Abstract
Background:
Some studies suggest better overall outcomes when right unilateral electroconvulsive therapy (RUL ECT) is given with an ultrabrief, rather than brief, pulse width.
Methods:
The aim of the study was to test if ultrabrief-pulse RUL ECT results in less cognitive side effects than brief- pulse RUL ECT, when given at doses which achieve comparable efficacy. One hundred and two participants were assigned to receive ultrabrief (at 8 times seizure threshold) or brief (at 5 times seizure threshold) pulse RUL ECT in a double-blind, randomized controlled trial. Blinded raters assessed mood and cognitive functioning over the ECT course.
Results:
Efficacy outcomes were not found to be significantly different. The ultrabrief group showed less cognitive impairment immediately after a single session of ECT, and over the treatment course (autobiographical memory, orientation).
Conclusions:
In summary, when ultrabrief RUL ECT was given at a higher dosage than brief RUL ECT (8 versus 5 times seizure threshold), efficacy was comparable while cognitive impairment was less.
Keywords: cognitive, depression, electroconvulsive therapy, pulse width, randomized controlled trial
Introduction
Shortening of the pulse width of the electroconvulsive (ECT) stimulus is a significant development in ECT treatment technique. The majority of randomized controlled trials and other studies have shown substantially reduced neurocognitive impairment when an ultrabrief pulse width of approximately 0.3 milliseconds (ms) was used compared with a brief pulse width (0.5–2.0ms; Pisvejc et al., 1998; Loo et al., 2008, 2012; Sackeim et al., 2008; Mayur et al., 2013; Spaans, Verwijk, et al., 2013). However, results from some studies also suggest that efficacy may be reduced, with lower response and remission rates, slower speed of improvement, and a greater number of treatments required (Loo et al., 2008, 2012, 2013; Niemantsverdriet et al., 2011; Spaans, Verwijk, et al., 2013). In this double-blind, randomized controlled trial, ultrabrief pulse right unilateral (RUL) ECT dosed at 8 times seizure threshold was compared with brief-pulse RUL ECT dosed at 5 times seizure threshold. The dose for RUL ECT was informed by our prior research (Loo et al., 2008) and we estimated that RUL ECT at 8 times seizure threshold would be required to achieve comparable efficacy. We hypothesized that, when given at a higher relative dosage level, ultrabrief pulse RUL ECT would result in comparable efficacy to brief-pulse RUL ECT, but retain cognitive benefits. A related but separate study of bilateral ECT using the same overall study design (also registered under NCT00870805) will be reported separately.
Methods
Study Design
Depressed inpatients from Wesley Hospital, the Melbourne Clinic, and St George Hospital (Sites) who were prescribed RUL ECT by their treating psychiatrists were assessed for trial inclusion. Written informed consent was obtained from all participants, and the trial was approved by the Human Research Ethics Committees of the University of New South Wales, the Melbourne Clinic, and South Eastern Sydney Illawarra Area Health Service. The trial followed a prospective, double-blind, parallel-group experimental design. Using a computer-generated random number sequence, stratified by trial site, participants were randomly assigned to receive RUL ECT with a brief (1.0ms) or ultrabrief pulse width (0.3ms). At doses of 500–1000 milliCoulombs, pulse width had to be increased to a maximum of 0.7ms in order to achieve higher absolute electrical doses because of machine limitations. ECT treatment condition was indicated on treatment sheets by a code, such that participants, their treating psychiatrist, and all clinical staff, other than the psychiatrist administering ECT, were blind to treatment assignment. All participants remained under the care of their own treating psychiatrist during the trial period, and decisions concerning the number of ECT treatments received, the decision to switch electrode placements, and concurrent medications were determined clinically by that psychiatrist.
Sample Size
The minimum sample size required for this study was calculated in two ways. Firstly, a non-inferiority analysis was undertaken, based on the assumption that a difference in end-of-treatment depression scores of 25% or less between the ultrabrief and brief pulse–width groups would not be of high clinical significance. End of ECT depression score for brief pulse RUL ECT was estimated at 10 (Hamilton Depression Rating Scale), based on data from a prior randomized, double-blind trial (McCall et al., 2002). If there were 42 subjects per group the upper limit of the 95% confidence interval would fall below the maximal value consistent with non-inferiority. Secondly, a power analysis based on expected differences in retrograde amnesia between the two groups was conducted. In the trial of Sackeim et al. (2008), brief and ultrabrief RUL ECT groups differed in outcomes on the Columbia Autobiographical Memory Interview by approximately 0.8 standardized units (z-scores). As our study examined ultrabrief ECT at higher doses relative to seizure threshold, a smaller (though still important) difference between the two groups in retrograde amnesia scores was expected. Power analysis based on a difference equivalent to an effect size of 0.6 found that 45 subjects per group would be required to have an 80% chance of showing this difference at p = 0.05. Allowing for 10% dropouts, the study aimed to recruit a minimum sample of 99 participants.
Participants
One hundred and two psychiatric inpatients were recruited for this study with the following inclusion criteria: aged ≥18 years; DSM-IV Major Depressive Episode; Montgomery-Åsberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979) score ≥25; no diagnosis of schizophrenia, schizoaffective disorder, or rapid cycling bipolar disorder; no ECT in the last 3 months; no drug or alcohol abuse or dependence in the last 6 months; no past or current neurological illness or injury; score ≧24 on Mini Mental State Examination; English proficiency sufficient to undertake cognitive testing.
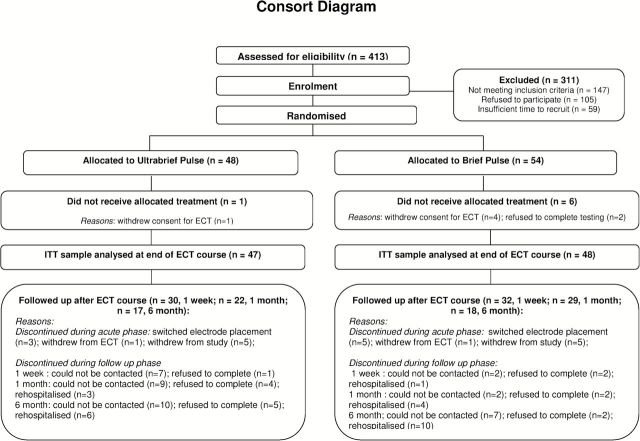
The intention-to-treat sample comprised 95 participants who received at least one ECT treatment and had at least one post-baseline mood rating (see Figure 1 Consort diagram). Five participants withdrew consent for ECT treatment independent of the clinical trial, and two refused to complete cognitive testing.
Figure 1.
CONSORT diagram.
Clinical and demographic details were collected by a research psychologist at baseline using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998; Table 1). In Table 1 and subsequent references, treatment resistance refers to the total score on the Maudsley scale (Fekadu et al., 2009).
Table 1.
Demographic, Clinical and ECT Treatment Data for the Two Treatment Groups Showing Numbers of Subjects or Means (Standard Deviations)
| Variable | Ultrabrief | Brief | t | χ2 | p |
|---|---|---|---|---|---|
| Age (years) | 47.72 (14.60) | 51.54 (14.00) | -1.3 | 0.196 | |
| Gender (male) | 17/47 | 17/48 | 0.0 | 0.939 | |
| Onset age (years) | 27.81 (13.95) | 32.98 (14.10) | -1.8 | 0.076 | |
| Current episode duration (weeks) | 31.87 (36.17) | 42.93 (57.91) | -1.1 | 0.273 | |
| Bipolar | 11/47 | 9/48 | 0.3 | 0.578 | |
| Psychotic | 4/47 | 4/48 | 0.0 | 0.975 | |
| Melancholic | 39/47 | 38/48 | 0.2 | 0.635 | |
| No. antidepressants failed (current episode) | 1.56 (1.06) | 1.49 (0.99) | 0.3 | 0.759 | |
| Treatment resistance total score | 7.02 (1.27) | 6.75 (1.66) | 0.2 | 0.388 | |
| MADRS pre ECT | 33.68 (6.09) | 33.77 (6.48) | -0.1 | 0.945 | |
| Predicted IQ | 107.80 (8.25) | 103.02 (8.57) | 2.7 | 0.009 | |
| Anaesthetic received (thiopentone) | 21/47 | 25/48 | 0.5 | 0.766 | |
| Increased Dose (at any point) | 28/47 | 20/48 | 3.0 | 0.081 | |
| Initial seizure threshold (mC) | 26.75 (11.64) | 74.08 (52.02) | -6.1 | <0.001 | |
| Retitrated seizure threshold | 42.19 (23.72) | 86.82 (41.35) | -4.0 | <0.001 | |
| First dose (mC) | 205.87 (97.65) | 328.81 (192.24) | -3.9 | <0.001 | |
| Last dose (mC) | 291.87 (180.30) | 393.13 (208.17) | -2.5 | 0.013 | |
| EEG seizure length (1st Treatment) | 43.55 (24.56) | 45.60 (17.59) | -0.5 | 0.642 | |
| EEG seizure length (final treatment) | 25.49 (14.94) | 27.85 (15.10) | -0.8 | 0.445 | |
| No. of ECT treatments1 | 8.62 (3.36) | 8.38 (3.16) | 0.4 | 0.718 | |
| Concurrent AD (Yes) | 39/47 | 41/48 | 0.3 | 0.562 | |
| Commenced new AD (Yes) | 30/47 | 31/48 | 0.0 | 0.939 | |
| Benzodiazepine taken during ECT (Yes) | 16/47 | 24/48 | 2.5 | 0.115 | |
| Responders (end of ECT) | 23/47 | 22/48 | 0.1 | 0.762 | |
| Number of ECT treatments (Responders) | 9.09 (3.50) | 8.77 (2.65) | 0.338 | 0.737 | |
| Remitters (end of ECT) | 11/47 | 14/48 | 0.4 | 0.524 | |
| Number of ECT treatments (Remitters) | 8.82 (2.04) | 8.64 (3.27) | 0.164 | .871 |
MADRS = Montgomery-Åsberg Depression Rating Scale; mc = milliCoulombs
1Number of right unilateral electroconvulsive therapy (RUL ECT) treatments as per assigned treatment condition (prior to any switch to another form of ECT)
ECT
ECT was administered three times a week using either a Mecta Spectrum 5000Q ECT machine (Mecta Corp.; at the Wesley Hospital) or a Thymatron System IV machine (Somatics; at the Melbourne Clinic and St George Hospital). ECT was dosed relative to seizure threshold, which was established using a titration method during the first session. Brief-pulse RUL ECT was administered at 5 times seizure threshold as in our previous study (Loo et al., 2008), as we had found in prior clinical experience that not all patients can be treated at 6 times seizure threshold, after allowing for ECT dose increases during the treatment course. Ultrabrief-pulse RUL ECT was administered at 8 times seizure threshold. The d’Elia placement of electrodes was used for all treatments. Participants were re-titrated at their seventh treatment, and ECT dose was adjusted as necessary to maintain dosing at the correct suprathreshold levels. For participants switched to a different ECT placement, study assessments were done following the final treatment of the first ECT placement and participants exited the trial without further follow-up.
Anaesthesia
Participants received either thiopentone (3–5mg/kg) or propofol (1–2mg/kg) for anaesthetic induction over the treatment course. Succinylcholine (1mg/kg) was given for muscle relaxation.
Cognitive Outcomes
A trained research psychologist blinded to treatment assignment assessed cognitive functioning prior to the ECT course, after the sixth ECT, 1–3 days after the last ECT, and at one- and six-month follow ups. The cognitive battery consisted of: a 9-item orientation scale (Orientation), Global Subjective Rating of Memory (McCall et al., 1995); Medical College of Georgia Complex Figure (MCG CFT; Meador et al., 1993); Hopkins Verbal Learning Test-Revised (HVLT-R; Benedict et al., 1998); Controlled Oral Word Association Test (Strauss et al., 2006); Symbol Digit Modalities Test (SDMT; Smith, 1991); Woodcock Johnson Cross-Out Test (Woodcock and Johnson, 1989); and the Columbia Autobiographical Memory Interview–Short Form (AMI-SF; McElhiney et al., 2001). Alternate forms of the MCG CFT, HVLT-R, and SDMT were used at subsequent test sessions to minimize practice effects. As an indicator of premorbid intellectual function, the Wechsler Test of Adult Reading Test (Wechsler, 2001) was administered at baseline. The neuropsychological battery was designed to test both memory and non-memory function. The specific tests were chosen because they have proven sensitive to ECT-induced neuropsychological impairment in prior research (e.g., Lerer et al., 1995; Neylan et al., 2001; Dubovsky et al., 2004) and successfully detected differences in neuropsychological outcome of brief-pulse and ultrabrief-pulse ECT in our prior study (Loo et al., 2008). They are also well tolerated by a severely depressed population.
At the third and sixth ECT treatments, participants also underwent brief cognitive testing to assess acute effects after ECT. Firstly, they were tested on time to reorientation (Sackeim et al., 2008). Secondly—once reoriented—they were tested on recall of six words learned 5–10 minutes prior to ECT treatment, and recognition of eight faces learned 5–10 minutes prior to ECT treatment. There were three sets of words and faces, for which the order was randomized and counterbalanced across participants.
Mood Outcomes
Mood was assessed by a trained research psychologist, blinded to treatment assignment, using the MADRS (Montgomery and Asberg, 1979) prior to the first ECT treatment, after every three ECT treatments, and 1–3 days after the final ECT. Response was defined as ≥50% improvement in MADRS scores over the treatment course and remission was defined as MADRS <10 after the final ECT. The MADRS was also administered at one-week, one-month, and six-month follow-ups.
Statistical Analysis
Differences between the ultrabrief pulse–width group and the brief pulse–width group in clinical and demographic features at baseline were examined using independent samples t-tests for dimensional variables and chi-square tests for categorical variables.
Between-group differences in mood outcomes (in particular linear and quadratic trend over the ECT course) were analyzed using a mixed-effects repeated-measures model of MADRS scores at baseline, after three and six ECT treatments, and after the final ECT, controlling for appropriate covariates (described below). A possible influence on the main results is that of missing data. For example, across weeks 1–4 some 34% of data points are missing, and of these 90% follow dropout, which, in almost all cases, is due to improvement. These data are not missing at random we cannot ignore the implications. Models for change over time which incorporate information about dropout have been proposed (Enders, 2011; Muthen et al., 2011). We were constrained to a series of simpler models, fitted to baseline and weeks 1–4, using Mplus (Muthén and Muthén, 1998). Estimates of the key regression—linear trend on type of ECT—remained quite similar in size and non-significance across the models, suggesting that the dropout due to improvement was not affecting the estimates.
Covariates were selected based on their predictive utility in univariate analyses of variance (ANOVAs) with MADRS scores after the third ECT, after the sixth ECT, and after the final ECT used as dependent variables. Variables were included as covariates if they were a significant predictor in these univariate analyses at a p < 0.1 level, or if they showed significant differences between the groups at baseline at a p < 0.1 level. Following this, age, study site, number of ECT treatments received, melancholic features, psychotic features, Maudsley treatment resistance score, whether participants were taking a benzodiazepine during their ECT course, and commencement of a new antidepressant during the course were included as covariates. Number of adequate antidepressant courses failed, whether participants were taking an antidepressant during their ECT course, commencement of a new antidepressant medication during the ECT course, and polarity of depression were assessed but not included.
To examine whether there were any differences in mood outcomes at specific time points, between-subject analyses of covariance (ANCOVAs) were used to examine change in MADRS scores at Week 1, Week 2, after the final ECT and at one week, one month, and six month follow-ups, controlling for the same covariates in addition to baseline MADRS score. The proportion of responders and remitters in each group were compared using chi-square tests.
To investigate differences in performance on the cognitive test battery at set time points, ANCOVAs were analyzed for all tests except the AMI-SF using scores after six ECT treatments, at the end of the treatment course, one month, and six months after the end of the treatment course, controlling for baseline test score, MADRS score at time of testing, and predicted premorbid IQ; ANCOVAs of test results after the end of the treatment course also controlled for the number of ECT treatments. AMI-SF scores were analyzed similarly, except that consistency scores (% consistency of recall of answers reported at pre-ECT baseline) were analyzed instead of raw scores controlling for baseline scores. Acute word recall and recognition of faces at the third and sixth ECT treatments were analyzed using ANCOVAs controlling for pre-ECT immediate word recall and face recognition. Finally, time to reorientation at the third and sixth ECT treatments was analyzed using ANOVAs. Analyses of cognitive test scores and analyses of acute cognitive effects after an ECT session were adjusted for multiple comparisons using Holm’s method (Aickin and Gensler, 1996).
All statistical analyses were two-tailed using alpha = 0.05 unless otherwise specified.
Results
Participants in the two treatment groups generally did not differ significantly in baseline clinical and demographic characteristics or in ECT treatment parameters, except for higher predicted premorbid IQ in the ultrabrief group (see Table 1). The Melbourne site sample differed from the two Sydney site samples by having fewer failed antidepressant trials in the current episode, lower electrical dosage at the final ECT for the ultrabrief group, and a higher likelihood of commencing a new antidepressant medication and taking a benzodiazepine during the ECT treatment course. The site samples did not differ significantly in age, gender, current episode duration, seizure threshold, initial ECT dose, or number of ECTs given.
Mood Outcomes
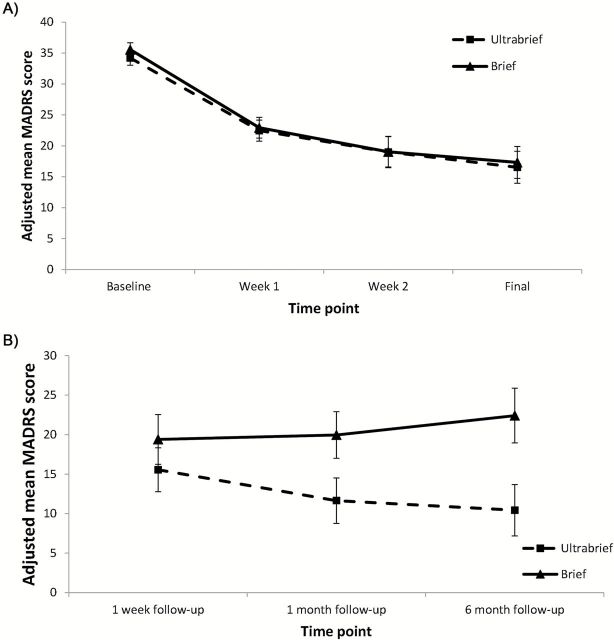
Mean MADRS scores over the treatment course, adjusted for the relevant covariates, are shown in Figure 2A. The mixed-effects repeated-measures model showed only a main effect for linear and quadratic changes over time for the group as a whole (t = -5.27, p < 0.001; t = 2.92, p = 0.004, respectively). There was no significant effect of treatment condition (pulsewidth; t = -0.68, p = 0.50), or interaction between treatment condition and time (t = 0.74, p = 0.46), or treatment condition and time squared (t = -0.72, p = 0.48). All other variables in the model were non-significant (p > 0.05), although the presence of melancholic features and higher treatment resistance scores showed trends towards higher MADRS scores (t = -1.79 and p = 0.08 and t = 1.74 and p = 0.09, respectively). In addition, the between-subjects ANCOVAs showed no significant differences between groups for MADRS score after three ECT treatments (F = 0.25, p = 0.62), after six ECT treatments (F = 1.07, p = 0.31), or after the final ECT (F = 0.01, p = 0.92). Finally, there were no significant differences in the proportion of responders or remitters between the ultrabrief pulse–width group and the brief pulse–width group (response 48.9% ultrabrief group, 45.8% brief group; remission 23.4% ultrabrief group, 29.2% brief group; see Table 1).
Figure 2.
(A) Mean Montgomery-Åsberg Depression Rating Scale (MADRS) scores at baseline, week 1, week 2, and after the final ECT, adjusted for covariates; (B) Mean MADRS scores at 1 week, 1 month, and 6 month follow-ups, adjusted for covariates.
The mean adjusted MADRS scores at follow-up are shown in Figure 2B. The groups did not differ significantly in MADRS scores at either the one-week follow-up (F = 0.03 p = 0.87) or the one-month follow-up (F = 0.59 p = 0.45). At the six-month follow-up, there was a trend for the ultrabrief-pulse group to show lower depression scores relative to the brief-pulse group (F = 3.71, p = 0.07); however, data was only available for a subset of participants (n = 35).
Cognitive Outcomes
The analyses of cognitive outcomes over the ECT course showed less impairment in the ultrabrief pulse–width group in both the orientation score (Orientation) and autobiographical memory (AMI-SF; after adjustment for multiple comparisons; see Table 2). The analyses of cognitive outcomes at the one-month and six-month follow-ups showed no significant differences between groups on any of the tests used (Supplementary Table).
Table 2.
Neuropsychological Test Scores for the Two Treatment Groups (Means and Standard Deviations)
| Assessment | Treatment Condition | Pre-ECT Mean (SD) | After 6 ECT Mean (SD) | After final ECT Mean (SD) | After 6 ECT1
F; p |
After final ECT2
F; p |
|---|---|---|---|---|---|---|
| Orientation | Brief | 8.63 (0.69) | 8.07 (1.20) | 7.98 (1.18) | F = 6.473; p = 0.013 |
F = 16.166; p < 0.001± |
| Ultrabrief | 8.87 (0.34) | 8.68 (0.53) | 8.76 (0.49) | |||
| GSRM | Brief | 3.16 (1.03) | 2.98 (0.94) | 3.05 (1.17) | F = 3.815; p = 0.055 |
F = 1.355; p = 0.248 |
| Ultrabrief | 2.91 (0.87) | 2.53 (0.98) | 2.77 (1.16) | |||
| MCG CFT Delayed Recall | Brief | 18.39 (7.29) | 13.82 (7.36) | 13.14 (6.93) | F = 0.528; p = 0.470 |
F = 0.593; p = 0.444 |
| Ultrabrief | 20.94 (6.97) | 16.74 (7.89) | 15.79 (7.97) | |||
| HVLT Total Learning | Brief | 22.12 (6.10) | 19.17 (6.46) | 19.51 (6.25) | F = 3.484; p = 0.066 |
F = 1.877; p = 0.175 |
| Ultrabrief | 24.11 (5.29) | 23.05 (5.70) | 22.32 (6.51) | |||
| HVLT Delayed Recall | Brief | 7.17 (3.27) | 4.05 (3.05) | 4.41 (3.27) | F = 4.811; p = 0.032 |
F = 4.325; p = 0.041 |
| Ultrabrief | 8.27 (2.76) | 6.21 (3.26) | 6.28 (3.15) | |||
| COWAT Letters | Brief | 30.77 (9.64)* | 23.32 (10.09) | 23.66 (10.53) | F = 0.144; p = 0.705 |
F = 0.269; p = 0.605 |
| Ultrabrief | 38.62 (9.18)* | 27.64 (10.86) | 29.02 (10.94) | |||
| COWAT Category | Brief | 15.65 (4.71) | 12.16 (4.19) | 12.10 (4.65) | F = 0.648; p = 0.423 |
F = 1.967; p = 0.165 |
| Ultrabrief | 18.16 (4.85) | 14.62 (4.35) | 14.85 (5.79) | |||
| SDMT | Brief | 35.29 (12.28) | 31.91 (11.26) | 32.25 (11.88) | F = 0.666; p = 0.417 |
F = 0.381; p = 0.539 |
| Ultrabrief | 42.42 (12.29) | 38.13 (12.83) | 37.22 (11.21) | |||
| Cross Out | Brief | 12.05 (4.21) | 11.31 (5.03) | 11.95 (4.88) | F = 0.992; p = 0.323 |
F = 0.581; p = 0.448 |
| Ultrabrief | 13.76 (4.57) | 13.49 (4.81) | 13.66 (4.63) | |||
| AMI-SF Consistency score ^ | Brief | 72.54 (15.87) | 70.50 (15.52) | F = 5.165; p = 0.026 |
F = 10.337; p = 0.002± |
|
| Ultrabrief | 81.39 (12.71) | 81.44 (12.55) |
AMI-SF: Autobiographical Memory Interview–Short Form; COWAT: Controlled Word Association Test; GSRM: Global Subjective Rating of Memory; HVLT: Hopkins Verbal Learning Tests; MCG CFT: Medical College of Georgia Complex Figure Test; SDMT: Symbol Digit Modalities Test;
1 Analyses of covariance (ANCOVA) controlling for predicted IQ, Montgomery-Åsberg Depression Rating Scale (MADRS) at time of testing, and baseline score on test (n = 71–78)
2 ANCOVA controlling for predicted IQ, MADRS at time of testing, baseline score on test and number of electroconvulsive therapy (ECT) treatments received (n = 76–83)
*ANCOVA controlling for MADRS significantly different between groups
±ANCOVA significant after using Holm’s procedure (p < 0.05)
^AMI-SF Consistency score refers to % retained from baseline.
Table 3 summarizes the acute cognitive effects data. At both ECT treatments three and six, after adjustments for multiple comparisons the ultrabrief group showed significantly superior recall of words and recognition of faces and a faster time to reorientation.
Table 3.
Acute Battery Scores for the Two Treatment Groups (Means and Standard Deviations)
| Assessment | Treatment Condition | Pre ECT3 Mean (SD) | Post ECT3 Mean (SD) | Pre ECT6 Mean (SD) | Post ECT6 Mean (SD) | ECT31 F; p | ECT 62 F; p |
|---|---|---|---|---|---|---|---|
| Faces (hits) | Brief | 6.73 (1.22) | 3.11 (2.39) | 6.44 (1.21) | 2.47 (1.87) | F = 6.64; p = 0.012± |
F = 10.06; p = 0.002± |
| Ultrabrief | 6.46 (1.70) | 4.45 (2.16) | 6.48 (1.60) | 4.12 (2.34) | |||
| Word recall | Brief | 5.33 (1.19) | 0.70 (1.39) | 5.03 (1.15) | 0.45 (0.87) | F = 15.14; p < 0.001± |
F = 11.61; p = 0.001± |
| Ultrabrief | 5.59 (0.74) | 2.28 (2.00) | 5.33 (1.16) | 1.82 (2.04) | |||
| Time to reorientation | Brief | 20.84 (10.49) | 22.29 (11.18) | F = 7.99; p = 0.006± |
F = 8.08; p = 0.006± |
||
| Ultrabrief | 15.26 (6.86) | 15.39 (8.07) |
±Analyses of covariance (ANCOVA) significant after Holm’s correction for multiple comparisons
1ANCOVA controlling for score at pre-ECT3
2ANCOVA controlling for score at pre-ECT6
Discussion
Mood Outcomes
This study tested the proposition that RUL ECT given with an ultrabrief pulse width delivers better overall outcomes than RUL ECT given with a brief pulse width, in terms of lesser cognitive side effects but comparable efficacy. To date, three randomized controlled trials have compared ultrabrief- and brief-pulse RUL ECT at the same dose relative to seizure threshold (Sackeim et al., 2008; Mayur et al., 2013; Spaans, Verwijk, et al., 2013). The largest study (Spaans, Verwijk, et al., 2013) found slightly lesser efficacy in the ultrabrief pulse group, whereas the two smaller studies (Sackeim et al., 2008; Mayur et al., 2013) found no significant difference. Possible explanations are that inadequate power in the smaller studies accounted for the lack of significant differences found, or that differences in study sample characteristics may explain apparent discrepancies between findings. For example, participants who are highly ECT responsive (older age, psychotic depression; Loo et al., 2011; Spaans, Koh, et al., 2013) are likely to respond well to either ultrabrief- or brief-pulse RUL ECT, with no difference in outcomes, whereas differences in treatment outcomes may be more evident in less ECT-responsive participants. Data from a prospective non-randomized study (Loo et al., 2008), naturalistic reports (Niemantsverdriet et al., 2011; Loo et al., 2012), and a large clinical sample (Galletly et al., 2013) also suggest slightly reduced efficacy for ultrabrief-pulse RUL ECT, even when it was given at a slightly higher dosage compared to brief-pulse RUL ECT. Finally, a formal speed-of-response analysis suggested that more treatments are required to attain response when an ultrabrief pulse was used in RUL ECT (Loo et al., 2013).
The current study used a different dosing approach for the two treatments, to test if the cognitive advantage for ultrabrief-pulse RUL ECT reported in earlier studies would still be demonstrated when it was given at a dose level likely to achieve comparable efficacy to brief-pulse RUL ECT: i.e., a proof-of-concept test of whether overall efficacy/cognitive effects outcomes are superior. The higher dosage relative to seizure threshold (eight times versus five times) did indeed lead to comparable efficacy between the groups, with no difference found in mean changes in depression scores, proportion of responders and remitters, or speed of response (number of ECT treatments required, comparison of depression scores at time points across the ECT course).
Overall, response and remission rates are lower than those reported in other randomized controlled trials of ultrabrief RUL ECT (Sackeim et al., 2008; Mayur et al., 2013; Spaans, Verwijk, et al., 2013). Several factors may explain this. The sample in this study may be less ECT responsive. For example, a lower percentage of this sample had psychotic depression, compared with the Sackeim and Spaans studies (Sackeim et al., 2008; Spaans, Verwijk, et al., 2013). Alternatively, the decision to end the ECT course based on clinical judgement rather than predetermined remission criteria (as in the Sackeim and Spaans studies) may have led to premature termination of ECT for some participants (Prudic et al., 2004). It is possible that the overall lower response and remission rates may have masked any efficacy differences between the treatment groups.
The follow-up data on mood outcomes should be interpreted with caution, given the attrition in sample numbers and presence of naturalistic clinical care over the follow-up period. Nevertheless, the data provide preliminary support for meaningful clinical improvement with ultrabrief-pulse RUL ECT that outlasts the immediate treatment period.
Cognitive Outcomes
Better memory functioning was demonstrated in the ultrabrief group. This was evident in testing of acute post-ECT effects at sessions three and six. The ultrabrief group was significantly better at recall of words and recognition of faces learnt prior to ECT, and faster in attaining reorientation after ECT.
Of all the domains of cognitive functioning tested in the main cognitive battery (orientation, processing speed, fronto-executive function, verbal and visual anterograde memory, retrograde autobiographical memory) the ultrabrief group had superior outcomes only in orientation and retrograde autobiographical memory. This is, however, an important finding, as loss of past personal memories can be persistent and often is the side effect of most concern to patients (Sackeim et al., 2007). It should be noted that the ultrabrief group had a slightly higher premorbid IQ at study outset, which may have reduced their susceptibility to cognitive impairment with ECT (Sackeim et al., 2007). The analyses accounted for this baseline difference, but it is nevertheless an important caveat in the interpretation of cognitive results.
Overall, this study supports prior reports that ultrabrief-pulse ECT is associated with better cognitive outcomes (Loo et al., 2008; Sackeim et al., 2008), though increasing the dosage of ultrabrief-pulse RUL ECT relative to brief-pulse RUL ECT diminished its overall cognitive advantage, as reflected in the lack of significant differences in the majority of cognitive outcomes, in contrast to earlier reports, which also found superior outcomes in global cognitive performance and anterograde memory function (Loo et al., 2008; Sackeim et al., 2008).
Seizure Threshold
Seizure threshold increased by 57% in the ultrabrief group and by 19% in the brief pulse group over the first six treatments. The difference may be related to the different dosage levels used for treatment (i.e., treating at a higher relative suprathreshold dose, eight times versus five times, may lead to a more rapid rise in seizure threshold). Importantly, this finding suggests that ECT practitioners may need to consider dose increases across a course of ultrabrief-pulse RUL ECT in order to maintain sufficiently suprathreshold dosing, as this study demonstrates that substantial increases in seizure threshold may occur.
Limitations and Strengths
A limitation of the study is that electrode placement, concurrent medications, and number of ECT treatments were determined clinically rather than by operationalized criteria. Although the statistical analyses controlled for these factors, it is possible that this may have biased study results. Loss to follow-up after the acute treatment period limits the study’s capacity to address potential differences in durability of benefit and in long-term cognitive effects. Strengths of the study include the relatively large sample size and the detailed cognitive testing.
Overall, this study supports the hypothesis that, when given at adequate dosing, ultrabrief-pulse RUL ECT may have comparable efficacy to brief-pulse RUL ECT, but with lesser cognitive side effects.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
All authors have no interests to declare with respect to any organization whose interests may be affected by publication of the paper. This study was funded by the Australian National Health and Medical Research Council (NHMRC).
Supplementary Material
Acknowledgments
The authors would like to thank Muireann Irish, Michelle Mahon, Joshua Garfield, Dr Jenny McGoldrick, Dr Nicholas Ingram, and clinical staff of ECT services at the Melbourne Clinic and Wesley hospitals. This study was supported by Australian National Health and Medical Research Council (NHMRC) Project Grant no. 568678, awarded to Drs Loo and Schweitzer. Clinical Trials Registration: www.clinicaltrials.gov, NCT00870805.
References
- Aickin M, Gensler H. (1996). Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health 86:726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. (1998). Hopkins Verbal Learning Test revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 12: 43–55. [Google Scholar]
- Dubovsky S, Buzan R, THomas M, Kassner C, Cullum C. (2004). Nicardipine improves the antidepressant action of ECT but does not improve cognition. J ECT 17:3–10. [DOI] [PubMed] [Google Scholar]
- Enders CK. (2011). Missing not at random models for latent growth curve analyses. Psychol Methods 16:1–16. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Wooderson SC, Markopoulou K, Cleare AJ. (2009). The Maudsley Staging Method for treatment-resistant depression: prediction of longer-term outcome and persistence of symptoms. J Clin Psychiatry 70:952–957. [DOI] [PubMed] [Google Scholar]
- Galletly C, Clarke P, Paterson T, Rigby A, Gill S. (2013). Practical considerations in the use of ultrabrief ECT in clinical practice. J ECT 30:10–14. [DOI] [PubMed] [Google Scholar]
- Lerer B, Shapira B, Calev A, Tubi N, Drexler H, Kindler S, Lidsky D, Schwartz JE. (1995). Antidepressant and cognitive effects of twice- versus three-times-weekly ECT. Am J Psych 152: 564–570. [DOI] [PubMed] [Google Scholar]
- Loo CK, Sainsbury K, Sheehan P, Lyndon B. (2008). A comparison of RUL ultrabrief pulse (0.3ms) ECT and standard RUL ECT. Int J Neuropsychop 11:883–890. [DOI] [PubMed] [Google Scholar]
- Loo CK, Mahon M, Katalinic N, Lyndon B, Hadzi-Pavlovic D. (2011). Predictors of response to ultrabrief right unilateral electroconvulsive therapy. J Affect Disord 130:192–197. [DOI] [PubMed] [Google Scholar]
- Loo CK, Katalinic N, Martin D, Schweitzer I. (2012). A review of ultrabrief pulse width electroconvulsive therapy. Ther Adv Chronic Dis 3:69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CK, Garfield JB, Katalinic N, Schweitzer I, Hadzi-Pavlovic D. (2013). Speed of response in ultrabrief and brief pulse width right unilateral ECT. Int J Neuropsychop 16:755–761. [DOI] [PubMed] [Google Scholar]
- Mayur P, Byth K, Harris A. (2013). Acute antidepressant effects of right unilateral ultra-brief ECT: a double-blind randomised controlled trial. J Affect Disord 149:426–429. [DOI] [PubMed] [Google Scholar]
- McCall WV, Farah BA, Reboussin D, Colenda CC. (1995). Comparison of the efficacy of titrated, moderate dose and fixed, high dose RUL ECT in the elderly. Am J Geriat Psychiatry 3:317–324. [DOI] [PubMed] [Google Scholar]
- McCall W, Dunn A, Rosenquist P, Hughes D. (2002). Markedly suprathreshold right unilateral ECT versus minimally suprathreshold bilateral ECT: antidepressant and memory effects. J ECT 18(3):126–129. [DOI] [PubMed] [Google Scholar]
- McElhiney M, Moody B, Sackeim H. (2001). The Autobiographical Memory Interview–Short Form, Version 3. New York: New York State Psychiatric Institute. [Google Scholar]
- Meador KJ, Moore EE, Nichols ME, Abney OL, Taylor HS, Zamrini EY, Loring DW. (1993). The role of cholinergic systems in visuospatial processing and memory. J Clin Exp Neuropsychol 15:832–842. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. (1979). A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Muthén B, Asparouhov T, Hunter AM, Leuchter AF. (2011). Growth modeling with nonignorable dropout: alternative analyses of the STAR*D antidepressant trial. Psychol Methods 16:17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO, eds (1998). MPlus User’s Guide, Seventh Edition Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Neylan TC, Canick JD, Hall SE, Reus VI, Sapolsky RM, Wolkowitz OM. (2001). Cortisol levels predict cognitive impairment induced by electroconvulsive therapy. Biol Psychiatry 50:331–336. [DOI] [PubMed] [Google Scholar]
- Niemantsverdriet L, Birkenhager TK, van den Broek WW. (2011). The efficacy of ultrabrief-pulse (0.25 millisecond) versus brief-pulse (0.50 millisecond) bilateral electroconvulsive therapy in major depression. J ECT 27:55–58. [DOI] [PubMed] [Google Scholar]
- Pisvejc J, Hyrman V, Sikora J, Berankova A, Kobeda B, Auerova M, Sochorova V. (1998). A comparison of brief and ultrabrief pulse stimuli in unilateral ECT. J ECT 14:68–75. [PubMed] [Google Scholar]
- Prudic J, Olfson M, Marcus SC, Fuller RB, Sackeim HA. (2004). Effectiveness of electroconvulsive therapy in community settings. Biol Psychiatry 55:301–312. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. (2007). The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology 32:244–254. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, Berman RM, Brakemeier EL, Perera T, Devanand DP. (2008). Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul 1:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33 [PubMed] [Google Scholar]
- Smith A. (1991). Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Spaans HP, Koh K, Verwijk R, Kok RM, Stek ML. (2013). Efficacy of ultrabrief pulse electroconvulsive therapy for depression: A systematic review. J Affect Disord 150:720–726. [DOI] [PubMed] [Google Scholar]
- Spaans HP, Verwijk E, Comijs HC, Kok RM, Sienaert P, Bouckaert F, Fannes K, Vandepoel K, Scherder EJ, Stek ML, Kho KH. (2013). Efficacy and cognitive side effects after brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy for major depression: a randomized, double-blind, controlled study. J Clin Psychiatry 74:e1029–1036. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman E, Spreen O. (2006). A compendium of neuropsychological tests: administration, norms, and commentary. Oxford, UK: Oxford Univerisity Press. [Google Scholar]
- Wechsler D. (2001). Wechsler Test of Adult Readng (WTAR). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Woodcock R, Johnson M. (1989). Woodcock-Johnson Psycho-Educational Battery, Revised. Chicago, IL: Riverside. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.