Abstract
Background:
The 5-HT4 receptor provides a novel potential target for antidepressant treatment. No studies exist to elucidate the 5-HT4 receptor’s in vivo distribution in the depressed state or in populations that may display trait markers for major depression disorder (MDD). The aim of this study was to determine whether familial risk for MDD is associated with cerebral 5-HT4 receptor binding as measured with [11C]SB207145 brain PET imaging. Familial risk is the most potent risk factor of MDD.
Methods:
We studied 57 healthy individuals (mean age 36 yrs, range 20–86; 21 women), 26 of which had first-degree relatives treated for MDD.
Results:
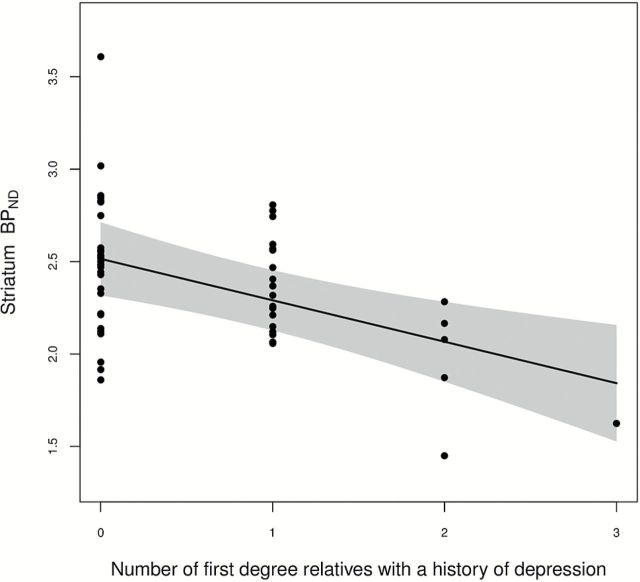
We found that having a family history of MDD was associated with lower striatal 5-HT4 receptor binding (p = 0.038; in individuals below 40 years, p = 0.013). Further, we found evidence for a “risk-dose effect” on 5-HT4 receptor binding, since the number of first-degree relatives with a history of MDD binding correlated negatively with 5-HT4 receptor binding in both the striatum (p = 0.001) and limbic regions (p = 0.012).
Conclusions:
Our data suggest that the 5-HT4 receptor is involved in the neurobiological mechanism underlying familial risk for depression, and that lower striatal 5-HT4 receptor binding is associated with increased risk for developing MDD. The finding is intriguing considering that the 5-HT4 receptor has been suggested to be an effective target for antidepressant treatment.
Keywords: 5-HT4 receptor, depression, MDD, PET, serotonin
Introduction
The serotonin system is involved in regulation of mood and is the predominant target for antidepressant treatment, primarily with selective serotonin reuptake inhibitors (SSRIs). The serotonin 4 (5-HT4) receptor is a Gs-coupled receptor and is believed to act by modulating other neurotransmitter systems. The heterogeneous cerebral distribution of the 5-HT4 receptor is illustrated in Figure 1A. The 5-HT4 receptor provides a new potential target for fast-acting antidepressant treatment (Vidal et al., 2013). Rodent experiments show that only 3 days of treatment with 5-HT4 agonists elicits actions similar to those induced by 2–3 weeks of treatment with classical antidepressants, including desensitization of 5-HT1A autoreceptors, increased tonus on hippocampal postsynaptic 5-HT1A receptors, enhanced phosphorylation of the CREB protein, and neurogenesis in the hippocampus (Lucas et al., 2007). Recent rodent work confirms a fast-acting antidepressant and anxiolytic effect of 5-HT4 receptor stimulation and also, notably, implicates 5-HT4 receptor activation in the behavioral and neurogenic effects of SSRIs (Mendez-David et al., 2013). In the Flinder sensitive line rat model for depression, decreased levels of hippocampal 5-HT4 receptor binding were reported (Licht et al., 2009), while regional changes in different directions were seen in two murine models of depression-related states, characterized by serotonin (5-HT) and hypothalamic-pituitary adrenal system changes of depression (Licht et al., 2010). Behaviorally, 5-HT4 agonists reverse effects of chronic mild stress on sucrose intake and reduce the effects of olfactory bulbectomy on mice locomotor activity, thereby displaying an antidepressant potential (Lucas et al., 2007). Accordingly, 5-HT4 receptor knock-out mice display a decreased reactivity to novelty seeking, which suggests a slight anxiety-like behavior (Compan et al., 2004).
Figure 1.
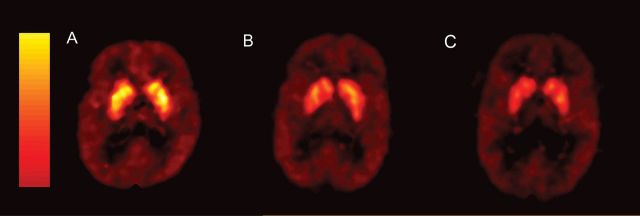
The distribution of [11C]SB207145 binding potentials in 3 young (24–30 yrs) males scanned at the HRRT scanner. Binding levels are high in the striatum, intermediate in the temporal and limbic areas, and low in the neocortex.
(A) The distribution in a subject without a family history of depression. (B) Lower binding potentials in a subject who reported to have one first-degree relative with MDD, and (C) even lower binding potentials in a subject who reported to have 2 first-degree relatives with MDD.
We have recently found evidence in humans that the cerebral 5-HT4 receptor is a biomarker for extracellular levels of serotonin (Haahr, Fisher, Jensen, et al., 2013), and that carriers of the short variant of the promoter serotonin transporter (SERT) gene have lower 5-HT4 receptor binding (Fisher et al., 2012). Thus, it seems that the 5-HT4 receptor is inversely regulated to the 5-HT tonus, albeit not responsive to acute changes in 5-HT (Licht et al., 2009; Marner et al., 2010). No in vivo studies of the cerebral 5-HT4 receptor binding in depressed patients or at-risk populations have been published so far, but a postmortem study of 19 depressed suicide victims showed increased 5-HT4 receptor density in the frontal cortex and caudate nucleus (Rosel et al., 2004).
Studying patients suffering from depression is intricate, and confounding effects of previous depressive episodes, co-morbidity with a current episode, and history of antidepressant treatment must be taken to account. An alternative approach is to study the serotonin system in relation to risk factors for depression. We have previously shown that personality risk factors associate positively with 5-HT2 receptor binding (Frokjaer et al., 2008), and a familial risk for depression may enhance the effect (Frokjaer et al., 2010). A relevant family history is the most potent risk factor for depression (Kendler et al., 1999). The inheritance of depression is polygenetic (Levinson, 2006), and twin studies have suggested that the heritability is around 40% (Kendler et al., 2006b). People with a family history of depression are more prone to develop depressive symptoms (Klaassen et al., 1999), and show compromised emotional processing after dietary depletion of the serotonin precursor protein tryptophan, which decreases synaptic serotonin transiently in CNS (Feder et al., 2010).
Whether healthy individuals with a family history of depression have different cerebral 5-HT4 receptor bindings compared to healthy individuals with no family history of depression may thus shed light on neurobiological mechanisms underlying risk for depression. The aim of this study was to investigate cerebral 5-HT4 receptor binding, measured with brain PET imaging, in healthy people with varying degrees of familial predisposition to depression. We hypothesized that a family history of major depressive disorder (MDD) is associated with lower cerebral 5-HT4 receptor binding based on the above-mentioned experimental studies.
Methods
Participants
Fifty-seven volunteers were included and gave a written informed consent for participation. The study was approved by the Copenhagen Region Ethics Committee ([KF]01-274821, [KF]01 2006-20 and H-D-2007-0067 with amendments). Demographic data are shown in Table 1. Exclusion criteria were significant medical history, drug or alcohol abuse, neurological or psychiatric disorders (also including depression and prior use of antipsychotics and antidepressants), pregnancy, or moderate-severe head trauma. All volunteers had a normal neurological examination, blood analyses within normal range, unremarkable brain magnetic resonance imaging (MRI) scans, and were screened for depressive symptoms using the MDI 10 questionaire (Bech et al., 2001; Olsen et al., 2003) on the day of the PET scan. The participants also completed the Danish version of the 240-item NEO Personal Inventory Revised self-report (NEO-PI-R) personality questionnaire, which evaluates the broad personality dimensions of neuroticism, extraversion, openness, agreeableness, and conscientiousness (Skovdahl-Hansen et al., 2004). Neuroticism is strongly related to lifetime prevalences of MDD, largely due to genetic factors (around 50%) that predispose to both neuroticism and MDD (Kendler et al., 1993, 2006a)
Table 1.
Data of Groups
| No familial risk | Familial risk | |
|---|---|---|
| Gender | 13 F / 18 M | 8 F / 18 M |
| Age (yrs) | 42 (20–86) | 32 (20–64) |
| BMI (kg/m2) | 26 (20–40) | 25 (20–38) |
| 5-HTTLPR status (ll/s-carrier) | 8/21 | 9/17 |
| Neuroticism (score) | 74 (34–139) | 66 (26–131) |
| Scanner (HRRT/Advance) | 16 / 15 | 12 / 14 |
| Mass dose SB207145 (µg) | 2.3 (0.23–5.1) | 2.7 (0.34–5.9) |
| Injected activity (MBq) | 511 (222–611) | 520 (226–617) |
Demographic data, risk factors for depression, and scanner data for individuals with and without familial risk for depression. Data listed as numbers or means (range). No significant between-group differences was found.
Fifty-one volunteers were recruited to the study by public advertisements or extracted from the civil registration system in Denmark. Six volunteers were included because they had a sibling or parent with MDD treated at an in- or out-patient hospital in Denmark; these volunteers also participated in a clinical trial study (Knorr et al., 2011). All volunteers were scanned in the period from 2006 to 2011, and some datasets have been included in previously-published studies regarding validation of the tracer (Marner et al., 2009; Madsen, Marner, et al., 2011) and studies of the 5-HT4 receptor in healthy volunteers to determine the association between the receptor binding and gender and age (Madsen, Haahr, et al., 2011), 5-HTTLPR genotype (Fisher et al., 2012), body mass index (BMI) (Haahr et al., 2012), memory (Haahr, Fisher, Holst, et al., 2013), effects of SSRI (Marner et al., 2010; Haahr, Fisher, Jensen, et al., 2013), and in relation to patients suffering from Alzheimer’s disease (Madsen, Neumann, et al., 2011). Relevant effects found in these studies were also evaluated in the statistical analysis of this study.
To identify participants with familial risk of depression, all participants were interviewed at the day of the PET scan using a Danish version of the Family History Assessment Module (FHAM) (Rice et al., 1995). This module is designed to assess major psychiatric disorders in relatives of the participant. For ethical reasons it was not possible to contact the affected relatives themselves. Instead, participants who reported to have one or more first-degree relatives with a major psychiatric disorder were subsequently interviewed by a trained physician with a structural interview regarding the symptoms and treatment of each affected relative. No participants had first-degree relatives diagnosed with schizophrenia or bipolar disorder.
In this enriched cohort, 26 out of 57 healthy volunteers (46%) reported to have one or more first-degree relatives diagnosed with depression according to the DSM-IV criteria, which were used as the diagnostic criteria in this study. A total of 34 affected relatives were identified: 97% had been treated for depression by a general physician or psychiatrist, 77% had been treated with antidepressants, 44% had been hospitalized, and 15% had attempted suicide. Consistent with the preponderance of women with depression, 68% of the affected relatives were women (14 mothers, 10 sisters, 8 fathers, 2 brothers, and 0 children).
Since short-allele carrier status of the 5-HTTLPR polymorphism in the promoter region of the SERT gene, SCL6A4, may modulate risk for developing depression (Caspi et al., 2003) and affect the 5-HT4 receptor levels (Fisher et al., 2012), blood samples were drawn to determine the 5-HTTLPR status as previously described (Kalbitzer et al., 2010).
MRI and Regions of Interest
MRI was conducted on a Siemens Magnetom Trio 3T MR scanner. T2-weighted sequences were acquired for brain-masking purposes. High-resolution 3D T1-weighted (matrix 256 x 256; 1 x 1 x 1mm voxels) images were segmented into grey matter, white matter, and cerebrospinal fluid using Statistical Parametric Mapping (SPM5; Wellcome Department of Cognitive Neurology). A set of 17 brain regions was automatically delineated with the Pvelab software package (Svarer et al., 2005) on each volunteer’s MRI in a user-independent fashion.
PET Imaging and Quantification of Non-Displaceable 5-HT4 Receptor Binding
All PET scans were based on a 120-minute dynamic acquisition starting with a bolus injection of [11C]SB207145 given over 20 seconds. Two different PET scanners were used over time, as the department added a scanner with a higher resolution. Twenty-nine volunteers had the PET scans performed with an 18-ring GE-Advance scanner (General Electric, Milwaukee, WI, USA) operating in 3D acquisition mode with an approximate in-plane resolution of 6mm. After acquisition, attenuation- and decay-corrected recordings were reconstructed by filtered back projection using a 6mm Hann filter. The remaining 28 volunteers completed the PET scans performed with a high-resolution research tomography (HRRT) Siemens PET scanner and the images were reconstructed with 3D-OSEM-PSF (Sureau et al., 2008) with a resolution of approximately 2mm (Olesen et al., 2009).
The scan consisted of 38 time frames (6 x 5 seconds [s], 10 x 15 s, 4 x 30 s, 5 x 120 s, 5 x 300 s, and 8 x 600 s). Mean voxel movement between frames was assessed with AIR 5.2.5 (Woods et al., 1992), and, only when exceeding 3mm, movement correction was applied as the rigid transformation of each frame to a selected single frame with sufficient structural information (frame 26: 15–20min. post injection) using the scaled least squares cost-function in AIR.
For automatic co-registration of the PET scan to the MRI, the AIR algorithm was applied for GE-Advance scans while SPM5 was applied for HRRT scans and the quality of each co-registration was evaluated by visual inspection in three planes.
The regional in vivo outcome measure for 5-HT4 receptor levels, the binding potential, BP ND, was modeled with the simplified reference tissue model as validated previously (Marner et al., 2009). From the set of regions, volume-weighted means of BP ND were calculated for three brain regions considered important for mood disorders: the striatum (high 5-HT4 receptor binding, including caudate nucleus and putamen), the limbic regions (intermediate 5-HT4 receptor binding, including hippocampus, amygdala, thalamus, and anterior and posterior cingulate gyrus), and the neocortex (low 5-HT4 receptor binding, including parietal cortex, occipital cortex, lateral temporal cortex, insula, and orbito-frontal and lateral-frontal cortex) as previously described (Madsen, Haahr, et al., 2011).
These regions were chosen since the striatum and the limbic regions previously have been shown to be involved in MDD (Price and Drevets, 2010), including findings of reduced grey-matter volumes, increased cerebral blood flow and metabolism, altered hemodynamic responses towards emotional stimuli, and reward-processing. The neocortex was included in our analysis as one large cortical region, as it has low 5-HT4 receptor binding, albeit frontal regions also may be involved in MDD.
Statistics
As the primary investigation, a multiple–linear regression model was employed to study the association between having a family history of depression (binary) and the 5-HT4 receptor bindings for each of the three selected brain regions. As expected, age, gender, and scanner type were significant covariates and were included in the regression model.
We also examined the effect of the number of affected relatives with a history of depression to investigate a possible “risk-dose effect” of family history of depression on 5-HT4 receptor binding. Also, the interaction of being female and having a female relative with a history of depression was investigated to see if the heritable effect could be sex-specific (Kendler et al., 2006b).
All statistical tests were two-sided, and p values were considered statistically significant when less than 0.05.
On one hand, the assessment of familial risk for depression may be biased by the age of the volunteers: e.g., being older increases the likelihood of a higher number of MDD-diagnosed first-degree relatives. On the other hand, remaining mentally healthy in spite of a family history may also result from being protected against depression. Also, the availability of efficient antidepressant treatment and more attention to the diagnosis in society over time may have an impact. Therefore we post hoc estimated the model in the subset of the cohort <40 years (n = 39).
Results
A family history of depression was associated with a significant decrease in striatal 5-HT4 receptor binding (p = 0.038, -0.20 BP ND, 95% CI: [-0.39; -0.012] BP ND), whereas 5-HT4 receptor binding in the limbic regions (p = 0.20) and in the neocortex (p = 0.87) did not differ significantly between groups (Table 2). The results were even more significant when only including individuals below 40 years in the model (n = 39; striatum p = 0.013; limbic regions p = 0.16; neocortex p = 0.99).
Table 2.
Multiple Linear Regression Model Analysis
| 5-HT4 Receptor Binding | |||
|---|---|---|---|
| Estimate ± SE | p value | R2 | |
| Neocortex constant = 0.468 | 0.86 | ||
| Age [/year] | -0.0019±0.0006 | 0.002 | |
| Sex [female] | -0.062±0.021 | 0.004 | |
| Scanner [HRRT] | 0.259±0.021 | <0.0001 | |
| Family history of MDD | -0.0031±0.019 | 0.87 | |
| Limbic regions constant = 0.689 | 0.71 | ||
| Age [/year] | -0.0017±0.0008 | 0.028 | |
| Sex [female] | -0.089±0.027 | 0.002 | |
| Scanner [HRRT] | 0.184±0.027 | <0.0001 | |
| Family history of MDD | -0.031±0.024 | 0.20 | |
| Striatum constant = 2.859 | 0.81 | ||
| Age [/year] | -0.012±0.003 | 0.0003 | |
| Sex [female] | -0.233±0.105 | 0.031 | |
| Scanner [HRRT] | 1.069±0.105 | <0.0001 | |
| Family history of MDD | -0.203±0.095 | 0.038 | |
Outcome of multiple–linear regression model analysis used to determine the effect of a family history of depression on 5-HT4 receptor binding. Age, gender, and scanner type were significant co-variates in all regions and are included in the model.
When considering the “risk-dose” of first-degree relatives with a history of depression, a significant negative correlation was observed with 5-HT4 receptor binding in both the striatum (p = 0.001, -0.22 BP ND/relative, 95% CI: [-0.352; -0.097] BP ND, Figure 2) and limbic regions (p = 0.012, -0.043 BP ND/relative, 95% CI: [-0.076; -0.010] BP ND), but no correlation was observed in the neocortex (p = 0.20, 0.017 BP ND/relative, 95% CI: [-0.044; 0.009] BP ND). The results are illustrated with examples of the distribution in different subjects in Figure 1. A leave-one-out sensitivity analysis showed that the estimated association was not strongly driven by any single observation in the data.
Figure 2.
The estimated linear association between 5-HT4 receptor binding in the striatum (corrected for age, gender, and scanner type) and the number of first-degree relatives treated for major depression (p = 0.001), with pointwise 95% confidence limits and partial residuals (reference: male, mean age 36 years; GE Advance). A leave-one-out analysis showed that the estimated association was not strongly driven by any single observation in the data (p values in the range 0.0003–0.005).
Consistent with a previous study in a subset of this cohort (Madsen, Haahr, et al., 2011), a decline is found with aging in all regions (Table 2). Female gender status was associated with reduced limbic 5-HT4 receptor binding in the previous study; however, in this larger study the association was significant in all regions (Table 2). As expected (Nilsson et al., 2010; Svarer et al., 2010), the HRRT PET scanner generated higher 5-HT4 receptor BPND than the GE scanner (Table 2). Inclusion of 5-HTTLPR genotype status, neuroticism score, and BMI in the model (all predictors were statistically insignificant in all three regions) resulted in very similar p values and parameter estimates of the associations between our primary predictor—familial history of MDD—and the regional 5-HT4 receptor binding.
Discussion
Consistent with our hypothesis, we found that familial risk of MDD was associated with lower striatal 5-HT4 receptor binding (p = 0.038), but no significant effect was found in the neocortex and limbic regions. The effect was even more significant in participants below 40 years (striatum p = 0.013) in spite of the reduced sample. Analysis using the same statistical model showed that the association to striatal 5-HT4 receptor binding was even more pronounced when considering the number of affected relatives (p = 0.001) and an association in the same direction was observed in the limbic region (p = 0.01), indicating that there may be a “risk-dose effect” of the heritability of depression on 5-HT4 receptor binding. The association towards the striatum and the limbic regions is in concordance with previous findings of involvement of these regions in MDD (Price and Drevets, 2010). Anhedonia is a common symptom in MDD and reward responsiveness (hedonic capacity) may be heritable (Bogdan and Pizzagalli, 2009). The striatum, particularly its ventral part, and the limbic regions are key structures in the reward system, and patients suffering from MDD have a reduced striatal activation response to rewards (Pizzagalli et al., 2009; Stoy et al., 2012). Reversal learning is also associated with striatal responses and has a negative bias in MDD (Robinson et al., 2011), and the 5-HT4 receptor has been suggested to be involved in cognitive function (King et al., 2008; Haahr, Fisher, Holst, et al., 2013). The involvement of the limbic and striatal regions in MDD has also been demonstrated by induction of mood changes from deep brain stimulation (including symptoms of hypomania, dysphoria, and anhedonia), and experimental investigations are currently being conducted in treatment-resistant MDD (Cusin and Dougherty, 2012).
Our finding of lower striatal and limbic 5-HT4 receptor binding in relation to the heritability of depression may reflect that decreased receptor availability is a trait marker of MDD. This is consistent with observations in a genetic rat model of depression (Licht et al., 2009) and a slight hyperanxiety-like behavior of the 5-HT4 receptor knock-out mice in studies of activity in the open field (Compan et al., 2004). Our finding of lower 5-HT4 receptor binding in mentally-healthy individuals at familial risk for developing depression could be interpreted as reflecting higher chronic endogenous serotonin levels, since the 5-HT4 receptor is inversely regulated to the 5-HT tonus (Haahr, Fisher, Jensen, et al., 2013). In vivo studies have reported associations between depression and altered SERT and elevated 5-HT1A binding (Meyer, 2007; Miller et al., 2009), and between behavioral phenotypes related to risk for depression and increased 5-HT2A binding (Frokjaer et al., 2008). We cannot determine whether the lower striatal 5-HT4 receptor binding found in our study represents a protective or compensatory mechanism for the included participants to remain mentally healthy, as part of being MDD resilient. It could be that those subjects at highest risk additionally down-regulate limbic 5-HT4 receptors to remain mentally healthy, maybe by modulation of the 5-HT tonus. The answer to this question can only be obtained through longitudinal follow-up studies, which would also reveal whether the low 5-HT4 receptor binding is predictive of development of depression later in life, or by examining unmedicated patients remitted from a depressed state.
The heritability of MDD is higher in women than in men and some genetic risk factors for MDD are sex-specific in their effect (Kendler et al., 2006b). A post hoc test showed no interaction between being a female and having a female relative with a history of depression (p = 0.51 in striatum, p = 0.84 in limbic regions, p = 0.74 in neocortex), even though females have lower limbic 5-HT4 receptor binding (Madsen, Haahr, et al., 2011). Thus, based on our data it seems that the effect of familial risk for depression on 5-HT4 receptor binding is not sex-specific.
Some potential limitations of our study should be considered. The number of first-degree relatives could bias our investigation, since some have more siblings and children than others. As being older increases the likelihood of a higher number of MDD-diagnosed first-degree relatives, age could be an important confounder. However, the participants were quite young, and none reported to have children who had suffered from depression. On the other hand, being elderly and having stayed healthy despite a family history of MDD may index protective factors. Yet, when we considered only individuals younger than 40 years old we continued to see a significant association between familial risk and striatal 5-HT4 receptor binding.
One could speculate whether participants with an affected relative had an overrepresentation of other neuropsychiatric disorders, which could potentially contribute to decreases in 5-HT4 receptor binding. However, absence of neuropsychiatric disorders was thoroughly assessed. In further support, participants were screened for depressive symptoms on the day of the PET scan.
Despite our inability to interview the affected relatives of the participants themselves, participants were able to give detailed information regarding their relatives. For example, they reported that 97% of the affected relatives had been treated for depression by a general physician or psychiatrist and 77% with antidepressant drugs, which we find underpins the validity of the MDD diagnosis. We experienced that the characteristics of the affected relatives were more difficult to clarify for elderly participants who reported a parent suffering from depression. This might explain why the association between familial risk and striatal 5-HT4 binding appeared weaker when including participants above 40 years of age. However, MDD is a heterogeneous disorder and 5-HT4 receptor binding could be more strongly related to a more homogenous phenotype of MDD (Bogdan et al., 2013), as, for example, in patients with predominant symptoms of anhedonia, anxiety, or suicidal behavior. However, we were not able to reliably characterize the affected relatives in such detail based on the interviews of the participants.
Conclusion
The finding of lower 5-HT4 receptor binding in healthy individuals with familial risk for MDD suggests that the 5-HT4 receptor is involved in the neurobiological mechanism underlying familial risk for depression. Our current finding is intriguing considering that the 5-HT4 receptor may be an effective target for antidepressant treatment (Lucas et al., 2007; Vidal et al., 2013). Future studies are needed to elucidate whether 5-HT4 receptor binding is changed in the depressed state of MDD, and clinical trials are needed to determine the effects of 5-HT4 agonists on depressive symptoms and cognitive performances in MDD.
Statement of Interest
Dr Knorr has been a consultant for Astrazeneca. Otherwise, the authors declare no conflicts of interest and no non-financial form of support has been given to the study.
Acknowledgments
This work was supported by The Lundbeck Foundation (Cimbi grant), the Toyota Foundation, and Savværksejer Jeppe Juhl og hustru Ovita Juhls Mindelegat. The John and Birthe Meyer Foundation is gratefully acknowledged for the donation of the Cyclotron and the PET scanner. Ling Feng is acknowledged for constructing Figure 1.
References
- Bech P, Rasmussen NA, Olsen LR, Noerholm V, Abildgaard W. (2001). The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. J Affect Disord 66:159–164. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Nikolova YS, Pizzagalli DA. (2013). Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiol Dis 52:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. (2009). The heritability of hedonic capacity and perceived stress: a twin study evaluation of candidate depressive phenotypes. Psychol Med 39:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389. [DOI] [PubMed] [Google Scholar]
- Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R. (2004). Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J Neurosci 24:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin C, Dougherty DD. (2012). Somatic therapies for treatment-resistant depression: ECT, TMS, VNS, DBS. Biol Mood Anxiety Disord 2:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Skipper J, Blair JR, Buchholz K, Mathew SJ, Schwarz M, Doucette JT, Alonso A, Collins KA, Neumeister A, Charney DS. (2010). Tryptophan depletion and emotional processing in healthy volunteers at high risk for depression. Biol Psychiatry 69:804–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Holst KK, Mc Mahon B, Haahr ME, Madsen K, Gillings N, Baare WF, Jensen PS, Knudsen GM. (2012). 5-HTTLPR status predictive of neocortical 5-HT4 binding assessed with [(11)C]SB207145 PET in humans. Neuroimage 62:130–136. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Mortensen EL, Nielsen FA, Haugbol S, Pinborg LH, Adams KH, Svarer C, Hasselbalch SG, Holm S, Paulson OB, Knudsen GM. (2008). Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry 63:569–576. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Vinberg M, Erritzoe D, Baare W, Holst KK, Mortensen EL, Arfan H, Madsen J, Jernigan TL, Kessing LV, Knudsen GM. (2010). Familial risk for mood disorder and the personality risk factor, neuroticism, interact in their association with frontolimbic serotonin 2A receptor binding. Neuropsychopharmacology 35:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahr ME, Rasmussen PM, Madsen K, Marner L, Ratner C, Gillings N, Baare WF, Knudsen GM. (2012). Obesity is associated with high serotonin 4 receptor availability in the brain reward circuitry. Neuroimage 61:884–888. [DOI] [PubMed] [Google Scholar]
- Haahr ME, Fisher P, Holst K, Madsen K, Jensen CG, Marner L, Lehel S, Baare W, Knudsen G, Hasselbalch S. (2013). The 5-HT4 receptor levels in hippocampus correlates inversely with memory test performance in humans. Hum Brain Mapp 34:3066–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahr ME, Fisher PM, Jensen CG, Frokjaer VG, Mahon BM, Madsen K, Baare WF, Lehel S, Norremolle A, Rabiner EA, Knudsen GM. (2013). Central 5-HT receptor binding as biomarker of serotonergic tonus in humans: a [C]SB207145 PET study. Mol Psychiatry. 19:427–432 [DOI] [PubMed] [Google Scholar]
- Kalbitzer J, Erritzoe D, Holst KK, Nielsen FA, Marner L, Lehel S, Arentzen T, Jernigan TL, Knudsen GM. (2010). Seasonal changes in brain serotonin transporter binding in short serotonin transporter linked polymorphic region-allele carriers but not in long-allele homozygotes. Biol Psychiatry 67:1033–1039. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. (1993). A longitudinal twin study of personality and major depression in women. Arch Gen Psychiatry 50:853–862. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. (1999). Clinical characteristics of major depression that predict risk of depression in relatives. Arch Gen Psychiatry 56:322–327. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. (2006a) Personality and major depression: a Swedish longitudinal, population-based twin study. Arch Gen Psychiatry 63:1113–1120. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. (2006b) A Swedish national twin study of lifetime major depression. Am J Psych 163:109–114. [DOI] [PubMed] [Google Scholar]
- King MV, Marsden CA, Fone KC. (2008). A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci 29:482–492. [DOI] [PubMed] [Google Scholar]
- Klaassen T, Riedel WJ, van Someren A, Deutz NE, Honig A, van Praag HM. (1999). Mood effects of 24-hour tryptophan depletion in healthy first-degree relatives of patients with affective disorders. Biol Psychiatry 46:489–497. [DOI] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Hansen A, Klose M, Feldt-Rasmussen U, Hilsted L, Hasselstrom J, Gether U, Winkel P, Gluud C, Wetterslev J, Kessing LV. (2011). Escitalopram and neuroendocrine response in healthy first-degree relatives to depressed patients--a randomized placebo-controlled trial. PLOS ONE 6:e21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF. (2006). The genetics of depression: a review. Biol Psychiatry 60:84–92. [DOI] [PubMed] [Google Scholar]
- Licht CL, Marcussen AB, Wegener G, Overstreet DH, Aznar S, Knudsen GM. (2009). The brain 5-HT4 receptor binding is down-regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J Neurochem 109:1363–1374. [DOI] [PubMed] [Google Scholar]
- Licht CL, Kirkegaard L, Zueger M, Chourbaji S, Gass P, Aznar S, Knudsen GM. (2010). Changes in 5-HT4 receptor and 5-HT transporter binding in olfactory bulbectomized and glucocorticoid receptor heterozygous mice. Neurochem Int 56:603–610. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, Lambas-Senas L, Wiborg O, Haddjeri N, Pineyro G, Sadikot AF, Debonnel G. (2007). Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 55:712–725. [DOI] [PubMed] [Google Scholar]
- Madsen K, Haahr MT, Marner L, Keller SH, Baaré W, Svarer C, Hasselbalch SG, Knudsen GM. (2011). Age and sex effects on 5-HT4 receptors in the human brain: a [11C]SB207145 PET study. J Cereb Blood Flow Metab. 31:1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen K, Marner L, Haahr M, Gillings N, Knudsen GM. (2011). Mass dose effects and in vivo affinity in brain PET receptor studies - a study of cerebral 5-HT(4) receptor binding with [(11)C]SB207145. Nucl Med Biol. 38:1085–1091. [DOI] [PubMed] [Google Scholar]
- Madsen K, Neumann WJ, Holst K, Marner L, Haahr MT, Lehel S, Knudsen GM, Hasselbalch SG. (2011). Cerebral Serotonin 4 Receptors and Amyloid-beta in Early Alzheimer’s Disease. J Alzheimers Dis. 26:457–466. [DOI] [PubMed] [Google Scholar]
- Marner L, Gillings N, Comley RA, Baare WF, Rabiner EA, Wilson AA, Houle S, Hasselbalch SG, Svarer C, Gunn RN, Laruelle M, Knudsen GM. (2009). Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo . J Nucl Med 50:900–908. [DOI] [PubMed] [Google Scholar]
- Marner L, Gillings N, Madsen K, Erritzoe D, Baare WF, Svarer C, Hasselbalch SG, Knudsen GM. (2010). Brain imaging of serotonin 4 receptors in humans with [11C]SB207145-PET. Neuroimage 50:855–861. [DOI] [PubMed] [Google Scholar]
- Mendez-David I, David DJ, Darcet F, Wu MV, Kerdine-Romer S, Gardier AM, Hen R. (2013). Rapid Anxiolytic Effects of a 5-HT Receptor Agonist Are Mediated by a Neurogenesis-Independent Mechanism. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH. (2007). Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci 32:86–102. [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Brennan KG, Ogden TR, Oquendo MA, Sullivan GM, Mann JJ, Parsey RV. (2009). Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology 34:2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson MS, Tóth M, Cselény Z, Karlsson P, Halldin C, Farde L, Varrone A. (2010). Quantification of serotonin transporter availability with [11C]MADAM - A comparison between the ECAT HRRT and HR systems. In: Neuroreceptor Mapping Congress Glasgow: Neuroimage. Abstract at congress in 2010. [DOI] [PubMed] [Google Scholar]
- Olesen OV, Sibomana M, Keller SH, Andersen F, Holm JJS, Svarer C, Højgaard L. (2009). Spatial Resolution of the HRRT PET Scanner Using 3D-OSEM PSF Reconstruction. In 2009 IEEE Nuclear Science Symposium Conference Record (MIC), IEEE. [Google Scholar]
- Olsen LR, Jensen DV, Noerholm V, Martiny K, Bech P. (2003). The internal and external validity of the Major Depression Inventory in measuring severity of depressive states. Psychol Med 33:351–356. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psych 166:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr., Schuckit MA, Begleiter H. (1995). Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res 19:1018–1023. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. (2011). Ventral Striatum Response During Reward and Punishment Reversal Learning in Unmedicated Major Depressive Disorder. Am J Psychiatry 169:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosel P, Arranz B, Urretavizcaya M, Oros M, San L, Navarro MA. (2004). Altered 5-HT2A and 5-HT4 postsynaptic receptors and their intracellular signalling systems IP3 and cAMP in brains from depressed violent suicide victims. Neuropsychobiology 49:189–195. [DOI] [PubMed] [Google Scholar]
- Skovdahl-Hansen H, Mortensen E, Scioetz H. (2004). Dokumentation for den danske udgave af NEO PI-R og NEO PI-R kort version. Copenhagen, Denmark: Dansk Psykologisk Forlag. [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hagele C, Suchotzki K, Schmack K, Wrase J, Ricken R, Knutson B, Adli M, Bauer M, Heinz A, Strohle A. (2012). Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol 26:677–688. [DOI] [PubMed] [Google Scholar]
- Sureau FC, Reader AJ, Comtat C, Leroy C, Ribeiro MJ, Buvat I, Trebossen R. (2008). Impact of image-space resolution modeling for studies with the high-resolution research tomograph. J Nucl Med 49:1000–1008. [DOI] [PubMed] [Google Scholar]
- Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbol S, Frokjaer VG, Holm S, Paulson OB, Knudsen GM. (2005). MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage 24:969–979. [DOI] [PubMed] [Google Scholar]
- Svarer C, Marner L, Madsen K, Keller SH, Haahr MT, Siboma M, Knudsen GM. (2010). Comparing HRRT and advance scanner data acquisition using a steady-state scan approach. In: Neuroreceptor Mapping Congress Glasgow: NeuroImage. Abstract at Neuroreceptor Mapping Congress in 2010. [Google Scholar]
- Vidal R, Castro E, Pilar-Cuellar F, Pascual-Brazo J, Diaz A, Rojo ML, Linge R, Martin A, Valdizan E, Pazos A. (2013). Serotonin 5-HT4 Receptors: a New Strategy for Developing Fast Acting Antidepressants? Curr Pharm Des 2014;20:3751–1762. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. (1992). Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 16:620–633. [DOI] [PubMed] [Google Scholar]