Abstract
Background:
Endocannabinoids modulate the glutamatergic excitatory transmission by acting as retrograde messengers. A growing body of studies has reported that both signaling systems in the mesocorticolimbic neural circuitry are involved in the neurobiological mechanisms underlying drug addiction.
Methods:
We investigated whether the expression of both endocannabinoid and glutamatergic systems in the prefrontal cortex (PFC) were altered by an acute and/or repeated cocaine administration schedule that resulted in behavioral sensitization. We measured the protein and mRNA expression of the main endocannabinoid metabolic enzymes and the cannabinoid receptor type 1 (CB1). We also analyzed the mRNA expression of relevant components of the glutamate-signaling system, including glutamate-synthesizing enzymes, metabotropic receptors, and ionotropic receptors.
Results:
Although acute cocaine (10mg/kg) produced no significant changes in the endocannabinoid-related proteins, repeated cocaine administration (20mg/kg daily) induced a pronounced increase in the CB1 receptor expression. In addition, acute cocaine administration (10mg/kg) in cocaine-sensitized mice (referred to as cocaine priming) induced a selective increase in the endocannabinoid-degrading enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL). These protein changes were accompanied by an overall decrease in the ratios of endocannabinoid synthesis/degradation, especially the N-acyl phosphatidylethanolamine phospholipase D/FAAH and diacylglycerol lipase alpha/MAGL ratios.
Regarding mRNA expression, while acute cocaine administration produced a decrease in CB1 receptors and N-acyl phosphatidylethanolamine phospholipase D, repeated cocaine treatment enhanced CB1 receptor expression. Cocaine-sensitized mice that were administered priming injections of cocaine mainly displayed an increased FAAH expression.
These endocannabinoid changes were associated with modifications in glutamatergic transmission-related genes. An overall decrease was observed in the mRNA expression of the glutamate-synthesizing gene kidney-type glutaminase (KGA), the metabotropic glutamate receptors (mGluR3 and GluR), and subunits of NMDA ionotropic receptors (NR1, NR2A, NR2B and NR2C) after acute cocaine administration, while mice repeatedly exposed to cocaine only displayed an increase in NR2C. However, in cocaine-sensitized mice primed with cocaine, this inhibition was reversed and a strong increase was detected in the mGluR5, NR2 subunits, and both GluR1 and GluR3.
Conclusions:
These findings indicate that cocaine sensitization is associated with an endocannabinoid downregulation and a hyperglutamatergic state in the PFC that, overall, contribute to an enhanced glutamatergic input into PFC-projecting areas.
Keywords: cannabinoid, cocaine, sensitization, glutamate, prefrontal cortex.
Introduction
Cocaine addiction is one of the most prevalent drug abuse disorders, and understanding its neurobiology remains a challenge. Unveiling the mechanisms associated with cocaine-induced neuroadaptions might help to improve the few effective therapeutic options available to date. Cocaine is a psychostimulant that activates, among others, the mesocorticolimbic dopaminergic system that projects to the nucleus accumbens (NAc) and the prefrontal cortex (PFC; Hearing et al., 2012). Pyramidal glutamatergic cells of the PFC are activated by cocaine and glutamate is released into projecting areas, such as the NAc. Therefore, these glutamatergic neurons regulate the organization of motor behaviors related to drug-associated responses, including locomotor sensitization, drug seeking, and conditioned responses to drug-associated stimuli (Cardinal and Everitt, 2004; Steketee and Kalivas, 2011). The control of the cocaine-activated glutamatergic output is achieved by multiple mechanisms comprised of gamma aminobutyric acidic neurons (Hearing et al., 2013) and retrograde messengers such as those belonging to the endocannabinoid system (Fourgeaud et al., 2004). Dysregulation of glutamate release–controlling mechanisms are likely part of the neuroadaptions that led to the enhanced behavioral output observed after repeated cocaine exposure (Hearing et al., 2012).
Although the role of the endocannabinoids in psychostimulant addiction has been a matter of active research (Gorriti et al., 1999; De Vries and Schoffelmeer, 2005; Maldonado et al., 2006; Martin et al., 2008), there are gaps in our understanding of their role in the PFC-NAc pathway. The endocannabinoid system is a modulatory system comprised of lipid mediators termed endocannabinoids (e.g., anandamide and 2-arachidonoylglycerol [2-AG]), the cannabinoid receptors (subtype 1 [CB1] and 2 [CB2]), and the enzymes that synthesize (diacylglycerol lipases alpha and beta [DAGLα/β] and N-acyl phosphatidylethanolamine phospholipase D [NAPE-PLD]) and degrade (monoacylglycerol lipase [MAGL] and fatty acid amide hydrolase [FAAH]) endocannabinoids. In the PFC-NAc pathway, endocannabinoids retrogradely modulate synaptic plasticity through the stimulation of presynaptic cannabinoid CB1 receptors (Freund et al., 2003; Piomelli, 2003), which are located in both the glutamatergic and the gamma aminobutyric acidic axon terminals. Endocannabinoids are formed upon the activation of postsynaptic glutamate receptors, including the ionotropic glutamate receptors (AMPA), NMDA-type receptors, and, especially, mGluR5 metabotropic glutamate receptors. Therefore, the postsynaptically-produced endocannabinoids reach the presynaptic glutamatergic terminals and inhibit the glutamate release. This retrograde endocannabinoid signaling is an essential element for the establishment of long-term neuroadaptations to drugs such as cocaine. In fact, a single administration of cocaine abolished the endocannabinoid-mediated, retrograde long-term depression at synapses between the PFC and NAc (Fourgeaud et al., 2004).
In addition to the interactions described above between endocannabinoids and cocaine-activated glutamatergic release in the PFC-NAc pathway, the endocannabinoid production is also promoted by the dopamine D2 receptor activation (Giuffrida et al., 1999) within the dorsal striatum, where it is linked to the regulation of dopamine-mediated behaviors (Rodriguez de Fonseca et al., 1994; Martin et al., 2008), psychostimulant-induced sensitization (Gorriti et al., 1999; Corbille et al., 2007; Mereu et al., 2013), cocaine-induced conditioned place-preference (Chaperon et al., 1998), cocaine self-administration and relapse (Adamczyk et al., 2009; Fattore et al., 1999; De Vries et al., 2001; Soria et al., 2005), and cocaine-induced conditioned locomotion (Luque-Rojas et al., 2013).
As can be deduced from these studies, the interplay between cortical glutamate, the ascending dopamine pathways, and endocannabinoid-mediated signaling is modified by the administration of cocaine. One of the most consistent models for studying this interplay is behavioral sensitization. Classical studies (Giuffrida et al., 1999; Gorriti et al., 1999) establish that both the blockade and desensitization of CB1 receptors facilitate the actions of psychostimulants, whereas recent studies (Mereu et al., 2013) have indicated that the endocannabinoid signaling is activated because the first cocaine administration to initiate the plastic changes leads to the onset of behavioral sensitization. However, there are no available data where the effects of a sensitization-inducing cocaine administration regimen on the endocannabinoid signaling system are linked to the hyperglutamatergic activity in the PFC. To this end, we investigated whether the gene and protein expression of relevant constituents of the endocannabinoid system and the glutamatergic signaling pathway may be altered by acute cocaine administration and/or following a sensitization regimen of cocaine administration in the PFC of mice.
Methods
Ethics Statements
The experiments and procedures were conducted under strict adherence to the European Directive 2010/63/EU on the protection of animals used for scientific purposes and with Spanish regulations (RD 53/2013 and 178/2004). All efforts were made to minimize unnecessary suffering. All protocols were approved by the Committee on Ethics of the University of Málaga and Hospital RU de Málaga.
Animals and Housing
Experimental research was performed using male C57BL/6J mice (25±5g; Charles River Laboratories International). All animals were maintained in the Animal Resource Center at the University of Málaga. All animals were individually housed in clear plastic cages in a temperature-controlled room (22±2°C) under a 12h light/dark cycle (lights off at 8:00 PM). Tap water and standard chow pellets were freely available throughout the course of the experiments.
Drugs
Cocaine-HCl was obtained from Alkaliber S.A.. Cocaine was dissolved in saline (0.9% NaCl) and administered at doses of 10mg/kg (acute) and 20mg/kg (repeated). Cocaine or vehicle (saline) was administered by an intraperitoneal injection in a volume of 10mL/kg body weight.
Apparatus and General Procedures
All experiments were carried out between 8:00 AM and 8:00 PM. To acclimate the animals to the test conditions, all mice were allowed to rest in the testing room for 30min prior to perfoming any behavioral assessment in the open field test during the cocaine sensitization paradigm. Performance in the open field test was recorded by computer-based video tracking system (Smart v2.5®, Panlab). The maximum light intensity in the center of the open field was 100 lux. Four open fields (50 x 50 x 50cm, Panlab) with grey backgrounds were used. The animals were placed in the center of the arena and their behavior was recorded for 30min. Horizontal locomotion was measured as the total distance traveled in cm.
Acquisition and Expression of Cocaine-Induced Sensitization
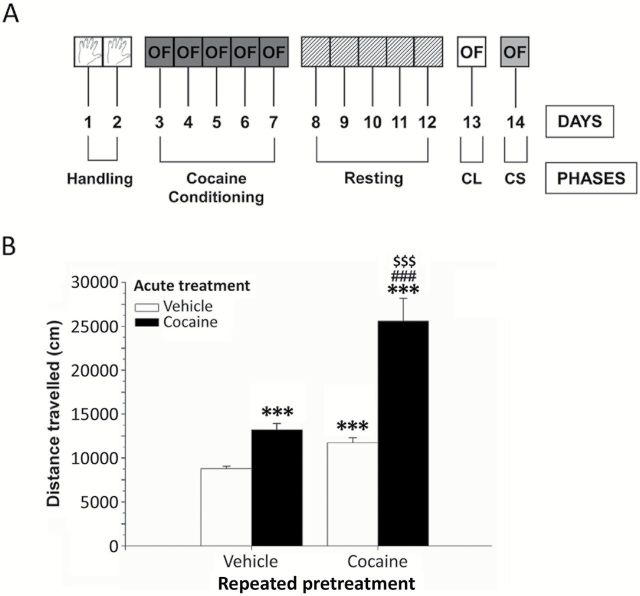
Cocaine sensitization was conducted as previously described (Blanco et al., 2012) following a consecutive 5-phase paradigm (Figure 1A): (1) handling: all animals were handled for 10min and weighed during 2 days for habituation (days 1 and 2) in the testing room; (2) cocaine conditioning: as commented, the mice were acclimated to the testing room for 30min prior to administration of vehicle (saline, intraperitoneally) or cocaine injection (20mg/kg, intraperitoneally, once daily for 5 consecutive days, days 3 to 7) and were tested in the open field immediately after each injection; (3) resting: during the next 5 days (days 8 to 12), the animals rested from the open field and the drug; (4) cocaine-conditioned locomotion: we evaluated the locomotor activity response induced by association between repeated administration of cocaine and the open field test using a simulated injection with vehicle (day 13); and (5) cocaine-induced sensitization: on the last day (day 14), the presence of cocaine sensitization was achieved by an acute administration of cocaine (10mg/kg) that was used as the priming dose.
Figure 1.
Acute and repeated cocaine injections on cocaine-induced sensitization in mice. (A) Schematic representation of the 5-phase-paradigm for acquisition and expression of cocaine-induced sensitization. (B) Effects of acute and repeated cocaine injections on the distance traveled by mice for 30min measured in the open field during cocaine sensitization. Bars represent the mean + SEM (n = 8 animals per group). Post hoc analysis: *p < 0.001 versus the vehicle-vehicle group; # p < 0.001 versus the vehicle-cocaine group; $ p < 0.001 versus the cocaine-vehicle group. OF: open field; CL: cocaine-conditioned locomotion; CS: cocaine-induced sensitization.
All animals were evaluated in the open field test to measure the distance traveled for 30min using a video tracking system, except in the drug-free period. The experimental groups were (n = 8 per group): (1) repeated vehicle pretreatment and acute vehicle treatment (vehicle-vehicle group); (2) repeated vehicle pretreatment and acute cocaine (10mg/kg) treatment (vehicle-cocaine group); (3) repeated cocaine pretreatment (20mg/kg) for 5 days and acute vehicle treatment (cocaine-vehicle group); and (4) repeated cocaine pretreatment and acute (in this case, the priming injection) cocaine (10 mg/kg) treatment (cocaine-cocaine group). The animals in these 4 groups were tested in the behavioral studies and their brains were collected and used for biochemical determinations.
Tissue collection
One hour after we performed acute treatment of mice who had already been pretreated repeatedly with cocaine or vehicle, all animals were euthanized by decapitation, and their brains were immediately dissected out, frozen on dry ice, and stored at -80ºC. The brains were dissected in coronal brain slice sections (1mm thick) on dry ice using razor blades and a mouse brain slicer matrix (Zivic Instruments). The PFC was precisely removed with fine surgical instruments (Paxinos and Franklin, 2004). The samples were stored at -80ºC until further use for gene and protein analyses.
Western Blot Analysis
Western blotting was used to measure the protein levels of the endocannabinoid signaling system (cannabinoid CB1 receptor, NAPE-PLD, DAGLα/β, MAGL, and FAAH) in the PFC, as previously described (Suarez et al., 2008). The samples were homogenized in 50mM Hepes buffer (pH 8) and 0.32M sucrose buffer to obtain the membrane protein extracts. For immunoblotting, the protein samples (40 μg) were separated on 10% (w/v) SDS-PAGE gels, transferred to nitrocellulose membranes (BioRad) and controlled by Ponceau Red staining. After blocking with 5% (w/v) bovine serum albumin in 0.1% Tween 20 in PBS at room temperature for 1h, the membranes were incubated with the primary antibodies overnight at 4ºC, as described previously (Suarez et al., 2008). The specific protein bands were visualized and quantified by chemiluminescence using an imaging AutoChemiTM UVP BioImaging System (LTF Labortechnik). β-actin was quantified and used as a loading control (anti-β-actin diluted 1:1000 from Sigma-Aldrich Co., cat. no. A5316).
RNA Isolation and Quantitative Polymerase Chain Analysis
Real-time quantitative polymerase chain reaction was used to measure the relative quantification of the mRNA levels of the relevant receptors and synthesis/degradation enzymes involved in endocannabinoid signaling (CB1 receptor, NAPE-PLD, DAGLα/β, MAGL, and FAAH) and glutamatergic signaling (liver-type [LGA] and kidney-type [KGA] isoforms of glutaminase, mGluR3/5 metabotropic receptors, and subunits of NMDA ionotropic receptors [NR1/2A/2B/2C-NMDA and GluR1/2/3/4-AMPA]) in the PFC. Total mRNA was isolated using the Trizol® method, according to the manufacturer’s instruction (Gibco BRL Life Technologies). Details on the RNA extraction and cDNA synthesis have been previously described (Blanco et al., 2012). The resultant cDNAs were used as templates for the real-time quantitative polymerase chain reaction with an iCycler system (BioRad) using the Quanti-Test SYBR Green PCR kit (Qiagen). The primers used are described in the supplementary Table S1. Oligonucleotides were provided by Sigma-Proligo.
Quantification was performed according to standard curves run simultaneously as the samples with each reaction run in duplicate, as previously described (Blanco et al., 2012). The absolute values from each sample were normalized with regard to β-actin mRNA (a housekeeping gene), which was used as a reference standard.
Statistical Analysis
Data are expressed as the mean ± standard error of the mean (SEM) of 8 determinations (animals) per experimental group. Statistical analyses of the behavioral data in studies with acute and repeated treatments were performed using (1) a two-way analysis of variance (ANOVA), with repeated pretreatment (conditioning with cocaine or vehicle for 5 days) and acute treatment (cocaine or vehicle) as the two factors and (2) multiple post hoc comparisons (Bonferroni test). P < 0.05 was considered significant.
Results
Cocaine Administration on the Acquisition and Expression of Sensitization
As shown in Figure 1A, cocaine sensitization was conducted following a consecutive 5-phase paradigm that was described previously. During the cocaine conditioning, the locomotor activity was evaluated daily in mice pretreated with repeated injections of vehicle or cocaine (20mg/kg). After resting, the mice were injected with an acute dose of vehicle or cocaine (10mg/kg), and the locomotor activity was assessed in the open field (Figure 1B). A two-way ANOVA revealed that the distance traveled in the open field was significantly affected by the repeated pretreatment (F 1,28 = 26.87, p <0.0001) and the acute treatment (F 1,28 = 37.89, p < 0.0001). Furthermore, a significant interaction between both repeated cocaine pretreatment and acute treatment was also detected (F 1,28 = 10.12, p = 0.0036). A post hoc comparison between the paired groups was subsequently performed. The vehicle-cocaine group displayed a significant increase in locomotion (p < 0.001) compared with the vehicle-vehicle group. Similarly, we observed that the cocaine-cocaine group displayed higher locomotion compared with the cocaine-vehicle mice (p < 0.001). Both repeated groups pretreated with cocaine—the cocaine-vehicle group and the cocaine-cocaine group—displayed a significant increase in locomotion compared with the vehicle-vehicle group (p < 0.001), which was used as a baseline for locomotor activity. Interestingly, repeated cocaine-conditioned mice that received cocaine priming (cocaine-cocaine group) showed a higher increase in the locomotor activity compared with the vehicle-cocaine group (p < 0.001).
Changes in the Endocannabinoid Signaling System During Cocaine-Induced Sensitization
To address whether cocaine treatment was associated with alterations in the endocannabinoid system, we analyzed protein and mRNA levels of the different components of this system in the PFC (CB1 receptor, endocannabinoid synthesis enzymes NAPE-PLD and DAGLα/β, and degradative enzymes FAAH and MAGL). A summary of these results can be found in Table S2.
Expression of Proteins Associated with the Endocannabinoid System
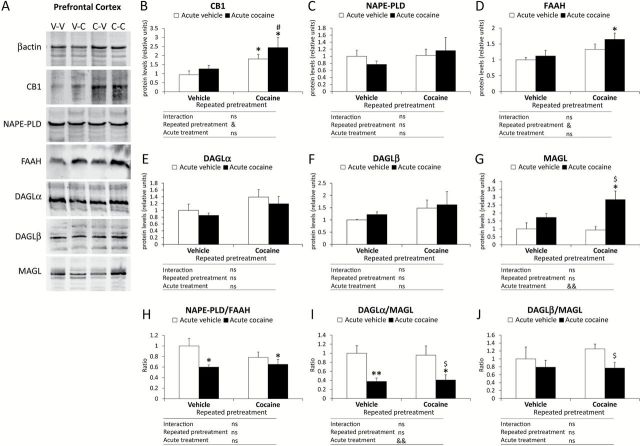
Western blot analysis showed that the antibodies used against the components of the endocannabinoid signaling system identified bands with the expected molecular weight in the PFC (Figure 2A), as previously described (Suarez et al., 2008; Marquez et al., 2009). Thus, CB1 receptor immunoblotting revealed a prominent band at approximately 60 kD, NAPE-PLD at 46 kD, FAAH at 63 kD, DAGLα at 120 kD, DAGLβ at 76 kD, and MAGL at 35–37 kD.
Figure 2.
Effects of cocaine-induced sensitization on the expression of endocannabinoid signaling-related proteins in the mouse PFC. (A) Representative immunoblots of endocannabinoid signaling-related proteins after repeated pretreatment (vehicle or cocaine) and acute treatment (vehicle or cocaine). Relative protein levels: (B) CB1 receptor; (C) NAPE-PLD; D) FAAH; (E) DAGLα; (F) DAGLβ; and (G) MAGL. Ratios between the synthesis and degradation enzymes: (H) NAPE-PLD/FAAH; (I) DAGLα/MAGL; and (J) DAGLβ/MAGL. Bars represent the mean + SEM (n = 8). Two-way ANOVA: & p < 0.05 and && p < 0.01. Post hoc analysis: *p < 0.05 and **p < 0.01 versus the vehicle-vehicle group; # p < 0.05 versus the vehicle-cocaine group; $ p < 0.05 versus the cocaine-vehicle group. V-V: vehicle-vehicle group; V-C: vehicle-cocaine group; C-V: cocaine-vehicle group; C-C: cocaine-cocaine group; ns: non-significant.
Two-way ANOVA analyses of the protein expressions were performed using the repeated pretreatment and acute treatment as factors. Thus, the repeated pretreatment (with vehicle or cocaine) had a significant effect on the protein expression of CB1 receptor (F 1,28 = 9.15, p = 0.01) and FAAH (F 1,28 = 6.94, p = 0.021), whereas the acute treatment only significantly affected the expression of MAGL (F 1,28 = 12.52, p = 0.004). No interaction between the factors was detected for these proteins.
Bonferroni post hoc tests showed the protein expression in the cannabinoid CB1 receptor was significantly increased in both groups pretreated with cocaine (the cocaine-vehicle and cocaine-cocaine groups) in comparison with the vehicle-vehicle group (p < 0.05). The CB1 receptor expression was also significantly enhanced in the cocaine-cocaine mice compared with the vehicle-cocaine mice (p < 0.05; Figure 2B). The protein expression of NAPE-PLD was unaltered when paired comparisons were performed between the groups with repeated pretreatment and acute treatment (Figure 2C). When the FAAH expression was analyzed, only a significant increase was observed in the cocaine-cocaine group compared with the vehicle-vehicle group (p < 0.05; Figure 2D). No changes were detected in the expression of the DAGLα/β proteins (Figure 2E and F). Finally, the MAGL expression was only affected by the administration of cocaine priming in repeated cocaine-conditioned animals (the cocaine-cocaine group) that displayed a significant increase in MAGL in comparison with the vehicle-vehicle mice and the cocaine-vehicle group (p < 0.05; Figure 2G).
We also calculated the ratios of the protein expressions in the PFC between NAPE-PLD and FAAH (Figure 2H), between DAGLα and MAGL (Figure 2I), and between DAGLβ and MAGL (Figure 2J), because these ratios could suggest changes in anandamide and 2-AG production. Two-way ANOVA analyses for these three ratios revealed that only the DAGLα/MAGL ratio was significantly affected by the acute treatment (F 1,28 = 15.2, p = 0.002). In comparison with the vehicle-vehicle group, post hoc tests showed that an acute injection of cocaine in both the vehicle-cocaine mice and the cocaine-cocaine mice induced a significant decrease in the protein expression of NAPE-PLD/FAAH (p < 0.05) and DAGLα/MAGL (p < 0.01 and p < 0.05, respectively) ratios, but not for the DAGLβ/MAGL ratio, which had a non-significant decrease. However, the cocaine-cocaine mice displayed significantly lower DAGLα/MAGL and DAGLα/β/MAGL ratios compared with the cocaine-vehicle mice (p < 0.05).
Changes in the Expression of Genes Associated with the Endocannabinoid System
The mRNA levels of the endocannabinoid-related genes were also assessed in the PFC (Figure 3). Two-way ANOVA analysis showed significant effects of the repeated pretreatment (with vehicle or cocaine) on the gene expression of endocannabinoid elements, specifically in the CB1 receptor (Cnr1, F 1,28 = 9.79, p = 0.0041), NAPE-PLD (Napepld, F 1,28 = 5.07, p = 0.032), FAAH (Faah, F 1,28 = 5.13, p = 0.031), and DAGLβ (Daglb, F 1,28 = 7.50, p = 0.01). However, the acute treatment did not produce significant effects in the mRNA of these cannabinoid components. The interaction between the factors was detected for the FAAH expression (F 1,28 = 5.94, p = 0.021).
Figure 3.
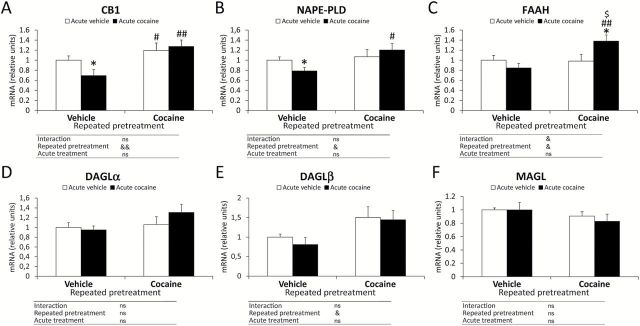
Effects of cocaine-induced sensitization on the gene expression of the endocannabinoid signaling-related components in the mouse PFC. Relative mRNA levels of the endocannabinoid signaling system were evaluated following a repeated pretreatment (vehicle or cocaine) and acute treatment (vehicle or cocaine): (A) CB1 receptor; (B) NAPE-PLD; (C) FAAH; (D) DAGLα; (E) DAGLβ; and (F) MAGL. Bars represent the mean + SEM (n = 8). Two-way ANOVA: & p < 0.05 and && p < 0.01. Post hoc analysis: *p < 0.05 versus the vehicle-vehicle group; # p < 0.05 and ## p < 0.01 versus the vehicle-cocaine group; $ p < 0.05 versus the cocaine-vehicle group. ns: non-significant.
Bonferroni post hoc tests showed the acute cocaine administration (the vehicle-cocaine group) showed a significant decrease in the mRNA levels of CB1 receptor and NAPE-PLD (p < 0.05; Figure 3A and B) compared with the vehicle-vehicle group. The pretreatment with repeated cocaine and acute vehicle (the cocaine-vehicle group) induced higher mRNA levels of CB1 receptor compared with the vehicle-cocaine group (p < 0.05; Figure 3A). The cocaine sensitization induced by the cocaine priming after repeated cocaine pretreatment (the cocaine-cocaine group) produced increased levels of CB1 receptor, NAPE-PLD, and FAAH mRNA (p < 0.01, p < 0.05, and p < 0.01, respectively) compared with the vehicle-cocaine group (Figure 3A–C). In addition, the mRNA levels of FAAH increased in comparison with the vehicle-vehicle group (p < 0.05) and the cocaine-vehicle group (p < 0.05; Figure 3C). No changes were detected in the mRNA levels of DAGLα, DAGLβ, and MAGL (Figure 3D–F) when the four experimental groups were compared.
Changes in the Expression of Genes During Cocaine-Induced Sensitization
To address whether the cocaine behavior-related changes observed in the endocannabinoid signaling components were associated with an alteration in the glutamatergic state, we analyzed the mRNA expression of glutamate-synthesizing genes LGA (Gls2) and KGA (Gls), mGluR3/5 (Gmr3/5) metabotropic receptors, and NR1/2A/2B/2C-NMDA (Grin1/2a/2b/2c) and GluR1/2/3/4-AMPA (Gria1/2/3/4) ionotropic receptor subunits in the PFC of acute versus repeated cocaine-exposed mice. The results are summarized in Table S3.
Changes in the Expression of Genes Associated with the Glutamatergic Signaling System
In the PFC (Figure 4), two-way ANOVA analyses showed significant effects of the repeated pretreatment on the gene expression of NR1 (F 1,28 = 8.25, p = 0.0077), NR2B (F 1,28 = 5.51, p = 0.026), NR2C (F 1,28 = 12.57, p = 0.0014), GluR2 (F 1,28 = 6.76, p = 0.014), GluR3 (F 1,28 = 6.9, p = 0.013), and GluR4 (F 1,28 = 7.38, p = 0.011). Acute treatment produced no effects on these glutamate components, but a significant interaction between both factors—the repeated pretreatment x the acute treatment—was observed in the mRNA levels of mGluR5 (F 1,28 = 5.36, p = 0.028), NR1 (F 1,28 = 5.15, p = 0.031), NR2A (F 1,28 = 5.44, p = 0.027), NR2B (F 1,28 = 7.8, p = 0.0093), and NR2C (F 1,28 = 4.27, p = 0.048). The interaction indicated that the acute treatment of the cocaine priming or vehicle affected the gene expression of mGluR5, NR1, NR2A, NR2B, and NR2C in an opposite manner (increase or decrease) according to the type of repeated pretreatment.
Figure 4.
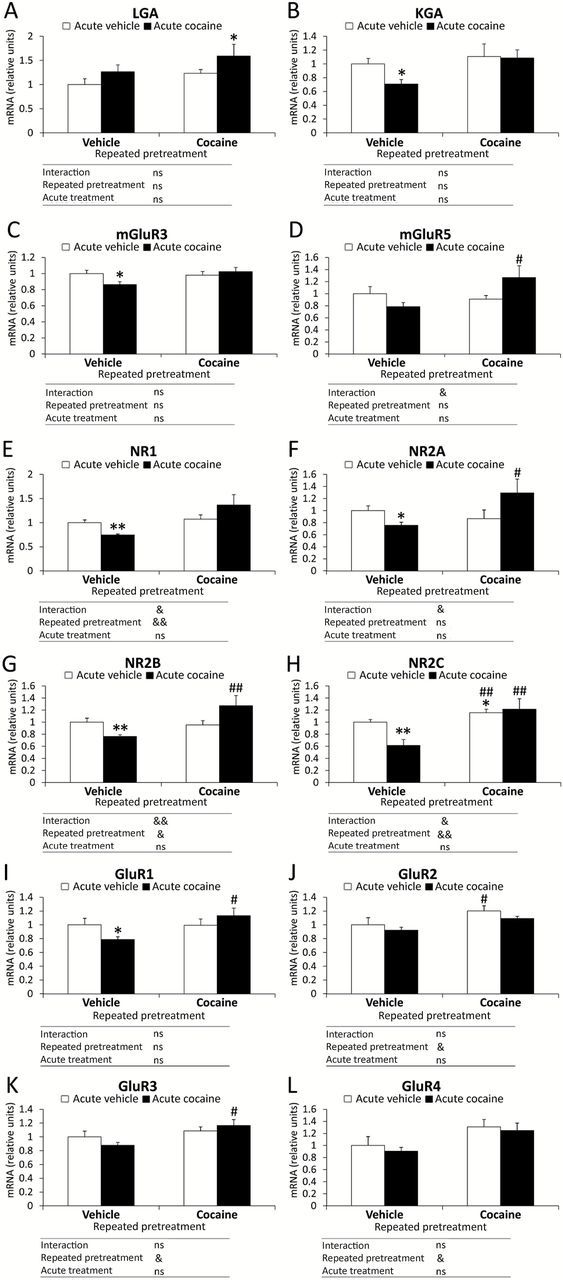
Effects of cocaine-induced sensitization on the gene expression of the glutamatergic signaling-related components in the mouse PFC. Relative mRNA levels of the glutamate signaling system were evaluated following a repeated pretreatment (vehicle or cocaine) and acute treatment (vehicle or cocaine): (A) LGA; (B) KGA; (C) mGluR3; (D) mGluR5; (E) NR1; (F) NR2A; (G) NR2B; (H) NR2C; (I) GluR1; (J) GluR2; (K) GluR3; and (L) GluR4. Bars represent the mean + SEM (n = 8). Two-way ANOVA: & p < 0.05 and && p < 0.01. Post hoc analysis: *p < 0.05 and **p < 0.01 versus the vehicle-vehicle group; # p < 0.05 and ## p < 0.01 versus the vehicle-cocaine group. ns: non-significant.
Bonferroni post hoc tests showed that repeated cocaine-conditioned animals that received cocaine (cocaine-cocaine group) displayed a significant increase in the mRNA level of LGA mRNA (p < 0.05) compared with the vehicle-vehicle group (Figure 4A). The vehicle-cocaine group displayed a significant decrease in the gene expression of KGA and mGluR3 in comparison with the vehicle-vehicle mice (p < 0.05; Figure 4B and C). The cocaine-cocaine group displayed a significant increase in the mRNA expression of mGluR5 compared with the vehicle-cocaine mice (p < 0.05; Figure 4D). An acute cocaine injection in the vehicle-cocaine mice produced a significant decrease in the gene expression of NR1 (p < 0.01), NR2A (p < 0.05), NR2B (p < 0.01), and NR2C (p < 0.01) in relation to the vehicle-vehicle mice (Figure 4E–H). The mRNA levels of NR2A (p < 0.05), NR2B (p < 0.01), and NR2C (p < 0.01) were also significantly enhanced in the cocaine-cocaine mice compared with the vehicle-cocaine mice. In addition, the repeated cocaine-conditioned animals that received vehicle (the cocaine-vehicle group) displayed a significant increase in the NR2C gene expression in comparison with the vehicle-vehicle mice and the vehicle-cocaine group (p < 0.05 and p < 0.01, respectively). Regarding the GluR1 mRNA level, an acute cocaine injection in the vehicle-cocaine mice produced a significant decrease in the gene expression in relation to the vehicle-vehicle mice (p < 0.05). In addition, the cocaine-pretreated group that received vehicle (the cocaine-vehicle group) showed a significant increase in the GluR1 mRNA level compared with the vehicle-cocaine group (p < 0.05; Figure 4I). A significant increase in the GluR2 mRNA level was induced in the cocaine-vehicle group in comparison with the vehicle-cocaine group (p < 0.05; Figure 4J), while the GluR3 mRNA level was increased in the cocaine-cocaine group in comparison with the vehicle-cocaine group (p < 0.05; Figure 4K). Finally, the mRNA expression of GluR4 was unaltered when paired comparisons were performed between the groups of the repeated pretreatment and the acute treatment (Figure 4L).
Discussion
The present study confirms that the endocannabinoid and glutamatergic systems are modulatory systems of the neurotransmission phenomena in cerebral areas involved in drug addiction that are susceptible to modification of their expression as the result of cocaine exposure. Overall, the data obtained in the mouse PFC suggest that during cocaine-sensitization, with a progressive increase in locomotor activity, the endocannabinoid signaling was affected; this fact could modify other signaling systems, including the cortical excitatory glutamatergic inputs in this area, given that the endocannabinoids act as retrograde messengers. This hypothesis is supported by previous studies that suggest that the first exposure to cocaine induced glutamate release in the PFC-NAc pathway, followed by a depression of the activity of glutamate PFC neurons. Also, a progressive sensitization to further cocaine administrations after a period of chronic exposure to the psychostimulant was seen (for review see Hearing et al., 2012). Further, our data revealed that cocaine exposure also altered the gene expression of the glutamate signal-related components. Overall, the present results suggest that, after repeated exposure to a cocaine regimen leading to sensitization, the enhanced glutamatergic response to cocaine is associated with a failure in the inhibitory action of the neuromodulatory endocannabinoid brake. This hypothesis links endocannabinoid plasticity to the described adaptations of PFC glutamate neurons to acute or chronic cocaine exposure.
As commented, an overall decrease in the ratios of the endocannabinoid synthesis/degradation was observed when the synthesizing-enzymes and the degrading-enzymes were measured under different behavioral paradigms of cocaine exposure, including both acute and repeated cocaine administration. We hypothesize that the reduced endocannabinoid-mediated inhibition might be partially responsible for the well-described hyperglutamatergic state associated with cocaine′s actions (Cornish and Kalivas, 2000). Moreover, we have observed that this hyperglutamatergic state was reflected in the expression of key signaling genes, such as glutaminases, metabotropic mGluR5 receptors, and AMPA-type receptor subunits. Of special interest is the mGLUR5 upregulation since this receptor triggers the formation of the retrograde endocannabinoid messenger 2-AG, indicating the existence of dissociation between endocannabinoid and glutamate cocaine-induced adaptations.
Because the PFC is involved in the control of the actions of psychostimulants through its projection to ventral and medio-dorsal striatum, we suggest that the adaptations of the endocannabinoid system to cocaine exposure are a relevant step towards the induction of plastic changes that lead to behavioral sensitization. Our hypothesis is that a deficit in endocannabinoid production could facilitate the dopamine-mediated activation of the excitatory glutamatergic transmission, which leads to the psychostimulant effects of cocaine exposure.
Decreased Endocannabinoid Signaling
Focusing on the endocannabinoid signaling system, we observed differences caused by the cocaine exposure, although the changes in the endocannabinoid components were more evident following the repeated cocaine administration, including the cocaine-conditioned locomotion and cocaine-induced behavioral sensitization. Thus, an acute cocaine injection in naïve mice produced little changes in the endocannabinoid-related proteins, although we detected an overall decrease in the ratios of NAPE-PLD/FAAH and DAGLα/MAGL. Even though the endocannabinoid-synthesizing enzymes displayed a trend to decrease, they were found to be unaltered in the present study. Previous studies with DAGL-deficient mice indicated that DAGLα is the major enzyme needed for retrograde synaptic 2-AG signaling in the nervous system (Gao et al., 2010). On the other hand, acute cocaine administration has been reported to increase the endocannabinoid formation via the activation of dopaminergic D2 receptors (Giuffrida et al., 1999; Centonze et al., 2004).
Concerning repeated cocaine exposure, during cocaine-conditioned locomotion we observed an increase in protein and gene expression of CB1 receptor in the PFC. In addition, cocaine priming after the repeated cocaine pretreatment enhanced the endocannabinoid-degrading profile, increasing FAAH and MAGL enzymes in the PFC, and the NAPE-PLD/FAAH, DAGLα/MAGL and DAGLβ/MAGL ratios were clearly reduced. CB1 receptor expression also increased after administration of cocaine priming injections. The upregulation of the CB1 receptor might be a compensatory mechanism to counteract the low availability of retrograde endocannabinoid inhibitory signals. Supporting this finding, a recent report by Orio and colleagues (2009) found that the anandamide and 2-AG release, as measured by microdialysis, was reduced in rats that were self-administering cocaine. This finding was associated with enhanced expression of CB1 receptors. In a previous study by our group (Rivera et al., 2013), we also demonstrated a similar finding in the hippocampus of Lewis rats self-administering cocaine. Although we did not measure the expression of the cannabinoid CB2 receptor in the present study, we cannot rule out a role for this receptor in the neuroadaptive responses to cocaine, as recently suggested (Xi et al., 2011; Aracil-Fernandez et al., 2012).
This combination of results in protein and gene expression is suggestive of a reduced availability of the endocannabinoids, and we consider that the repeated administration of cocaine could lead to a desensitization of dopaminergic D2 receptor-driven endocannabinoid inhibitory signal by adapting the machinery needed for its production and degradation (Giuffrida et al., 1999; Centonze et al., 2004).
Increased Glutamatergic Signaling
In addition to the endocannabinoid changes, an increase in the gene expression of the glutamatergic signaling was also observed in these sensitized mice, mainly in synthesis enzymes and glutamate receptors. Glutamate, but not dopamine neurotransmission, seems to be a primary mediator of cocaine-induced behaviors associated with drug seeking (Cornish and Kalivas, 2000). The stimulation of glutamate receptors in the NAc augments the reinforcing effects of cocaine, and the increased glutamatergic neurotransmission may be involved in facilitating the relapse to cocaine-seeking behavior (Cornish et al., 1999). The activation of group I (mGluR1/5) metabotropic receptors resulted in an increase in the extracellular glutamate levels in the NAc (Swanson et al., 2001). Moreover, the stimulation of mGluR5 in the PFC is sufficient to induce cocaine sensitization (Timmer and Steketee, 2012), whereas the administration of mGluR5 antagonists attenuated cocaine priming- and cue-induced reinstatement of cocaine seeking (Platt et al., 2008; Kumaresan et al., 2009). Previously, Chiamulera and colleagues (2001) showed that mGluR5-null mice do not display increased locomotor activity following cocaine treatment. Our present data are aligned with these findings, suggesting the induction of hyperactive glutamatergic signaling after repeated cocaine treatment. Thus, we found an increase in the expression of the glutamate-synthesizing enzymes LGA and KGA, which was associated with enhanced mRNA expression of mGluR3 and mGluR5, as well as that of the subunits of the AMPA receptors GluR1, GluR2, and GluR3. Of special relevance was the activation of mGluR5 expression, considering its relevant role in activating the endocannabinoid (2-AG-mediated) retrograde signaling (Gregg et al., 2012).
In summary, repeated cocaine administration modulates the expression of the endocannabinoid signaling machinery in the PFC. These changes are compatible with a situation in which the inhibitory actions of endocannabinoids on glutamatergic terminals are affected. This scenario might contribute to the induction of behavioral sensitization, a process thought to be relevant for psychostimulant addiction.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
Dr Fernando Rodriguez de Fonseca is a Founder of VIVIA Biotech Inc., but he did not receive funds or support for the present study. The remaining authors state no competing financial interests.
Supplementary Material
Acknowledgments
This work was supported by Ministerio de Ciencia e Innovación (PI13/02261 and SAF 2010–20521), Instituto de Salud Carlos III (ISCIII), Ministerio de Economía y Competitividad, Red de Trastornos Adictivos (RD12/0028/0001), Plan Nacional Sobre Drogas, Ministerio de Sanidad y Consumo (PNSD2013/049), Consejería de Economía, Innovación y Ciencia, Junta de Andalucía, UE/ERDF (CTS-433 and P-11-CVI-07637), Consejería de Salud, and Junta de Andalucía (PI0232/2008, PI0029/2008 and SAS111224). Dr Suárez is the recipient of a Miguel Servet research contract from ISCIII (CP12/03109). We thank Mariam Vázquez for English language assistance.
References
- Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M. (2009). The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behavior in rats. J Physiol Pharmacol 60:119–125. [PubMed] [Google Scholar]
- Aracil-Fernandez A, Trigo JM, Garcia-Gutierrez MS, Ortega-Alvaro A, Ternianov A, Navarro D, Robledo P, Berbel P, Maldonado R, Manzanares J. (2012). Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB(2) receptors. Neuropsychopharmacology 37:1749–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco E, Bilbao A, Luque-Rojas MJ, Palomino A, Bermudez-Silva FJ, Suarez J, Santin LJ, Estivill-Torrus G, Gutierrez A, Campos-Sandoval JA, Alonso-Carrion FJ, Marquez J, de Fonseca FR. (2012). Attenuation of cocaine-induced conditioned locomotion is associated with altered expression of hippocampal glutamate receptors in mice lacking LPA1 receptors. Psychopharmacology (Berl) 220:27–42. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. (2004). Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol 14:156–162. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agro A, Bernardi G, Calabresi P, Maccarrone M. (2004). A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic Transmission. Neuropsychopharmacology 29:1488–1497. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Soubrie P, Puech AJ, Thiebot MH. (1998). Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl) 135:324–332. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. (2001). Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci 4:873–874. [DOI] [PubMed] [Google Scholar]
- Corbille AG, Valjent E, Marsicano G, Ledent C, Lutz B, Herve D, Girault JA. (2007). Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci 27:6937–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. (1999). A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience 93:1359–1367. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. (2000). Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN. (2005). Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci 26:420–426. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. (2001). A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7:1151–1154. [DOI] [PubMed] [Google Scholar]
- Fattore L, Martellotta MC, Cossu G, Mascia MS, Fratta W. (1999). CB1 cannabinoid receptor agonist WIN 55,212-2 decreases intravenous cocaine self-administration in rats. Behav Brain Res 104:141–146. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. (2004). A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci 24:6939–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. (2003). Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066. [DOI] [PubMed] [Google Scholar]
- Gao Y, et al. (2010). Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci 30:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. (1999). Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2:358–363. [DOI] [PubMed] [Google Scholar]
- Gorriti MA, Rodriguez de Fonseca F, Navarro M, Palomo T. (1999). Chronic (-)-delta9-tetrahydrocannabinol treatment induces sensitization to the psychomotor effects of amphetamine in rats. Eur J Pharmacol 365:133–142. [DOI] [PubMed] [Google Scholar]
- Gregg LC, Jung KM, Spradley JM, Nyilas R, Suplita RL, 2nd, Zimmer A, Watanabe M, Mackie K, Katona I, Piomelli D, Hohmann AG. (2012). Activation of type 5 metabotropic glutamate receptors and diacylglycerol lipase-alpha initiates 2-arachidonoylglycerol formation and endocannabinoid-mediated analgesia. J Neurosci 32:9457–9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Lujan R, Wickman K. (2013). Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron 80:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Zink AN, Wickman K. (2012). Cocaine-induced adaptations in metabotropic inhibitory signaling in the mesocorticolimbic system. Rev Neurosci 23:325–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. (2009). Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res 202:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque-Rojas MJ, Galeano P, Suarez J, Araos P, Santin LJ, de Fonseca FR, Calvo EB. (2013). Hyperactivity induced by the dopamine D2/D3 receptor agonist quinpirole is attenuated by inhibitors of endocannabinoid degradation in mice. Int J Neuropsychopharmacol 16:661–676. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. (2006). Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29:225–232. [DOI] [PubMed] [Google Scholar]
- Marquez L, Suarez J, Iglesias M, Bermudez-Silva FJ, Rodriguez de Fonseca F, Andreu M. (2009). Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLOS ONE 4:e6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AB, Fernandez-Espejo E, Ferrer B, Gorriti MA, Bilbao A, Navarro M, Rodriguez de Fonseca F, Moratalla R. (2008). Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology 33:1667–1679. [DOI] [PubMed] [Google Scholar]
- Mereu M, Tronci V, Chun LE, Thomas AM, Green JL, Katz JL, Tanda G. (2013). Cocaine-induced endocannabinoid release modulates behavioral and neurochemical sensitization in mice. Addict Biol. Advance online publication. Retrieved 4 Aug 2013. 10.1111/adb.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. (2009). A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci 29:4846–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. (2004). The mouse brain in stereotaxic coordinates, Compact 2nd Edition. Boston: Elsevier Academic Press. [Google Scholar]
- Piomelli D. (2003). The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2008). Attenuation of cocaine self-administration in squirrel monkeys following repeated administration of the mGluR5 antagonist MPEP: comparison with dizocilpine. Psychopharmacology (Berl) 200:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera P, Miguens M, Coria SM, Rubio L, Higuera-Matas A, Bermudez-Silva FJ, de Fonseca FR, Suarez J, Ambrosio E. (2013). Cocaine self-administration differentially modulates the expression of endogenous cannabinoid system-related proteins in the hippocampus of Lewis vs. Fischer 344 rats. Int J Neuropsychopharmacol 16:1277–1293. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Martin Calderon JL, Mechoulam R, Navarro M. (1994). Repeated stimulation of D1 dopamine receptors enhances (-)-11-hydroxy-delta 8-tetrahydrocannabinol-dimethyl-heptyl-induced catalepsy in male rats. Neuroreport 5:761–765. [DOI] [PubMed] [Google Scholar]
- Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, Maldonado R, Valverde O. (2005). Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology 30:1670–1680. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. (2011). Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 63:348–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR. (2008). Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol 509:400–421. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. (2001). Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci 21:9043–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer KM, Steketee JD. (2012). Examination of a role for metabotropic glutamate receptor 5 in the medial prefrontal cortex in cocaine sensitization in rats. Psychopharmacology (Berl) 221:91–100. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. (2011). Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci 14:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.