Abstract
Background:
N-methyl-d-aspartate receptor (NMDAR) dysfunction is thought to contribute to the pathophysiology of schizophrenia. Accordingly, NMDAR antagonists such as phencyclidine (PCP) are used widely in experimental animals to model cognitive impairment associated with this disorder. However, it is unclear whether PCP disrupts the structural integrity of brain areas relevant to the profile of cognitive impairment in schizophrenia.
Methods:
Here we used high-resolution magnetic resonance imaging and voxel-based morphometry to investigate structural alterations associated with sub-chronic PCP treatment in rats.
Results:
Sub-chronic exposure of rats to PCP (5mg/kg twice daily for 7 days) impaired sustained visual attention on a 5-choice serial reaction time task, notably when the attentional load was increased. In contrast, sub-chronic PCP had no significant effect on the attentional filtering of a pre-pulse auditory stimulus in an acoustic startle paradigm. Voxel-based morphometry revealed significantly reduced grey matter density bilaterally in the hippocampus, anterior cingulate cortex, ventral striatum, and amygdala. PCP-treated rats also exhibited reduced cortical thickness in the insular cortex.
Conclusions:
These findings demonstrate that sub-chronic NMDA receptor antagonism is sufficient to produce highly-localized morphological abnormalities in brain areas implicated in the pathogenesis of schizophrenia. Furthermore, PCP exposure resulted in dissociable impairments in attentional function.
Keywords: 5-CSRTT, anterior cingulate cortex, hippocampus, PCP, VBM
Introduction
Deficits in cognition are a hallmark of schizophrenia. The Measurement and Treatment Research to Improve Cognitive in Schizophrenia Initiative (MATRICS) and the CNTRICS group (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia) seek to further understand the nature of cognitive impairment in schizophrenia and identify new targets for treatment (Nuechterlein et al., 2004; Carter et al., 2008; Lustig et al., 2012). Both MATRICS and CNTRICS demonstrate the current focus on cognitive impairment associated with schizophrenia and highlight deficits in attention as a core feature of this disorder (Nuechterlein et al., 2008, 2009). Attentional impairments are resistant to treatment, precede episodes of psychosis, and may contribute to further, higher-order cognitive impairments (an der Heiden and Häfner, 2000; Riedel et al., 2006). There is, therefore, an important unmet need to understand the neural mechanisms that contribute to attentional impairment in schizophrenia.
Structural and functional brain abnormalities are commonly observed in patients with schizophrenia (Meyer-Lindenberg, 2010), including ventricular enlargement (Van Horn and McManus, 1992), reduced total brain volume (Wright et al., 2000), cortical thinning (Schultz et al., 2010), and decreased grey matter density (Honea et al., 2005). While the regional specificity of structural alterations in schizophrenia are heterogeneous (Honea et al., 2005), the more consistent morphological alterations include enlargement of the ventricles together with grey matter loss in amygdala, hippocampus, and parahippocampus gyrus (Shenton et al., 2001). In addition, schizophrenia patients show impaired attentional performance on continuous performance tests and reduced activation of the dorsolateral prefrontal cortex compared with unaffected controls (Chen and Faraone, 2001; Yoon et al., 2008).
Dysfunction of the glutamatergic system, specifically N-methyl-d-aspartate receptor (NMDAR) hypofunction, has been hypothesised to play a central role in schizophrenia pathogenesis (Olney et al., 1999; Kantrowitz and Javitt, 2010). This hypothesis was developed from the observation that NMDAR antagonists (e.g., ketamine and phencyclidine [PCP]) could mimic various aspects of disease symptomatology in humans (Cosgrove and Newell, 1991; Krystal et al., 1994) and exacerbate symptoms in treatment-stabilized schizophrenia patients (Lahti et al., 1995; Malhotra et al., 1997). In addition, chronic administration of ketamine in humans leads to structural brain abnormalities and reduces functional connectivity (Liao et al., 2010, 2011, 2012), while acutely administered ketamine impairs regional activation during cognitive and perceptual testing (Honey et al., 2005a; Deakin et al., 2008). Collectively, these studies suggest a causal contribution of NMDA-receptor blockade in mediating many of the morphological and functional alterations that underlie the pathophysiology of schizophrenia (Fusar-Poli et al., 2007; Ellison-Wright and Bullmore, 2010).
Several behavioral paradigms are commonly used to assess cognitive function in animal models of schizophrenia (Young et al., 2009). One widely-used task is the 5-choice serial reaction time task (5-CSRTT; Carli et al., 1983; Robbins, 2002), which was selected by the CNTRICS group as a task with high construct validity in the assessment of attentional impairment in schizophrenia (Lustig et al., 2012). The 5-CSRTT requires animals to actively detect and respond to brief visuo-spatial stimuli to obtain rewards, thus recruiting elements of sustained and divided attention. In contrast, assessment of sensorimotor gating (prepulse inhibition [PPI]) reflects a passive form of attention that enables filtering of irrelevant sensory information (Young et al., 2009). Both dimensions of attention are disrupted in schizophrenia patients, as well as in experimental animals treated with acute PCP (Swerdlow et al., 1994; Chen and Faraone, 2001; Geyer et al., 2001; Amitai and Markou, 2010).
Previous imaging studies in rats have assessed functional correlates of acutely administered NMDAR antagonists (Gozzi et al., 2007; Gozzi et al. 2008a, 2008b; Herdon et al., 2008; Schwarz et al., 2008; Hodkinson et al., 2012). However, repeated drug exposure is thought to more closely model cognitive deficits, negative symptoms, and neuropathological processes relevant to schizophrenia (Jentsch and Roth, 1999; Morris et al., 2005; Neill et al., 2010, 2013). The present study therefore used a well-validated sub-chronic dosing regimen to investigate the effects of PCP on passive (PPI) and active (5-CSRTT) forms of attention in the drug-free state. In addition, ex-vivo high-resolution microscopy was carried out with voxel-based morphometry to investigate perturbations in regional brain morphology and cortical thickness in PCP-treated animals.
Methods and Materials
Animals
Male hooded-Lister rats (n = 40; Charles River; weighing 350±10g at the start of the experiment) were housed in groups of four on a 12 hour reversed light cycle (lights on at 19:00h) in a temperature-controlled (21±2oC) and humidity-controlled (55±5%) environment. Behavioral testing took place during the dark cycle, under red lighting. Animals undergoing behavioral experiments had free access to food (Special Diet Services) and water until one week prior to the beginning of training, where food restriction reduced animal weights to 90% of their free-feeding body weight (approximately 16-18g rat chow/rat/day). Food restriction continued throughout training and testing, with water available ad libitum in the home cage. Experiments were conducted in accordance with the UK Animals (Scientific Procedures) 1986 Act and local university ethical guidelines.
Apparatus
Twelve 5-hole boxes (25cm x 25cm aluminium chambers), each enclosed within a wooden sound-attenuating cubicle (Med Associates) were used. The rear wall of each testing chamber was concavely curved and contained five response apertures. Each aperture was 2.5cm2, 4cm deep, and set 2cm above floor level. Located at the rear of each aperture was a yellow LED providing the visual stimulus. An infrared photocell beam fixed horizontally at the entrance of each aperture registered nose-poke responses. Located on the front wall of the test chamber was a food magazine that allowed retrieval of the food reward (45mg Rodent Pellet, Sandown Scientific). Interruption of an infrared photocell beam at the entrance to the food magazine reported the collection of food rewards and triggered the next trial. A wall-mounted fan provided ventilation and background noise. All twelve chambers were connected to a PC and data was acquired using the Whisker control system v2.5 (Cambridge Cognition; Cardinal and Aitken, 2010)
PPI of the acoustic startle was assessed in startle chambers (Kinder Scientific). Animals were placed in a confinement chamber, beneath which was placed an accelerometer to assess the magnitude of the startle response. Confinement chambers were contained within a sound-attenuating chamber, with startle stimulus and pre-pulse being delivered by a speaker fixed on the sound-attenuating chamber.
Behavioral Procedures
Rats were trained to detect and report the occurrence of a brief, 1 s visual stimulus presented pseudo-randomly in one of the five available apertures following an inter-trial interval (ITI; 5 s). The procedure was conducted with the house light on, as described previously (Bari et al., 2008). Correct detection of the visual stimulus within the limited hold (LH; time period following the stimulus during which animals can make a response, 5 s) was reported via a nose-poke in the illuminated aperture (correct response), resulting in the delivery of food reward. Reward collection initiated the next ITI, during which the animal was required to attend to the array of visual apertures and detect the presentation of the next visual stimulus. A failure to respond to the stimulus (error of omission) or responding to an aperture where the light was not presented (incorrect response) resulted in a 5 s time-out period, whereby the house light was extinguished and no food reward was delivered. A failure to wait for the stimulus presentation—i.e. a nose-poke during the ITI—was recorded as a premature response and initiated a time-out period. Repeat responses in any of the apertures (perseverative response), while recorded, were not punished. The session ended following 100 trials or 30min, whichever occurred first. In previous 5-CSRTT studies, response accuracy and percent correct responding have been used to describe the same measure. These two measures are distinguishable, as accuracy is calculated independently of omissions while percent correct responding is derived from correct, incorrect, and omitted trials. We have used this method of calculating accuracy and percent correct responding, as previously described (Amitai et al., 2007; Barnes et al., 2012). Manipulations of basic task parameters are an accepted method of increasing 5-CSRTT difficulty (Amitai and Markou, 2011). Sessions that included either a variable stimulus duration (1.0, 0.75, 0.5, 0.25 s) or a variable ITI (8, 6, 4, 2 s) were presented as acute attentional challenges. These were presented in a pseudorandom order with 25 trials for each variable level.
The acoustic startle reactivity paradigm (see Geyer and Swerdlow, 2001) began with a 5min acclimation period consisting of 65 dB background white noise. Following acclimation, a series of 6 pulse-alone (40 msec burst of 120 dB noise) trials were presented. This was followed by 25 trials comprising four distinct trial types: pulse alone (described above) and 3 prepulse trials. The prepulse trial included a prepulse stimulus (3, 6, or 12 dB above background for 20ms) that preceded a 120sec pulse trial with an inter-stimulus interval of 100 msec. These four trial types were presented in a pseudorandom order, with 10 pulse-alone trials and 5 of each prepulse trial. The session was concluded by the presentation of 5 consecutive pulse-alone trials. Each trial was interspersed by a variable ITI that averaged 15 s. Percent PPI was calculated as follows: 100-(pp/po) x 100 in which pp = response during prepulse intensity and po = response during pulse only trial.
Behavioral Training
Rats were habituated to the 5-CSRTT chambers for two days prior to training, and apertures were illuminated and baited to encourage exploration. The training schedule (6 days per week, previously described in Bari et al., 2008) started with a stimulus duration (SD) of 120 s. The SD was progressively reduced to the final testing criteria of 1 s. Animals progressed when they reached a stable level of performance >80% accuracy and <20% omissions. The LH was also reduced as the animal acquired the task in the same manner as the SD, with the exception that the LH reduction stopped at 5 s, therefore giving testing criteria of 1 s SD and 5 s LH. Throughout the training period, the ITI and time-out period remained at 5 s. Rats were deemed trained when they had reached stable performance at the final protocol (1 s SD, 5 s ITI, 5 s LH) for three consecutive days. The entire training procedure required approximately 12 weeks to complete.
Sub-Chronic PCP Administration
PCP was purchased from Sigma-Aldrich. Animals were administered saline (0.9% saline, n = 10) or PCP (5mg/kg, n = 10) via the intra-peritoneal route. The sub-chronic PCP treatment regimen consisted of 7 twice-daily (9 AM and 5 PM) injections followed by a 7-day washout period, previously shown to produce robust, lasting cognitive and social behavioral deficits (reviewed in Neill et al., 2010, 2013). During this 14-day period, animals received no behavioral training or testing. A separate cohort of animals was administered saline (0.9% saline, n = 10) or PCP (5mg/kg intra-peritonealy, n = 10) and perfused after a 15-day washout period to enable ex-vivo magnetic resonance imaging to be carried out.
Experimental Design
A summary of the timeline of experimental procedures is shown in Figure 1. Once trained, all 20 animals were tested on the standard version of the 5-CSRTT (SD 1 s; ITI 5 s) followed by two behavioral challenge sessions. The variable stimulus duration and ITI challenge sessions were interspersed with two baseline (i.e., non-challenge) sessions. Following 5-CSRTT testing, PPI was assessed. After the baseline behavioral testing was completed, the sub-chronic PCP treatment schedule commenced. To determine treatment groups, animals were group-matched based on baseline 5-CSRTT performance as well as task-related performance. Animals were re-tested on the 5-CSRTT, using standard task parameters, 8 days after the last PCP injection. Thereafter, the acute behavioral challenges (variable SD and variable ITI; 10 and 13 days post-PCP) were interspersed with baseline sessions (days 11 and 12) and PPI (14 days post-PCP). A separate cohort of 20 animals was administered PCP or saline in an identical manner and perfused with 4% PFA 15 days post-PCP, prior to high-resolution ex-vivo microscopy.
Figure 1.
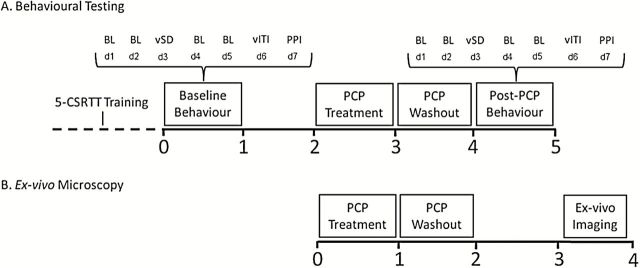
Experimental timeline. Rats were trained for approximately 3 months on the five-choice serial reaction time task (5-CSRTT). Once trained, behavioral assessment involved standard (SD = 1 s; ITI = 5 s) and challenge (variable SD and ITI) sessions on the 5-CSRTT and PPI. Animals were treated with PCP (n = 10) or saline (n = 10) twice daily for 7 days, followed by a 7-day washout period. After the 1-week treatment regimen, animals were re-assessed in the 5-CSRTT during baseline sessions and challenge sessions, followed by PPI (A). A separate cohort of animals was administered PCP (n = 10) or saline (n = 10) and perfused with 4% PFA to enable ex-vivo microscopy to be carried out. Animals were perfused 15-days post-PCP treatment to coincide with the completion of behavioral testing in the first group (B). Values shown are in weeks. BL, baseline 5-CSRTT session; vSD, variable stimulus duration challenge session; vITI, variable inter-trial interval challenge session; PPI, prepulse inhibition; d1 - d7, day 1 - day 7.
Magnetic Resonance Imaging
Brains were immersed in FluorInert solution (FC-70, 3M) as a susceptibility-matching fluid without any MR signal and scanned at 4.7 T using a Bruker PharmaScan 47/16 system with a 20mm birdcage transmit/receive coil (Sawiak et al., 2009). The pulse sequence used was a rapid acquisition with relaxation enhancement sequence with TR/TE 1500/36ms. Other scan parameters included: field of view (3.1cm × 1.6cm × 1.2cm) with a matrix of 384×200×150 for an isotropic resolution of 80 µm with 8 averages and an echo train length of 10 echoes. Parameters were chosen for optimal contrast between grey and white matter for morphometry.
Voxel Based Morphometry
Scans were analyzed using SPM8 following previously established methods (Draganski et al., 2004). The unified segmentation algorithm of SPM8 with DARTEL was used as modified by SPM Mouse for rat brains (Ashburner and Friston, 2005; Ashburner, 2007; Sawiak et al., 2013). MR images were corrected for radio-frequency coil inhomogeneity and registered to a stereotactic template of the rat brain using SPM8 and SPMMouse (Sawiak et al., 2009). The resulting maps of grey matter were smoothed with a 400 µm Gaussian kernel. Treated and control brains were compared using a two-tailed, two-group student’s t-test on a voxel-by-voxel basis. Regions of the brain with a low grey matter score (below 30%) were excluded from the analysis, and areas in the ex-vivo data where damage had occurred in extraction were masked before analysis. To control the type I error rate due to multiple comparisons, a voxel-wise height threshold of p < 0.001 was used, together with an empirically-derived extent threshold of 603 voxels for a corrected family-wise error probability of p < 0.05.
Cortical Thickness
To specifically investigate alterations in cortical morphology, we used a voxel-based cortical thickness method (Hutton et al., 2008), as adapted and validated previously for the rodent brain (Sawiak et al., 2012). Briefly, DARTEL registration fields for each subject were used to warp a cortical mask drawn on the template image to the native space of each animal, where cortical thickness values were calculated by integrating streamlines found from Laplace’s equation. The resulting maps were then assessed using a general linear model. A two-group student’s t-test was used, significant voxels were identified using a height threshold of p < 0.001, and type I errors were controlled with a family-wise error-adjusted p-value of p < 0.05, using a cluster extent threshold established with random field theory (SPM). Two-tailed tests were used throughout.
Behavioral Data Analysis
Behavioral data are displayed as mean ± SEM and analyzed using Statistica v8 (Statsoft). 5-CSRTT data were analyzed by a two-way repeated-measures ANOVA, with day, stimulus duration, ITI, or trial bin (first half of session vs. second half of session) as the repeated measures, where appropriate, and treatment as a fixed factor. To specifically assess post-treatment, between-subject performance, a one-way ANCOVA with treatment as the fixed factor and pre-treatment performance as the covariate was conducted. PPI data were analyzed using a three-way repeated measures ANOVA (with day and treatment the fixed factors and prepulse intensity the repeated measure). Following global analysis of the behavioral data, planned comparisons (Snedecor and Cochran, 1989) were used to assess longitudinal within-subject and/or between-subject effects. For interactions at p < 0.1, we also examined whether lower-order main effects were detectable after subdivision of the interactive variables (Snedecor and Cochran, 1967). The threshold for statistical significance was set at α = 0.05.
Results
5-CSRTT: Baseline Session
Sub-chronic PCP significantly impaired performance on the standard version of the 5-CSRTT (SD 1 s; ITI 5 s; see Table 1). However, these effects were less pronounced compared with those observed during the challenge sessions reported below, and mainly affected the latency of animals to respond correctly (day x treatment interaction, F(1,18) = 6.95, p < 0.05). Specifically, animals treated with PCP responded more slowly than control rats (p < 0.05). Apart from significant main effects of day for correct responses (F(1,18) = 6.11, p < 0.05) and omissions (F(1,18) = 6.17, p < 0.05), no other significant effects were found.
Table 1. Summary of Behavioral Performance of Rats on the 5-CSRTT During Baseline (BL), Variable Stimulus Duration (vSD), and Variable ITI(vITI) Challenge Sessions.
).
| Pre-treatment | Post-treatment | |||
|---|---|---|---|---|
| Saline (n = 10) | PCP (n = 10) | Saline (n = 10) | PCP (n = 10) | |
| BL Performance | ||||
| % Accuracy | 84.5±1.6 | 83.7±2.3 | 84.5±1.0 | 80.3±3.2 |
| % Correct | 76.4±1.8 | 77.6±3.4 | 75.0±1.7 | 69.7±3.3 |
| % Incorrect | 14.1±1.3 | 14.8±2.0 | 13.7±0.9 | 17.0±2.8 |
| % Omission | 9.6±1.7 | 7.6±2.2 | 11.3±1.6 | 13.3±1.5 |
| % Premature | 8.4±1.8 | 5.9±1.3 | 11.2±1.8 | 9.6±3.3 |
| Correct Lat (s) | 0.72±0.06 | 0.69±0.04 | 0.68±0.05 | 0.76±0.05 *,# |
| Magazine Lat (s) | 1.44±0.10 | 1.40±0.06 | 1.37±0.09 | 1.29±0.06 |
| Perseverative | 103.2±9.6 | 157.8±28.0 | 131.2±11.4 | 161.8±26.9 |
| vSD Performance | ||||
| % Accuracy | 75.8±1.4 | 74.8±2.1 | 77.4±2.1 | 67.8±2.2 **, ## |
| % Correct | 68.3±1.6 | 66.2±2.3 | 67.3±2.3 | 58.2±3.3 |
| % Incorrect | 21.9±1.4 | 22.3±1.9 | 19.9±2.1 | 27.3±1.7 **, ## |
| % Omission | 9.8±1.7 | 11.5±1.6 | 12.9±2.8 | 14.5±2.8 |
| % Premature | 10.2±2.2 | 6.9±1.4 | 4.8±1.0 | 6.3±2.4 |
| Correct Lat (s) | 0.69±0.05 | 0.71±0.04 | 0.71±0.05 | 0.77±0.05 |
| Magazine Lat (s) | 1.36±0.09 | 1.37±0.04 | 1.37±0.09 | 1.36±0.07 |
| Perseverative | 128.0±15.7 | 167.4±32.5 | 120.0±12.4 | 149.5±30.7 |
| vITI Performance | ||||
| % Accuracy | 79.8±2.4 | 79.8±1.7 | 80.0±2.0 | 71.8±3.1 *, # |
| % Correct | 63.4±4.0 | 58.2±3.2 | 60.1±3.2 | 52.0±3.5 |
| % Incorrect | 15.69±1.7 | 14.9±1.3 | 14.7±1.3 | 19.8±2.0 *, # |
| % Omission | 20.9±3.6 | 22.1±2.2 | 25.2±2.6 | 26.3±3.7 |
| % Premature | 30.2±4.1 | 21.7±2.9 | 24.3±2.8 | 28.6±2.7 |
| Correct Lat (s) | 0.84±0.03 | 0.79±0.06 | 0.84±0.07 | 0.91±0.05 |
| Magazine Lat (s) | 1.35±0.10 | 1.35±0.4 | 1.35±0.85 | 1.30±0.05 |
| Perseverative | 144.5±13.9 | 181.0±36.4 | 130.2±16.6 | 171.7±35.5 |
5-CSRTT: Variable Stimulus Duration
PCP-treated rats were markedly impaired with respect to attentional accuracy (%Accuracy) during the variable stimulus duration challenge session (day x treatment interaction: F(1,18) = 7.79, p < 0.05). Thus, the attentional accuracy of PCP rats significantly decreased relative to pre-treatment performance and saline control animals (p < 0.01; Figure 2A). ANCOVA confirmed a significant reduction in response accuracy between control and PCP animals (F(1,17) = 9.85, p < 0.01). This impairment was driven by a significant increase in incorrect responses (day x treatment interaction, F(1,18) = 8.66, p < 0.01), revealed by a significant within-subject increase in incorrect responding in PCP-treated animals but not saline-treated animals (p < 0.01; Figure 2B), and a significant post-treatment group difference between saline and PCP rats (ANCOVA: F(1,17) = 9.66, p < 0.01). No other significant effects were found during this intervention (see Table 1).
Figure 2.
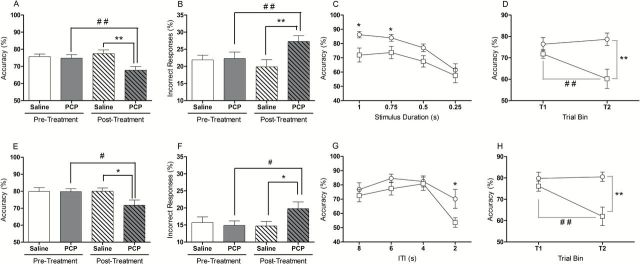
Attentional impairment in rats treated sub-chronically with PCP. Rats were challenged with variable SD (top row) and variable ITI (bottom row) sessions. Response accuracy was reduced in PCP-treated animals in the variable SD session (A). This was mainly attributed to an increase in incorrect responding (B). Analysis of performance, as a function of stimulus duration, revealed a reduction in accuracy of PCP-treated animals when presented with stimuli of 1 and 0.75 s in duration (C). These deficits were restricted to the last half of the session (D). Response accuracy was also reduced in PCP-treated animals when challenged during an acute variable ITI session (E), an effect largely driven by an increase in incorrect responding (F). When performance was analyzed as a function of ITI, a reduction in accuracy was only evident when animals were presented with a 2 s ITI (G). Accuracy impairments were only evident during the second half of the session (H).,Between-subject differences: *p < 0.05 and **p < 0.01. Within-subject differences: #p < 0.05 and ##p < 0.01. Open white columns represent saline control animals; open shaded columns represent PCP animals before sub-chronic PCP treatment. Dashed white columns represent saline animals and dashed shaded columns represent PCP treated animals after PCP treatment. Open circles represent saline-treated animals and open-squares denote PCP-treated animals. Data are mean ± SEM.
Further analysis demonstrated that response accuracy declined as the stimulus duration decreased (F(3,54) = 12.47, p < 0.001). ANOVA revealed a main effect of treatment (F(1,18) = 9.22, p < 0.01) and planned comparisons demonstrated that PCP-treated animals showed significantly reduced attentional accuracy when presented with stimuli of 1 and 0.75sec in duration (p < 0.05; Figure 2C). Moreover, attentional accuracy declined over the course of the challenge session (trial x treatment interaction, F(1,18) = 5.95, p < 0.05), with response accuracy significantly reduced in PCP rats compared with controls during the second half of the session (p < 0.01; Figure 2D). Thus, PCP treatment significantly impaired the ability of animals to maintain accurate performance over the full duration of the session.
5-CSRTT: Variable Inter-Trial Interval
PCP rats also exhibited impaired attentional accuracy when the inter-trial interval was made unpredictable, a manipulation that taxes sustained attention. Decomposing the near-significant day x treatment interaction (F(1,17) = 4.23, p = 0.055) revealed a within-subject reduction in response accuracy in PCP-treated animals but not saline controls (p < 0.05), and a significant post-treatment impairment between saline- and PCP-treated animals (F(1,16) = 5.35, p < 0.05; Figure 2E). Similar to the effects reported above, PCP-treated rats made more incorrect responses than saline-treated animals (day x treatment interaction, F(1,17) = 3.92, p = 0.06), revealed by a significant post-treatment group effect (F(1,16) = 4.75, p < 0.05; Figure 2F). No other behavioral variable was significantly affected by sub-chronic PCP during this challenge session (Table 1).
Attentional accuracy also significantly declined during the variable ITI session (F(3,54) = 8.58, p < 0.001) and was accompanied by a significant main effect of treatment (F(1,18) = 4.90, p < 0.05). Based on previous findings (Dalley et al., 2004), a planned comparison revealed that response accuracy was significantly decreased in PCP-treated rats compared with controls when the ITI was 2 s (p < 0.05; Figure 2G). Similar to the findings reported for the variable SD session, PCP-treated rats showed a decrement in attentional accuracy during the second half of the session compared with control animals (trial x treatment interaction:, F(1,15) = 8.15, p < 0.05), demonstrated by significant within- and between-subject effects during the second block of trials (p < 0.01; Figure 2H).
Prepulse Inhibition
Analysis of startle magnitude between the two acoustic startle sessions indicated that both saline and PCP rats exhibited habituation to the startle response (F(1,18) = 8.09, p < 0.05; data not shown). However, although the level of PPI increased predictably as the prepulse intensity increased (F(2,72) = 50.36, p < 0.001), this was no different between saline and PCP animals (Figure 3A and B).
Figure 3.
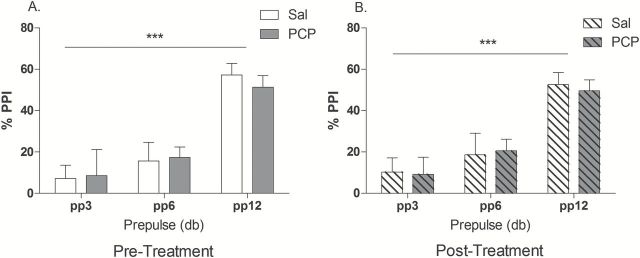
Pre-pulse inhibition of the startle response before (A) and after (B) sub-chronic PCP treatment. It can be seen that PCP had no significant effect on prepulse inhibition of the acoustic startle response. A main effect of prepulse effect: *p < 0.001. Open white columns represent saline control animals (n = 10); open shaded columns represent PCP animals (n = 10) before sub-chronic PCP treatment. Dashed white columns represent saline control animals; shaded dashed columns represent PCP-treated animals after PCP. Data are mean ± SEM.
High-Resolution Ex-Vivo Microscopy
The mean cortical thickness of control and PCP rats is shown in Figure 4. Significant clusters demonstrating a reduction in cortical thickness in PCP rats were found in the lateral somatosensory cortex and insula cortex anteriorly and posteriorly bordering the ectorhinal cortex (Figure 4B and C). Qualitatively similar differences were observed bilaterally, though the significance scores were lower on the right side and did not survive correction for multiple comparisons.
Figure 4.
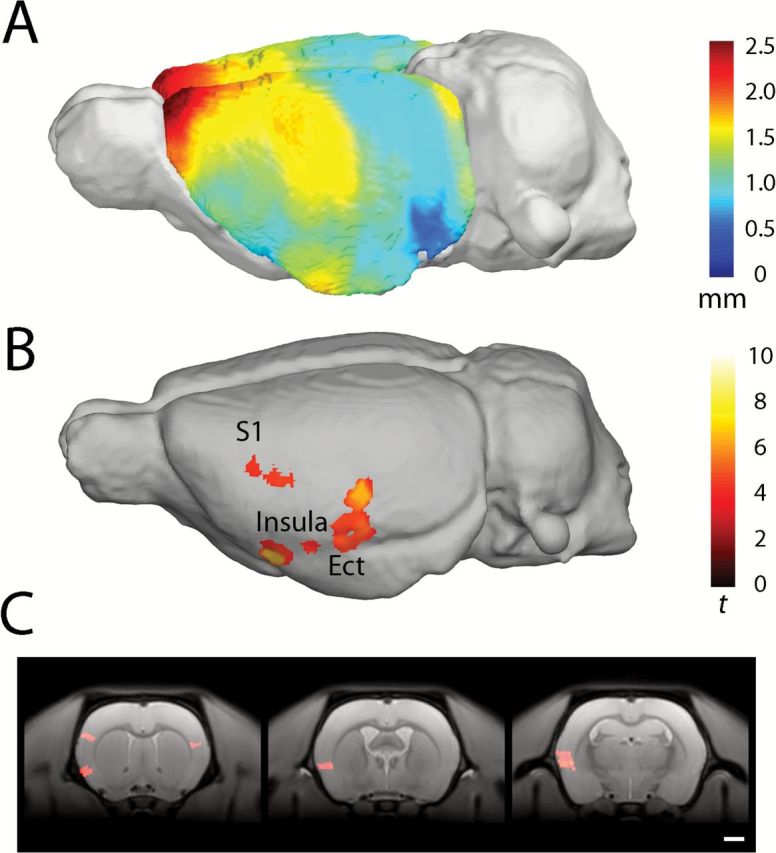
Average voxel-based cortical thickness in rats treated with PCP. Scale bar in mm (A). Areas of significantly reduced cortical thickness in PCP-treated animals compared with saline-treated animals; the scale bar shows student’s t-score based on 14 degrees of freedom. Results surviving a cluster-extent correction for family-wise error p < 0.05 are shown (B). Coronal sections indicating reduced cortical thickness in the insular cortex, left somatosensory cortex (S1; C; panel 1), granular and dysgranular insular cortex (panel 2), granular, dysgranular insular cortex, and ectorhinal cortex (panel 3). Regions identified using Paxinos and Watson (1997).
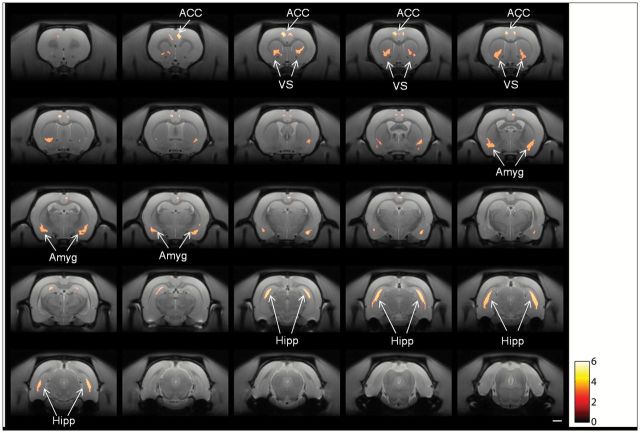
Significant reductions in grey matter density were observed bilaterally in the anterior cingulate cortex (ACC), ventral striatum (VS), amygdaloid nucleus, and, most prominently, in the hippocampal formation of PCP-treated rats compared with saline-treated rats (Figure 5). No areas of increased grey matter were observed.
Figure 5.
Coronal sections showing areas of significant differences in grey matter between rats treated with saline and PCP. The horizontal scale bar is 2mm; the color scale shows the student’s t-score. Results shown are significant at a cluster-level corrected family-wise error of p < 0.05. The test performed was two-tailed, but only grey matter reductions were observed. ACC, anterior cingulate cortex; VS, ventral striatum; Amyg, amydaloid regions; Hipp, hippocampus.
Discussion
These findings demonstrate that sub-chronic PCP administration causes a range of structural abnormalities that localize to the hippocampus, ACC, amygdala, insular cortex, and VS. These abnormalities were detected up to 15 days after the last PCP injection and may contribute to impaired attentional function, notably affecting active sustained attention whilst sparing passive attention in terms of sensorimotor gating. Collectively, these findings add specificity to the sequelae of effects produced by protracted NMDAR antagonism whilst also revealing significant overlaps with the spectrum of cognitive and neural abnormalities found in schizophrenia. In particular, our findings implicate NMDAR hypofunction within cortico-amygdalo-hippocampal circuitry, encompassing the VS and ACC as putative key loci underlying attentional dysfunction in schizophrenia.
The present study demonstrates that sub-chronic PCP treatment impairs 5-CSRTT performance in the drug-free state when performance is challenged. During the variable SD challenge session, PCP rats were significantly less accurate than control animals during long SDs. In general, short SDs demand greater attention to facilitate target detection (Bushnell, 1998), and this manipulation predictably reduced discriminative accuracy in both control and PCP rats. The deficit when longer (but not shorter) SDs were presented likely represented a floor effect with performance in control animals impaired. However, the increased attentional demands of this manipulation likely exacerbated deficits during the longer SDs, impairing performance in the second half of the session. This deficit could perhaps best be described as a vigilance decrement, as corroborated by recent findings (Barnes et al., 2012). Consistent with this interpretation, attentional accuracy also declined in PCP rats on trials where the opportunity to respond prematurely was significantly reduced. Deficits following this manipulation were only present when shortest ITI was presented and again emerged during the second half of the session. This variant of the task requires the continuous allocation of finite attentional resources and previously has been shown to reveal a vigilance decrement in rats depleted of acetylcholine in the prefrontal cortex (PFC) (Dalley et al., 2004). In support of our findings, increasing task difficulty by increasing perceptual load has been shown to temporally degrade continuous attentional performance in schizophrenia patients (Nestor et al., 1990; Mass et al., 2000). Time-on-task deficits have also been reported in schizophrenia patients on related tasks of sustained attention (Hahn et al., 2012; Young et al., 2013). Our findings thus indicate that sub-chronic PCP produces deficits in sustained attention that resemble a significant component of the attentional impairment present in schizophrenia.
Pre-attentive sensorimotor gating, as assessed by PPI, is also impaired in schizophrenia (Braff et al., 2001). However, the neural correlates of PPI are complex, with interactions between sub-cortical and brainstem circuitry being modulated by a number of forebrain structures (Fendt et al., 2001; Swerdlow et al., 2001). Grey matter volume changes in several limbic-striatal structures have been linked to PPI (Kumari et al., 2005). Our results suggest, however, that while the structural integrity of many of these regions may be vulnerable to disruption by sub-chronic PCP, this alone is not sufficient to impair PPI. Our findings thus replicate previous studies showing PCP to have no effect on PPI in adult rats (Martinez et al., 1999; Egerton et al., 2008). However, when administered to neonatal rats, PCP has been shown to impair PPI in adulthood (Le Pen et al., 2003). The mechanism underlying this presumed neurodevelopmental influence is unclear but may involve disruption of GABAergic remodelling during adolescence (O’Donnell, 2012). Despite GABAergic deficits being evident in rats following both neonatal and adult PCP administration (Jones et al., 2011), the absence of PPI deficits in the present study may be attributable to PCP being administered after this critical period of neurodevelopment (Powell, 2010). Unlike PPI, active attentional processing is less reliant on sensory filtering, and this may explain why sub-chronic antagonism of NMDAR during adulthood selectively impaired sustained, effortful attention.
Our findings show that sub-chronic PCP results in reduced grey matter density in several limbic cortico-striatal structures, including the amygdala, VS, hippocampus, insular cortex, and ACC. Comparable structural abnormalities have been reported in schizophrenia (Honea et al., 2005), affecting the amygdala, hippocampus, parahippocampus gyrus, caudate, striatum, and ACC (Seidman et al., 1999; Shenton et al., 2001; Chua et al., 2007; Williams, 2008; Rametti et al., 2010; Stegmayer et al., 2013). Cortical thinning in frontal regions (Schultz et al., 2010) and insular cortexes (Pujol et al., 2013) are evident. In addition, grey matter deficits in temporal lobe structures, together with structural and functional deficits in the ACC, predict impaired continuous attentional performance in schizophrenia (Volz et al., 1999; Salgado-Pineda et al., 2004). Moreover, reduced cingulate, caudate, and hippocampus volume is evident in patients with attentional deficits (Salgado-Pineda et al., 2003), while decreased amygdala volume has been linked to first-episode psychosis patients (Aas et al., 2012). Interestingly, decreased hippocampal and caudate volume has also been reported in relatives of schizophrenia patients (Bhojraj et al., 2011) whilst structural deficits that also include the amygdala underlie attentional dysfunction in mania (Sax et al., 1999; Fleck et al., 2012). Along with structural deficits, schizophrenia is associated with functional disconnectivity of several regions, including the ACC, and attentional deficits (Honey et at. 2005b). Sustained NMDAR hypofunction has recently been shown to compromise functional integration between several brain regions in the rat, with connectivity between frontal and hippocampal structures impaired (Dawson et al., 2014). These observations encourage the conclusion that the observed structural deficits associated with sub-chronic PCP, localized within discrete regions implicated in schizophrenia pathology and attentional processing, contributed to impaired sustained attention in our study. In addition, our findings implicate NMDAR hypofunction in the spectrum of morphological and attentional deficits found in schizophrenia.
Structural brain abnormalities are widely reported in ketamine abusers (Liao et al., 2010, 2011; Wang et al., 2013). Although these structural alterations appear to be less pronounced than those observed in the current study, heterogeneity in terms of age, years of ketamine use, and poly-drug abuse may account for this variance. Nevertheless, the effects of acute ketamine administration and structural deficits associated with chronic NMDAR antagonism suggest similar regional specificity to the regions implicated in the present study. While we cannot rule out PCP-induced neurotoxicity (Olney et al., 1989) as a contributing mechanism for the structural deficits we observed, several investigations suggest this may not be the case. High doses of PCP (up to 50mg/kg) produced neurotoxicity in regions including the posterior cingulate/retrosplenial cortex, piriform cortex, olfactory tubercle, and cerebellum (Nakki et al., 1995; Corso et al., 1997; Johnson et al., 1998). Animals administered 5mg/kg were indistinguishable from saline-treated animals (Nakki et al., 1995), or alterations indicative of neurotoxicity were transient and restricted to the retrosplenial cortex (Corso et al., 1997). Moreover, female rats displayed increased sensitivity to NMDAR neurotoxicity (Olney et al., 1989) and were used in several of the studies described above, in contrast to male rats used in the current investigation. Importantly, previous investigations with lower PCP doses (including one using male rats and an identical regimen to ours) did not observe any evidence of PCP-induced neurotoxicity (Hajszan et al., 2006; Fattorini et al., 2008). Given the treatment regimen used and the absence of structural deficits in the posterior cingulate/retrosplenial cortex, piriform cortex, olfactory tubercle, or cerebellum, it is unlikely the structural changes presently described were due to PCP-induced neuronal degeneration or apoptosis. A potential mechanism underpinning our findings may lie with NMDAR antagonists producing excitatory disinhibition via blockade of NMDAR located on GABAergic interneurons (Nakazawa et al., 2012). This effect results in increasing activity within limbic cortico-thalamic circuits (Morris et al., 2005). Persistent GABAergic impairments are evident following repeated PCP treatments (Neill et al., 2010), which are associated with reductions in GAD67 and parvalbumin expression (Abdul-Monim et al., 2007; Bullock et al., 2009; Jenkins et al., 2010). Furthermore, dendritic spine density reductions have also been reported using an identical treatment regimen (Hajszan et al., 2006; Elsworth et al., 2011). Therefore, reductions in both GABAergic markers and dendritic spine density may have contributed to reductions in grey matter and attentional deficits observed in the current investigation.
Sustained attentional performance on the 5-CSRTT depends on the functional integrity of distinct striatal and frontal brain regions coupled with dissociable modulatory influences by the monoamine transmitter systems (Robbins, 2002). Thus, neurotoxic lesions of the ACC in rats selectively impair the accuracy of responding on this task (Passetti et al., 2002; Chudasama et al., 2003), a deficit that is notably sensitive to modulation at the level of the nucleus accumbens (Pezze et al., 2009). Moreover, lesions localized to the medial striatum, which together with the nucleus accumbens overlap with the region of reduced grey matter density in the present study, also impair attentional performance on this task (Rogers et al., 2001). Although initial reports suggested that the hippocampus was not required for 5-CSRTT performance (Kirkby and Higgins, 1998), subsequent investigations in neonatal and adult rats have demonstrated a convincing role of the ventral hippocampus in maintaining performance during increased attentional load (Le Pen et al., 2003; Abela et al., 2013). These findings resonate with the profile of PCP-induced structural abnormalities observed in the present study, which likely therefore contributed to the decrement in attentional performance on the 5-CSRTT.
In addition to attentional impairment, sub-chronic PCP administration produces robust impairments in a number of cognitive processes relevant to schizophrenia (for review see Neill et al., 2010). For example, PCP disrupts novel object recognition (Grayson et al., 2007), spatial memory on the Morris water maze (Didriksen et al., 2007), attentional set-shifting (McLean et al., 2008), reversal learning (McLean et al., 2010), and social behavior (Snigdha and Neill, 2008). Key neural loci responsible for these impairments include the hippocampus (Broadbent et al., 2004; Squire et al., 2004), amgydala (Lee et al., 2005), and interactions with the fronto-striatal systems (Chudasama and Robbins, 2006; Brooks et al., 2012). The spectrum of structural deficits reported in the present study may, as a result, have broader consequences for impaired cognitive processing after sub-chronic exposure to PCP and other NMDAR antagonists.
In summary, our findings reveal a collection of structural and attentional abnormalities associated with sub-chronic PCP administration in rats that may have relevance for schizophrenia. In particular, our results implicate NMDAR dysfunction as a candidate neural mechanism in the emergence of regional volumetric deficits in schizophrenia that may contribute to cognitive deficits in schizophrenia.
Statement of Interest
Dr Neill has received expenses to attend conferences and fees for lecturing and consultancy work (including attending advisory boards) from the manufacturers of various neuropsychiatric drugs.
Acknowledgments
This work was supported by Medical Research Council grants to Dr Dalley (G0701500; G0802729) and by a joint core award from the MRC and Wellcome Trust in support of the Behavioural and Clinical Neuroscience Institute at Cambridge University (MRC G1000183; WT 093875/Z/10/Z). This work was completed within the MRC Imperial College-Cambridge University-Manchester University (ICCAM) strategic cluster (G1000018). Dr Jupp was supported by fellowships from the National Health and MRC of Australia and the AXA research fund. Dr Mar has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement #115008, whose resources are composed of EFPIA in-kind contributions and financial contribution from the European Union’s Seventh Framework Program (FP7/2007–2013). Dr Fletcher has received consultancy fees from GlaxoSmithKline unrelated to this work. Dr Barnes was partly supported by a DTA award to Dr Neill and by b-neuro (b-neuro.com). The authors would like to thank David Theobald for technical assistance and Dr James Kesby for insightful comments during the preparation of the manuscript.
References
- Aas M, Navari S, Gibbs A, Mondelli V, Fisher HL, Morgan C, Morgan K, MacCabe J, Reichenberg A, Zanelli J, Fearon P, Jones PB, Murray RM, Pariante CM, Dazzan P. (2012). Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first-episode psychosis? Schizophr Res 137:73–79. [DOI] [PubMed] [Google Scholar]
- Abdul-Monim Z, Neill JC, Reynolds GP (2007). Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol 21:198–205. [DOI] [PubMed] [Google Scholar]
- Abela AR, Dougherty SD, Fagen ED, Hill CJ, Chudasama Y. (2013). Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb Cortex 23:1396–1409. [DOI] [PubMed] [Google Scholar]
- Amitai N, Markou A. (2010). Disruption of Performance in the Five-Choice Serial Reaction Time Task Induced By Administration of N-Methyl-D-Aspartate Receptor Antagonists: Relevance to Cognitive Dysfunction in Schizophrenia. Biol Psychiatry 68:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. (2011). Comparative effects of different test day challenges on performance in the 5-choice serial reaction time task. Behav Neurosci 125:764 –774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. (2007). Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology 193:521–537. [DOI] [PubMed] [Google Scholar]
- an der Heiden W, Häfner H. (2000). The epidemiology of onset and course of schizophrenia. Eur Arch Psychiatry Clin 250:292–303. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. (2005). Unified segmentation. Neuroimage. 26:839–851. [DOI] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. (2008). The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc 3:759–767. [DOI] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. (2012). Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology 62:1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Francis AN, Montrose DM, Keshavan MS. (2011). Grey matter and cognitive deficits in young relatives of schizophrenia patients. Neuroimage 54(S1):S287–S292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. (2001). Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 156:234–258. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. (2004). Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA 101:14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JM, Pershing ML, Thomsen MS, Mikkelsen JD, Sarter M, Bruno JP. (2012). Transient Inactivation of the Neonatal Ventral Hippocampus Impairs Attentional Set-Shifting Behavior: Reversal with an [alpha]7 Nicotinic Agonist. Neuropsychopharmacology 37:2476–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock WM, Bolognani F, Botta P, Valenzuela CF, Perrone-Bizzozero NI. (2009). Schizophrenia-like GABAergic gene expression deficits in cerebellar Golgi cells from rats chronically exposed to low-dose phencyclidine. Neurochem Int 55:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ. (1998). Behavioral approaches to the assessment of attention in animals. Psychopharmacology (Berl) 138:231–259 [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins T, Evenden J, Everitt B. (1983). Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Aitken MRF. (2010) Whisker: a client–server high-performance multimedia research control system. Behav Res Methods 42:1059–1071. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Buchanan RW, Bullmore E, Krystal JH, Cohen J, Geyer M, Green M, Nuechterlein KH, Robbins TW, Silverstein S, Smith EE, Strauss M, Wykes T, Heinssen R (2008) Identifying cognitive mechanisms target for treatment development in schizophrenia: An overview of the first meeting of the cognitive neuroscience treatment research to improve cognition in schizophrenia initiative. Biol Psychiatry 64:4–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Faraone SV. (2001). Sustained attention deficits as markers of genetic susceptibility to schizophrenia. Am J Med Genet 97:52–57. [DOI] [PubMed] [Google Scholar]
- Chua SE, Cheung C, Cheung V, Tsang JTK, Chen EYH, Wong JCH, Cheung JPY, Yip L, Tai K-s, Suckling J, McAlonan GM. (2007). Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res 89:12–21. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. (2003). Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146:105–119. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. (2006). Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychology 73:19–38. [DOI] [PubMed] [Google Scholar]
- Corso TD, Sesma MA, Tenkova TI, Der TC, Wozniak DF, Farber NB, Olney JW. (1997). Multifocal brain damage induced by phencyclidine is augmented by pilocarpine. Brain Research 752:1–14. [DOI] [PubMed] [Google Scholar]
- Cosgrove J, Newell TG. (1991). Recovery of neuropsychological functions during reduction in use of phencyclidine. J Clin Psychol 47:159–169. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. (2004). Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784. [DOI] [PubMed] [Google Scholar]
- Dawson N, Xiao X, McDonald M, Higham DJ, Morris BJ, Pratt JA. (2014). Sustained NMDA receptor hypofunction induces compromised neural systems integration and schizophrenia-like alterations in functional brain networks. Cereb Cortex 24:452–464. [DOI] [PubMed] [Google Scholar]
- Deakin J, Lees J, McKie S, Hallak JC, Williams SR, Dursun SM. (2008). Glutamate and the neural basis of the subjective effects of ketamine: A pharmaco–magnetic resonance imaging study. Arch Gen Psychiatry 65:154–164. [DOI] [PubMed] [Google Scholar]
- Didriksen M, Skarsfeldt T, Arnt J. (2007). Reversal of PCP-induced learning and memory deficits in the Morris’ water maze by sertindole and other antipsychotics. Psychopharmacology 193:225–233. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. (2004). Neuroplasticity: changes in grey matter induced by training. Nature 427:311–312. [DOI] [PubMed] [Google Scholar]
- Egerton A, Reid L, McGregor S, Cochran SM, Morris BJ, Pratt JA. (2008). Subchronic and chronic PCP treatment produces temporally distinct deficits in attentional set shifting and prepulse inhibition in rats. Psychopharmacology (Berl) 198:37–49. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. (2010). Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res 117:1–12. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Morrow BA, Hajszan T, Leranth C, Roth RH. (2011). Phencyclidine-induced loss of asymmetric spine synapses in rodent prefrontal cortex is reversed by acute and chronic treatment with olanzapine. Neuropsychopharmacology 36:2054–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattorini G, Melone M, Bragina L, Candiracci C, Cozzi A, Pellegrini Giampietro DE, Torres-Ramos M, Perez-Samartin A, Matute C, Conti F. (2008). GLT-1 expression and Glu uptake in rat cerebral cortex are increased by phencyclidine. Glia 56:1320–1327. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. (2001). Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 156:216–224. [DOI] [PubMed] [Google Scholar]
- Fleck DE, Eliassen JC, Durling M, Lamy M, Adler CM, DelBello MP, Shear PK, Cerullo MA, Lee JH, Strakowski SM. (2012). Functional MRI of sustained attention in bipolar mania. Mol Psychiatry 17:325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Perez J, Broome M, Borgwardt S, Placentino A, Caverzasi E, Cortesi M, Veggiotti P, Politi P, Barale F. (2007). Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev 31:465–484. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. (2001). Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 156:117–154. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. (2001). Measurement of startle response, prepulse inhibition, and habituation. Curr Protoc Neurosci 3:8.7:8.7.1–8.7.15 [DOI] [PubMed] [Google Scholar]
- Gozzi A, Large CH, Schwarz A, Bertani S, Crestan V, Bifone A. (2007). Differential effects of antipsychotic and glutamatergic agents on the phMRI response to phencyclidine. Neuropsychopharmacology 33:1690–1703. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Herdon H, Schwarz A, Bertani S, Crestan V, Turrini G, Bifone A. (2008a). Pharmacological stimulation of NMDA receptors via co-agonist site suppresses fMRI response to phencyclidine in the rat. Psychopharmacology 201:273–284. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Schwarz A, Crestan V, Bifone A. (2008b). Drug–anaesthetic interaction in phMRI: the case of the psychotomimetic agent phencyclidine. Magn Reson Imaging 26:999–1006. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. (2007). Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res 184:31–38. [DOI] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Matveeva TM, Harvey AN, Luck SJ, Gold JM. (2012). Kraepelin and Bleuler had it right: people with schizophrenia have deficits sustaining attention over time. J Abnorm Psychol 121:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Leranth C, Roth RH. (2006). Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol Psychiatry 60:639–644. [DOI] [PubMed] [Google Scholar]
- Hodkinson DJ, de Groote C, McKie S, Deakin JF, Williams SR. (2012). Differential Effects of Anaesthesia on the phMRI Response to Acute Ketamine Challenge. Br J Med Med Res 2:373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. (2005). Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiat 162:2233–2245. [DOI] [PubMed] [Google Scholar]
- Honey GD, Honey RAE, O’Loughlin C, Sharar SR, Kumaran D, Suckling J, Menon DK, Sleator C, Bullmore ET, Fletcher PC. (2005a). Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic,emory: an fMRI study. Cereb Cortex 15:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RAE, Mckenna PJ, Bullmore ET, Fletcher PC. (2005b). Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain 128:2597–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C, De Vita E, Ashburner J, Deichmann R, Turner R. (2008). Voxel-based cortical thickness measurements in MRI. Neuroimage 40:1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TA, Harte MK, Reynolds GP. (2010). Effect of subchronic phencyclidine administration on sucrose preference and hippocampal parvalbumin immunoreactivity in the rat. Neurosci Lett 471:144–147. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. (1999). The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20:201–225. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Phillips M, Wang C, Kevetter GA. (1998). Chronic phencyclidine induces behavioral sensitization and apoptotic cell death in the olfactory and piriform cortex. J Neurosci Res 52:709–722. [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJ, Fone KC. (2011). Animal models of schizophrenia. Br J Pharmacol 164:1162–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. (2010). N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull 83:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby DL, Higgins GA. (1998). Characterization of perforant path lesions in rodent models of memory and attention. Eur J Neurosci 10:823–838. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP. (1994). Subanesthetic effects of the noncompetitive nmda antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Zachariah E, Galea A, Aasen I, Ettinger U, Mitterschiffthaler MT, Sharma T. (2005). Structural brain correlates of prepulse inhibition of the acoustic startle response in healthy humans. Neuroimage 26:1052–1058. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. (1995). Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13:9–19. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Grottick AJ, Higgins GA, Moreau JL. (2003). Phencyclidine exacerbates attentional deficits in a neurodevelopmental rat model of schizophrenia. Neuropsychopharmacology 28:1799–1809. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. (2005). Social Interaction Deficits Caused by Chronic Phencyclidine Administration are Reversed by Oxytocin. Neuropsychopharmacology 30:1883–1894. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Corlett PR, Wang X, Yang M, Chen H, Liu T, Chen X, Hao W, Fletcher PC. (2011). Reduced dorsal prefrontal gray matter after chronic ketamine use. Biol Psychiatry 69:42–48. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Fornito A, Liu T, Chen X, Chen H, Xiang X, Wang X, Hao W. (2012). Alterations in regional homogeneity of resting-state brain activity in ketamine addicts. Neurosci Lett. 522:36–40. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X, Liu T, Chen X, Fletcher PC, Hao W. (2010). Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain 133:2115–2122. [DOI] [PubMed] [Google Scholar]
- Lustig C, Kozak R, Sarter M, Young JW, Robbins TW. (2012). CNTRICS final animal model task selection: control of attention. Neurosci Biobehav Rev. 37:2099–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A. (1997). Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology 17:141–150. [DOI] [PubMed] [Google Scholar]
- Martinez ZA, Ellison GD, Geyer MA, Swerdlow NR. (1999). Effects of sustained phencyclidine exposure on sensorimotor gating of startle in rats. Neuropsychopharmacology 21:28–39. [DOI] [PubMed] [Google Scholar]
- Mass R, Wolf K, Wagner M, Haasen C. (2000). Differential sustained attention/vigilance changes over time in schizophrenics and controls during a degraded stimulus Continuous Performance Test. Eur Arch Psych Clin Neurosci 250:24–30. [DOI] [PubMed] [Google Scholar]
- McLean SL, Beck JP, Woolley ML, Neill JC. (2008). A preliminary investigation into the effects of antipsychotics on sub-chronic phencyclidine-induced deficits in attentional set-shifting in female rats. Behav Brain Res 189:152–158. [DOI] [PubMed] [Google Scholar]
- McLean SL, Neill JC, Idris NF, Marston HM, Wong EH, Shahid M. (2010). Effects of asenapine, olanzapine, and risperidone on psychotomimetic-induced reversal-learning deficits in the rat. Behav Brain Res 214:240–247. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. (2010). From maps to mechanisms through neuroimaging of schizophrenia. Nature 468:194–202. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. (2005). PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol 5:101–106. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. (2012). GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 62:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakki R, Koistinaho J, Sharp FR, Sagar SM. (1995). Cerebellar toxicity of phencyclidine. J Neurosci 15:2097–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. (2010). Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther 128:419–432. [DOI] [PubMed] [Google Scholar]
- Neill JC, Harte MK, Haddad PM, Lydall ES, Dwyer DM. (2013). Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: A translational link to humans. Eur Neuropsychop. 24:822–835. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Faux SF, McCarley RW, Shenton ME, Sands SF. (1990). Measurement of visual sustained attention in schizophrenia using signal detection analysis and a newly developed computerized CPT task. Schizophr Res 3:329–332. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K, Green M, Kern R, Baade L, Barch D, Cohen J, Essock S, Fenton W, Frese F, Gold J. (2008). The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiat 165:203–213. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. (2004). Identification of separable cognitive factors in schizophrenia. Schizophr Res 72:29–39. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M. (2009). CNTRICS final task selection: control of attention. Schizophr Bull 35:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P. (2012). Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: new vistas on possible therapeutic approaches. Pharmacol Ther 133:19–25. [DOI] [PubMed] [Google Scholar]
- Olney JW, Labruyere J, Price MT. (1989). Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science 244:1360–1362. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. (1999). NMDA receptor hypofunction model of schizophrenia. J Psychiatric Res 33:523–533. [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. (2002). The frontal cortex of the rat and visual attentional performance: dissociable functions of distinct medial prefrontal subregions. Cereb Cortex 12:1254–1268. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1997). The rat brain instereotaxic coordinates. San Diego, CA: Academic Press. [Google Scholar]
- Pezze M, Dalley J, Robbins T. (2009). Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D2/3 receptor antagonist sulpiride. Psychopharmacology 202:307–313. [DOI] [PubMed] [Google Scholar]
- Powell SB. (2010). Models of neurodevelopmental abnormalities in schizophrenia. Curr Top Behav Neurosci 4:435–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol N, Penades R, Rametti G, Catalan R, Vidal-Pineiro D, Palacios E, Bargallo N, Bernardo M, Junque C. (2013). Inferior frontal and insular cortical thinning is related to dysfunctional brain activation/deactivation during working memory task in schizophrenic patients. Psychiatry Res 214:94–101. [DOI] [PubMed] [Google Scholar]
- Rametti G, Junque C, Bartres-Faz D, Zubiaurre-Elorza L, Catalan R, Penades R, Bargallo N, Bernardo M. (2010). Anterior cingulate and paracingulate sulci morphology in patients with schizophrenia. Schizophr Res 121:66–74. [DOI] [PubMed] [Google Scholar]
- Riedel WJ, Mehta MA, Unema PJ. (2006). Human cognition assessment in drug research. Curr Pharm Des 12:2525–2539. [DOI] [PubMed] [Google Scholar]
- Robbins T. (2002). The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology 163:362–380. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. (2001). Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav Neurosci 115:799–811. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Baeza I, Pérez-Gómez M, Vendrell P, Junqué C, Bargalló N, Bernardo M. (2003). Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage 19:365–375. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Junque C, Vendrell P, Baeza I, Bargallo N, Falcon C, Bernardo M. (2004). Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. Neuroimage 21:840–847. [DOI] [PubMed] [Google Scholar]
- Sawiak SJ, Wood NI, Carpenter TA, Morton AJ. (2012). Huntington’s disease mouse models online: high-resolution MRI images with stereotaxic templates for computational neuroanatomy. Plos One 7:e53361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawiak SJ, Wood NI, Williams GB, Morton AJ, Carpenter TA. (2009). Use of magnetic resonance imaging for anatomical phenotyping of the R6/2 mouse model of Huntington’s disease. Neurobiol Dis 33:12–19. [DOI] [PubMed] [Google Scholar]
- Sawiak SJ, Wood NI, Williams GB, Morton AJ, Carpenter TA. (2013). Voxel-based morphometry with templates and validation in a mouse model of Huntington’s disease. Magn Reson Imaging 31:1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck PE, Jr., Hawkins JM. (1999). Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry 156:139–141. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, Nenadic I, Reichenbach JR, Sauer H, Schlosser RG. (2010). Reduced cortical thickness in first episode schizophrenia. Schizophr Res 116:204–209. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Faraone SV, Goldstein JM, Goodman JM, Kremen WS, Toomey R, Tourville J, Kennedy D, Makris N, Caviness VS, Tsuang MT. (1999). Thalamic and amygdala–hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry 46:941–954. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. (2001). A review of MRI findings in schizophrenia. Schizophr Res 49:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. (1967). Statistical methods. Ames, IA: Iowa State University Press. [Google Scholar]
- Snedecor GW, Cochran WG. (1989). Statistical methods. Ames, IA: Iowa State University Press. [Google Scholar]
- Snigdha S, Neill JC. (2008). Efficacy of antipsychotics to reverse phencyclidine-induced social interaction deficits in female rats—a preliminary investigation. Behav Brain Res 187:489–494. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. (2004). The medial temporal lobe. Ann Rev Neurosci 27:279–306. [DOI] [PubMed] [Google Scholar]
- Stegmayer K, Horn H, Federspiel A, Razavi N, Bracht T, Laimbock K, Strik W, Dierks T, Wiest R, Muller TJ, Walther S. (2013). Ventral striatum gray matter density reduction in patients with schizophrenia and psychotic emotional dysregulation. Neuroimage 4:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. (1994). Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry 51:139. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. (2001). Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 156:194–215. [DOI] [PubMed] [Google Scholar]
- Van Horn JD, McManus IC. (1992). Ventricular enlargement in schizophrenia. A meta-analysis of studies of the ventricle:brain ratio (VBR). Br J Psychiatry 160:687–697. [DOI] [PubMed] [Google Scholar]
- Volz H, Gaser C, Hager F, Rzanny R, Ponisch J, Mentzel H, Kaiser WA, Sauer H. (1999). Decreased frontal activation in schizophrenics during stimulation with the continuous performance test--a functional magnetic resonance imaging study. Eur Psychiatry 14:17–24. [DOI] [PubMed] [Google Scholar]
- Wang C, Zheng D, Xu J, Lam W, Yew DD. (2013). Brain damages in ketamine addicts as revealed by magnetic resonance imaging. Front Neuroanat 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM. (2008). Voxel-based morphometry in schizophrenia: implications for neurodevelopmental connectivity models, cognition and affect. Expert Rev Neurother 8:1049–1065. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. (2000). Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiat 157:16–25. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Tamir D, Minzenberg MJ, Ragland JD, Ursu S, Carter CS. (2008). Multivariate pattern analysis of functional magnetic resonance imaging data reveals deficits in distributed representations in schizophrenia. Biol Psychiatry 64:1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. (2009). Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther 122:150–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard GL, Light GA (2013) Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Transl Psychiatry 3:e324. [DOI] [PMC free article] [PubMed] [Google Scholar]