Abstract
Background:
Binge drinking is prevalent during adolescence and may have effects on the adult brain and behavior. The present study investigated whether adolescent intermittent ethanol exposure alters adult risky choice and prefrontal dopaminergic and forebrain cholinergic neuronal marker levels in male Wistar rats.
Methods:
Adolescent (postnatal day 28–53) rats were administered 5g/kg of 25% (vol/vol) ethanol 3 times/d in a 2-days–on/2-days–off exposure pattern. In adulthood, risky choice was assessed in the probability discounting task with descending and ascending series of large reward probabilities and after acute ethanol challenge. Immunohistochemical analyses assessed tyrosine hydroxylase, a marker of dopamine and norepinephrine in the prelimbic and infralimbic cortices, and choline acetyltransferase, a marker of cholinergic neurons, in the basal forebrain.
Results:
All of the rats preferred the large reward when it was delivered with high probability. When the large reward became unlikely, control rats preferred the smaller, safe reward, whereas adolescent intermittent ethanol-exposed rats continued to prefer the risky alternative. Acute ethanol had no effect on risky choice in either group of rats. Tyrosine hydroxylase (prelimbic cortex only) and choline acetyltransferase immunoreactivity levels were decreased in adolescent intermittent ethanol-exposed rats compared with controls. Risky choice was negatively correlated with choline acetyltransferase, implicating decreased forebrain cholinergic activity in risky choice.
Conclusions:
The decreases in tyrosine hydroxylase and choline acetyltransferase immunoreactivity suggest that adolescent intermittent ethanol exposure has enduring neural effects that may lead to altered adult behaviors, such as increased risky decision making. In humans, increased risky decision making could lead to maladaptive, potentially harmful consequences.
Keywords: adolescence, cholinergic neurons, dopaminergic neurons, ethanol, probability discounting
Introduction
Heavy alcohol exposure is common among adolescents. Nearly one-quarter of American 12th grade students report consuming more than 5 drinks in a row at least once in the preceding 2 weeks (Johnston et al., 2013). Adolescence is a time of extensive neurodevelopment, with continuing maturation throughout the brain, including frontal cortical regions that mediate higher order cognitive and executive functions, impulsivity, and risky behavior (Gogtay et al., 2004; Ernst et al., 2005; Casey et al., 2008; Steinberg, 2008; Jacobus et al., 2013). Heavy alcohol exposure during adolescence may damage the developing brain and alter the assessment of risks and rewards, leading to increased impulsivity (White et al., 2011) and risky decision making (Goudriaan et al., 2007; Cantrell et al., 2008; Xiao et al., 2009; Gullo and Stieger, 2011). Although such studies demonstrate a link between risky decision making and early alcohol use, they cannot determine causality. That is, a tendency toward risky decision making may predate or predispose an individual to heavy alcohol use during adolescence. Alternatively, excessive risky decision making may result from neuroadaptations engendered by adolescent alcohol exposure.
Risk-based decision making can be assessed in rodents using the probability discounting task (Cardinal and Howes, 2005; St. Onge and Floresco, 2009). In this discrete-trial choice procedure, rats choose between a small reward (a single food pellet) delivered with perfect certainty (ie, safe reward or choice) and a large reward (4 food pellets) that is increasingly less likely to be delivered as the session progresses (risky choice). Continued preference for the large reward when it is unlikely (risky choice) is maladaptive and irrational and results in fewer food pellets overall.
Limited work has investigated the effects of adolescent ethanol exposure on risky choice in adulthood in rodents. In a variant of the probability discounting task, adult rats given continuous unlimited access to ethanol-containing sweetened gelatin during adolescence exhibited increased preference for the large risky reward compared with control rats (Nasrallah et al., 2009). However, the reported greater preference for the large risky reward may not necessarily indicate increased risky choice. In this study, the rate that preference for the large risky reward changed as a function of its probability was similar in control and ethanol-exposed rats. That is, adolescent ethanol-exposed rats were biased to the large reward at all probabilities, whereas control rats were biased to the small reward at all large reward probabilities.
The present study investigated the long-term effects of adolescent intermittent ethanol (AIE) exposure on risky choice using the probability discounting task. Rats were exposed to AIE throughout adolescence (postnatal day [PND] 28–53). AIE exposures similar or identical to those used in the present study have had long-term effects on ethanol self-administration (Alaux-Cantin et al., 2013), brain pathology (Vetreno and Crews, 2012), impulsivity (Mejia-Toiber et al., 2014), and brain reward function (Boutros et al., 2014) in adulthood. Risky choice was assessed in adulthood under baseline conditions with both ascending and descending probability series to eliminate the potential effects of perseverative responding. Risky choice was also assessed in response to ethanol challenges.
Multiple neurotransmitter systems are involved in risky choice in the probability discounting task. Hyperactive dopaminergic activity in prefrontal brain areas and the nucleus accumbens accompanies increased risky choice (Cardinal and Howes, 2005; Floresco and Whelan, 2009; St. Onge and Floresco, 2009; Rokosik and Napier, 2012), and dopaminergic neurotransmission is altered after adolescent ethanol exposure (Badanich et al., 2007; Pascual et al., 2009; Philpot et al., 2009). Additionally, systemic manipulations that decrease cholinergic receptor activation increase risky choice (Mitchell et al., 2011; Ryan et al., 2013), while decreased cholinergic cell density has been reported in the basal forebrain after adolescent ethanol exposure (Coleman et al., 2011; Ehlers et al., 2011). Thus, in the present study, immunohistochemical analyses were performed to assess alterations in prefrontal dopaminergic and cholinergic neurons in the basal forebrain. These neuronal markers were correlated with risky choice.
Methods
Subjects
Timed-pregnant female Wistar rats (Charles River, Raleigh, NC) arrived in the vivarium on gestational day 13. Male pups were weaned on PND 21 and pair-housed in a humidity- and temperature-controlled vivarium on a 12-h/12-h reverse light/dark cycle. During behavioral training, the rats were food restricted and received 20g of rat chow per day approximately 1 hour after behavioral testing in addition to the food pellet rewards obtained during behavioral testing. All of the procedures were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council’s Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. A timeline of the experimental events, including AIE exposure, behavioral testing, and brain sample collection, is presented in Figure 1.
Figure 1.
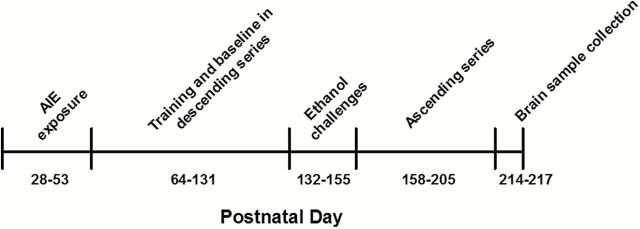
Timeline of experimental design showing the sequence of adolescent intermittent ethanol (AIE) exposure, training and testing in the probability discounting task, and brain sample collection across the lifespan of the rats. See text for details.
AIE Exposure
To ensure ethanol exposure during developmentally sensitive periods, rats were exposed to AIE throughout the adolescent period (PND 28–53), which has been broadly defined to start on PND 28 (Spear, 2000) and extend up to PND 60 in rats (Brenhouse and Andersen, 2011). Adolescent rats were administered 5g/kg of 25% (vol/vol) ethanol or an equivalent volume of water intragastrically via oral gavage 3 times/d at 8 am, 12 pm, and 4 pm in a 2-days–on/2-days–off pattern. In our previous work, this AIE exposure produced long-term effects on behavior in adulthood, including increased ethanol-induced reward enhancement and diminished reward deficits during ethanol withdrawal (Boutros et al., 2014) and increased impulsive choice during ethanol challenges (Mejia-Toiber et al., 2014). The rats were observed for behavioral intoxication signs before each ethanol administration. The doses were adjusted to maintain a high level of intoxication while avoiding administration of a lethal dose, as previously described (Majchrowicz, 1975; Morris et al., 2010; Boutros et al., 2014; see details in supplementary Materials). Blood samples (200 μL) were taken from the tip of the tail for the analysis of blood ethanol concentrations (BECs) 60 to 90 minutes after the final ethanol administration on the second binge day of binge days 2 (PND 33), 4 (PND 41), and 7 (PND 55). Blood samples were immediately centrifuged at 1500rpm for 15 minutes. Plasma was then extracted and stored at −80°C until further analysis. Plasma samples (5 μL) were analyzed using an Analox AM 1 analyzer (Analox Instruments LTD, Lunenberg, MA). This AIE exposure resulted in high mean BECs (291±21, 387±19, and 460±23mg/dL on binge days 2, 4, and 7, respectively; see supplementary Materials for details). The higher BECs after later binges may reflect changes in body weight and composition (ie, increased body fat) that occurred throughout maturation and may suggest changing ethanol metabolism throughout adolescence.
Apparatus
Testing was conducted in 12 identical standard 9-hole operant chambers (25.5×28.4×28.7cm) enclosed in sound-attenuating boxes (Med Associates, St. Albans, VT). Each chamber was equipped with a ventilator fan to provide air circulation and ambient low-level noise. The rear wall of each chamber was curved with 9 nosepoke holes. Every other response aperture was blocked, and only the center and 2 far-side nosepoke holes were operative. A photocell beam within each hole detected nosepoke responses. A magazine was connected to a food dispenser located on the opposite wall. All of the experimental events were recorded by an adjacent computer that ran Med-PC software.
Behavioral Procedures
Training in the probability discounting task consisted of several stages and was based on the procedure described by Cardinal and Howes (2005). Response training procedures are described in the supplementary Methods. Training commenced 10 days after the final ethanol/water administration to ensure that the signs of early ethanol withdrawal completely dissipated and animals had reached adulthood (PND 60).
Probability discounting (descending series)
Each session began in the intertrial interval (ITI) state with all cue lights and houselight turned off. After this 40-second ITI, the center nosepoke hole was illuminated. Failure to respond within 10 seconds (a response omission) initiated a 40-second ITI and restarted the same trial. Responses during the ITI were also recorded but had no scheduled consequences. A nosepoke response within 10 seconds turned off the cue light in the central nosepoke aperture and illuminated either one (forced trials) or both (choice trials) side nosepoke alternatives. One side was designated the large risky reward, and the other was designated the small safe reward alternative. Left and right nosepoke apertures were counterbalanced across rats and remained unchanged throughout the experiment.
Each session consisted of 5 blocks that differed in the probability that the large reward (4 pellets) would be delivered. The large reward probabilities were 1.0, 0.5, 0.25, 0.125, and 0.0625 across successive blocks within a session. The small reward (1 food pellet) probability was always 1.0. Sixteen forced trials were conducted at the beginning of each block. During the forced trials, only 1 side aperture was illuminated after a central nosepoke response. Of the 16 forced trials, 8 included presentations of the large risky alternative, and 8 included presentations of the small safe alternative, presented pseudorandomly with no more than 2 presentations of the same alternative occurring in a row. The 16 forced trials were followed by 10 choice trials in which the rats were presented with both side alternatives, and a response to either of these resulted in the consequence specific to that alternative, either one food pellet with 100% certainty or 4 food pellets according to the probability that was active in that block.
Each session lasted until all of the trials were completed (130 trials) or 180 minutes elapsed, whichever occurred first. The sessions could extend beyond 130 trials, because a response omission at either the orienting response or choice phase restarted that trial and did not advance the session. The rats were tested in the probability discounting descending probability series 5d/wk until preference in all free choice blocks was stable (<10% variability in the proportion of large reward selections across the last 5 consecutive sessions). In total, 36 sessions were required for choice in all blocks to reach stability in the descending probabilities series.
Ethanol challenges
The rats were tested in the descending probability series after tap water or ethanol administration (1, 2, and 3g/kg of 25% vol/vol ethanol administered via oral gavage 5 minutes before testing) according to a within-subjects Latin-square experimental design. After testing in the probability discounting procedure, tail-tip blood was taken to analyze BECs 180 minutes post ethanol. The ethanol challenge sessions were conducted once per week, with testing continuing in an ethanol-free state in sessions between each ethanol challenge.
Probability discounting (ascending series)
The rats began testing in the ascending probability series 1 week after completing the ethanol challenges to ensure that the effects of ethanol challenges were dissipated. The probability of large reward delivery increased across trial blocks according to the sequence 0.0625, 0.125, 0.25, 0.5, and 1.0. For each rat, the side position of the large risky and small certain reward alternatives remained unchanged from the descending probability series. In total, 30 sessions were required for preference in all blocks to reach stability in the ascending series.
Perfusion, Brain Tissue Preparation, Immunohistochemistry, and Quantification
After completing the experiments, the rats were left undisturbed for 1 week to ensure measurement of the long-term effects of AIE exposure on brain neurochemistry without potential confounds of continuous behavioral testing on the brain. All of the rats were humanely euthanized between PND 214 and PND 217. The rats were first anesthetized with sodium pentobarbital (100mg/kg, intraperitoneal). When nonresponsive, the rat was transcardially perfused first with ice-cold phosphate-buffered saline (pH 7.4) and then with ice-cold 4% paraformaldehyde in phosphate-buffered saline. The brains were post fixed in 4% paraformaldehyde for 24 hours at 4°C as previously described (Ehlers et al., 2011). The brains were sliced coronally on a vibratome into 40-µm thick sections after cryoprotection with 30% sucrose. Every 12th section was used for each of the following antigens. For choline acetyltransferase (ChAT) staining, free-floating sections were incubated in mouse anti-ChAT monoclonal antibody (1:200; MAB 305, Millipore, Temecula, CA) for 2 hours at room temperature and then for 16 hours at 4°C. For TH staining, the sections were incubated in rabbit anti-TH polyclonal antibody (1:500; AB152, Millipore) overnight at 4°C. On the second day, the sections were incubated with biotinylated horse anti-mouse (ChAT) or goat anti-rabbit (TH) secondary antibody (1:200; Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. Subsequently, avidin-biotin complex (Vector ABC kit, Vector Laboratories) was applied for 1 hour at room temperature, and ChAT- and TH-positive cells were visualized using nickel-enhanced diaminobenzidine reaction. The number of positive neurons was quantified using a modified stereological procedure for labeled cells within a specified region of interest (Crews et al., 2004). Bioquant Nova Advanced Image Analysis (R&M Biometric, Nashville, TN) was used for image capture and analysis. The images were captured using an Olympus BX50 Microscope and Sony DXC-390 video camera linked to a computer. For ChAT+ immunoreactivity (IR), ChAT-positive neurons were counted within the region of interest and are expressed as the number of cells per square millimeter. Ch1 and Ch2 are contained in the medial septal nucleus and the nucleus of the vertical limb of the diagonal band, respectively. Ch3 is mostly in the lateral portion of the horizontal limb nucleus of the diagonal band, and Ch4 is in the nucleus basalis and parts of diagonal band nuclei. For the Ch1 and Ch2 sectors, the coronal sections were 0.70 to 0.20mm from bregma. For the Ch3 and Ch4 sectors, the coronal sections were from 0.70 to −0.40mm (Figure S1). For TH+ IR, the prelimbic and infralimbic cortices (from 3.20 to 2.20mm from bregma) were outlined using anatomical markers, and the pixel density was measured from the outlined area (Figure S2). Both the left and right hemispheres of an individual brain subregion from each animal were counted, and the average value was used (Figures S1 and S2).
Statistical Analyses
For the preference data, the risky response probability in the free-choice trials was calculated as the following in each block: responses to risky alternative / (responses to risky alternative + responses to safe alternative). The 5-day means in each block for each rat were calculated under descending and ascending probability conditions. The win-stay/lose-shift response ratios were also calculated (supplementary Materials). One-sample t tests were used to compare the large reward probabilities obtained by each subject in each block with the arranged large reward probabilities. Additionally, the Pearson correlation coefficient was calculated to determine whether decision making in a block of choice trials correlated with the number of large rewards obtained in the preceding forced trials.
All of the group data were subjected to univariate analysis of variance (ANOVA) using SPSS 18 (SPSS, Chicago, IL). The level of significance was set to 0.05. Significant main and interaction effects were followed by simple-effects ANOVAs and t tests using a Šidák adjustment for multiple comparisons. For repeated-measures analyses, Mauchly’s test of sphericity of the covariance matrix was applied. When the sphericity assumption was violated, the degrees of freedom for any term that involved that factor were adjusted to more conservative values by applying the Huynh-Feldt correction. We report the uncorrected degrees of freedom.
For the immunohistochemical data, the levels of TH and ChAT in AIE-exposed and control rats were compared using between-subject t tests. To further explore the relationship between risky choice and markers of neural functioning, the Pearson correlation between the area under the curve (AUC) of the discounting function in the descending probability series and measures of TH and ChAT were computed. We used the AUC as a composite of preference across blocks instead of preference in any or all of the individual blocks to avoid an inflated Type I error rate and minimize the number of correlations. This approach resulted in the computation of 3 correlations: AUC and TH; AUC and ChAT 1,2; and AUC and ChAT 3,4. The AUC was calculated by summing the areas of each successive trapezoid created by interpolating a straight line from each data point on the probability discounting function to the origin of the graph.
Results
The ANOVAs of behavioral intoxication scores and BECs during AIE exposure are reported in the supplementary Results. To reach stable preference levels, control and AIE-exposed rats required 38.93±0.42 and 36.92±0.49 sessions, respectively, in the descending series and 25.33±0.99 sessions (control rats) and 24.54±1.21 sessions (AIE-exposed rats) in the ascending series, with no significant differences between the groups.
Probability discounting in descending and ascending probability conditions
In the forced trials for both AIE-exposed and control rats, the obtained reward probabilities did not differ from the arranged reward probabilities in any block (1.0, 0.5, 0.25, 0.125, and 0.0625) in either the descending or the ascending probability series (all P>.1). No correlation was found between the number of large rewards obtained in each block of forced trials and large reward choice in the subsequent block of choice trials (all Pearson correlation coefficients <0.20, all P>.05) indicating that, within each block, there was no effect of the number of large rewards obtained during the forced trials on choice performance in the subsequent choice trials.
In the choice trials, all of the rats, independent of AIE or water exposure, showed near-exclusive preference for the large reward when there was a high probability (1.0 or 0.5) that the large reward would be delivered. When the risky reward probability decreased throughout the session (Figure 2A), the ANOVAs indicated significant main effects of session block (F 4,108=93.44, P<.001) and AIE exposure (F 1,27=6.31, P<.05) and a significant session block × AIE exposure interaction (F 4,108=5.78, P<.01). As the large reward became less likely, control rats showed decreasing preference for the large reward. This pattern of results was less prevalent in AIE-exposed rats. The post hoc tests revealed that AIE-exposed rats showed significantly greater preference for the risky reward when the probability that the reward would be delivered was 0.25 (Block 3) and 0.0625 (Block 5) compared with controls. Identical results were obtained when the large reward probability increased throughout the session (Figure 2B). In the ascending probability series, the ANOVA revealed significant main effects of session block (F 4,104=69.80, P<.001) and AIE exposure (F 1,26=5.28, P<.05) and a significant session block × AIE exposure interaction (F 4,104=3.92, P<.05). The post hoc tests revealed that AIE-exposed rats showed a significantly (P<.05) greater preference for the risky reward when the reward was delivered with low probability (0.125 in Block 2 and 0.0625 in Block 1) compared with controls (Figure 2B). These findings suggest that AIE increased risky choices into adulthood long after the last exposure to ethanol.
Figure 2.
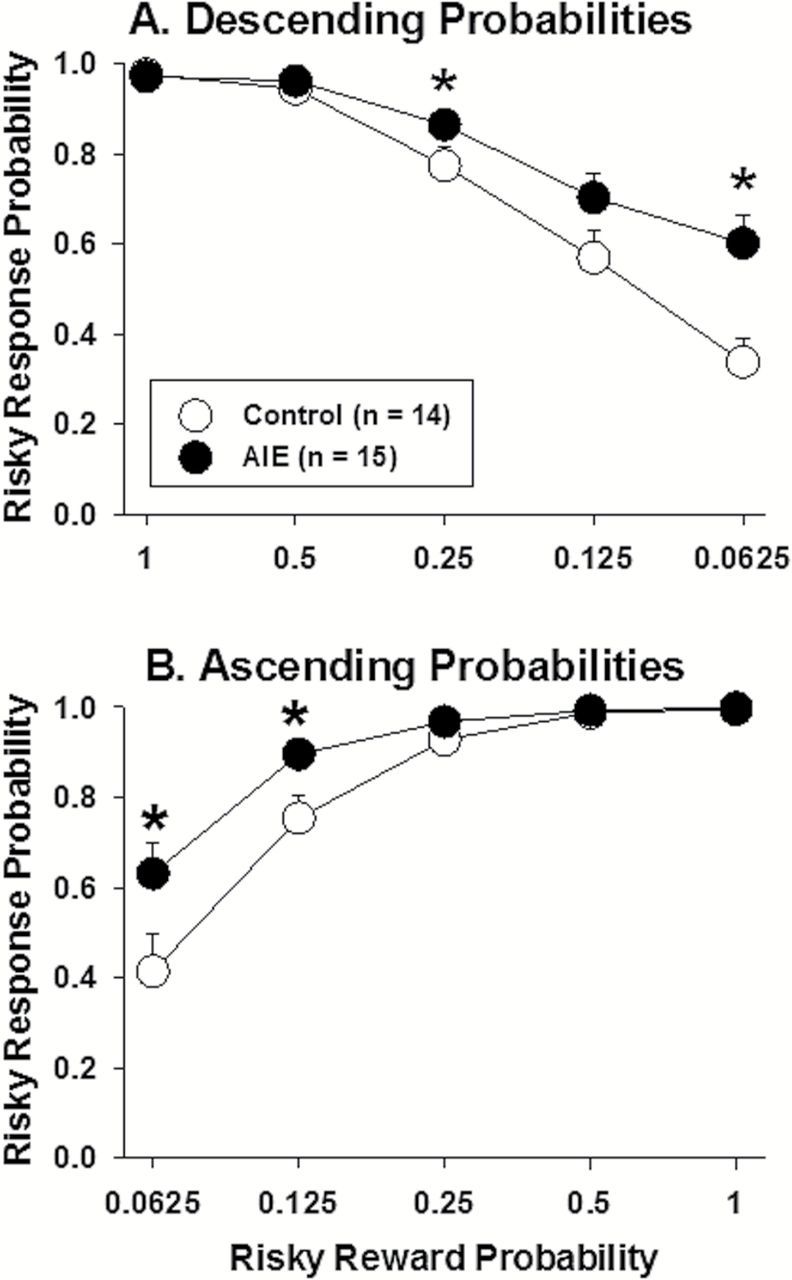
Risky response probability as a function of risky reward probability across blocks of trials in the descending (A) and ascending (B) probability conditions. The data are expressed as the mean ± SEM of the last 5 baseline sessions in each condition.
Responding during the ITI did not differ between AIE-exposed and control rats during either descending or ascending series (see supplementary Results). Win-stay and lose-shift response strategies also did not differ between AIE-exposed and control rats (see supplementary Results).
Ethanol challenges
To investigate how ethanol affected adult responses, acute ethanol challenges were conducted. No effect of ethanol challenges on risky choice was found in either control (Figure 3A) or AIE-exposed (Figure 3B) rats. The ANOVA revealed a significant main effect of session block (F 4,80=66.59, P<.001) and a significant session block × AIE interaction (F 4,80=2.86, P<.05) but no effect of ethanol dose, indicating that the group differences apparent in the baseline data (Figure 2A) were maintained with no effect of acute ethanol administration on probability discounting. Ethanol impaired performance on this task, independent of AIE exposure, reflected by a significant main effect of ethanol dose on response omissions (F 3,78=8.68, P<.001; data not shown) but no effect of AIE exposure or interactions. The post hoc tests revealed more response omissions after the highest ethanol dose of 3g/kg (43±7 omissions) compared with 1g/kg (22±6 omissions) and vehicle (16±5 omissions) in all rats. Control rats had higher BECs than AIE-exposed rats after 3g/kg ethanol only (see supplementary Materials for detailed analyses). Thus, acute ethanol challenge at any of the doses did not alter risky choice in either control rats or AIE-treated rats. Furthermore, the AIE-induced increase in risky choice was maintained in adults during acute ethanol challenges.
Figure 3.
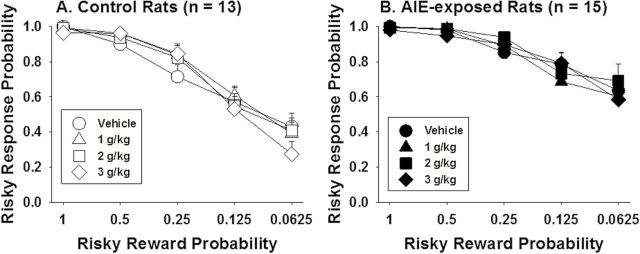
Risky response probability as a function of risky reward probability across blocks of trials after acute ethanol administration (g/kg via oral gavage) for control (A) and adolescent intermittent ethanol (AIE)-exposed (B) rats. The data are expressed as group means ± SEM.
Immunohistochemistry
To investigate changes in brain chemistry after AIE, markers of acetylcholine (ie, ChAT+ IR), dopamine, and norepinephrine (ie, TH+ IR) were determined. Some samples were lost during brain dissection and neurochemical analyses, resulting in the following sample sizes for each region included in the analyses: ChAT+ IR in cholinergic nuclei Ch1,2: control, n=10; AIE, n=12; ChAT+ IR in cholinergic nuclei Ch3,4: control, n=9, AIE; n=12; TH+ IR in the prelimbic cortex: control, n=13; AIE, n=14; TH+ IR in the infralimbic cortex: control, n=13; AIE, n=15.
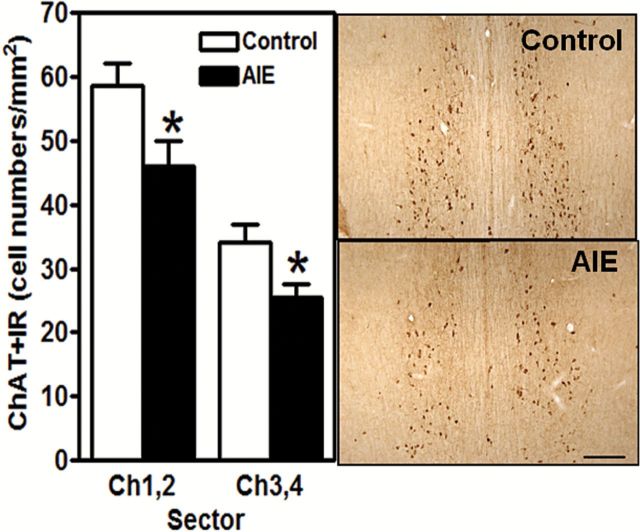
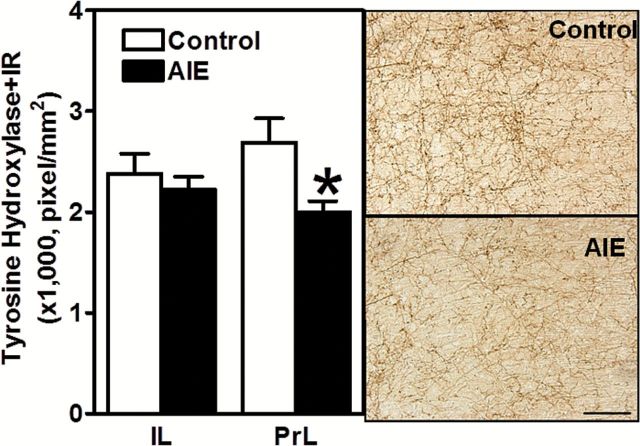
AIE-exposed rats exhibited significantly lower levels of ChAT+ IR in cholinergic nuclei Ch1,2 (medial septal nucleus and nucleus of the vertical limb of the diagonal band, respectively; t 20=2.39, P<.05) and Ch3,4 (lateral portion of the horizontal limb nucleus of the diagonal band and nucleus basalis and parts of diagonal band nuclei, respectively; t 19=2.51, P<.05) of the basal forebrain (Figure 4). AIE-exposed rats also showed significantly lower levels of TH+ IR in the prelimbic cortex (t 26=4.41, P<.05) but not in the infralimbic cortex (Figure 5). These findings demonstrate the long-term effects of AIE exposure on key neurotransmitters involved in risky choice.
Figure 4.
Choline acetyltransferase-positive immunoreactivity (ChAT+IR) in the forebrain is decreased in adult rats after adolescent intermittent ethanol (AIE) exposure. ChAT is the synthetic enzyme for acetylcholine and marks cholinergic neurons. Left, Quantification of ChAT+ IR cell density. Right, Representative photomicrograph of ChAT+ IR neurons in Ch1-Ch2 nuclei from adult control and AIE-exposed rats (Ch1-Ch2 from 0.70 to 0.20mm from bregma; Ch3-Ch4 from 0.7 to 0.30mm from bregma; Paxinos and Watson, 1998). Scale bar=100 μm. *P<.05 (Student’s t test). Control rats, n=9 to 10; AIE-exposed rats, n=12.
Figure 5.
Left, Pixel density of tyrosine hydroxylase (TH)-positive immunoreactivity (IR) is significantly decreased in the prelimbic but not infralimbic cortex after adolescent intermittent ethanol (AIE) exposure. Right, Representative photomicrograph of TH-positive IR neurons in the prelimbic cortex in adult control and AIE-exposed rats (from 3.2 to 2.2mm from bregma; Paxinos and Watson, 1998). Scale bar=50 µm. *P<.05 (Student’s t test). Control rats, n=13; AIE-exposed rats, n=14–15).
To investigate possible associations between the neurochemical alterations and probability discounting, correlational analyses were performed between histochemical staining and risky choice. For each individual rat, ChAT+ IR and TH+ IR levels were correlated with the AUC of the discounting functions generated during the descending probability series (Figure 2A). A significant correlation was found between AUC and Ch1,2 (r 20=−0.499, P<.05) (Figure 6A), and a nearly significant correlation was found between the AUC and Ch3,4 (r 19=−0.433, P=.05) (Figure 6B), suggesting that the loss of Chat+ IR may contribute to increased risky decision making. The correlations between the AUC and TH+ IR in the prelimbic cortex (r 27=−0.024, P=.91) and infralimbic cortex (r 26=−0.17, P=.41) were not significant (data not shown).
Figure 6.
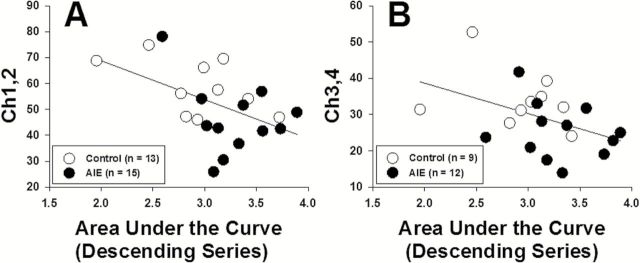
Correlations between choline acetyltransferase (ChAT) immunoreactivity cell counts and area under the curve (AUC) of the probability discounting function in the Ch1 and Ch2 nuclei (medial septal nucleus and nucleus of the vertical limb of the diagonal band) (A) and Ch3 and Ch4 nuclei (lateral portion of the horizontal limb nucleus of the diagonal band, nucleus basalis, and parts of diagonal band nuclei) (B) of the basal forebrain. The correlation between ChAT+ IR in the Ch1 and Ch2 nuclei and AUC (left) was significant (p<0.05), and the correlation between ChAT+ IR in the Ch3 and Ch4 nuclei (right) was nearly significant (p=0.05; see text for details).
Discussion
The results of the present study demonstrate that AIE exposure had long-term effects on risky decision making and brain neurochemistry. In the probability discounting task, adult rats with a history of AIE exposure showed increased risky choice compared with controls. Increased risky choice was observed in both the descending probability series, when the large reward probability decreased throughout the session, and the ascending probability series, when the large reward probability increased throughout the session. Additionally, compared with controls, AIE-exposed rats had decreased levels of TH+ IR in the prelimbic cortex and decreased levels of ChAT+ IR in the basal forebrain. Moreover, the area under the discounting curve was negatively correlated with ChAT+ IR in the basal forebrain, suggesting that the loss of ChAT+ IR may contribute to increased risky decision making.
The increased risky choice after AIE exposure that was first observed when the large reward probability decreased within each session (descending probability series) may indicate that AIE-exposed rats were risk-prone relative to control rats. During task acquisition, AIE-exposed and control rats required an equal number of sessions to reach criterion performance in both the ascending and descending series, eliminating a general learning deficit as a cause of the increased preference for the large risky reward in AIE-exposed rats. The increased risky choice may indicate that AIE-exposed rats were exhibiting a perseverative behavioral profile and were incapable of switching preference to the small safe reward when the large reward became unlikely within the same session. Previous studies demonstrated that intermittent ethanol exposure during adolescence (Coleman et al., 2011; Vetreno and Crews, 2012) or adulthood (Badanich et al., 2011) had no effect on task acquisition but resulted in reversal learning deficits in both spatial and food-motivated tasks. However, the increase in risky choice observed after AIE exposure in the present study cannot be attributed to deficits in reversal learning or behavioral inflexibility, because AIE-induced increases in risky choice were observed in both the descending and ascending probability series of trials. The ascending probabilities series demonstrates that the AIE-exposed rats were capable of changing preference for the large reward and showed no deficits in reversal learning or cognitive flexibility in the probability discounting task. In contrast, chronic intermittent ethanol exposure during adulthood resulted in performance deficits in an operant set-shifting task that specifically assesses reversal learning and behavioral flexibility (Trantham-Davidson et al., 2014). These findings suggest that the effects of AIE exposure on reversal learning and cognitive flexibility may be revealed in specific behavioral tasks.
There are a few limitations in the present work. The present study cannot determine whether the increased preference for the large risky reward was due to deficits in decision making, deficits in the assessment of low reward probabilities, or some combination of the two. In addition, it is not clear whether AIE-induced increases in risky choice are age specific, because, to our knowledge, the effects of intermittent binge ethanol exposure during adulthood on risky decision making have not been investigated.
Our findings of increased risky choice in adulthood after experimenter-administered ethanol during adolescence extend earlier findings by Nasrallah and colleagues (2009, 2011) showing that access to ethanol-containing gelatin during adolescence increased risky choice in adulthood in a similar probability discounting task. Both the earlier work and present study showed increased risky choice after adolescent ethanol exposure. However, there are differences between the present study and previously published work, which, although small, have important implications for interpretation of the data. Specifically, in the present study, both control and AIE-exposed rats showed near-exclusive preference for the large reward when there was a high probability that it would be delivered, and differences only emerged when the large reward became unlikely. In contrast, in the previous work (Nasrallah et al., 2009, 2011), control rats were consistently risk averse (ie, always preferring the small certain reward even when it had a lower overall expected payoff), whereas adolescent ethanol-exposed rats were consistently risk prone (ie, always preferring the large risky reward even when it had the lower overall expected payoff). The degree to which preference changed as the large reward probability changed was similar in control and adolescent ethanol-exposed rats in the earlier studies (Nasrallah et al., 2009, 2011). This pattern of results suggests that the rates of probability discounting were similar across the 2 groups, although bias to the large reward may have differed. In contrast, our findings showed group differences only when the large reward was unlikely, providing strong evidence that AIE exposure alters the rate of probability discounting without altering bias for the larger reward. Considering that the large reward probability changed throughout the session in the present study while the large reward probability varied across sessions in the earlier studies (Nasrallah et al., 2009, 2011), the discrepancies in findings may be attributed to methodological differences between studies.
Whereas intermittent ethanol exposure throughout adolescence increased risky choice in adulthood, acute ethanol challenges during adulthood had no effect on risky choice. Regardless of AIE exposure, risky choice in adult rats was unaltered by acute ethanol challenges. Similar to our findings, no effect of acute ethanol on risky choice has been reported in rats without a history of previous ethanol exposure (Mitchell et al., 2011) or in healthy humans (Richards et al., 1999; Balodis et al., 2006). Interestingly, in the delay discounting task that assesses impulsive choice, there was no effect of ethanol exposure either during adolescence or adulthood on baseline impulsive choice, whereas acute ethanol challenges increased impulsive choice in adolescent-exposed rats (Mejia-Toiber et al., 2014). These opposing results suggest that impulsive choice and risky choice may be mediated by different neurobiological mechanisms.
The present work investigated several markers to determine neurochemical differences that may have contributed to the increased risky choice in AIE-exposed rats. Adult AIE-exposed rats (PND 215) had reduced levels of ChAT+ IR, a marker of cholinergic neurons, in the basal forebrain, including the medial septal nucleus, vertical limb nucleus, and lateral portion of the horizontal limb nucleus of the diagonal band, and nucleus basalis. Similarly, reduced ChAT+ IR has been reported in young adult rats (PND 72) exposed to ethanol vapor during adolescence (Ehlers et al., 2011) and young adult mice (PND 88) exposed to intragastric ethanol during adolescence (Coleman et al., 2011). Altogether, these findings indicate long-term decreases in cholinergic function after AIE exposure that is independent of the route of ethanol administration during adolescence (intragastric vs vapor) or species (rats vs mice).
The basal forebrain, through widespread projections to the cerebral cortex, may be involved in learning and memory, executive function, and impulsivity (Sarter and Paolone, 2011). In adult rats, reduced basal forebrain cholinergic neuronal density after prolonged ethanol exposure has been associated with cognitive and behavioral deficits (Arendt et al., 1987, 1988; Hodges et al., 1991; Floyd et al., 1997; Savage et al., 2000). In the present study, AIE-induced decreases in ChAT levels were correlated with increased risky choice, suggesting that the basal forebrain may be involved in the regulation of risky decision making. Similar to our findings, decreased ChAT+ IR after AIE exposure was associated with reversal learning deficits in mice (Coleman et al., 2011) and behavioral disinhibition in rats (Ehlers et al., 2011). Interestingly, behavioral disinhibition is characteristic of human and rodent adolescence and has been suggested to contribute to the increased risky behavior seen during adolescence (Steinberg, 2008, 2010). Pharmacological studies showing decreased risky choice after activation of cholinergic receptors with nicotine further support a role for cholinergic neurotransmission in risky decision making (Mitchell et al., 2011; Ryan et al., 2013; but see Mendez et al., 2012).
Findings indicate that dopamine transmission in prefrontal cortical areas, including the infralimbic and prelimbic cortices, may be involved in decision making in the probability discounting task (St. Onge and Floresco, 2009; St. Onge et al., 2010). In the present study, AIE-exposed rats had reduced levels of TH+ IR in the prelimbic but not infralimbic cortex compared with control rats. Decreased TH+ IR in the prelimbic cortex in AIE-exposed rats may suggest decreased dopamine or norepinephrine transmission. However, TH levels in either the prelimbic or infralimbic cortex did not correlate with increased risky choice. Previous research in the probability discounting task found that reversible inactivation of the prelimbic medial frontal cortex caused preference for the large reward to remain unchanged even as the large reward probability decreased in both ascending and descending probability conditions, implicating the prelimbic cortex in the reappraisal of changing reward probabilities (St. Onge and Floresco, 2010). Studies with central administration of receptor-specific dopamine agonists or antagonists into the medial prefrontal cortex showed that changes in preference for the large reward depend on dopamine receptor subtype, suggesting a complex mechanism for the effects of prefrontal dopamine on performance in the probability discounting task (St. Onge et al., 2011).
In summary, our findings provided strong evidence that exposure to intermittent ethanol binges throughout adolescence led to increased risky decision making in adulthood. Immunohistochemical analyses demonstrated the long-term effects of AIE exposure on the brain. Specifically, decreased TH+ IR in the prelimbic cortex and decreased ChAT+ IR in the basal forebrain were observed in adult AIE-exposed rats. Decreased ChAT levels were negatively correlated with increased risky choice, implicating decreased cholinergic function in risky decision making. These results suggest that binge-like ethanol exposure during adolescence may interfere with the development of dopaminergic and cholinergic neurotransmission in ways that lead to persistent deficits in risky behavior, creating a risk proneness in adulthood that resembles adolescent-typical behavioral tendencies (Steinberg, 2008, 2010; Zoratto et al., 2013). Such a prolongation of adolescent-typical risk proneness may extend the period of heightened vulnerability to the negative consequences likely to follow risky behaviors (Casey et al., 2008).
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
A.M. has received contract research support from Bristol-Myers Squibb Co. and Astra-Zeneca and consulting fees from Abbott GmbH&Co and Astra-Zeneca during the past 3 years. Other authors have nothing to report.
Supplementary Material
Acknowledgments
This work was supported by NIH grants U01-AA019970-Neurobiology of Adolescent Drinking in Adulthood (A.M.), U01-AA020022 (F.T.C.), and U01-AA020023 (F.T.C.).
The authors would like to thank members of the Markou and Crews research groups for assistance and input and the NADIA core for overall directorship and Michael Arends for excellent editorial assistance.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naasila M. (2013). Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology 67:521–531. [DOI] [PubMed] [Google Scholar]
- Arendt T, Marchbanks R, Gray JA. (1987). Effects of prolonged ethanol-consumption on cholinergic function in the basal forebrain and memory. Biochem Soc T 15:499–500. [Google Scholar]
- Arendt T, Hennig D, Gray JA, Marchbanks R. (1988). Loss of neurons in the rat basal forebrain cholinergic projection system after prolonged intake of ethanol. Brain Research Bull 21:563–569. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. (2007). Chronic ethanol exposure during adolescence increases basal dopamine in the nucleus accumbens septi during adulthood. Alcohol Clin Exp Res 31:895–900. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Becker HC, Woodward JJ. (2011). Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav Neurosci 125:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, MacDonald TK, Olmstead MC. (2006). Instructional cues modify performance on the Iowa Gambling Task. Brain Cognition 60:109–117. [DOI] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Markou A. (2014). Adolescent intermittent ethanol exposure diminishes anhedonia during ethanol withdrawal in adulthood. Eur Neuropsychopharm 24:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. (2011). Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev 35:1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell H, Finn PR, Rickert ME, Lucas J. (2008). Decision making in alcohol dependence: insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcohol Clin Exp Res 32:1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ. (2005). Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. (2008). The adolescent brain. Dev Rev 28:62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, He J, Lee J, Styner M, Crews FT. (2011). Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res 35:671–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Collins MA, Dlugos C, Littleton J, Wilkins L, Neafsey EL, Petney R, Snell LD, Tabakoff B, Noronha A. (2004). Alcohol-induced neurodegeneration: when, where and why? Alcohol Clin Exp Res 28:350–364. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT. (2011). Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience 199:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. (2005). Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med 36:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Whelan JM. (2009). Perturbations in different forms of cost/benefit decision making induced by repeated amphetamine exposure. Psychopharmacology 205:189–201. [DOI] [PubMed] [Google Scholar]
- Floyd EA, Young-Seigler AC, Ford BD, Reasor JD, Moore EL, Townsel JG, Rucker HK. (1997). Chronic ethanol ingestion produces cholinergic hypofunction in rat brain. Alcohol 14:93–98. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. P Natl Acad Sci USA 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. (2007). Decision making and binge drinking: a longitudinal study. Alcohol Clin Exp Res 31:928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullo MJ, Stieger AA. (2011). Anticipatory stress restores decision-making deficits in heavy drinkers by increasing sensitivity to losses. Drug Alcohol Depen 117:204–210. [DOI] [PubMed] [Google Scholar]
- Hodges H, Allen Y, Sinden J, Mitchell SN, Arendt T, Lantos PL, Gray JA. (1991). The effects of cholinergic drugs and cholinergic-rich foetal neural transplants on alcohol-induced deficits in radial maze performance in rats. Behav Brain Res 43:7–28. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. (2013). White matter integrity, substance use, and risk taking in adolescence. Psychol Addict Behav 27:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Backman JG, Schulenberg JE. (2013). Monitoring the future: national results on adolescent drug use: 2012. Overview of key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan. [Google Scholar]
- Majchrowicz E. (1975). Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia 43:245–254. [DOI] [PubMed] [Google Scholar]
- Mejia-Toiber J, Boutros N, Markou A, Semenova S. (2014). Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behav Brain Res 266:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Gilbert RJ, Bizon JL, Setlow B. (2012). Effects of acute administration of nicotinic and muscarinic cholinergic agonists and antagonists on performance in different cost-benefit decision making tasks in rats. Psychopharmacology 224:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B. (2011). Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology 218:703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. (2010). Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol 44:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TWH, Bernstein IL. (2009). Long-term risk preference and suboptimal decision making following adolescent alcohol use. P Natl Acad Sci USA 106:17600–17604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL. (2011). Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. P Natl Acad Sci USA 108:5466–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. (2009). Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem 108:920–931. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1998). The rat brain in stereotaxic coordinates. New York: Academic Press, Spiral Bound. [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL. (2009). Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int J Dev Neurosci 27:805–815. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. (1999). Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav 71:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokosik SL, Napier TC. (2012). Pramipexole-induced increased probabilistic discounting: comparison between a rodent model of Parkinson’s disease and controls. Neuropsychopharmacol 37:1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Dube SL, Potter AS. (2013). Rate dependent effects of acute nicotine on risk taking in young adults are not related to ADHD diagnosis. Pharmacol Biochem Beh 103:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Paolone G. (2011). Deficits in attentional control: cholinergic mechanisms and circuitry-based treatment approaches. Behav Neurosci 125:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Candon PM, Hohmann HL. (2000). Alcohol-induced brain pathology and behavioral dysfunction: using an animal model to examine sex differences. Alcohol Clin Exp Res 24:465–475. [PubMed] [Google Scholar]
- Spear LP. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Floresco SB. (2009). Dopaminergic modulation of risk-based decision making. Neuropsychopharmacol 34:681–697. [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Floresco SB. (2010). Prefrontal cortical contribution to risk-based decision making. Cereb Cortex 20:1816–1828. [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Chiu YC, Floresco SB. (2010). Differential effects of dopaminergic manipulations on risky choice. Psychopharmacology 211:209–221. [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Abhari H, Floresco SB. (2011). Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci 31:8625–8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2008). A social neuroscience perspective on adolescent risk-taking. Dev Rev 28:78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2010). A dual systems model of adolescent risk-taking. Dev Psychobio 52:216–224. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ. (2014). Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci 34:3706–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. (2012). Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and toll-like receptors in the adult prefrontal cortex. Neuroscience 226:475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Marmorstein NR, Crews FT, Bates ME, Mun EY, Loeber R. (2011). Associations between heavy drinking and changes in impulsive behavior among adolescent boys. Alcohol Clin Exp Res 35:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Grenard LJ, Stacy WA, Palmer P, Wei Y, Jia Y, Fu X, Johnson CA. (2009). Affective decision-making predictive of Chinese adolescent drinking behaviors. J I Neuropsych Soc 15:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoratto F, Laviola G, Adriani W. (2013). Gambling proneness in rats during the transition from adolescence to young adulthood: a home-cage method. Neuropharmacology 67:444–454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.