Abstract
Background:
Depression and related disorders are characterized by deficits in behavioral activation, exertion of effort, and other psychomotor/motivational dysfunctions. Depressed patients show alterations in effort-related decision making and a bias towards selection of low effort activities. It has been suggested that animal tests of effort-related decision making could be useful as models of motivational dysfunctions seen in psychopathology.
Methods:
Because clinical studies have suggested that inhibition of catecholamine uptake may be a useful strategy for treatment of effort-related motivational symptoms, the present research assessed the ability of bupropion to increase work output in rats responding on a test of effort-related decision-making (ie, a progressive ratio/chow feeding choice task). With this task, rats can choose between working for a preferred food (high-carbohydrate pellets) by lever pressing on a progressive ratio schedule vs obtaining a less preferred laboratory chow that is freely available in the chamber.
Results:
Bupropion (10.0–40.0 mg/kg intraperitoneal) significantly increased all measures of progressive ratio lever pressing, but decreased chow intake. These effects were greatest in animals with low baseline levels of work output on the progressive ratio schedule. Because accumbens dopamine is implicated in effort-related processes, the effects of bupropion on markers of accumbens dopamine transmission were examined. Bupropion elevated extracellular dopamine levels in accumbens core as measured by microdialysis and increased phosphorylated dopamine and cyclic-AMP related phosphoprotein 32 kDaltons (pDARPP-32) immunoreactivity in a manner consistent with D1 and D2 receptor stimulation.
Conclusion:
The ability of bupropion to increase exertion of effort in instrumental behavior may have implications for the pathophysiology and treatment of effort-related motivational symptoms in humans.
Keywords: dopamine, nucleus accumbens, depression, fatigue, animal models
Introduction
Depression is marked by various emotional and cognitive symptoms but is also characterized by effort-related motivational and psychomotor symptoms, including psychomotor retardation, anergia, lassitude, and fatigue (Stahl 2002; Caligiuri et al., 2003; Demyttenaere et al., 2005; Treadway and Zald 2011; Fava et al., 2013). The severity of effort-related symptoms in depression is correlated with problems with employment, social function, and treatment outcomes (Tylee et al., 1999; Stahl 2002), and these motivational symptoms are highly resistant to treatment in many people (Stahl 2002; Fava et al., 2013). Moreover, recent papers emphasize that many people with major depression have fundamental deficits in exertion of effort in reward seeking that do not depend simply upon problems with experiencing pleasure in response to a primary motivational stimulus (Treadway and Zald, 2011; Treadway et al., 2012a; Argyropoulos and Nutt, 2013). The neural basis of the effort-related dysfunctions in depression is still being characterized, but evidence implicates central dopamine (DA), striatal areas, and cortical mechanisms (Hickie et al., 1999; Caligiuri and Ellwanger et al., 2000; Schmidt et al., 2001; Volkow et al., 2001; Salamone et al., 2006, 2007; Tellez et al., 2008; Treadway and Zald, 2011). Moreover, effort-related motivational symptoms appear to be some of the most common psychiatric symptoms observed in general medicine (Demyttenaere et al., 2005) and are seen in multiple disorders, including schizophrenia, Parkinsonism, and multiple sclerosis (Friedman et al., 2007; Tellez et al., 2008; Gold et al., 2013). Because of the clinical significance of these activational or effort-related motivational symptoms and the growing emphasis on identifying neural circuits related to specific psychiatric symptoms (ie, the Research Domain Criterion approach), it is critical to develop animal models that assess effort-related processes.
It has been suggested that animal tests of effort-related decision-making could be useful for modeling motivational dysfunctions seen in psychopathology (Salamone et al., 2006, 2007; Salamone and Correa, 2012). Effort-based decision-making is studied with tasks offering choices between high-effort options leading to highly valued reinforcers vs low-effort/low-reward choices. With animals, such tasks include a T-maze barrier climbing task (Salamone et al., 1994; Cousins et al., 1996; Hauber and Sommer 2009; Mott et al., 2009; Pardo et al., 2012; Mai et al., 2012), effort discounting (Floresco et al., 2008; Bardgett et al., 2009), and operant procedures offering choices between responding on ratio schedules for preferred reinforcers vs approaching and consuming less preferred ones (Salamone et al., 1991, 2002; Schweimer and Hauber 2005; Randall et al., 2012). Research has highlighted the effort-related functions of DA systems, particularly accumbens DA. Across multiple tasks, low doses of DA antagonists and accumbens DA depletions or antagonism shift choice behavior, decreasing selection of high-effort/high-reward options and increasing selection of low-effort/low-reward choices (Salamone et al., 1994, 2007; Nowend et al., 2001; Mai et al., 2012; Pardo et al., 2012). Moreover, these effects of dopaminergic manipulations are not due to reinforcer devaluation or suppression of primary food motivation or appetite (Salamone et al., 1991, 2002; Koch et al. 2000; Sink et al., 2008; Randall et al., 2012; Nunes et al., 2013b). Recent studies have shown that reduced selection of high-effort activities in rats is induced by manipulations associated with depression, including muscarinic receptor stimulation (Nunes et al., 2013a; see also Janowski et al. 1994; Chau et al. 2001; Rada et al. 2006 for discussion of acetylcholine and depression), stress (Shafiei et al., 2012), injections of the proinflammatory cytokine interleukin-1β (Nunes et al., 2014), and administration of the catecholamine-depleting agent tetrabenazine (Nunes et al., 2013b). These observations are consistent with recent clinical data demonstrating that people with major depression show a reduced likelihood of selecting high-effort alternatives when assessed in human tests of effort-related choice (Treadway et al., 2012a).
Animal models of effort-based choice are also useful for the assessment of drug treatment strategies, and recent studies have investigated the effects of muscarinic acetylcholine and adenosine A2A receptor antagonists (Nunes et al., 2013a, 2013b, 2014) for their ability to reverse effort-related impairments. Another compound that has been assessed is the antidepressant bupropion, which is a drug that can inhibit catecholamine uptake and facilitate vesicular uptake of DA (Dwoskin et al., 2006). Bupropion has been shown to reverse the effects of tetrabenazine on effort-related choice behavior in rats tested on the T-maze barrier choice task (Yohn et al., in press), the concurrent fixed ratio 5/chow feeding choice task (Nunes et al., 2013b), and the concurrent progressive ratio (PROG)/chow feeding choice task (Randall et al., 2014). The evaluation of catecholamine uptake inhibitors on tests of effort-related function is important because of clinical research indicating that catecholamine uptake blockade can be a relatively effective strategy for the treatment of psychomotor/motivational symptoms (Rampello et al., 1991; Stahl, 2002; Demyttenaere et al., 2005; Papakostas et al., 2006; Pae et al., 2007; Fava et al., 2013). Thus, the present studies evaluated the ability of bupropion to enhance responding on a PROG/chow feeding choice task. There are several variants of this task (Schweimer and Hauber, 2005; Beeler et al., 2012), and the specific procedure used was one that has previously been employed to assess drug effects in our laboratory (Randall et al., 2012, 2014). This specific procedure was chosen, because the PROG schedule gradually increases the lever-pressing requirement and therefore presents the animal with a gradually incrementing work-related cost. In addition, this procedure generates enormous individual differences in performance (Randall et al., 2012, 2014), which allows for the differential assessment of drug effects in high vs low performers. Because of the literature implicating accumbens DA in effort-related processes (Salamone et al., 1997, 2003, 2007; Salamone and Correa 2012; Nunes et al., 2013a, 2013b), including performance on the PROG/chow feeding choice task (Randall et al., 2012, 2014), the effects of bupropion on pre- and postsynaptic markers of accumbens DA transmission also were examined (ie, microdialysis to measure extracellular DA and phosphorylated dopamine and cyclic-AMP related phosphoprotein 32 kDaltons (pDARPP-32) immunoreactivity as a marker of DA-related signal transduction).
Methods
Animals
Seventy-two adult male Sprague-Dawley rats were housed in a colony at 23°C with 12-h–light/–dark cycles (lights on at 7:000 am). Rats in experiment 1 (n=42) weighed 300 to 350g at the beginning of the study and were initially food restricted to 85% of their free-feeding body weight for training; they were fed supplemental chow to maintain weight throughout the study, with water available ad libitum in home cages, and were allowed modest weight gain throughout the experiment. All other rats (n=30) weighed 300 to 350g at the beginning of the study and had ad libitum access to laboratory chow and water in home cages. Animal protocols were approved by the University of Connecticut Institutional Animal Care and Use Committee and followed NIH guidelines (DHEW Publications, NIH, 80-23).
Pharmacological Agents and Dose Selection
Bupropion hydrochloride (Alfa Aesar, Ward Hill, MA) was dissolved in 0.9% saline solution that also served as the vehicle control. Doses were selected based on previous papers (Bruijnzeel and Markou, 2003; Nunes et al., 2013b).
Behavioral Procedures
Behavioral sessions were conducted in operant chambers (28×23×23cm3; Med Associates, Putney, VT) with 30-minute sessions 5d/wk. Rats were initially trained to lever press on a continuous reinforcement schedule (high-carbohydrate 45-mg pellets, Bio-serv, Frenchtown, NJ) and then shifted to the PROG schedule (Randall et al., 2012). For PROG sessions, the ratio started at fixed ratio (FR)1 and was increased by 1 additional response every time 15 reinforcements were obtained (FR1×15, FR2×15, etc.). A “time-out” feature deactivated the response lever if 2 minutes elapsed without a completed ratio. After 9 to 10 weeks of PROG training, chow was introduced. Weighed amounts of laboratory chow (Laboratory Diet, 5P00 Prolab RMH 3000, Purina Mills, St. Louis, MO; typically 15–20g) were concurrently available on the floor of the chamber during the PROG sessions. Chow intake was determined by weighing the remaining food (including spillage). Rats were trained on the PROG/chow feeding choice procedure for 4 to 5 weeks, after which drug testing began. On baseline and drug treatment days, rats consumed all the operant pellets that were delivered during each session.
Microdialysis and High-Performance Liquid Chromatography
Rats were anesthetized with a 1.0-mL/kg intraperitoneal (IP) injection of a solution containing 10.0mL of 100mg/mL ketamine plus 0.75mL of 20.0mg/mL xylazine (Phoenix Scientific, Inc., St. Joseph, MO). While in the stereotax (Kopf, Tujunga, CA; incisor bar 5.0mm above interaural line), rats received unilateral implantations of a 10.0-mm probe guide cannula (Bioanalytical Systems, Indianapolis, IN). The tips of the guide cannulae were implanted 2.0mm above accumbens core (anterior/posterior: +2.8mm, medial/lateral: ±1.4mm, dorsal/ventral: −5.8mm from bregma; counterbalanced left vs right) and secured to the skull with stainless steel screws and cement. Stainless steel stylets were inserted into the guide cannulae to maintain patency. Animals were housed in separate cages and allowed 7 days postsurgical recovery.
Rats with implanted cannulae were habituated in Plexiglas chambers (28×28×23cm3) the day before sampling for 8 hours with infusion pumps running. The following day, probes were inserted through the cannula, and artificial cerebrospinal fluid was pumped through at a rate of 2 μL/min (syringe pump, Harvard Apparatus, Cambridge, MA). Two hours postinsertion, sampling began and continued for 6 hours. After sampling, the probe was removed, and after euthanasia, histological analyses were performed to verify placements. Samples were frozen and analyzed for DA content using reverse-phase high-performance liquid chromatography with electrochemical detection (ESA, New Bedford, MA; Segovia et al., 2011; Nunes et al. 2013b). Each liter of mobile phase contained 27.6g sodium phosphate monobasic monohydrate, 8% methanol, 750 μL 0.1M ethylenediaminetetraacetic acid, and 2000 μL 0.4M sodium octyl sulfate dissolved in dH2O (pH=4.5). DA standards were assayed before, during, and after the dialysis samples.
Immunocytochemistry for Phosphorylated DA and c-AMP Related Phophoprotein-32 kDaltons (pDARPP-32)
Two hours after injection, rats were perfused with 3.7% formaldehyde and brains were extracted. Tissue was fixed in 3.7% formaldehyde overnight and moved to 30% sucrose cryo-protectant. Then 50-μm sections were cut using a cryostat. Tissue was washed in phosphate buffered saline (PBS) and bathed in blocking solution (0.1% Triton-X, 5.0% normal donkey serum, PBS) on a rotating shaker for 60 minutes, then washed for 15 minutes in PBS, and incubated in primary antibody (donkey anti-rabbit Thr34, donkey anti-rabbit Thr75, 1:500, Santa Cruz Bioscience, CA) with 0.1% Triton-X, 5.0% normal donkey serum, PBS, on a refrigerated rotating shaker for 24 hours. Next, tissue was washed for 15 minutes in PBS and incubated in secondary antibody (Alexaflour anti-rabbit 488 for pDARPP-32(Thr34) and pDARPP-32(Thr75), 1:200, Life Scientific) with 0.1% Triton-X, 5.0% normal donkey serum, PBS, on a rotating shaker for 120 minutes. Tissue was then washed for 15 minutes in PBS, wet mounted on slides, and dried overnight. Tissue was imaged on an Axio Imager-M2 fluorescence microscope. Images used for counting cells were 20× magnification. Cells were counted using a custom macro for ImageJ.
Experiment 1: The Effects of Bupropion on PROG/Chow Performance
Rats (n=42) were trained as described above and were run on the PROG/chow feeding choice procedure 5d/wk. On drug test days, they received IP injections of either saline vehicle or 10.0, 20.0, or 40.0mg/kg bupropion, 30 minutes before testing, using within-subjects design with each subject receiving all drug treatments, once per week, in a random order. Baseline training days were conducted on the other 4 days each week.
Experiment 2: Effects of Bupropion on nucleus accumbens Core DA in Untrained Rats as Measured by in-Vivo Microdialysis
Rats were implanted with dialysis guide cannulae and had dialysis probes inserted on the morning of the drug test as described above. On test days, rats (n=5/group) received injections of 20.0 or 40.0mg/kg bupropion or vehicle following the second baseline sample, and 6 additional dialysis samples were collected.
Experiment 3: Effects of Bupropion on NAc and Neostriatal DA Signaling in Untrained Rats as Measured by pDARPP-32 Immunoreactivity
Rats (n=5/group) received IP injections of saline vehicle or 20.0 or 40.0mg/kg bupropion 2 hours before perfusion for tissue analysis.
Statistical Analyses
Animals in experiment 1 were separated into high- and low-performance groups by a median split, and total lever presses, highest ratio achieved, active lever time, and chow consumption on the PROG/chow were analyzed with a 2 (performance group) × 4 (drug treatment) factorial analysis of variance (ANOVA) with repeated measures on the drug treatment factor. Based on the performance group × treatment interactions for all 4 behavioral variables analyzed, separate ANOVAs and nonorthogonal planned comparisons (Keppel, 1991) of each performance group were used to determine differences between each drug treatment vs vehicle. Previous research has shown that there is considerable variability in performance of the PROG/chow choice task (Randall et al., 2012), and the present work as well as recent studies indicate that baseline performance is a stable characteristic across many weeks of training (data not shown). Additionally, total lever presses under each drug treatment condition were analyzed between high and low performers using analysis of simple effects, and effect sizes (partial ε2) for each variable and performance group across all doses were calculated to determine the magnitude of the drug treatment effects across each variable and group. For the microdialysis experiment, DA levels were analyzed as percentage change from baseline, with the mean of the 2 samples immediately preceding the lever-pressing session serving as the 100% baseline level. Animals that had a variability of >35% from the mean during the 2 baseline samples were excluded from further analyses (2 animals were excluded that failed to meet this criterion). Factorial ANOVA with repeated measures on the time factor was used to analyze the DA data. In experiment 3, mean number of pDARPP-32(Thr34) and pDARPP-32(Thr34) positive cells across each region of interest were analyzed using simple ANOVA with posthoc analysis (Tukey) to determine differences between treatment levels.
Results
Experiment 1: Bupropion Increases PROG/Chow Responding
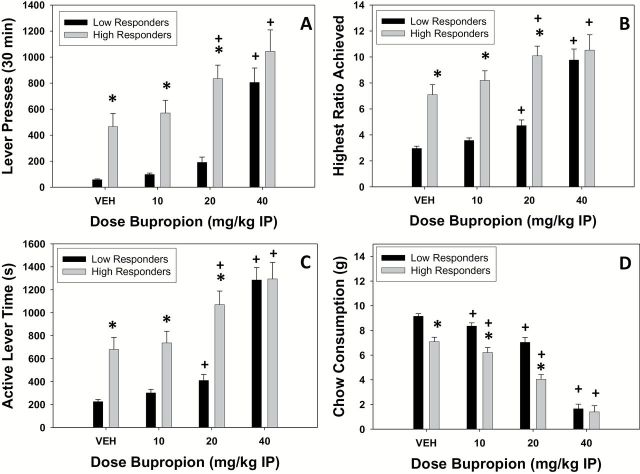
Rats were split into 2 performance groups (high vs low) using a median split of total lever presses under the vehicle condition and analyzed with ANOVA. There were significant performance group × drug treatment interactions for all 4 variables (total lever presses: F[3,120]=2.726, P<.05), highest ratio achieved (F[3,120]=7.201, P<.05), active lever time (F[3,120]=7.038, P<.05), and chow consumption (F[3,120]=7.904, P<.05). Based on these findings, high performers and low performers were separately analyzed with repeated-measures ANOVA to characterize performance in each group. There was a significant effect of treatment in high performers on total lever presses (F[3,60]=9.468, P<.05), highest ratio achieved (F[3,60]=7.015, P<.05), active lever time (F[3,60]=12.366, P<.05), and chow consumption (F[3,60]=66.461, P<.05). Planned comparisons demonstrated that total lever presses, highest ratio achieved, and active lever time were all increased at 20.0 and 40.0mg/kg bupropion compared with vehicle (P<.05). Furthermore, chow consumption was decreased in high performers at all doses compared with vehicle (P<.05). For low performers, there also were significant effects of treatment in low performers on total lever presses (F[3,60]=40.359, P<.05), highest ratio achieved (F[3,60]=46.128, P<.05), active lever time (F[3,60]=63.096, P < 0.05), and chow consumption (F[3,60]=160.489, P<.05). The highest ratio achieved and active lever time in low performers were both significantly increased at 20.0 and 40.0mg/kg bupropion compared with vehicle (P<.05; planned comparisons), whereas total lever presses were increased only at 40.0mg/kg compared with vehicle (P<.05). Furthermore, chow consumption in low performers was decreased at all doses of bupropion compared with vehicle (P<.05).
To further analyze differences between performance groups on each measure, individual drug treatment levels were analyzed. For lever pressing, ANOVA revealed that low performers responded less than high performers on vehicle (F[1,40]=15.822, P<.05), 10.0mg/kg (F[1,40]=22.796, P <.05), and 20.0mg/kg (F[1,40]=33.295, P<.05) but did not differ from high performers at 40.0mg/kg (F[1,40]=1.449, not significant). Similarly, for highest ratio achieved for low performers reached a lower ratio than high performers on vehicle (F[1,40]=27.644, P<.05), 10.0mg/kg (F[1,40]=36.370, P<.05), and 20.0mg/kg (F[1,40]=39.729, P<.05) but were not different from high performers at 40.0mg/kg bupropion (F[1,40]=0.274, n.s.). Moreover, low performers showed less active lever time compared with high performers on vehicle (F[1,40]=18.068, P<.05), 10.0mg/kg (F[1,40]=16.628, P<.05), and 20.0mg/kg (F[1,40]=25.698, P<.05) but not at 40.0mg/kg bupropion (F[1,40]=0.002, n.s.). Finally, with chow intake, low performers consumed significantly more chow compared with high performers on vehicle (F[1,40]=25.160, P<.05), 10.0mg/kg (F[1,40]=19.637, P<.05), and 20.0mg/kg (F[1,40]=30.121, P<.05) but not at 40.0mg/kg bupropion (F[1,40]=0.162, n.s.). Moreover, these performance group differences in the effects of bupropion were supported by measures of effect size (partial ε2), in which low performers showed a greater effect of bupropion on total lever presses, highest ratio achieved, active lever time, and chow consumption compared with high performers (Table 1).
Table 1.
Effect Sizes (Partial ε2): Low Performers Show Greater Effect of Bupropion on All Measures Compared with High Performers
| High Performers | Low Performers | |
|---|---|---|
| Total lever presses | 0.321 | 0.669 |
| Highest ratio achieved | 0.260 | 0.698 |
| Active lever time | 0.382 | 0.759 |
| Chow consumption | 0.769 | 0.889 |
Experiment 2: Bupropion Increases Extracellular DA in NAc Core in Untrained Animals
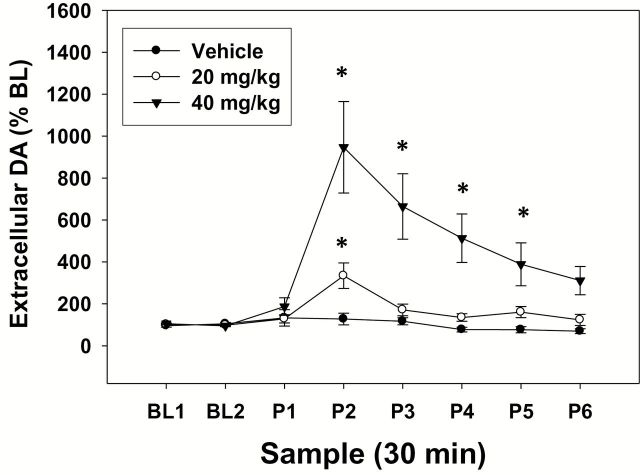
The results of the microdialysis study are shown in Figure 2. Factorial ANOVA with repeated measures on the sample factor demonstrated that there was a significant overall difference across samples (F[7,77]=12.815, P<.001) and a significant overall difference across treatment groups (ie, the different groups of rats receiving vehicle, 20.0 or 40.0mg/kg; F[2,11]=7.978, P<.01). Moreover, there was a significant sample × treatment interaction (ie, across the different dialysis samples and treatment groups; F[14,77]=6.656, P<.001). As a result of the significant interaction, each treatment group was separately analyzed with repeated-measures ANOVA. There was no difference across samples in vehicle-treated rats (F[7,21]=1.561, n.s.). However, there was a significant effect of treatment in both 20.0mg/kg (F[7,28]=7.669, P<.05) and 40.0mg/kg treated rats (F[7,28]=10.657, P<.05). Planned comparisons revealed that the postdrug sample 2 was significantly different from baseline in rats treated with 20.0mg/kg bupropion (P<.05) and that samples 2, 3, 4, and 5 were significantly different from baseline in rats treated with 40.0mg/kg bupropion (P<.05).
Figure 2.
Bupropion increases extracellular dopamine (DA) in NAc core. Mean (±SEM) extracellular DA (expressed as percent baseline) in 30-minute samples. Two baseline samples were collected prior to injection of vehicle or bupropion. Six samples were collected following injection. (* P<.05, different from baseline).
Experiment 3: Bupropion Increases Phosphorylated DARPP-32 Expression at Both Thr34 and Thr75 Residues
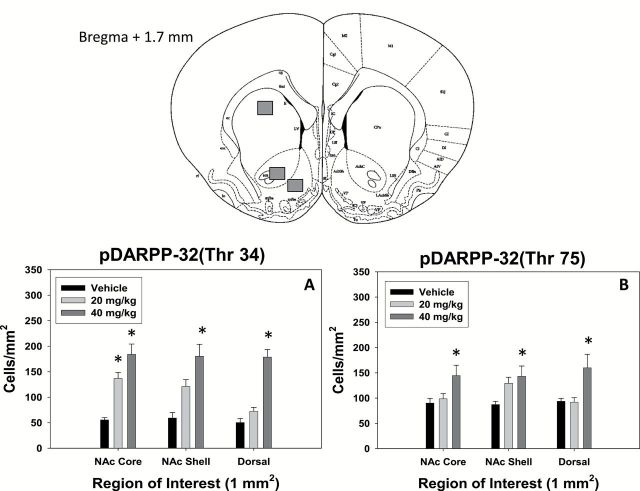
Figures 3 to 5 depict the effects of bupropion treatment on expression of pDARPP-32(Thr34) and pDARPP-32(Thr75) in accumbens core, accumbens shell, and overlying dorsal striatum (neostriatum). Bupropion increased signs of DA-related signal transduction at both D1 and D2 family receptors (Figures 3 and 4). ANOVA revealed a significant effect of drug treatment on pDARPP-32(Thr34) expression in accumbens core (F[2,12]=22.093, P<.05), shell (F[2,12]=12.862, P<.05), and dorsal striatum (F[2,12]=39.989, P<.05). Posthoc analysis (Tukey) revealed that pDARPP-32(Thr34) expression in the accumbens core was significantly increased at 20.0 and 40.0mg/kg (P<.05) compared with vehicle. These doses did not significantly differ from each other. In the accumbens shell and dorsal striatum, only 40.0mg/kg bupropion increased pDARPP-32(Thr34) expression over vehicle (P<.05). In addition, there was a significant effect of treatment on pDARPP-32(Thr75) expression in accumbens core (F[2,12]=4.191, P<.05), shell (F[2,12]=4.343, P<.05), and dorsal striatum (F[2,12]=5.473, P<.05). pDARPP-32(Thr75) expression in accumbens core, shell, and dorsal striatum was significantly increased by 40.0mg/kg bupropion (P<.05).
Figure 3.
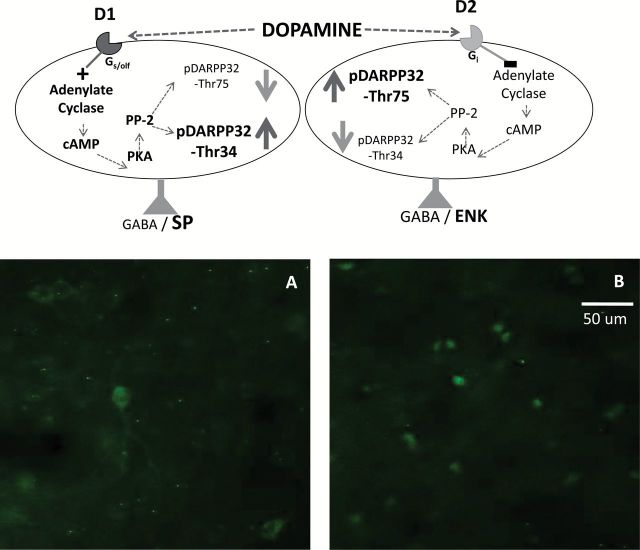
Top, DARPP-32 biochemistry in accumbens neurons containing dopamine (DA) D1 and D2 family receptors (see Bateup et al., 2008 for details). D1 receptor stimulation increases c-AMP production and protein kinase A (PKA) activity, which phosphorylates DARPP-32 to yield pDARPP-32(Thr34). D2 receptor stimulation decreases c-AMP production and protein kinase A activity, which decreases the dephosphorylation of pDARPP-32(Thr34) by protein phosphatase 2A (PP-2A), and therefore increases pDARPP-32(Thr75) expression. Bottom, High magnification photomicrographs of pDARPP-32(Thr34) (A) and pDARPP-32(Thr75) (B) staining in nucleus accumbens core, showing representative rats treated with 40.0mg/kg bupropion. Images were taken at 40× magnification. Scale bar=50 μm.
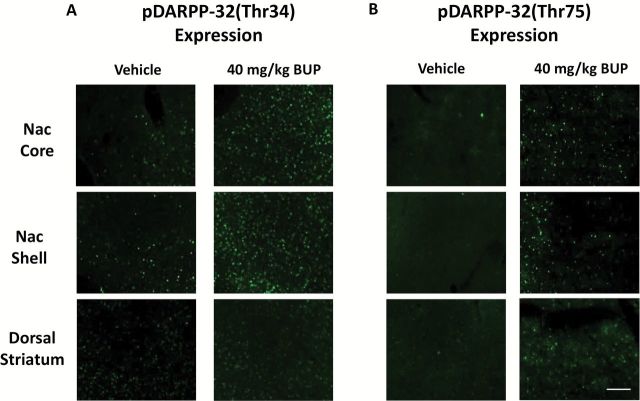
Figure 5.
Photomicrographs of pDARPP-32(Thr34) (A) and pDARPP-32(Thr75) (B) staining in each region of interest, showing representative vehicle treated (left column) and rats treated with 40.0mg/kg (right column).
Figure 4.
Top, Schematic diagrams of the relevant coronal section and specific brain regions depicted in bottom figures (based on the atlas of Paxinos and Watson, 1998). The squares indicate the placement of optical dissectors for counting pDARPP-32 positive cells. Bottom, Bupropion increases expression of pDARPP-32 at both Thr34 and Thr75 residues. A, Mean (±SEM) DARPP-32(Thr34) positive cells counted in each region of interest for each treatment group. B, Mean (±SEM) DARPP-32(Thr75) positive cells counted in each region of interest for each treatment group. (* P<.05, different from vehicle in each region of interest).
Discussion
Bupropion is a catecholamine uptake inhibitor that has been used for many years as an antidepressant (Dwoskin et al., 2006). Traditional rodent tests, such as forced swim and tail suspension, are sensitive to the effects of bupropion (Yamada et al., 2004; Kitamura et al., 2010). Bupropion (Wellbutrin) is frequently prescribed, and in 2010 it was reported to be the most commonly prescribed antidepressant in the United States (Milea et al., 2010). Moreover, this drug is particularly interesting because of evidence indicating that inhibition of DA uptake may be relatively effective for treating anergia, fatigue, or psychomotor symptoms observed in many depressed patients (Rampello et al., 1991; Stahl, 2002; Demyttenaere et al., 2005; Papakostas et al., 2006; Pae et al., 2007). Bupropion is capable of reversing the effort-related impairments induced by the vesicular monoamine transporter (VMAT)-2 inhibitor and DA-depleting agent tetrabenazine in rats (Nunes et al., 2013b; Randall et al., 2014). In contrast, the current studies investigated the effects of bupropion administered on its own to assess its ability to alter effort-based choice as measured by the PROG/chow feeding choice procedure. Bupropion shifted choice behavior and increased the tendency to work for food reinforcement, as marked by increases in all measures of PROG lever pressing (total lever presses, highest ratio achieved, and active lever time) (Figure 1) while decreasing consumption of the concurrently available chow. These data are consistent with a previous study showing that bupropion could increase food-reinforced responding on a conventional PROG schedule (Bruijnzeel and Markou, 2003). Nevertheless, as previously described (Randall et al., 2012), the PROG/chow feeding choice procedure provides additional information compared with conventional schedules, because the animal is given an explicit choice between lever pressing for food reinforcement and intake of an alternative food source (chow) as opposed to the choice between responding and not responding. In addition, the specific version of the PROG/chow feeding choice procedure used generates enormous individual variability, which is related to differences in drug response and markers of DA-related signal transduction (Randall et al., 2012). In the present study, the effects of bupropion were greater in low performers than high performers on all 4 behavioral measures, as indicated by the larger effect sizes seen with the low performers (Table 1). Moreover, despite the substantial differences in baseline performance on all variables, after injection of 40.0mg/kg bupropion, there were no significant differences between high and low performers on any behavioral measure, indicating that this dose of bupropion made the 2 performance groups roughly equal. These findings demonstrate the utility of the PROG/chow feeding choice procedure as a model for assessing the effort-related effects of antidepressant drugs.
Figure 1.
Effects of bupropion on progressive ratio (PROG)/chow performance in high and low responders. Mean (±SEM) number of total lever presses (A), highest ratio achieved (B), active lever time (in seconds; C), and chow consumption (in grams; D). (* P<.05, high responders different from low responders; + P<.05, bupropion different from vehicle in that specific performance group).
To better understand the effects of bupropion on DA signaling, 2 different neurochemical measures were employed. Experiment 2 assessed the effects of bupropion on extracellular DA concentrations in accumbens core using microdialysis. Accumbens core was studied, because it is the DA terminal area in the striatal complex of rats that appears to be most consistently linked to the regulation of effort-related choice behavior (Cousins et al., 1993; Font et al., 2008; Mingote et al., 2008; Farrar et al., 2010; Ghods-Sharifi et al., 2010; Hauber and Sommer, 2010; Randall et al., 2012). Bupropion significantly increased accumbens extracellular DA at both 20.0 and 40.0mg/kg, which were the behaviorally active doses in experiment 1. Furthermore, these increases were maximal during the same time span that an operant session would be taking place (30–60 minutes postinjection). The 20.0 dose of bupropion, which increased extracellular DA in nucleus accumbens by approximately 3-fold, significantly increased lever pressing in the high-performance group in experiment 1 but not the low-performance group. The 40.0-mg/kg dose of bupropion was needed to increase PROG lever pressing in the low-performance group, and the microdialysis study indicated that this dose produced a very large increase in extracellular DA (ie, 9- to 10-fold). These large increases in extracellular DA that were induced by the highest dose of bupropion are comparable with the increases seen with administration of other drugs that block DA uptake, including stimulants such as cocaine (Lenoir et al., 2007).
Experiment 3 assessed the effects of bupropion on accumbens and neostriatal pDARPP-32 immunoreactivity in untrained rats. The greatest effects were seen at 40.0mg/kg, though pDARPP-32(Thr34) expression in the accumbens core was significantly increased at both 20.0 and 40.0mg/kg, which was the same dose range as the behavioral effects observed in experiment 1. Both phosphorylated forms of DARPP-32 (ie, at the Thr34 and Thr75 amino acid residues) were responsive to bupropion treatment, although the magnitude of effects on pDARPP-32(Thr75) tended to be smaller than those seen with pDARPP-32(Thr34). Based on the microdialysis results showing increased extracellular DA after bupropion administration and on papers describing the role of DARPP-32 as a signaling protein (Svenningson et al., 2004; Bateup et al., 2008; Yger and Girault, 2011; Santerre et al., 2012; Segovia et al., 2012), it is likely that pDARPP-32(Thr34) increased after bupropion administration because of increased D1 receptor stimulation in substance P positive neurons, while pDARPP-32(Thr75) increased because of increased D2 receptor stimulation in enkephalin positive neurons (Figures 3 and 4). Thus, it appears that the doses of bupropion used in the present experiment increased DA transmission at multiple subtypes of medium spiny neurons (ie, those predominantly expressing D1 receptors and those expressing D2 receptors). However, double-labeling and tract-tracing methods would be necessary to confirm the efferent projection targets of these neurons.
Consistent with the hypothesis that bupropion is shifting choice behavior and increasing PROG lever pressing through actions on DA, previous studies have shown that interfering with DA transmission produces the opposite effect and decreases PROG lever pressing in rats responding on the PROG/chow feeding choice task. Administration of either D1 or D2 family antagonists, as well as the DA depleting agent tetrabenazine, all decrease PROG lever pressing in rats responding on the PROG/chow choice task (Randall et al., 2012, 2014). Although the effects of bupropion on preference between high-carbohydrate pellets and laboratory chow have not been studied, previous research has shown that other dopaminergic manipulations did not alter preference between the 2 different foods (Salamone et al., 1991; Nunes et al., 2013b). In addition, recent unpublished data from our laboratory indicate the high lever-pressing performance on the PROG/chow feeding choice task is not simply related to a higher degree of preference for high-carbohydrate food pellets relative to chow. Future research should also use choice tasks that involve selection of nonfeeding activities (eg, wheel running).
With increasing interest in the effort-related symptoms of depression (eg, Salamone et al., 2006; Treadway et al., 2011, 2012a), there is a growing need for treatments that effectively improve these symptoms. Bupropion can increase both DA and norepinephrine transmission (Hudson et al., 2012), but at this point, there is little evidence implicating norepinephrine in effort-related choice behavior, whereas considerable evidence supports a role for DA (Salamone et al., 2007; Salamone and Correa, 2012). Thus, the current findings showing that bupropion increases the tendency to work for food at doses that increase DA transmission are consistent with the suggested use of drugs that augment DA transmission as therapeutic treatments for effort-related motivational symptoms (Argyropoulos and Nutt, 2013; Soskin et al., 2013). As demonstrated here and in previous work (Nunes et al., 2013b; Randall et al., 2014), bupropion increases exertion of effort in otherwise untreated animals (experiment 1), and attenuates the effort-related deficits induced by low doses of tetrabenazine that deplete accumbens DA and reduce DA-related signal transduction in rats. These findings are consistent with clinical studies reporting that patients treated with bupropion show improvements in effort-related motivational symptoms (Papakostas et al., 2006; Pae et al., 2007) and that clinically relevant doses of bupropion occupy DA transporters in vivo (Learned-Coughlin et al., 2003). Furthermore, in the current studies, bupropion increased lever pressing in low responders, bringing them up to the same level as high responders; this finding is particularly important in view of the recent report indicating that poor performers on this task show lower levels of DA-related signal transduction [ie, pDARPP-32(Thr34) expression] in accumbens core compared with high responders (Randall et al., 2012). Moreover, the present results are consistent with previous studies indicating that amphetamine increases exertion of cognitive effort in animals with low baseline performance (Cocker et al., 2012). Future research should assess a wider variety of drugs for their ability to enhance exertion of effort, including novel DA uptake inhibitors, monoamine oxidase inhibitors such as deprenyl (Randall et al., 2014), adenosine A2A receptor antagonists (Randall et al., 2012; Nunes et al., 2013b, 2014), and drugs that inhibit norepinephrine and 5-HT uptake.
In summary, bupropion can increase the motivation to work for food reinforcement, particularly in animals with poor baseline performance. Together with studies using other behavioral procedures and drug treatments (Randall et al., 2012, 2014; Nunes et al., 2013a, 2013b), the present studies indicate that the PROG/chow feeding choice procedure is useful for the preclinical assessment of drug treatment of effort-related motivational symptoms in psychopathology. Moreover, bupropion exerts its behavioral effects in rats at doses that augment pre- and postsynaptic markers of DA transmission. These observations are consistent with the extensive body of evidence linking DA transmission to effort-related processes in animals (Salamone et al., 1994, 1997, 2003, 2007; Cagniard et al., 2006; Floresco et al., 2008; Mai et al., 2012; Salamone and Correa, 2012a; Nunes et al., 2013b; Trifilieff et al., 2013) and humans (Wardle et al., 2011; Treadway et al., 2012b) and with clinical studies indicating that DA transmission regulates effort-related motivational symptoms in depression and other disorders (Rampello et al., 1991; Brown and Gershon, 1993; Treadway and Zald, 2011; Argyropoulos and Nutt, 2013; Treadway and Pizzigali, 2014). Although motivational impairments are very common in depressed patients, they also are present across a wide spectrum of psychopathologies, including schizophrenia, Parkinsonism, multiple sclerosis, and immune system challenges (Salamone et al., 2006; Winograd-Gurvich et al., 2006; Dantzer et al., 2012; Gold et al., 2013; Markou et al., 2013; Nunes et al., 2014). Therefore, tests of effort-related choice behavior should not be viewed simply as animal models of depression per se. Instead, they are probably modeling a class of motivational symptoms that is characteristic of depression but also spans multiple disorders and conditions. This suggestion is consistent with the Research Domain Criteria approach, which places less emphasis on traditional diagnostic categories and instead focuses on the neural circuits mediating specific pathological symptoms (Cuthbert and Insel, 2013).
Statement of Interest
J. Salamone has received grants from Pfizer, Roche, Shire, and Prexa.
Acknowledgments
Many thanks to Hector Contreras for his help with conducting these experiments and to Regina Vontell, Nicole Segovia, Matthew Eastman, and Joanne Conover for assistance with the immunocytochemistry. This work was supported by a grant to J.D.S. from the National Institute of Mental Health (MH094966) and to M.C. from U. Jaume I (P1.1 A 2013-01) and UCONN SURF grants to M.R. and M.J.
References
- Argyropoulos SV, Nutt DJ. (2013). Anhedonia revisited: is there a role for dopamine-targeting drugs for depression? J Psychopharmacol 27:869–877. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. (2009). Dopamine modulates effort-based decision making in rats. Behav Neurosci 123:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwas M, Gong S, Nishi A, Heintz N, Greengard P. (2008). Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and anti-psychotic drugs. Nat Neuroscience 11:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, McCutcheon JE, Cao ZF, Murakami M, Alexander E, Roitman MF, Zhuang X. (2012). Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurosci 36:2533–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Gershon S. (1993). Dopamine and depression. J Neural Transm Gen Sect 91:75–109. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. (2003). Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse 50:20–28. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. (2006). Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology 31:1362–1370. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Ellwanger J. (2000). Motor and cognitive aspects of motor retardation in depression. J Affect Disord 57:83–93. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP, Gentili V, Eberson S, Kelsoe J, Rapaport M, Gillin JC. (2003). A quantitative neuromotor predictor of antidepressant non-response in patients with major depression. J Affect Disord 77:135–141. [DOI] [PubMed] [Google Scholar]
- Chau D, Rada PV, Kosloff RA, Taylor JL, Hoebel BG. (2001). Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonists in the Porsolt swim test. Neuroscience 104:791–798. [DOI] [PubMed] [Google Scholar]
- Cocker PJ, Hosking JG, Benoit J, Winstanley CA. (2012). Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacol 37:1825–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD. (1996). Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res 74:189–197. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. (2013). Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Meagher MW, Cleeland CS. (2012). Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol 9:414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K, De Fruyt J, Stahl SM. (2005). The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacology 8:93–105. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. (2006). Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 12:178–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar AM, Segovia KN, Randall PA, Nunes EJ, Collins LE, Stopper CM, Port RG, Hockemeyer J, Müller CE, Correa M, Salamone JD. (2010). Nucleus accumbens and effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience 166:1056–1067. [DOI] [PubMed] [Google Scholar]
- Fava M, Ball S, Nelson JC, Sparks J, Konechnik T, Classi P, Dube S, Thase ME. (2013). Clinical relevance of fatigue as a residual symptom in major depressive disorder. Depress Anxiety 31:250–257. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. (2008). Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology 33:1966–1979. [DOI] [PubMed] [Google Scholar]
- Font L, Mingote S, Farrar AM, Pereira M, Worden L, Stopper C, Port RG, Salamone JD. (2008). Intra-accumbens injections of the adenosine A(2A) agonist CGS 21680 affect effort-related choice behavior in rats. Psychopharmacology 199:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JH, Brown RG, Cornella C, Garber CE, Krupp LB, Lou JS, Marsh L, Nail L, Shulman L., Taylor CB. (2007). Fatigue in Parkinson’s disease: a review. Mov Disord 22:297–308. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Floresco SB. (2010). Differential effects on effort discounting induced by inactivations of the nucleus accumbens core or shell. Behav Neurosci 124:179–191. [DOI] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. (2013). Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry 74:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W, Sommer S. (2009). Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex 19:2240–2247. [DOI] [PubMed] [Google Scholar]
- Hickie I, Ward P, Scott E, Haindl W, Walker B, Dixon J, Turner K. (1999). Neo-striatal rCBF correlates of psychomotor slowing in patients with major depression. Psychiatry Res 92:75–81. [DOI] [PubMed] [Google Scholar]
- Hudson AL, Lalies MD, Silverstone P. (2012). Venlafaxine enhances the effect of bupropion on extracellular dopamine in rat frontal cortex. Can J Physiol Pharmacol 90:803–809. [DOI] [PubMed] [Google Scholar]
- Janowski DS, Overstreet DH, Nurnberger JI. (1994). Is cholinergic sensitivity a genetic marker for the affective disorders. Am J Med Genet 54: 335–344. [DOI] [PubMed] [Google Scholar]
- Keppel (1991). Design and analysis: a researcher’s handbook. 3rd ed. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Kitamura Y, Yagi T, Kitagawa K, Shinomiya K, Kawasaki H, Asanuma M, Gomita Y. (2010). Effects of bupropion of the forced swim test and release of dopamine in the nucleus accumbens in ACTH-treated rats. Naunyn Schmiedebergs Arch Pharmacol 382:151–158. [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. (2000). Role of nucleus accumbens (DA) D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology 152:67–73. [DOI] [PubMed] [Google Scholar]
- Learned-Coughlin SM, Bergström M, Savitcheva I, Ascher J, Schmith VD, Långstrom B. (2003). In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol Psychiat 54:800–805. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. (2007). Intense sweetness surpasses cocaine reward. PLoS One 2:e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai B, Sommer S, Hauber W. (2012). Motivational states influence effort-based decision making in rats: the role of dopamine in the nucleus accumbens. Cogn Affect Behav Neurosci 12:74–84. [DOI] [PubMed] [Google Scholar]
- Markou A, Salamone JD, Bussey TJ, Mar AC, Brunner D, Gilmour G, Balsam P. (2013). Measuring reinforcement learning and motivation constructs in experimental animals: relevance to the negative symptoms of schizophrenia. Neurosci BiobehavRev 37:2149–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milea D, Guelfucci F, Bent-Ennakhil N, Toumi M, Auray JP. (2010). Antidepressant monotherapy: a claims database analysis of treatment changes and treatment duration. Clin Ther 32:2057–2072. [DOI] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, Port RG, Sink KS, Bunce JG, Chrobak JJ, Salamone JD. (2008). Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci 28:9037–9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J, Muller CE, Salamone JD. (2009). The adenosine A2A antagonist MSX-3 reverses the effects of the dopamine antagonist haloperidol on effort-related decision making in a T-maze cost/benefit procedure. Psychopharmacology 204:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. (2001). D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav 69:373–382. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD. (2013a) Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neurosci Biobehav Rev 37:2015–2025. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Hart EE, Freeland C, Yohn S, Baqi Y, Müller CE, Lopez-Cruz L, Correa M, Salamone JD. (2013b) Effort-related motivational effects of the VMAT-2 inhibitor tetrabenazine: implications for animal models of the motivational symptoms of depression. J Neurosci 33:19120–19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Estrada A, Epling B, Hart EE, Lee CA, Baqi Y, Müller CE, Correa M, Salamone JD. (2014). Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: studies with the concurrent fixed ratio 5/ chow feeding choice task. Psychopharmacology 231:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae CU, Lim HK, Han C, Patkar AA, Steffens DC, Masand PS, Lee C. (2007). Fatigue as a core symptom in major depressive disorder: overview and the role of bupropion. Expert Rev Neurother 7:1251–1263. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M. (2006). Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry 60:1350–1355. [DOI] [PubMed] [Google Scholar]
- Pardo M, Lopez-Cruz L, Valverde O, Ledent C, Baqi Y, Müller CE, Salamone JD, Correa M. (2012). Adensoine A2A receptor antagonism and genetic deletion attenuate the effects of dopamine D2 antagonism on effort-related decision making in mice. Neuropharmacology 62:2068–2077. [DOI] [PubMed] [Google Scholar]
- Rada P, Colasante C, Skirzewski M, Hernandez L, Hoebel L. (2006). Behavioral depression in the swim test causes a biphasic, long-lasting change in accumbens acetylcholine release, with partial compensation by acetylcholinesterase and muscarinic-1 receptors. Neuroscience 141:67–76. [DOI] [PubMed] [Google Scholar]
- Rampello L, Nicoletti G, Raffaele R. (1991). Dopaminergic hypothesis for retarded depression: a symptom profile for predicting therapeutical responses. Acta Psychiatr Scand 84:552–554. [DOI] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, López Cruz L, Vemuri VK, Makriyannis A, Baqi Y, Müller CE, Correa M, Salamone JD. (2012). Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow task: pharmacological studies and role of individual differences. PLoS One 7:e47934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Nunes EJ, Yohn SE, Nowak V, Khan B, Shah P, Pandit S, Vemuri VK, Makriyannis A, Baqi Y, Müller CE, Correa M, Salamone JD. (2014) The VMAT-2 inhibitor tetrabenazine affects effort-related decision making in a progressive ratio/chow feeding choice task: reversal with antidepressant drugs. PLoS One 9:e99320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. (1991). Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology 104:515–521. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. (1994). Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res 65:221–229. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. (2002). Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology 160:371–380. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. (2006). Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: implications for understanding anergia and psychomotor slowing in depression. Curr Psychiat Rev 2:267–280. [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191:461–482. [DOI] [PubMed] [Google Scholar]
- Santerre JL, Nunes EJ, Randall PA, Baqi Y, Müller CE, Salamone JD. (2012). Behavioral studies with the novel adenosine A2A antagonist MSX-4: reversal of the effects of (DA) D2 antagonism. Pharmacol Biochem Behav 102:477–487. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nolte-Zenker B, Patzer J, Bauer M, Schmidt LG, Heinz A. (2001). Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry 32:66–72. [DOI] [PubMed] [Google Scholar]
- Schweimer J, Hauber W. (2005). Involvement of the rat anterior cingulate cortex in control of instrumental responses guided by reward expectancy. Learn Mem 12:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia KN, Correa M, Salamone JD. (2011). Slow phasic changes in nucleus accumbens dopamine release during fixed ratio acquisition: a microdialysis study. Neuroscience 196:178–188. [DOI] [PubMed] [Google Scholar]
- Segovia KN, Correa M, Lennington JB, Conover JC, Salamone JD. (2012). Changes in nucleus accumbens and neostriatal c-Fos and DARPP-32 immunoreactivity during different stages of food-reinforced instrumental training. Eur J Neurosci 35:1354–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei N, Gray M, Viau V, Floresco SB. (2012). Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology 37:2194–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. (2008). Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology 196:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soskin DP, Holt DJ, Sacco GR, Fava M. (2013). Incentive salience: novel treatment strategies for major depression. CNS Spectrums 18:307–314. [DOI] [PubMed] [Google Scholar]
- Stahl SM. (2002). The psychopharmacology of energy and fatigue. J Clin Psychiat 63:7–8. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. (2004). DARPP32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol 44:269–296. [DOI] [PubMed] [Google Scholar]
- Tellez N, Alonso J, Rio M, Tintore M, Nos C, Montalban X, Rovira A. (2008). The basal ganglia: a substrate for fatigue in multiple sclerosis. Neuroradiology 50:17–23. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Pizzagalli DA. (2014). Imaging the pathophysiology of major depressive disorder–from localist models to circuit-based analysis. Biol Mood Anxiety Disord 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. (2011). Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 35:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. (2012a) Effort-based decision making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol 121:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. (2012b) Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci 32:6170–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, Martinez D, Moore H, Balsam PD, Simpson EH, Javitch JA. (2013). Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry 18:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J. (1999). Identification of depressed patient types in the community and their treatment needs: findings from the DEPRES II (Depression Research in European Society II) survey. DEPRES Steering Committee. Int Clin Psychopharmacol 14:153–165. [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzermann R, Ding YS, Logan J. (2001). Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21:9414–9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. (2011). Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci 31:16597–16602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis, Bradshaw JL, White OB. (2006). Negative symptoms: a review of schizophrenia, melancholic depression and Parkinson’s disease. Brain Res Bull 70:312–321. [DOI] [PubMed] [Google Scholar]
- Yamada J, Sugimoto Y, Yamada S. (2004). Involvement of dopamine receptors in the anti-immobility effects of dopamine re-uptake inhibitors in the forced swimming test. Eur J Pharmacol 504:207–211. [DOI] [PubMed] [Google Scholar]
- Yohn SE, Thompson C, Randall PA, Lee CE, Muller CE, Baqi Y, Correa M, Salamone JD. (in press) The VMAT-2 inhibitor tetrabenazine alters effort-related decision making as measured by the T-maze barrier choice task: reversal with the adenosine A2A antagonist MSX-3 and the catecholamine uptake blocker bupropion. Psychopharmacology. [DOI] [PubMed] [Google Scholar]
- Yger M, Girault JA. (2011). DARPP-32, jack of all trades… master of which? Front Behav Neurosci 5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]