, an intracellular target of Wnt transcriptional activation (Fancy et al., 2011). In addition, synthetic and natural cannabinoids have been shown to protect oligodendrocytes and oligodendrocyte progenitor cells and to enhance myelination by promoting oligodendrocyte maturation in vivo and vitro (Molina-Holgado et al., 2002; Gomez et al., 2010; Solbrig et al., 2010; Mecha et al., 2012). They are currently being tested for efficacy in multiple sclerosis clinical trials (Zajicek and Apostu, 2011; Velayudhan et al., 2013) and could be evaluated for the treatment of SZ and BD (Deiana, 2012).
We also found that ANK3 protein levels were reduced in affective psychosis, supporting the finding on a wider population scale applying the expanded model. The ANK3 protein is involved in neuronal scaffolding and the formation and maintenance of the axon initial segment of neurons and nodes of Ranvier (Bennett and Lambert, 1999). Recently, a meta-analysis of the major psychiatric disorders suggested that the ANK3 locus represents a shared risk gene for a number of psychiatric disorders (Smoller et al., 2013). ANK3 polymorphisms have been associated with cognitive and behavioral dysfunction, including increased risk taking (Ruberto et al., 2011) and startle response (Roussos et al., 2011a), decreased sustained attention (Ruberto et al., 2011; Hatzimanolis et al., 2012), impaired set-shifting (Linke et al., 2012), and increased novelty seeking and anhedonia (Athanasiu et al., 2010; Roussos et al., 2011a). Interestingly, common ANK3 risk alleles in BD and SZ have been linked to both increased and decreased mRNA expression, dependent on the brain region under investigation (Roussos et al., 2011b; Rueckert et al., 2012). The identification of ANK3 in our study further supports the pathological relevance of this gene/protein in multiple psychiatric disorders and especially in affective psychosis. Neuroimaging studies have associated ANK3 risk alleles with decreased white matter integrity in line with our findings of decreased oligodendrocyte markers in affective psychosis. BD is clinically characterized by a higher frequency of psychotic symptoms compared with MDD (Mondimore, 2005), whereas other studies have suggested an affective-psychotic continuum between MDD, BD, and schizoaffective disorder (Gershon et al., 1982).
Another interesting finding of our study was a 40% decrease in the levels of SEPT5 in SZ patients only, which was supported by the population-based model. SEPT5 belongs to a family of evolutionarily well-conserved polymerizing GTP-binding proteins that contribute to the lateral compartmentalization of membranes, cortical rigidity, and regulation of membrane trafficking (Kinoshita and Noda, 2001). Notably, SEPT5 has been colocalized with presynaptic vesicles (Beites et al., 1999; Kinoshita et al., 2000), plays a role in membrane fusion during neuronal exocytosis (Amin et al., 2008), and is critical for dendritic spine morphology (Xie et al., 2007). Decreased levels of SEPT5 in SZ prefrontal cortex may be associated with abnormal neurotransmitter secretion, for example glutamatergic hypofunction (Paz et al., 2008) and abnormal dendritic spine morphology in the prefrontal cortex of SZ patients (Glantz and Lewis, 2000; Broadbelt et al., 2002). In terms of pathophysiology, SEPT5 is a strong new candidate gene, as it is located on chromosome 22q11.2. In this chromosomal region, heterozygous deletions comprising either 3 or 1.5 Mbp (affecting 90% or 10% of patients, respectively) cause the 22q11.2 Deletion Syndrome. The phenotype of this syndrome is variable, but characteristic signs and symptoms include learning disabilities, dysfunctional social behavior and SZ, and autism spectrum disorders. Approximately one-third of adults with 22q11.2 Deletion Syndrome develop SZ-like symptoms (Karayiorgou et al., 1995; Ivanov et al., 2003; Horowitz et al., 2005). Interestingly, 3 major SZ susceptibility genes (catechol O-methyltransferase, probable palmitoyltransferase, and mitochondrial proline dehydrogenase 1) are located in this chromosome region. However, it is still unclear which of the deleted genes or gene interactions contribute to the risk of developing the neuropsychiatric phenotype. The current study provides evidence that SEPT5 may represent a novel risk gene for psychiatric disorders. As the SEPT5 protein is phosphorylated by cyclin-dependent kinase 5 to modulate exocytotic secretion (Amin et al., 2008), drugs targeting this kinase might represent a novel treatment approach for SZ. A SEPT5 knockout mouse (Dent et al., 2002) and a patient case report of SEPT5 deficiency (Bartsch et al., 2011) demonstrated reduced dense granule secretion in platelets, suggesting that there may be a potential link of this protein to the periphery. Three independent studies showed reduced platelet-dense granule secretion in first-episode psychosis individuals and SZ patients (Yao et al., 1994, 1996; Reddy et al., 2007).
In summary, we have employed a hypothesis-driven, label-based SRM approach and generated the largest quantitative targeted proteomics data set to date in human postmortem brain tissue and specifically for neuropsychiatric disorders. We were able to confirm changes in protein levels of previously reported risk factors for psychiatric disorders and identified novel putative risk factors for SZ and affective psychosis (SEPT5 and ANK3). Testing a wide range of previously described molecular risk factors for neuropsychiatric disorders, this is the first study to follow up risk genes and validate findings at the protein and functional level. We were able to demonstrate that myelination abnormalities are prominent in SZ, BD, and affective psychosis, as implicated by overlapping changes in several oligodendrocyte protein markers. We have also shown that GSK3b and Wnt signalling is altered in BD and SZ, and CamK2 abnormalities are prominently changed in BD and affective psychosis postmortem brain, indicating potential novel drug targets.
Our study highlights the potential of SRM to analyze protein abundance levels of candidate markers of neuropsychiatric spectrum disorders in a highly quantitative manner, thus providing a high-throughput multiplex method to validate and quantify potential disease markers and drug targets. So far, targeted proteomics has been employed in only a small number of clinical studies ranging from oncology (Cerciello et al., 2013) to neurology (Jia et al., 2012) and covering a range of biofluids (Shi et al., 2013). Because of increased throughput and sensitivity compared with shotgun approaches, SRM will enable the evaluation and validation of protein biomarkers during the hypothesis-generating phase of drug discovery. Universally applicable SRM assays for disease-associated proteins will also accelerate preclinical biomarker discovery and validation studies, facilitating the transfer of pathophysiological hypotheses towards clinical application and translation.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Conflicts of Interest
S.B. is a consultant for Myriad Genetics Inc and Psynova Neurotech Ltd. H.W. and M.G.G. declare no conflicts of interest.
Supplementary Material
Acknowledgments
This research was supported by the Stanley Medical Research Institute (SMRI) and the donations of the Stanley brain collection courtesy of Drs. Michael B. Knable, E. Fuller Torrey, Maree J. Webster, Serge Weis, and Robert H. Yolken. We gratefully acknowledge SMRI support. We thank all other members of the Bahn laboratory for intellectual and practical input, especially Drs. Paul Guest and Jason Cooper for their helpful suggestions and discussions throughout the project and proofreading of the final manuscript. Michael G. Gottschalk is supported by a Gonville & Caius College/Cambridge Home and European Scholarship Scheme EU Maintenance Bursary and an EPSRC Doctoral Training Grant studentship.
References
- Amin ND, Zheng YL, Kesavapany S, Kanungo J, Guszczynski T, Sihag RK, Rudrabhatla P, Albers W, Grant P, Pant HC. (2008). Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis. J Neurosci 28:3631–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. (2011). Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 70:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiu L, et al. (2010). Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res 44:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim K, Butt AM. (2011). GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 59:540–553. [DOI] [PubMed] [Google Scholar]
- Bartsch I, Sandrock K, Lanza F, Nurden P, Hainmann I, Pavlova A, Greinacher A, Tacke U, Barth M, Busse A, Oldenburg J, Bommer M, Strahm B, Superti-Furga A, Zieger B. (2011). Deletion of human GP1BB and SEPT5 is associated with Bernard-Soulier syndrome, platelet secretion defect, polymicrogyria, and developmental delay. Thromb Haemost 106:475–483. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Amar CP, Raven EP, Detore NR, Altshuler LL, Mintz J, Ventura J, Casaus LR, Luo JS, Subotnik KL, Nuechterlein KH. (2011). Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res 132:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS. (1999). The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci 2:434–439. [DOI] [PubMed] [Google Scholar]
- Benes FM. (1989). Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull 15:585–593. [DOI] [PubMed] [Google Scholar]
- Bennett V, Lambert S. (1999). Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol 28:303–318. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Bellani M, Yeh PH, Soares JC. (2009). Myelination in bipolar patients and the effects of mood stabilizers on brain anatomy. Curr Pharm Des 15:2632–2636. [DOI] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. (2002). Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res 58:75–81. [DOI] [PubMed] [Google Scholar]
- Cerciello F, Choi M, Nicastri A, Bausch-Fluck D, Ziegler A, Vitek O, Felley-Bosco E, Stahel R, Aebersold R, Wollscheid B. (2013). Identification of a seven glycopeptide signature for malignant pleural mesothelioma in human serum by selected reaction monitoring. Clin Proteomics 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Picotti P, Huttenhain R, Heinzelmann-Schwarz V, Jovanovic M, Aebersold R, Vitek O. (2011). Protein significance analysis in selected reaction monitoring (SRM) measurements. Mol Cell Proteomics 11:M111 014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Sklar P. (2013). Bipolar Disorder 1 Genetics of bipolar disorder. Lancet 381:1654–1662. [DOI] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. (2003). White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry 60:443–456. [DOI] [PubMed] [Google Scholar]
- Deiana S. (2012). Medical use of cannabis. Cannabidiol: a new light for schizophrenia? Drug Test Anal 5:46–51. [DOI] [PubMed] [Google Scholar]
- Dent J, Kato K, Peng XR, Martinez C, Cattaneo M, Poujol C, Nurden P, Nurden A, Trimble WS, Ware J. (2002). A prototypic platelet septin and its participation in secretion. Proc Natl Acad Sci U S A 99:3064–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwork AJ, Mancevski B, Rosoklija G. (2007). White matter and cognitive function in schizophrenia. Int J Neuropsychopharmacol 10:513–536. [DOI] [PubMed] [Google Scholar]
- Ernst A, Ma D, Garcia-Perez I, Tsang TM, Kluge W, Schwarz E, Guest PC, Holmes E, Sarnyai Z, Bahn S. (2012). Molecular validation of the acute phencyclidine rat model for schizophrenia: identification of translational changes in energy metabolism and neurotransmission. J Proteome Res 11:3704–3714. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH. (2011). Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci 14:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah T, Deutsch EW, Omenn GS, Campbell DS, Sun Z, Bletz JA, Mallick P, Katz JE, Malmstrom J, Ossola R, Watts JD, Lin BAY, Zhang H, Moritz RL, Aebersold R. (2011). A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteomics 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick LR, Williams K, Pittenger C. (2013). Microglial dysregulation in psychiatric disease. Clin Dev Immunol 2013:608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon ES, Hamovit J, Guroff JJ, Dibble E, Leckman JF, Sceery W, Targum SD, Nurnberger JI, Jr., Goldin LR, Bunney WE., Jr. (1982). A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry 39:1157–1167. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. (2000). Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry 57:65–73. [DOI] [PubMed] [Google Scholar]
- Gomez O, Arevalo-Martin A, Garcia-Ovejero D, Ortega-Gutierrez S, Cisneros JA, Almazan G, Sanchez-Rodriguez MA, Molina-Holgado F, Molina-Holgado E. (2010). The constitutive production of the endocannabinoid 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia 58:1913–1927. [DOI] [PubMed] [Google Scholar]
- Hatzimanolis A, Smyrnis N, Avramopoulos D, Stefanis CN, Evdokimidis I, Stefanis NC. (2012). Bipolar disorder ANK3 risk variant effect on sustained attention is replicated in a large healthy population. Psychiatr Genet 22:210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Shifman S, Rivlin N, Pisante A, Darvasi A. (2005). A survey of the 22q11 microdeletion in a large cohort of schizophrenia patients. Schizophr Res 73:263–267. [DOI] [PubMed] [Google Scholar]
- Ivanov D, Kirov G, Norton N, Williams HJ, Williams NM, Nikolov I, Tzwetkova R, Stambolova SM, Murphy KC, Toncheva D, Thapar A, O’Donovan MC, Owen MJ. (2003). Chromosome 22q11 deletions, velo-cardio-facial syndrome and early-onset psychosis. Molecular genetic study. Br J Psychiatry 183:409–413. [DOI] [PubMed] [Google Scholar]
- Jia Y, Wu T, Jelinek CA, Bielekova B, Chang L, Newsome S, Gnanapavan S, Giovannoni G, Chen D, Calabresi PA, Nath A, Cotter RJ. (2012). Development of protein biomarkers in cerebrospinal fluid for secondary progressive multiple sclerosis using selected reaction monitoring mass spectrometry (SRM-MS). Clin Proteomics 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec PS, Lasseter VK, et al. (1995). Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci U S A 92:7612–7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Zerwas S, Trace SE, Sullivan PF. (2011). Schizophrenia genetics: where next? Schizophr Bull 37:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Noda M. (2001). Roles of septins in the mammalian cytokinesis machinery. Cell Struct Funct 26:667–670. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Noda M, Kinoshita M. (2000). Differential localization of septins in the mouse brain. J Comp Neurol 428:223–239. [DOI] [PubMed] [Google Scholar]
- Klengel T, Binder EB. (2013). Gene-Environment Interactions in Major Depressive Disorder. Can J Psychiatry 58:76–83. [DOI] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. (2013). Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry 19: 699–709. [DOI] [PubMed] [Google Scholar]
- Lange V, Malmstrom JA, Didion J, King NL, Johansson BP, Schafer J, Rameseder J, Wong CH, Deutsch EW, Brusniak MY, Buhlmann P, Bjorck L, Domon B, Aebersold R. (2008). Targeted quantitative analysis of Streptococcus pyogenes virulence factors by multiple reaction monitoring. Mol Cell Proteomics 7:1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF. (2006). The genetics of depression: a review. Biol Psychiatry 60:84–92. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C. (2013). A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61:732–749. [DOI] [PubMed] [Google Scholar]
- Linke J, Witt SH, King AV, Nieratschker V, Poupon C, Gass A, Hennerici MG, Rietschel M, Wessa M. (2012). Genome-wide supported risk variant for bipolar disorder alters anatomical connectivity in the human brain. Neuroimage 59:3288–3296. [DOI] [PubMed] [Google Scholar]
- MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. (2010). Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26:966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E, Catena Dell’Osso M. (2012). Psychiatric disorders and mitochondrial dysfunctions. Eur Rev Med Pharmacol Sci 16:270–275. [PubMed] [Google Scholar]
- Mcintosh AM, Hall J, Lymer GKS, Sussmann JED, Lawrie SM. (2009). Genetic risk for white matter abnormalities in bipolar disorder. Int Rev Psychiatry 21:387–393. [DOI] [PubMed] [Google Scholar]
- Mecha M, Torrao AS, Mestre L, Carrillo-Salinas FJ, Mechoulam R, Guaza C. (2012). Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis 3:e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, Guaza C. (2002). Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci 22:9742–9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondimore FM. (2005). Unipolar depression/bipolar depression: connections and controversies. Int Rev Psychiatry 17:39–47. [DOI] [PubMed] [Google Scholar]
- Oberg AL, Vitek O. (2009). Statistical design of quantitative mass spectrometry-based proteomic experiments. J Proteome Res 8:2144–2156. [DOI] [PubMed] [Google Scholar]
- Paz RD, Tardito S, Atzori M, Tseng KY. (2008). Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur Neuropsychopharmacol 18:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard B. (2011). Progress in defining the biological causes of schizophrenia. Expert Rev Mol Med 13:e25. [DOI] [PubMed] [Google Scholar]
- Picotti P, Aebersold R. (2012). Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat Methods 9:555–566. [DOI] [PubMed] [Google Scholar]
- Picotti P, Rinner O, Stallmach R, Dautel F, Farrah T, Domon B, Wenschuh H, Aebersold R. (2010). High-throughput generation of selected reaction-monitoring assays for proteins and proteomes. Nat Methods 7:43––U45. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. (2004). Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry 9:684–697, 643. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Keshavan MS, Yao JK. (2007). Reduced platelet serotonergic responsivity as assessed by dense granule secretion in first-episode psychosis. Clin Biochem 40:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Georgakopoulos A, Robakis NK, Bitsios P. (2011a) The CACNA1C and ANK3 risk alleles impact on affective personality traits and startle reactivity but not on cognition or gating in healthy males. Bipolar Disorders 13:250–259. [DOI] [PubMed] [Google Scholar]
- Roussos P, Katsel P, Davis KL, Bitsios P, Giakoumaki SG, Jogia J, Rozsnyai K, Collier D, Frangou S, Siever LJ, Haroutunian V. (2011b) Molecular and genetic evidence for abnormalities in the nodes of Ranvier in schizophrenia. Arch Gen Psychiatry 69:7–15. [DOI] [PubMed] [Google Scholar]
- Ruberto G, Vassos E, Lewis CM, Tatarelli R, Girardi P, Collier D, Frangou S. (2011). The cognitive impact of the ANK3 Risk variant for bipolar disorder: initial evidence of selectivity to signal detection during sustained attention. Plos One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert EH, Barker D, Ruderfer D, Bergen SE, O’Dushlaine C, Luce CJ, Sheridan SD, Theriault KM, Chambert K, Moran J, Purcell SM, Madison JM, Haggarty SJ, Sklar P. (2012). Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Mol Psychiatry 18:922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Gao Y, Quek SI, Fillmore TL, Nicora CD, Su D, Zhao R, Kagan J, Srivastava S, Rodland KD, Liu T, Smith RD, Chan DW, Camp DG II, Liu AY, Qian WJ. (2013). A highly sensitive targeted mass spectrometric assay for quantification of AGR2 protein in human urine and serum. J Proteome Res 13:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, Ripke S, Santangelo S, Sullivan PF, Consortium PG. (2013). Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbrig MV, Fan Y, Hermanowicz N, Morgese MG, Giuffrida A. (2010). A synthetic cannabinoid agonist promotes oligodendrogliogenesis during viral encephalitis in rats. Exp Neurol 226:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surinova S, Huttenhain R, Chang CY, Espona L, Vitek O, Aebersold R. (2013). Automated selected reaction monitoring data analysis workflow for large-scale targeted proteomic studies. Nat Protoc 8:1602–1619. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. (2003). Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 362:798–805. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. (2000). The Stanley Foundation Brain Collection and Neuropathology Consortium. Schizophr Res 44:151–155. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. (2009). Schizophrenia. Lancet 374:635–645. [DOI] [PubMed] [Google Scholar]
- Velayudhan L, Van Diepen E, Marudkar M, Hands O, Suribhatla S, Prettyman R, Murray J, Baillon S, Bhattacharyya S. (2014). Therapeutic potential of cannabinoids in neurodegenerative disorders: a selective review. Curr Pharm Des 20: 2218–2230. [DOI] [PubMed] [Google Scholar]
- Wesseling H, Guest PC, Lago SG, Bahn S. (2014). Technological advances for deciphering the complexity of psychiatric disorders: merging proteomics with cell biology. Int J Neuropsychopharmacol:1–15. [DOI] [PubMed] [Google Scholar]
- Whiteaker JR, et al. (2011). A targeted proteomics-based pipeline for verification of biomarkers in plasma. Nat Biotechnol 29:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YL, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. (2007). The GTP-binding protein septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol 17:1746–1751. [DOI] [PubMed] [Google Scholar]
- Yao JK, Vankammen DP, Gurklis J, Peters JL. (1994). Platelet-aggregation and dense granule secretion in schizophrenia. Psychiatry Res 54:13–24. [DOI] [PubMed] [Google Scholar]
- Yao JK, vanKammen DP, Moss HB, Sokulski DE. (1996). Decreased serotonergic responsivity in platelets of drug-free patients with schizophrenia. Psychiatry Res 63:123–132. [DOI] [PubMed] [Google Scholar]
- Zajicek JP, Apostu VI. (2011). Role of cannabinoids in multiple sclerosis. CNS Drugs 25:187–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.