Abstract
Background:
Evidence suggests that individuals with social anxiety demonstrate vigilance to social threat, whilst the peptide hormone oxytocin is widely accepted as supporting affiliative behaviour in humans.
Methods:
This study investigated whether oxytocin can affect attentional bias in social anxiety. In a double-blind, randomized, placebo-controlled, within-group study design, 26 healthy and 16 highly socially anxious (HSA) male volunteers (within the HSA group, 10 were diagnosed with generalized social anxiety disorder) were administered 24 IU of oxytocin or placebo to investigate attentional processing in social anxiety. Attentional bias was assessed using the dot-probe paradigm with angry, fearful, happy and neutral face stimuli.
Results:
In the baseline placebo condition, the HSA group showed greater attentional bias for emotional faces than healthy individuals. Oxytocin reduced the difference between HSA and non-socially anxious individuals in attentional bias for emotional faces. Moreover, it appeared to normalize attentional bias in HSA individuals to levels seen in the healthy population in the baseline condition. The biological mechanisms by which oxytocin may be exerting these effects are discussed.
Conclusions:
These results, coupled with previous research, could indicate a potential therapeutic use of this hormone in treatment for social anxiety.
Keywords: attention, attentional bias, emotion, facial expression, oxytocin, social anxiety
Introduction
Research has indicated oxytocin as a key neuromodulator of social and emotional processing across phyla. Oxytocin has a long evolutionary lineage linked to social approach and recognition behaviour (Lee et al., 2009). Moreover, oxytocin is widely accepted as supporting affiliative behaviour in humans by modulating emotional processing. Oxytocin has been shown to be centrally involved in the recognition and memory of facial identity (Savaskan et al., 2008; Rimmele et al., 2009) and also fundamental in the processing of facial emotion (Schulze et al., 2011), higher level mental states using cues from the eyes (Domes et al., 2007), and social reward learning from emotional faces (Bethlehem et al., 2014; Clark-Elford et al., 2014).
At a neural level, oxytocin receptors are expressed in areas of the brain associated with emotion and attention, namely the amygdala (Huber et al., 2005). In healthy volunteers, oxytocin attenuates amygdala activity to threatening social stimuli (Kirsch et al., 2005). Oxytocin has also been shown to normalize hyperactive amygdala response to threat-related cues and hyperactive medial prefrontal response to negative social cues, and to reduce amygdala-frontal resting-state functional connectivity in patients with social anxiety disorder (SAD; Labuschagne et al., 2010, 2012; Dodhia et al., 2014). These findings demonstrate that oxytocin modulates a neural circuit known for social threat processing and emotion regulation, suggesting a neural mechanism underlying oxytocin’s behavioral effects. Accordingly, in healthy volunteers behavioral findings suggest that oxytocin may have direct effects on social attention. Oxytocin has been shown to increase attention to the eye region (Guastella et al., 2008)—although this is disputed (Domes et al., 2013)—increase attention toward emotional faces that shift gaze (Tollenaar et al., 2013), and increase vigilance to happy faces at early stages of attentional processing (Domes et al., 2013).
Maladaptive allocation of attention in socio-emotional processing is also associated with clinical conditions such as anxiety (Bar-Haim et al., 2007). According to Rapee and Heimberg’s (1997) influential model of social phobia, a bias to allocate attention—preferentially to social threat cues—may maintain the disorder, with enhanced threat processing exacerbating anxiety symptoms (Rapee and Heimberg, 1997). Several studies demonstrate initial orientating and vigilance toward threat-related social stimuli in highly socially anxious (HSA) individuals (Mogg and Bradley, 2002; Mogg et al., 2004; Gamble and Rapee, 2010; Staugaard, 2010). However, this bias is not invariably detected, and sometimes may operate for a wider range of emotional social cues, including happy faces (Staugaard, 2010). Attentional bias has potential therapeutic importance, as clinical trials indicate that attention training interventions which reduce attention bias to social threat cues are effective in reducing social anxiety symptoms (Schmidt et al., 2009). Studies of the neural correlates of attentional bias to threat indicate that the bias occurs at early stages of information processing (Rossignol et al., 2013) and is likely modulated by the amygdala (Carlson et al., 2009). Coupled with evidence of hyperactivity of the amygdala to faces in patients with SAD (Yoon et al., 2007), which is reduced by oxytocin to similar activity levels as seen in healthy controls (Labuschagne et al., 2010), this provides ground to investigate the effects of oxytocin on attentional bias to facial stimuli in social anxiety.
The primary aims of the current study were to investigate the effects of oxytocin on attentional bias in HSA individuals, using a dot-probe task. Considering prior theory (Rapee and Heimberg, 1997) and evidence of increased attention to threat faces in social anxiety (Mogg and Bradley, 2002; Mogg et al., 2004), we predicted that, at placebo baseline, HSA individuals would show greater attentional bias to threat faces compared to healthy controls (Hypothesis 1). Moreover, given findings that oxytocin attenuates hyperactivity of the amygdala to fear cues in HSA individuals (Labuschagne et al., 2010), that the amygdala mediates attention to fear cues in a dot-probe paradigm (Carlson et al., 2009), and that oxytocin increases attention to positive and negative emotional cues in healthy volunteers (Domes et al., 2013; Tollenaar et al., 2013), we also predicted that oxytocin would reduce the difference in attentional bias between the HSA and control groups for all emotional stimuli (Hypothesis 2).
Materials and methods
Participants
Twenty-six controls, average age 26.00 years (standard deviation = 6.32, age range 18–42 years old) and 16 HSA participants, average age 27.13 (standard deviation = 9.25, age range 19–51 years old) were included in the study. Of the 16 HSA participants, 10 were diagnosed with generalized SAD by the study psychiatrists (see Procedure for details). All participants were recruited via the Internet and advertisements at the University of Cambridge and surrounding area.
Control participants were excluded from participation if they had any current or previous history of DSM IV–Axis I disorders (as verified by the Mini International Neuropsychiatric Interview; Sheehan et al., 1998; administered by a psychiatrist), any other physical medical condition, history of alcohol or substance abuse (within 12 months of study entry), smoked, and/or were currently prescribed medication known to affect brain function.
HSA participants were excluded if they were currently being prescribed medication, smoked, were suffering from a current depressive episode (evident ≤6 months) or alcohol/substance abuse (within 12 months of study entry), or another anxiety disorder (e.g. generalized anxiety disorder, specific phobia, or panic disorder) that was more clinically salient or preceded their social anxiety. Participants were also excluded if they had a history of obsessive-compulsive disorder, body dysmorphic disorder, post-traumatic stress disorder, bipolar disorder, psychotic disorder, mental retardation, developmental disorder, or any other physical medical condition.
All participants provided written informed consent and the study was conducted in accordance with the UK National Research Ethics Committee (REC Reference: 10/H0308/77) and NHS Research and Development guidelines. This study was exempt from clinical trials status by the Medicines and Healthcare products Regulatory Agency.
Study Design
This study utilized a double-blind, randomized, placebo-controlled, within-group study design whereby participants were tested under two acute treatment conditions separated by at least one week. Treatment conditions included an active intranasal oxytocin spray (24IU, 40.32 micrograms, Syntocinon-spray, Novartis) and a placebo (containing all ingredients except for the peptide), both administered with three actuations to each nostril (4IU, 6.72 micrograms each). Oxytocin and placebo administration were fully counterbalanced across both control and HSA groups.
Procedure
All participants attended a screening session followed by two nasal-spray study visits. Prior to or during screening sessions all participants who presented as socially anxious were assessed using the Liebowitz Social Anxiety Scale (Liebowitz, 1987). A score of >70 (including >30 on the social situations subscale) was required to be included in the study as a HSA participant. Any participant who scored below these thresholds was excluded, as in previous research (Dodhia et al., 2014). Those participants that reached criteria on the Liebowitz Social Anxiety Scale were then assessed using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998) for diagnosis of generalized SAD at the screening session; however, a diagnosis of generalized SAD was not required to take part in the study. Finally, participants could not reach criteria for body dysmorphic disorder as assessed by the Body Dysmorphic Disorder Questionnaire-Dermatology Version (Dufresne et al., 2001), verified by the study psychiatrist. Participants were also administered the Beck Depression Inventory–II (Beck et al., 1996) and the National Adult Reading Test (Nelson and Willison, 1991) at screening sessions. Control participants were assessed using the same measures and procedures; however, study psychiatrists ensured that these participants did not suffer from any DSM IV–Axis I disorders and were fit for participation.
Prior to study visits all participants were asked to refrain from caffeine on the day of testing and alcohol was not permitted 24 hours prior to the session. At study visits participants completed three pre-drug questionnaires: the Bond and Lader Visual Analogue Mood Scale (VAS; Bond and Lader, 1974), State-Trait Anxiety Inventory (STAI; Spielberg et al., 1983), and the Positive and Negative Affective Schedule (PANAS; Crawford and Henry, 2004). The VAS consists of 16 visual analogue scales that assess current mood. Items were combined into three main scores: alertness, contentedness, and calmness. The PANAS is a 20 item self-report measure that assesses the extent to which a person feels positive affect or negative affect at that moment. The state and trait versions of the STAI measure how anxious the participant is feeling at that moment and in general, respectively, rated on a four-point Likert scale. Following a brief medical assessment, nasal sprays were self-administered—supervised by a medical professional—and participants were then advised to rest for 45 minutes, consistent with previous studies (Bethlehem et al., 2013) and physiological evidence (Born et al., 2002). After this break, participants were again medically assessed and task administration began. Following cognitive testing, participants completed post-drug questionnaires (STAI, PANAS, and VAS) and asked whether they thought they received oxytocin or placebo at the session.
Experimental Task
The dot-probe task (MacLeod et al., 1986) is a well-established paradigm for measuring attentional bias. The current task was modeled on that used in previous research (Mogg and Bradley, 2002; Mogg et al., 2004). In brief, participants were presented with face pairs comprising emotional faces (angry, fearful, and happy) each paired with a neutral face of the same identity. Following the offset of the face pair a probe replaced the central location of a previously presented face. Participants responded, via button press, to the location of the probe stimulus (either left or right; see Figure 1).
Figure 1.
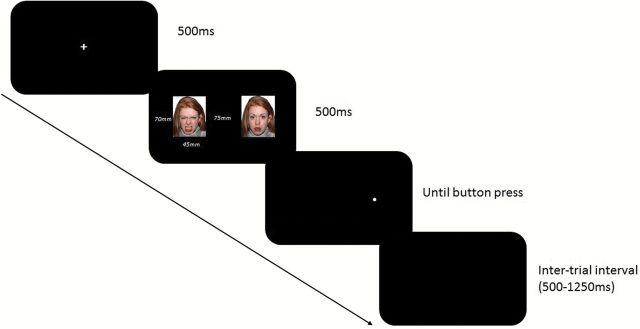
Illustration of trial events on dot-probe task, showing an angry-neutral face pair displayed prior to the probe, which on this trial replaces the neutral face (not drawn to scale).
Color photographs of 24 individuals were selected from the NimStim stimulus set (Tottenham et al., 2009; contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set) to construct the face pairs for the emotional conditions. In addition, a further 4 individuals were used to construct distractor face pairs, where two neutral expressions of the same individual were paired. Each face pair was presented 8 times.
Each trial began with a fixation cross-followed by a face pair (emotion paired with a neutral face). Face stimuli were presented in color on a black background of approximately 55mm x 70mm, with a 75mm distance between them. After the face pair disappeared, a dot then appeared in the central location of one of the face stimuli, either congruent (same location as emotion face) or incongruent with the emotion face (same location as the neutral face), and was displayed until button press response. Distance between dot-probe positions was 125mm. Participants then indicated the location of the probe (either on the left- or the right-hand side of the screen). A blank screen was then presented at randomly varying inter-trial intervals (500-1250ms, averaging 1000ms). The task was fully counterbalanced for probe congruency to emotional faces and for probe location.
In total there were 16 practice trials (different stimuli, including feedback on error trials), 224 experimental trials (presented in a new, random order for each participant; no feedback) and four buffer trials (two at the beginning of the experimental trials and two after a short mid-task break of one minute), for a total of 244 trials, taking under 14 minutes to complete. The experiment was run on a Sahara Slate PC i400 Series using Presentation and a Cedrus RB-830 Response Pad was utilized for participants to indicate left or right.
Statistical Analysis
Median reaction times were calculated for each experimental condition as a function of emotional face type, face location, and probe location, as in previous studies (Browning et al., 2010). For each emotional face type, the attentional bias score is calculated by subtracting the median reaction times when the probe replaces the emotional face from the median reaction time when the probe replaces the neutral face. Positive values of an attentional bias score indicate that participants are quicker to respond to the probe that replaces an emotional face (vigilance to emotional face, relative to neutral face), and negative values indicate that participants are quicker to respond to probes that replace the neutral face (avoidance of emotional face, relative to neutral face). Bias scores were calculated for each condition: happy, fearful, and angry faces for both oxytocin and placebo sessions. Erroneous responses were excluded from analysis, comprising 1.2% of the data, and did not differ significantly across groups or study visits. Hypothesis 1 was evaluated using an independent t-test to compare groups on attentional bias scores for threat (angry and fearful) faces averaged in the placebo baseline condition. To test Hypothesis 2, attentional bias scores were entered into a 2 group (HSA, control) x 2 drug (oxytocin, placebo) x 3 facial emotion (angry, fearful, happy) x 2 drug order (oxytocin first, placebo first) mixed ANOVA. Drug order is entered as a between-subjects factor to account for learning and order effects. If interactions were significant, post-hoc comparisons were implemented and were corrected using an adjusted p value of .01 to account for multiple comparisons.
Groups were compared on age, National Adult Reading Test scores, and Beck Depression Inventory–II scores using independent t-tests. Subjective mood and anxiety (PANAS, VAS, and STAI) were assessed twice: pre- and post-drug administration. Separate analyses were conducted for PANAS positive and negative affect, each VAS score, and STAI state and trait anxiety scores. All study visit mood and anxiety questionnaires were analyzed using 2 drug (oxytocin, placebo) x 2 group (HSA, control) x 2 time (pre, post) mixed ANOVAs. For all questionnaires, if main interactions were significant post-hoc pairwise comparisons were made and Bonferroni corrected for multiple comparison.
RESULTS
Demographics, Mood, and Anxiety Questionnaires
Groups did not significantly differ in age (p = .64) or IQ (p = .42). Compared with controls the HSA group demonstrated significantly greater trait (F[1,40] = 20.13, p < .01) and state (F[1,40] = 17.02, p < .01) anxiety across the sessions as measured by the STAI, and lower mood as demonstrated by the Beck Depression Inventory–II, t(40) = -3.89, p < .01. Moreover, oxytocin did not affect participants’ subjective ratings of mood or anxiety. See Supplemental Materials S1 for more detail and means tables.
Attentional Bias Scores
Mean attentional bias scores for each condition are outlined in Table 1. To test Hypothesis 1 we used a planned independent t-test, which demonstrated that, post placebo, HSA participants showed increased attentional bias toward threat cues (averaged across angry and fearful faces) compared to controls (t[40] = 2.13, p =.04, d = .67, 2-tail uncorrected for hypothesis-driven test). The 2 x 2 x 3 x 2 ANOVA of bias scores to test Hypothesis 2 revealed a significant group x drug interaction, which did not interact with type of facial emotion (F[1,38] = 10.58, p < .01, η2 = .22). There were no significant main effects of drug (p = .57), emotion (p = .62), or group (p = .32). Furthermore, there was no main effect of drug order (p = .70) and were no interactions of drug order with any other variables. To clarify the group x drug interaction, post-hoc independent sample t-tests revealed that, in the placebo condition, HSA participants demonstrate significantly increased attentional bias toward emotional faces in general (averaged across angry, fearful, and happy faces) compared to controls (t[40] = 2.63, p = .01, d = .83; see Figure 2, which illustrates this significant group difference in the placebo condition). However, following oxytocin there is no significant difference between the two groups in attentional bias scores for emotional faces (p = .41). Moreover, mean attentional bias scores of HSA participants following oxytocin were not significantly different to bias scores of the control group following placebo (p = .18). Furthermore, a paired-sample t-test revealed that attentional bias scores of the control group were significantly higher post-oxytocin than post-placebo (p = .01, d = .59). In the HSA group, attentional bias scores tended to reduce post-oxytocin compared with post-placebo, although this trend did not reach significance (p = .10, d = .43; Figure 2).
Table 1.
Mean attentional bias scores for each condition.
| Placebo | Oxytocin | ||||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | ||
| HSA Group | Happy | 3.23 | 14.11 | -1.67 | 13.98 |
| Fearful | 3.50 | 14.48 | 4.02 | 15.70 | |
| Angry | 5.83 | 10.09 | -0.78 | 8.95 | |
| Control Group | Happy | -5.86 | 15.47 | 5.53 | 14.33 |
| Fearful | -1.14 | 8.61 | 2.91 | 11.82 | |
| Angry | -0.63 | 12.56 | -0.31 | 17.24 | |
S.D., standard deviation; HSA, highly socially anxious
Figure 2.
Mean attentional bias scores for drug x group interaction. * indicates significant difference (p < .01); ns indicates non-significant.
Discussion
This study investigated the effects of oxytocin on attentional biases in highly socially anxious individuals and healthy controls. At placebo baseline, HSA individuals demonstrate greater attentional bias toward threat cues, as well as toward emotion faces in general, compared to healthy controls. However, following oxytocin administration the groups did not differ in their overall attentional bias to emotional faces. Indeed, following oxytocin HSA individuals demonstrated similar levels of attentional bias to emotional faces as controls at placebo baseline, suggesting normalization of attentional bias.
Our results indicate that, at placebo baseline, social anxiety was associated with significantly greater attentional bias to threat cues, consistent with previous research (Staugaard, 2010). The amygdala and prefrontal cortex modulate initial orientating of attention to threat-related stimuli in dot-probe paradigms (Carlson et al., 2009) and dysfunction of this network could underlie clinical disorders, such as anxiety. Indeed, evidence suggests that limbic hyperactivity to social stimuli could indicate a biological marker for SAD (Yoon et al., 2007), and this hyperactivity is hypothesised to drive attentional bias. Furthermore, areas including the prefrontal cortex have been shown to modulate attentional vigilance in a top-down fashion (Browning et al., 2010). Moreover, anxiety is associated with reduced recruitment of the prefrontal cortex in top-down control over threat (Bishop et al., 2004). Therefore, increased initial attention toward emotional stimuli coupled with difficulty in top-down modulation of attention in social anxiety could explain the observed attentional bias to emotional faces.
An attentional bias in social anxiety for emotional faces is not frequently reported (Staugaard, 2010), in general, although some research suggests that social anxiety symptoms are associated with increased attention towards both threat and happy faces (Bradley et al., 1999). Other evidence suggests that hyperactivity of the amygdala in social anxiety generalizes across different types of emotional valence (Yoon et al., 2007). Given that under conditions of uncertainty HSA individuals show greater difficulty classifying emotion (Button et al., 2013) and recognizing happy faces (Silvia et al., 2006), the experimental context may have contributed to this general emotional effect. Further research is needed to clarify the specific conditions under which attentional bias in social anxiety operates specifically for threat or for a more general range of emotional social cues.
This is the first study to suggest that oxytocin may have the potential to normalize attentional bias in HSA individuals, to levels seen in the healthy population. There is promising evidence for the therapeutic effects of oxytocin in social anxiety (Guastella et al., 2009). Oxytocinergic pathways in the brain project to several areas, specifically the emotion-regulating limbic system (McCarthy and Altemus, 1997) and amygdala (Huber et al., 2005). Moreover, intranasal oxytocin administration has been shown to increase central amygdala oxytocin levels (Neumann et al., 2013). Therefore it is hypothesised that attentional bias may be modulated by oxytocinergic increases in central sites, including the amygdala, which facilitates subsequent connectivity with prefrontal regions in HSA participants. Indeed oxytocin has been shown to normalize hyperactive amygdala response to threat-related cues (Labuschagne et al., 2010) and hyperactive medial prefrontal response to negative social cues (Labuschagne et al., 2012), as well as to reduce amygdala-frontal resting-state functional connectivity (Dodhia et al., 2014) in SAD patients. The latter studies are consistent with our findings and suggest a neural basis for the normalization of attentional bias observed in this study.
Oxytocin supports a wide range of central systems, including serotoninergic and dopaminergic function (Bethlehem et al., 2013). Therefore it is likely that oxytocin is interacting with these systems to facilitate normalization of attentional bias, as seen in this study. For instance, serotonin manipulations have been shown to effect attentional bias (Merens et al., 2007). Moreover, oxytocin has indeed been shown to mediate central serotonin production (Bethlehem et al., 2013). Therefore oxytocin could be exerting behavioral effects through interactions with other key neurotransmitters. It is further questioned whether the behavioral effects of oxytocin are indeed due to oxytocin receptor interactions; for example, oxytocin has been shown to exert effects through interactions with arginine vasopressin V1a receptors (Bowen and McGregor, 2014). The role of oxytocin in mediating behavioral effects in humans is clearly complex, considering its supporting role in many neural systems. These interactions could mediate the effects of oxytocin on attentional bias, as seen in the HSA group and typical volunteers in the current study.
It is noteworthy that in healthy controls, oxytocin increased attention to emotional faces. This is consistent with recent evidence demonstrating that oxytocin can increase vigilance to positive cues (Domes et al., 2013) or emotional cues, in general, in healthy volunteers (Tollenaar et al., 2013), as well as induce pro-social effects (Kirsch et al., 2005; Rimmele et al., 2009). In the current sample the effect of oxytocin increasing attentional bias in controls was significant, whereas its effect of decreasing attention bias in the HSA group was a trend that did not reach significance. This is likely a power issue and a methodological limitation of our study that warrants discussion. The sample for the HSA group was relatively small, although similar sample sizes have been used in previous studies (Mogg et al., 2004). Furthermore, we do demonstrate medium to large effect sizes across the study and that the results are not due to any learning or order effects, further substantiating the results. The sample was also exclusively male, in common with most other research investigating effects of oxytocin (Bethlehem et al., 2013). Exclusively male samples prevent assessment of sex differences in oxytocinergic function in humans (Carter, 2007) and side effects associated with the use of intranasal oxytocin in females. This limits generalizability of the present results to female HSA individuals.
A strength of this study is that it examined the effect of oxytocin on attentional biases for different types of emotional stimuli. In addition to happy faces, fearful and angry expressions were presented as they have previously been found to elicit consistent amygdala hyper-activation in SAD patients (Labuschagne et al., 2010). Previous studies investigating the modulation of oxytocin on attentional bias have used differing combinations of happy, angry, fearful, or sad faces (Ellenbogen et al., 2012; Domes et al., 2013). Researchers have noted that different negative expressions may elicit different action tendencies resulting in oxytocinergic expression-specific effects: e.g. sad faces primarily elicit empathic responses, whilst happy faces elicit approach response tendencies (Putman et al., 2004; Tollenaar et al., 2013). This highlights the importance of further research elucidating the effects of oxytocin on attentional bias, depending on which emotional cues are presented.
The results of this study have potential relevance for a therapeutic use of oxytocin in individuals who experience high levels of persistent social anxiety. Indeed, oxytocin has been identified as a potential treatment for social anxiety disorder (Guastella et al., 2009); however, there is evidence that oxytocin detrimentally affects people with borderline personality disorder (Bartz et al., 2011), and administering oxytocin has been shown to hinder processing rewards from social stimuli in the healthy population (Clark-Elford et al., 2014). There is also evidence that chronic peripheral infusion of oxytocin causes a reduction in central oxytocin receptor binding in mice (Peters et al., 2014). Further clinical trials are critically needed to assess the effects of oxytocin in such clinical conditions after consecutive doses and how this affects social functioning.
In conclusion, this is the first study to demonstrate that an acute administration of oxytocin reduces the difference between HSA and non-HSA individuals in attentional bias for emotional faces. We hypothesise that this effect is mediated by the modulatory effects of oxytocin on the amygdala–prefrontal cortex network, which supports emotion processing (Bethlehem et al., 2013). Further imaging and behavioral studies are required to assess the effects of repeated oxytocin administration on both social functioning and social information processing, including attentional biases, in socially anxious individuals.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
This study was co-funded by a PhD Studentship from the BBSRC and GSK awarded to Dr Clark-Elford. Dr Nathan is involved in funded grants and projects from UCB Pharma and GSK. Drs Baron-Cohen and Auyeung were supported by the MRC, in association with the NIHR CLAHRC EoE, during the period of this work. Drs Bradley and Mogg have received consultancy fees from GSK for research work, which is unrelated to this study. Dr Dudas has received speaker’s fees from GSK. Dr Müller has received consultancy fees from Eli Lilly, Heptares, and Shire. Dr Sahakian consults for Cambridge Cognition, Servier, and Lundbeck, holds a grant from Janssen/J&J, and has share options in Cambridge Cognition. No other authors have any conflicts of interest to disclose.
Supplementary Material
Acknowledgments
This work was supported by the BBSRC and GlaxoSmithKline (GSK). We acknowledge the Newcastle Specials Pharmacy Production Unit and the Addenbrooke’s Hospital Pharmacy for the supply and dispensation of oxytocin and placebo nasal sprays, and the Addenbrooke’s Clinical Research Centre and the Herchel Smith Building Clinical Suite. We also thank Dr Dane Rayment for his support. Finally, we are grateful to all the participants that took part in the study.
References
- Bar-Haim Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol Bull 133:1–24. [DOI] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. (2011). Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci 6:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. (1996). Manual for Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bethlehem RA, Baron-Cohen S, van Honk J, Auyeung B, Bos PA. (2014). The oxytocin paradox. Front Behav Neurosci 8:48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S. (2013). Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology 38:962–974. [DOI] [PubMed] [Google Scholar]
- Bishop Duncan J, Brett M, Lawrence AD. (2004). Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci 7:184–188. [DOI] [PubMed] [Google Scholar]
- Bond A, Lader M. (1974). The use of analogue scales in rating subjective feelings. Br J Med Psychol 47:211–217. [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. (2002). Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5:514–516. [DOI] [PubMed] [Google Scholar]
- Bowen MT, McGregor IS. (2014). Oxytocin and vasopressin modulate the social response to threat: a preclinical study. Neuropsychopharmacologicum 17:1621–1633. [DOI] [PubMed] [Google Scholar]
- Bradley Mogg K, White J, Groom C, de Bono J. (1999). Attentional bias for emotional faces in generalized anxiety disorder. Br J Clin Psychol 38(3):267–278. [DOI] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. (2010). Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biol Psychiatry 67:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K, Lewis G, Penton-Voak I, Munafo M. (2013). Social anxiety is associated with general but not specific biases in emotion recognition. Psychiat Res 210:199–207. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Reinke KS, Habib R. (2009). A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia 47:1386–1389. [DOI] [PubMed] [Google Scholar]
- Carter CS. (2007). Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behavioural Brain Research 176:170–186. [DOI] [PubMed] [Google Scholar]
- Clark-Elford R, Nathan PJ, Auyeung B, Voon V, Sule A, Muller U, Dudas R, Sahakian BJ, Phan KL, Baron-Cohen S. (2014). The effects of oxytocin on social reward learning in humans. Int J Neuropsychopharmacol 17:199–209. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. (2004). The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol 43:245–265. [DOI] [PubMed] [Google Scholar]
- Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, Phan KL. (2014). Modulation of Resting-State Amygdala-Frontal Functional Connectivity by Oxytocin in Generalized Social Anxiety Disorder. Neuropsychopharmacology 39:2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. (2007). Oxytocin improves “mind-reading” in humans. Biol Psychiatry 61:731–733. [DOI] [PubMed] [Google Scholar]
- Domes G, Sibold M, Schulze L, Lischke A, Herpertz SC, Heinrichs M. (2013). Intranasal oxytocin increases covert attention to positive social cues. Psychol Med 43:1747–1753. [DOI] [PubMed] [Google Scholar]
- Dufresne RG, Phillips KA, Vittorio CC, Wilkel CS. (2001). A screening questionnaire for body dysmorphic disorder in a cosmetic dermatologic surgery practice. Dermatol Surg 27:457–462. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Linnen AM, Grumet R, Cardoso C, Joober R. (2012). The acute effects of intranasal oxytocin on automatic and effortful attentional shifting to emotional faces. Psychophysiology 49:128–137. [DOI] [PubMed] [Google Scholar]
- Gamble AL, Rapee RM. (2010). The time-course of attention to emotional faces in social phobia. J Behav Ther Exp Psychiatry 41:39–44. [DOI] [PubMed] [Google Scholar]
- Guastella, Howard AL, Dadds MR, Mitchell P, Carson DS. (2009). A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology 34:917–923. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. (2008). Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry 63:3–5. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. (2005). Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308:245–248. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25:11489–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35:2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. (2012). Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int J Neuropsychopharmacol 15:883–896. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd (2009). Oxytocin: the great facilitator of life. Prog Neurobiol 88:127–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR. (1987). Social phobia. Mod Probl Pharmacopsychiatry 22:141–173. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. (1986). Attentional bias in emotional disorders. J Abnorm Psychol 95:15–20. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Altemus M. (1997). Central nervous system actions of oxytocin and modulation of behavior in humans. Mol Med Today 3:269–275. [DOI] [PubMed] [Google Scholar]
- Merens W, van der Does AJW, Spinhoven P. (2007). The effects of serotonin manipulations on emotional information processing and mood. J Affect Disorders 103:43–62. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. (2002). Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther 40:1403–1414. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. (2004). Selective attention to angry faces in clinical social phobia. J Abnorm Psychol 113:160–165. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison J. (1991). National adult reading test (NART), 2nd ed. Windsor, UK: NFER-Nelson. [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38:1985–1993. [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. (2014). Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology 42:225–236. [DOI] [PubMed] [Google Scholar]
- Putman P, Hermans E, van Honk J. (2004). Emotional stroop performance for masked angry faces: it’s BAS, not BIS. Emotion 4:305–311. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG. (1997). A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther 35:741–756. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. (2009). Oxytocin makes a face in memory familiar. J Neurosci 29:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol M, Campanella S, Bissot C, Philippot P. (2013). Fear of negative evaluation and attentional bias for facial expressions: an event-related study. Brain Cogn 82:344–352. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. (2008). Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology 33:368–374. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Richey JA, Buckner JD, Timpano KR. (2009). Attention training for generalized social anxiety disorder. J Abnorm Psychol 118:5–14. [DOI] [PubMed] [Google Scholar]
- Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, Domes G. (2011). Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology 36:1378–1382. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- Silvia PJ, Allan WD, Beauchamp DL, Maschauer EL, Workman JO. (2006). Biased recognition of happy facial expressions in social anxiety. J Soc Clinical Psychol 25:585–602 [Google Scholar]
- Spielberg CD, Gorsuch R. L, Lushene R. E. (1983). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologist Press. [Google Scholar]
- Staugaard SR. (2010). Threatening faces and social anxiety: a literature review. Clin Psychol Rev 30:669–690. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Chatzimanoli M, van der Wee NJ, Putman P. (2013). Enhanced orienting of attention in response to emotional gaze cues after oxytocin administration in healthy young men. Psychoneuroendocrinology 38:1797–1802. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res 168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. (2007). Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Res 154:93–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



