Abstract
Background:
Recent studies revealed that bipolar disorder may be associated with deficits of neuroplasticity. Additionally, accumulating evidence has implicated alterations of the intracellular signaling molecule protein kinase C (PKC) in mania.
Methods:
Using sleep deprivation (SD) as an animal model of mania, this study aimed to examine the possible relationship between PKC and neuroplasticity in mania. Rats were subjected to SD for 72h and tested behaviorally. In parallel, SD-induced changes in hippocampal cell proliferation were evaluated with bromodeoxyuridine (BrdU) labeling. We then examined the effects of the mood stabilizer lithium, the antipsychotic agent aripiprazole, and the PKC inhibitors chelerythrine and tamoxifen on both behavioral and cell proliferation impairments induced by SD. The antidepressant fluoxetine was used as a negative control.
Results:
We found that SD triggered the manic-like behaviors such as hyperlocomotion and increased sleep latency, and reduced hippocampal cell proliferation. These alterations were counteracted by an acute administration of lithium and aripiprazole but not of fluoxetine, and only a single administration of aripiprazole increased cell proliferation on its own. Importantly, SD rats exhibited increased levels of phosphorylated synaptosomal-associated protein 25 (SNAP-25) in the hippocampus and prefrontal cortex, suggesting PKC overactivity. Moreover, PKC inhibitors attenuated manic-like behaviors and rescued cell proliferation deficits induced by SD.
Conclusions:
Our findings confirm the relevance of SD as a model of mania, and provide evidence that antimanic agents are also able to prevent SD-induced decrease of hippocampal cell proliferation. Furthermore, they emphasize the therapeutic potential of PKC inhibitors, as revealed by their antimanic-like and pro-proliferative properties.
Keywords: bipolar disorder, hippocampal cell proliferation, manic-like behaviors, protein kinase C, sleep deprivation
Introduction
Bipolar disorder is a devastating long-term disease characterized by recurrent episodes of depression and mania. The symptoms of manic episodes include psychomotor agitation, little need for sleep, hyper-sexuality, and increased reward-seeking behaviors (American Psychiatric Association, 2000). With a lifetime prevalence of 1% (Kessler et al., 2005), bipolar disorder is cited among the leading causes of disability worldwide by the World Health Organization’s most recent global burden of disease study (Lopez et al., 2006). Although the underlying mechanisms of bipolar disorder are still unknown, recent data have linked this disorder to impairments of neuroplasticity and cellular resilience (Schloesser et al., 2008; Machado-Vieira et al., 2014).
Current antimanic pharmacotherapy—which often relies on the use of a mood stabilizer, such as lithium—remains unsatisfactory because of its limited adherence and partial therapeutic efficacy (Goodwin and Geddes, 2003). However, the development of novel antimanic agents and the understanding of the pathophysiology of this disease have been hampered by the lack of suitable animal models. Currently, there is still a need for animal models that reflect the oscillating nature of bipolar disorder; the majority of current preclinical research utilizes separate models to measure facets of bipolar disorder, typically either mania or depression (Einat and Manji, 2006). Amphetamine-induced hyperactivity is the most widely used rodent animal model to test the efficacy of antimanic therapeutics (Young et al., 2011). A 72h rapid eye movement (REM) sleep deprivation (SD) produced in rats a transient period of hyperactivity and insomnia, two clinical symptoms of manic episodes, and has been suggested as a putative model of mania by Gessa et al. (1995). Nevertheless, and despite its obvious etiopathogenic relevance—given that SD is a triggering factor of manic episodes in bipolar patients (Barbini et al., 1996)—SD has been less studied in the context of mania modeling, especially in terms of its predictive validity. Interestingly, more recent studies have shown that 96h of REM SD reduced neurogenesis in the dentate gyrus of the hippocampus of adult rats (Guzman-Marin et al., 2005). Since neuroplasticity deficits have been associated with bipolar disorder (Machado-Vieira et al., 2014), this SD model may lead to progress in understanding the neurobiological aspects of mania and to identification of new treatments.
Increasing evidence suggests that the intracellular signaling molecule protein kinase C (PKC) may be involved in the etiology of bipolar disorder (Abrial et al., 2011). Thus, abnormally-elevated PKC activity has been found in platelets from medication-free bipolar patients experiencing a manic episode (Friedman et al., 1993; Hahn et al., 2005). Moreover, mood-stabilizers such as lithium and valproate have been reported to inhibit PKC activity both in vitro and in vivo (Jensen and Mørk, 1997; Manji and Lenox, 1999). In rodents, the non-selective PKC inhibitor tamoxifen has been shown to reduce the hyperlocomotion elicited by amphetamine (Einat et al., 2007; Sabioni et al., 2008). In addition, preliminary clinical trials demonstrating that tamoxifen rapidly improved manic symptoms of bipolar patients (Bebchuk et al., 2000; Kulkarni et al., 2006; Zarate et al., 2007; Yildiz et al., 2008; Amrollahi et al., 2010) suggest that PKC inhibition might be a relevant antimanic strategy.
In view of these elements, this study aimed to investigate the antimanic-like action of PKC inhibition in the SD model in rats. We first verified the validity of SD as a model of mania by assessing the effects of clinically-effective agents on behavioral consequences of SD. Second, we explored impaired adult hippocampal cell proliferation as a possible cellular mechanism underlying manic-like behaviors and its recovery by antimanic agents. And third, we examined the antimanic potential of both selective (chelerythrine) and non-selective (tamoxifen) PKC inhibitors and their effects on hippocampal cell proliferation in the SD model.
Methods
Animals
Male Sprague-Dawley rats (Charles River), ranging in weight from 200–225g upon arrival, were housed four per cage under a 12h light/dark cycle (lights on at 7:00 AM; room temperature 22°C), with free access to food and water. All rats were allowed to acclimate for at least one week prior to experiments, and were gently handled three times before behavioral testing. All experiments were conducted in accordance with the European Community Council Directive (86/609/EEC) and the French guidelines (Act. 87–848, Ministère de l’Agriculture) for the care and use of laboratory animals.
Drugs and Treatments
Tamoxifen citrate (Alexis Biochemicals) was prepared in 4% Tween 80/saline and administered i.p. at 80mg/kg (5mL/kg). Chelerythrine chloride (LC Labs) was dissolved in water and injected s.c. at 3mg/kg (1mL/kg). Lithium chloride (Sigma-Aldrich) was dissolved in saline and administered i.p. at 100mg/kg (1mL/kg). Aripiprazole (Sequoia Research Products Ltd) was prepared in 4% Tween 80/water and injected i.p. at 1mg/kg (1mL/kg). Fluoxetine hydrochloride (LKT Laboratories) was dissolved in water and administered i.p. at 10mg/kg (1mL/kg), either acutely or chronically for 21 days. The control groups received vehicle injections. Acute injections were done during SD, 30min (aripiprazole) or 1h (lithium, fluoxetine, tamoxifen, chelerythrine) before behavioral testing, or 24h before sacrifice for evaluation of hippocampal cell proliferation. Chronic treatment with fluoxetine (10mg/kg/day i.p. for 21 days) began 18 days before the SD procedure and continued throughout SD; the last injection of fluoxetine occurred 24h before behavioral testing. The doses of drugs were chosen based on their previously reported effects in similar paradigms in rats: tamoxifen and chelerythrine (Abrial et al., 2013), lithium (Mavrikaki et al., 2009), aripiprazole (Steed et al., 2011), and fluoxetine (Callaway et al., 1990; Mnie-Filali et al., 2011). There was no difference between the results obtained in rats treated with the different vehicles used in this study. Therefore, vehicle groups were pooled together for the sake of clarity.
Sleep Deprivation Procedure
Sleep deprivation (SD) was performed by the standard flower pot procedure (Jouvet et al., 1964). Rats were individually placed (2:30 PM) in a standard container (30 H x 30cm diameter), on a small platform (8.6 H x 6.6cm diameter) surrounded by 2cm of warm water (≈33°C) for 72h. Using this SD procedure, each time the animal engaged in REM sleep, it fell into the water because of the muscular atonia accompanying REM sleep onset. Previous studies in similar experimental conditions showed that rats exhibited a 30–35% decrease of slow wave sleep and a 99% decrease of REM sleep (Verret et al., 2005; Sapin et al., 2009). Food and water were available ad libitum, and the container was cleaned daily. During cleaning time, rats were placed in a dry cage for 5min where they stayed awake (grooming and exploratory behavior). Control rats (home-caged controls, CC) remained in their home cages (4 rats per cage) for the duration of the experiment. In some experiments, an additional control group, namely the isolated control (IC) group, was exposed for 72h to the same conditions as SD animals, except that the water was replaced by sawdust.
Experimental Design
Each rat was submitted to a single 72h period of SD and behaviorally tested or sacrificed immediately afterwards. Separate rats were used for each behavioral test, and for all other experiments. For behavioral experiments, animals were transferred and kept in the experimental room, within their SD containers, for a 60min habituation period.
Experiment 1. Evaluation of the Behavioral Impairments Elicited by 72h of SD and Effects of Antimanic and Antidepressant Drugs
CC, IC, or SD rats were behaviorally tested (locomotor activity, sleep latency, penile erections, forced swim test, and Y-maze) or sacrificed for dissection of adrenal glands. Other groups of animals, treated with vehicle, lithium, aripiprazole, or fluoxetine, were assessed for locomotor activity and sleep latency.
Experiment 2. Effects of SD, Antimanic and Antidepressant Drugs on Adult Hippocampal Cell Proliferation
CC or SD rats treated with vehicle, lithium, aripiprazole, or fluoxetine were sacrificed for assessment of cell proliferation in the hippocampus (bromodeoxyuridine [BrdU]-immunolabeling).
Experiment 3. Effects of PKC Inhibitors on SD-Induced Behavioral and Cell Proliferation Deficits
CC or SD rats treated with tamoxifen or cheleyrthrine were behaviorally tested (locomotor activity, sleep latency, and penile erections) or sacrificed for evaluation of PKC activity (quantification of phospho-synaptosomal-associated protein 25 (SNAP-25) by Western blot) or cell proliferation in the hippocampus (BrdU-immunolabeling).
Behavioral Testing
Locomotor Activity
Monitoring of locomotion was performed in an actimeter (Imetronic) consisting of 8 independent chambers (25 lux) equipped with infrared beams positioned 4cm and 12cm above the floor.
Each rat was removed from its container, placed in a Plexiglas activity chamber (41 x 26 x 20cm), and its locomotor activity (number of beam breaks) was recorded for 30 min. Sleep latency was determined as the first occurrence of 5 or fewer beam breaks during a period of at least 5 min. We showed that this criterion was well correlated (r = 0.73, p < 0.001) to the sleep latency measured by behavioral observations (data not shown).
Y-maze Task
Spontaneous alternation was assessed in the Y-maze task. Each arm of the maze was 45cm long, 33cm high, and 15cm wide, and converged to an equal angle (5 lux in the center of the apparatus). Each rat was placed at the end of one arm and allowed to move freely through the maze during an 8min session. The total number of arm entries and alternations (defined as consecutive entries into all three arms without repetitions) was scored. The percent alternation was calculated as the ratio of actual to possible alternations defined as the (total number of arm entries - 2) x100.
Penile Erection Assessment
Experiments were carried out in a dimly lit room (5 lux) in Plexiglas cages (41 x 26 x 20cm) with a mirror placed under the floor at a 45° angle to allow an unobstructed view of the rat from underneath. Rats were placed individually in the cages, and observations were carried out for 60min. Penile erection was counted only when the rat stood on its hind limbs, leant its body forward, bent its head down to reach the genital area, and held and licked its penis in full erection and/or displayed hip movements (Andersen et al., 2007). The number and latency to the first penile erection were counted.
Forced Swim Test
The forced swim test (FST) was performed as previously described by Porsolt et al. (1978). Rats were individually placed in a plastic cylinder (50cm high by 20cm in diameter) filled with tap water (25±0.5°C) at a depth of 35cm. Two swim sessions were conducted in a dimly lit room (12 lux): a 15min pretest at 48h of SD, and a 5min test at 72h of SD. Following both swim sessions, rats were removed from the cylinders, dried with towels, and kept warm for 30min before returning to their home cage. The 5min test was recorded and analyzed by a video-tracking system (Viewpoint), allowing measurement of the immobility, swimming, and climbing times. Immobility, defined as the animal floating without struggling and only making movements necessary to maintain its head above water, was used as an indicator of depression-like behavior.
Evaluation of Hippocampal Cell Proliferation
BrdU Labeling
For analysis of BrdU-positive cells, rats were administered 4 injections of BrdU (50mg/kg i.p. every 2h, starting at 9:00 AM; Fluka), with the last injection occurring 24h before the end of the SD. Twenty-four hours after the last BrdU injection, rats were deeply anesthetized with pentobarbital (50mg/kg, i.p.) and transcardially perfused (150mL of 0.9% NaCl followed by 500mL of 4% paraformaldehyde). After perfusion, all brains were post-fixed overnight in paraformaldehyde at 4°C and stored at 4°C in 30% sucrose. Coronal sections (30 µm-thick) were cut through the entire hippocampus, between -1.60 to -6.72, from Bregma (Paxinos and Watson, 2007) on a freezing microtome, and stored at 4°C in 10mM phosphate-buffered saline (PBS) pH 7.4 containing 0.1% NaN3.
Immunohistochemistry
Free-floating sections were incubated with 50% formamide and 50% 2x saline sodium citrate buffer (pH 7.4; 0.3M sodium citrate, 0.03M NaCl) for 90min at 65°C, and then immersed in 2x saline sodium citrate buffer, followed by 30min in 2M hydrochloric acid (HCl) to denature deoxyribonucleic acid (DNA). After neutralization with 0.1M sodium borate (pH 8) and pre-incubation with PBS containing 0.3% Triton X-100 (PBST), sections were processed for 3min in pepsin (0.45U/mL in 0.1M HCl) and transferred for 15min to 3% H2O2 to eliminate endogenous peroxidases. Then, sections were incubated at room temperature overnight with a primary antibody against BrdU (rat monoclonal IgG, 1/400, AbCys) in PBST 1% bovine serum albumin. Sections were then washed with PBST and incubated with a secondary antibody for 90min (biotinylated rabbit anti-rat IgG, 1/250, Vector Laboratories), followed by amplification with an avidin-biotinylated horseradish peroxidase complex (Elite ABC kit, Vector Laboratories). Peroxidase activity was revealed by incubating sections with 0.02% 3,3′-diaminobenzidine and 0.8% NiCl in 0.5mol/L Tris-HCl (pH 7.4). After several rinses, sections were mounted on gelatin-coated slices, dehydrated in graded alcohols, and cover-slipped in DePeX mounting medium (VWR).
Counting Procedure
The number of BrdU-labeled cells was quantified with a light microscope (Zeiss) at 40x magnification on a series of every sixth section of the entire hippocampus. The hippocampus was segregated into two halves, with the dorsal hippocampus defined as the anterior region (-1.60 to -5.30 Bregma) and the ventral hippocampus defined as the posterior region (-5.30 to -6.72 Bregma). About 10 sections in the dorsal hippocampus and 5 sections in the ventral hippocampus were examined per animal to estimate the total number of BrdU-labeled cells per structure. BrdU-labeled cells were counted in the granule cell layer and the subgranular zone, and cells that were located more than two cells away from the subgranular zone were omitted.
Quantitative Western Blotting
After euthanasia, brains were rapidly removed. The whole hippocampus and prefrontal cortices were quickly dissected on an ice-cold plate and they were snap-frozen in liquid nitrogen and kept at -80°C until further processing. Tissue was lysed in 400 µL-800 µL lysis buffer (25mM HEPES pH 7.4, 500mM NaCl, 2mM EDTA, 1mM dithiothreitol, 1mM phenylmethylsulfonyl fluoride, 20mM NaF, 1mM sodium orthovanadate, and Roche cOmplete mini protease inhibitor cocktail) in 0.5–2mL tubes containing ceramic beads (Precellys soft tissue homogenizing CK14) and processed three times 30sec each at 6 800 rpm using a Precellys®24 apparatus, interspaced by 5min pauses on ice to prevent sample overheating. Protein concentrations were estimated by standard Bradford protein assay and samples were boiled 10min in 5X Laemmli buffer. 20 µg of samples were loaded on Bio-Rad 12% Mini-Protean® TGXTM Precast gels, run 1 h at 105V, transferred 45 min at 18V on a nitrocellulose membrane, and blocked in Tris Buffer Saline 0.1% Tween 20 (TBST) containing 5% non-fat dry milk, 20mM NaF, and 2mM sodium orthovanadate. After blocking, membranes were incubated with primary antibodies in 1% milk TBST containing 20mM NaF and 2mM sodium orthovanadate: β-actin (1/100 000, mouse, Sigma-Aldrich), SNAP-25 (1/3 000, mouse, Sigma-Aldrich), or phospho-SNAP-25 at Ser187 (1/270, mouse; kindly provided by Professor M Takahashi, Kitasato University School of Medicine, Kitasato, Japan). After overnight incubation at 4°C, membranes were washed extensively in TBST and incubated 2 h in TBST with CF680 or CF770 secondary antibodies (Biotium, 1/10 000) at room temperature. After extensive washing in TBST, followed by TBS washes, laser scanning and quantitative analyses of the blots were performed using the Odyssey Infrared Imaging System (Li-Cor). Quantification of protein phosphorylation was carried out by measuring the intensity of fluorescence of SNAP-25 or phospho-SNAP-25 bands normalized by β-actin expression. Ratios of normalized phospho-SNAP-25 to normalized SNAP-25 were calculated and results were expressed as the percentage of increase over the mean of caged controls ratios, which was set to 100%.
Statistical Analysis
Statistical analysis was performed using StatView (Abacus Concepts Inc.). For experiments having a 2 x 2 design, two-way ANOVA was used, followed by Fisher PLSD post-hoc test if significant main (sleep condition and treatment) or interaction effects were found. For the other experiments, a student’s t-test was used. Probability values of less than 5% were considered statistically significant. Statistical analysis for each figure is shown in Supplementary Tables S1 and S2.
Results
Sleep Deprivation Induces a Manic-Like Syndrome Characterized by Hyperlocomotion, Insomnia, and Increased Penile Erections
As previously reported by Gessa et al. (1995) by using the single platform technique, rats submitted to 72h of SD displayed enhanced locomotor activity (p < 0.001) and delayed sleep onset latency (p < 0.001) compared to CC rats (Figure 1A, Supplementary Table S1). These behavioral effects were likely not due to isolation or the context in which rats were placed during the 72h, since IC and CC groups were not statistically different in either locomotion (IC: 192±31 beam breaks/30min, n = 8, vs CC: 194±18 beam breaks/30min, n = 8) or in sleep latency (IC: 17±3min, n = 8, vs CC: 14±1min, n = 8). Moreover, SD rats presented a significantly higher number of penile erections (p < 0.01) and a shorter latency to the first erection (p < 0.01) than CC rats (Figure 1A). These results indicate that SD induced hyperlocomotion, insomnia, and increased penile erections. On the other hand, SD did not affect immobility, swimming, and climbing time in the FST, nor spontaneous alternation score in a Y-maze, suggesting that SD rats did not exhibit changes in depression- or cognition-related behaviors in the behavioral tests used (Figure 1B). Furthermore, SD rats had a greater relative adrenal gland weight compared to CC rats (p < 0.05), suggesting a hyperactivity of the hypothalamic-pituitary-adrenal axis. Besides, SD rats had a significantly lower body weight than CC rats (Figure 1C; p < 0.01).
Figure. 1.
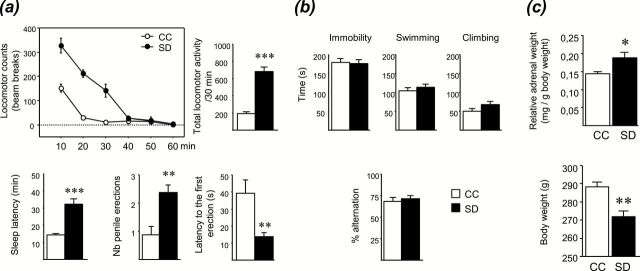
Behavioral phenotype of rats submitted to a sleep deprivation (SD). (A) 72h of SD increased locomotor activity, sleep latency, and penile erections. (B) SD had no effect on behaviors in the forced swim test or on spontaneous alternation in a Y-maze. (C) SD increased adrenal glands and decreased body weights. Data represent the mean ± S.E.M. of 7–8 rats/group. *p < 0.05, **p < 0.01, ***p < 0.001 vs control (CC) group.
SD-Induced Hyperlocomotion and Insomnia are Sensitive to Lithium, Aripiprazole, but not Fluoxetine
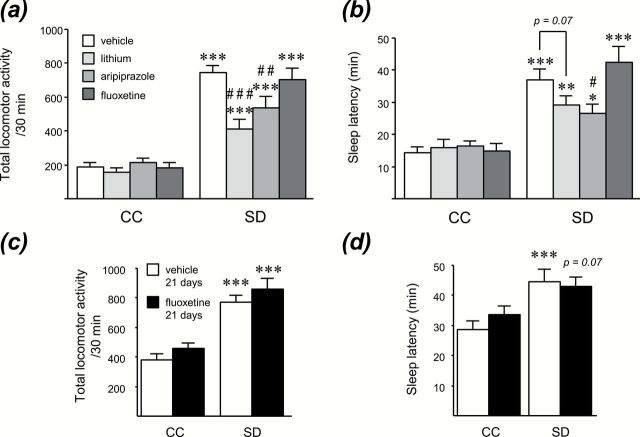
To assess the pharmacological validity of this model, we evaluated whether lithium, a mood stabilizer, and aripiprazole, an atypical antipsychotic showing antimanic efficacy in bipolar patients, were able to reverse SD-induced manic-like behaviors. Fluoxetine, an antidepressant which is devoid of antimanic properties, was used as a negative control. Acute administration of lithium (100mg/kg, i.p.) or aripiprazole (1mg/kg, i.p.) to CC rats had no significant effect on locomotion, but significantly attenuated the hyperlocomotor effect of SD (p < 0.001 and p < 0.01, respectively; Supplementary Table S2), whereas fluoxetine was without any effect in CC or SD rats either acutely (10mg/kg, i.p.) or chronically for 21 days (10mg/kg/day, i.p.; Figure 2A and C). Besides, in SD rats, aripiprazole significantly reduced sleep latency (p < 0.05) and lithium resulted in a trend towards decreased sleep latency (p = 0.07), while acute or chronic fluoxetine did not modify this parameter (Figure 2B and D).
Figure. 2.
Hyperlocomotion and insomnia induced by sleep deprivation (SD) are attenuated by antimanic agents (lithium and aripiprazole), but not by the antidepressant fluoxetine. Locomotor activity and sleep latency were assessed in control (CC) and SD rats after (A and B) an acute injection of lithium (100mg/kg, i.p.), aripiprazole (1mg/kg, i.p.), fluoxetine (10mg/kg, i.p.), or (C and D) a chronic treatment with fluoxetine (10mg/kg/day i.p., for 21 days). Data represent the mean ± S.E.M. of 7–8 rats/group. *p < 0.05, **p < 0.01, ***p < 0.001 vs respective CC group. #p < 0.05, ##p < 0.01, ###p < 0.001 vs SD-vehicle.
Sleep Deprivation Decreases Cell Proliferation in the Dorsal and Ventral Hippocampus, an Effect Rescued by Lithium, Aripiprazole, but not Fluoxetine
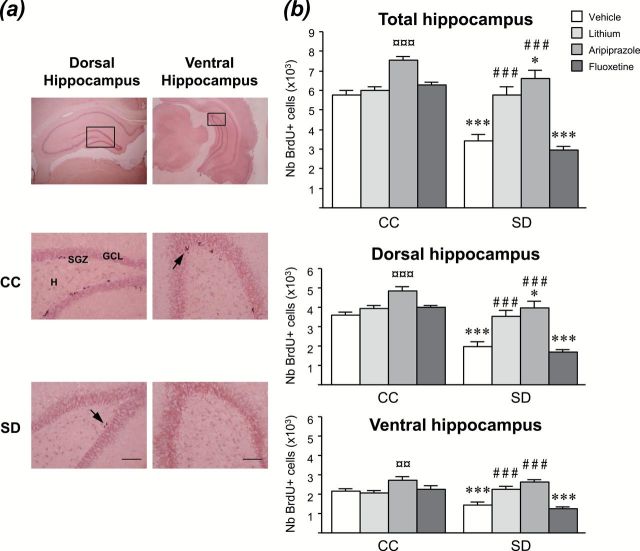
To investigate the cellular mechanisms associated with the behavioral effects of SD, we evaluated the impact of SD on adult hippocampal cell proliferation. As shown in Figure 3, 72 h of SD reduced the number of BrdU-positive cells in the hippocampus (p < 0.001). When looking at subdivisions of the hippocampus, significant effects were found in both dorsal (p < 0.001) and ventral (p < 0.001) regions. The number of BrdU-positive cells counted in IC rats (dorsal: 3608±272; ventral: 2060±31; total: 5668±295; n = 5) was not statistically different from that of CC rats (dorsal: 3593±161; ventral: 2160±120; total: 5753±230; n = 6).
Figure. 3.
Sleep deprivation (SD) decreases hippocampal cell proliferation, which is rescued by antimanic but not antidepressant treatments. (A) Representative photomicrographs of dorsal and ventral hippocampi (bottom: enlarged view of boxed area in top) of control (CC) and SD rats. Arrows indicate examples of clusters of BrdU positive cells (scale bar = 20 μm). The majority of the BrdU-labeled cells are located in the subgranular zone (SGZ) of the dentate gyrus at the border between the granule cell layer (GCL) and hilus (H), and appeared in irregular shapes as clusters of 3–4 cells. (B) 72h of SD decreased cell proliferation in dorsal and ventral hippocampi. Acutely injected lithium (100mg/kg, i.p.) and aripiprazole (1mg/kg, i.p.), but not fluoxetine (10mg/kg, i.p.), counteracted the SD-induced hippocampal cell proliferation decrease. Values plotted are mean ± S.E.M. of 5–6 rats/group. *p < 0.05, ***p < 0.001 vs respective CC group; ¤¤p < 0.01, ¤¤¤p < 0.001 vs CC-vehicle; ##p < 0.001 vs SD-vehicle.
To address the possibility that the antimanic-like behavioral effects of current bipolar medications may be associated with a recovery of cell proliferation impairments, we examined the ability of lithium, aripiprazole, and fluoxetine to increase hippocampal cell proliferation in the SD model (Figure 3B). In SD rats, an injection of lithium or aripiprazole significantly increased the number of BrdU-positive cells in the hippocampus (p < 0.001), whereas fluoxetine was without any effect. It can be noted that aripiprazole was also able to increase hippocampal cell proliferation in CC animals (p < 0.001). Similar effects of these drugs were found in both dorsal and ventral parts of the hippocampus.
Sleep Deprivation Provokes an Enhancement of SNAP-25 Phosphorylation in the Prefrontal Cortex and the Hippocampus
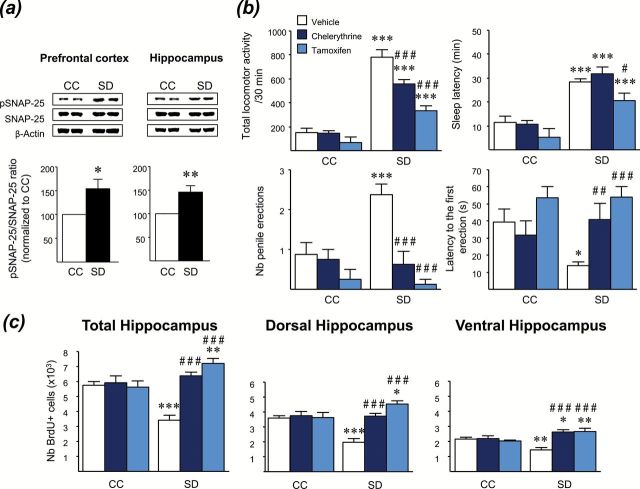
To assess the brain PKC activity in the SD model, we examined the phosphorylation level of synaptosomal-associated protein of 25kDa (SNAP-25; Figure 4A). Immunoblotting analysis with a specific antibody to Ser187, a PKC-specific phosphorylation site of SNAP-25 (Shimazaki et al., 1996), revealed a significant increase of the phosphorylated SNAP-25/unphosphorylated form ratio in both the prefrontal cortex (p < 0.05) and hippocampus (p < 0.01) of SD rats compared with CC, indicating a PKC activation in these brain regions after SD.
Figure. 4.
PKC inhibition prevents manic-like behaviors and rescues hippocampal cell proliferation in the sleep deprivation (SD) model. (A) Western blot, showing representative results of protein levels of phosphorylated and non-phosphorylated forms of SNAP-25 in the prefrontal cortex and hippocampus of control (CC) and SD rats. Densitometric analysis performed on 8 rats/group showed an increase of phospho-SNAP-25/SNAP-25 ratio in SD rats. Results are expressed as a percentage of increase relative to the response of CC, which was set at 100%. (B) The PKC inhibitors chelerythrine (3mg/kg, s.c.) and tamoxifen (80mg/kg, i.p.) reduced manic-like behavioral alterations (hyperlocomotion, insomnia, and increased penile erections) in SD rats. Data represent the mean ± S.E.M. of 7–8 rats/group. (C) An acute injection of chelerythrine (3mg/kg, s.c.) or tamoxifen (80mg/kg, i.p.) rescued hippocampal cell proliferation in SD rats. Values plotted are mean ± S.E.M. of 5–6 rats/group. *p < 0.05, **p < 0.01, ***p < 0.001 vs respective CC group; #p < 0.05, ##p < 0.01, ###p < 0.001 vs SD-vehicle.
PKC Inhibitors Attenuates Manic-Like Behaviors and Rescues Cell Proliferation Deficits Triggered by Sleep Deprivation
Subsequently, we investigated whether PKC inhibition may reverse the manic-like behaviors elicited by SD (Figure 4B). SD rats acutely treated with the selective PKC inhibitor chelerythrine (3mg/kg, s.c.) or the dual selective estrogen-receptor modulator/PKC inhibitor tamoxifen (80mg/kg, i.p.) displayed lower locomotion than those treated with vehicle (p < 0.001). SD-induced insomnia was significantly reduced by tamoxifen (p < 0.05), but not by chelerythrine. Moreover, both PKC inhibitors decreased the number of penile erections (p < 0.001) and delayed the first erection (respectively, p < 0.01 and p < 0.001) in SD rats. None of these parameters were significantly altered by PKC inhibitors in CC animals. Taken together, these results suggest that PKC inhibitors have antimanic-like properties in the SD model.
Thereafter, we examined whether PKC inhibitors may be able to rescue the cell proliferation impairments displayed in SD rats (Figure 4C). A single injection of chelerythrine or tamoxifen did not produce any effect on cell proliferation in CC rats, but counteracted the cell proliferation deficits in SD animals in both the dorsal (p < 0.001) and ventral (p < 0.001) areas of the hippocampus.
Discussion
The results of the present study revealed that 72h of SD in rats is a relevant animal model of mania. First, SD rats displayed behavioral alterations analogous to manic symptoms of bipolar disorder. Second, the antimanic agents (lithium and aripiprazole), but not the antidepressant (fluoxetine), yielded rapid antimanic-like effects in rats exposed to SD. Additionally, we demonstrated in this SD rat model that PKC inhibitors are able to produce antimanic-like effects and to reverse the SD-related decrease of hippocampal cell proliferation.
The SD model provides convincing support for construct validity to the extent that a relationship between sleep loss and the onset of mania has been reported in human. Thus, reduced sleep represents a reliable prodrome of a manic episode (Plante and Winkelman, 2008), and sleep disruptions, subsequent to a stress or a change in sleep schedule, increase the risk of switching from depression to mania (Wehr et al., 1987; Barbini et al., 1996; Salvadore et al., 2010). Moreover, alterations of sleep architecture, such as REM sleep abnormalities, have been observed in manic subjects (Hudson et al., 1988, 1992). In line with previous studies (Gessa et al., 1995; Benedetti et al., 2008; Armani et al., 2012), we showed that rats displayed enhanced locomotor activity immediately after 72h of SD. Because psychomotor agitation has been described as a cardinal feature of mania (Young et al., 2007), hyperlocomotion has been used as the primary outcome to assess manic-like behavior. Yet, mania is characterized by a broad set of symptoms, and hyperactivity does not represent the only behavioral feature of bipolar disorder. We thus assessed other symptoms of mania and we found that, besides hyperlocomotion, SD rats exhibited behavioral alterations having face validity with manic symptoms, such as insomnia and increased penile erections, which have been linked with enhanced sexual performance (Bernardi et al., 2011). Furthermore, other manic-like behaviors have been previously reported in rodents following total or partial SD of varying lengths, such as increased aggressive behavior (Benedetti et al., 2008), hedonic behavior to sucrose solution (Andersen et al., 2009), and irritability (Frau et al., 2008). Predictive validity of the SD model was supported by the present data showing the ability of two clinically-efficient antimanic agents from distinct classes, namely the mood stabilizer lithium and the atypical antipsychotic aripiprazole, to attenuate the SD-induced hyperlocomotion and insomnia. Importantly, these behaviors were not modified by an acute or chronic treatment with fluoxetine, an antidepressant which is given in combination with lithium for depressive episodes but is devoid of antimanic efficacy. It can be noted that an acute lithium treatment produced antimanic-like effects in the SD model, whereas lithium’s early improvement of mania is not observed before at least one week of treatment in bipolar patients (Machado-Vieira et al., 2013). However, acute lithium also produces an antimanic-like effect in the amphetamine-induced hyperlocomotion test (Gould et al., 2007). Besides, acutely administered antidepressants are effective in the FST, a well-used paradigm of antidepressant efficacy (Cryan and Lucki, 2005).
A recurring matter of debate in the field of sleep research is how many of the molecular and cellular changes observed after SD can be attributed to stress, rather than to sleep loss itself. Our single-platform method, that involves enforced immobilization and social isolation, actually induced stress, as shown by the adrenal hypertrophy observed in SD rats. However, this stress, also encountered with the multiple-platform SD method (Coenen and van Luijtelaar, 1985), is not a concern in the context of a model of mania, but rather an advantage in term of construct validity, as stressful life events may contribute to the onset of bipolar disorder (Tsuchiya et al., 2003). Overall, the present SD model that associates mild stress with sleep disturbance, in which REM SD is a major component, meets most of the validity criteria, and can therefore be considered as a valuable tool to further explore the underlying mechanisms of mania.
Following this perspective, and given the proposed role of PKC in mania (Abrial et al., 2011), we investigated the possible occurrence of PKC changes in the brain after SD. Our results indicate that 72h of SD increased the phosphorylation of a major PKC substrate, SNAP-25, in the prefrontal cortex and hippocampus, suggesting an overactivation of the PKC pathway. As other stressors were demonstrated to affect PKC activity (Yamamori et al., 2014), it should be noted that this enhancement of PKC activity might not be SD specific, but more likely is a combination of stresses induced by the SD procedure. In accordance with our results, Szabo et al. (2009) observed increased phosphorylation of MARCKS, another PKC substrate, in the frontal cortexes of SD rats. Together, these results are consistent with studies revealing a hyperactivity of the PKC pathway in platelets of bipolar patients in a manic phase (Friedman et al., 1993; Hahn and Friedman, 1999; Wang et al., 1999). We subsequently investigated whether inhibition of PKC may be able to block the manic-like behaviors in the SD model. We evidenced that the PKC inhibitors chelerythrine and tamoxifen acutely attenuated the hyperlocomotion of SD rats. Contrary to tamoxifen, an estrogen receptor modulator with PKC inhibitory properties, chelerythrine is a selective PKC inhibitor, and thus it is assumed that this antimanic effect can likely be attributed to PKC inhibition. In agreement with the present results, we and others have previously shown that these PKC inhibitors also decreased the hyperlocomotion of animals injected with amphetamine (Einat et al., 2007; Sabioni et al., 2008; Abrial et al., 2013). Moreover, we showed that tamoxifen reduced the insomnia, and both inhibitors decreased the penile erections displayed by rats submitted to SD. These results emphasize the potential of PKC inhibition as an efficient antimanic strategy.
Adult hippocampal neurogenesis is thought to be increased by interventions that are associated with beneficial effects on mood, such as chronic treatment with antidepressants (Santarelli et al., 2003). However, except for the mood stabilizer lithium, for which several studies have reported neurogenic effects (Chen et al., 2000; Son et al., 2003; O’Leary et al., 2012), the ability of drugs used for the treatment of bipolar disorder to modulate the proliferation and survival of newly-born cells in the adult hippocampus remains less explored. Thus, we hypothesized that the antimanic-like effects obtained in the present study may be related to changes of hippocampal cell proliferation. Our results evidenced that 72h of SD markedly decreased the proliferation of new cells in the dentate gyrus. These data are consistent with previous findings showing that disruptions of sleep exceeding 48h achieved by different techniques significantly inhibit cell proliferation (Guzman-Marin et al., 2005; Tung et al., 2005). Interestingly, hippocampal cell proliferation reduction in SD rats was reversed by a single injection of tamoxifen or chelerythrine, suggesting that cell proliferation events in the adult hippocampus following SD are under a potent control mediated by PKC. In apparent contradiction with the present data, we previously found that chronic low-dose tamoxifen (10mg/kg/day for 14 days) and chelerythrine (0.3mg/kg/day for 14 days) decreased adult cell proliferation in the dentate gyrus in naive rats (Abrial et al., 2013). A possible explanation for these opposite effects could be that low doses of these inhibitors may not be sufficient to completely block the PKC activity, and would thus promote compensatory mechanisms which would lead in fine to up-regulation of the PKC pathway. Further studies are necessary to confirm this hypothesis. Otherwise, it seems possible that the counteracting effects observed with lithium and aripiprazole on SD-induced decreases of hippocampal cell proliferation are mediated, at least in part, by PKC inhibition. Indeed, the PKC pathway is one of the downstream targets of lithium (Lenox and Wang, 2003), and a recent study revealed that aripiprazole could also inhibit PKC (Kato et al., 2011). Interestingly, besides recovering hippocampal cell proliferation in SD rats, an acute administration of aripiprazole in naive animals also stimulated cell proliferation. Aripiprazole is an atypical antipsychotic drug that possesses dual D2/5-HT1A properties, acting as a partial agonist at both receptors (Kikuchi et al., 1995; Dahan et al., 2009). Given that acute administration of 5-HT1A receptors agonists is known to produce significant elevation of hippocampal neurogenesis (Banasr et al., 2004; Klempin et al., 2010), it is likely that the 5-HT1A receptor agonist properties of aripiprazole contribute to its pro-proliferative effect in naive and/or SD rats.
In line with previous data suggesting that adult hippocampal neurogenesis—and more broadly, neuroplasticity and cellular resilience—may be a significant factor in the pathophysiology and/or treatment of affective disorders (Schloesser et al., 2008; Machado-Vieira et al., 2014), we propose that the positive outcomes of acute lithium, aripiprazole, and PKC inhibitors on cell proliferation in the dentate gyrus could account for their ability to reduce manic-like behaviors. However, a direct relationship between adult hippocampal neurogenesis and affective disorders has been challenged. Whilst earlier studies highlighted that neurogenesis integrity is a requirement for the behavioral effects of antidepressants (Santarelli et al., 2003; Surget et al., 2008), recent reports have rejected this possibility (Bessa et al., 2009) or depicted a more nuanced picture by suggesting the existence of neurogenesis-dependent and -independent mechanisms of antidepressant action (David et al., 2009). Thus, although other studies are necessary to determine whether adult neurogenesis is critical for the behavioral effects of antimanic agents, it is noteworthy that any increase in cell proliferation may not be sufficient to produce antimanic effects. Indeed, in our study fluoxetine, which is known to increase cell proliferation when chronically administrated to naive rats (Malberg et al., 2000) or to mice submitted to a chronic mild stress (Tanti et al., 2013), was not able to produce antimanic effects in our SD model.
An interesting finding of the present study is that a short-term drug treatment was able to increase adult hippocampal cell proliferation. So far, treatments that induce neurogenic effects such as antidepressants (Malberg et al., 2000) or the mood stabilizer lithium (Son et al., 2003) typically do so after chronic, but not acute, administration. In our study, all the agents tested had both antimanic and pro-proliferative effects in response to a single administration. This result highlights the relevance of a rapid cell proliferation increase in the dentate gyrus as a common feature of antimanic agents. In conclusion, we have demonstrated that 72h of SD induced manic-like behaviors and hippocampal cell proliferation deficits, which are sensitive to classic antimanic drugs, therefore supporting the SD model as a good alternative to existing models of mania. Our data suggest a possible role of hippocampal neurogenesis in mediating antimanic effects, even after a single administration. Finally, the present results emphasize the therapeutic potential of PKC inhibitors by revealing their antimanic-like properties and their effects on hippocampal cell proliferation. Overall, these findings provide new insights on the pathophysiology of bipolar disorder and on the mechanisms underlying the antimanic response.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
None.
Supplementary Material
Acknowledgments
The authors would like to thank Magali Novo-Perez and Renaud Rovera for their technical assistance in experiments, Professor Jean-Pierre Abrial from Keio University for his technical expertise in photo editing, and Dr Daniel Gonzalez-Dunia for providing technical and material support on Western blot experiments.
References
- Abrial E, Etievant A, Bétry C, Scarna H, Lucas G, Haddjeri N, Lambás-Señas L. (2013). Protein kinase C regulates mood-related behaviors and adult hippocampal cell proliferation in rats. Prog Neuropsychopharmacol Biol Psychiatry 43:40–48. [DOI] [PubMed] [Google Scholar]
- Abrial E, Lucas G, Scarna H, Haddjeri N, Lambás-Señas L. (2011). A role for the PKC signaling system in the pathophysiology and treatment of mood disorders: involvement of a functional imbalance? Mol Neurobiol 44:407–419. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders, Revised 4th ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- Amrollahi Z, Rezaei F, Salehi B, Modabbernia A–H, Maroufi A, Esfandiari G–R, Naderi M, Ghebleh F, Ahmadi-Abhari S–A, Sadeghi M, Tabrizi M, Akhondzadeh S. (2010). Double-blind, randomized, placebo-controlled 6-week study on the efficacy and safety of the tamoxifen adjunctive to lithium in acute bipolar mania. J Affect Disord 129:327–331. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Antunes IB, Tufik S. (2007). Cocaine-induced genital reflexes in paradoxical sleep deprived rats: Indications of mediation by serotonin receptors. Prog Neuropsychopharmacol Biol Psychiatry 31:496–502. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Hoshino K, Tufik S. (2009). Increased susceptibility to development of anhedonia in rats with chronic peripheral nerve injury: involvement of sleep deprivation? Prog Neuropsychopharmacol Biol Psychiatry 33:960–966. [DOI] [PubMed] [Google Scholar]
- Armani F, Andersen ML, Andreatini R, Frussa-Filho R, Tufik S, Galduróz JCF. (2012). Successful combined therapy with tamoxifen and lithium in a paradoxical sleep deprivation-induced mania model. CNS Neurosci Ther 18:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Hery M, Printemps R, Daszuta A. (2004). Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 29:450–460. [DOI] [PubMed] [Google Scholar]
- Barbini B, Bertelli S, Colombo C, Smeraldi E. (1996). Sleep loss, a possible factor in augmenting manic episode. Psychiatry Res 65:121–125. [DOI] [PubMed] [Google Scholar]
- Bebchuk JM, Arfken CL, Dolan-Manji S, Murphy J, Hasanat K, Manji HK. (2000). A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Arch Gen Psychiatry 57:95–97. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Fresi F, Maccioni P, Smeraldi E. (2008). Behavioural sensitization to repeated sleep deprivation in a mice model of mania. Behav Brain Res 187:221–227. [DOI] [PubMed] [Google Scholar]
- Bernardi MM, Kirsten TB, Lago JH, Giovani TM, Massoco C, de O. (2011). Nepeta cataria L. var. citriodora (Becker) increases penile erection in rats. J Ethnopharmacol 137:1318–1322. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. (2009). The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14:764–773. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. (1990). Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharm Exp Ther 254:456–464. [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. (2000). Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75:1729–1734. [DOI] [PubMed] [Google Scholar]
- Coenen AM, van Luijtelaar EL. (1985). Stress induced by three procedures of deprivation of paradoxical sleep. Physiol Behav 35:501–504. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–569. [DOI] [PubMed] [Google Scholar]
- Dahan L, Husum H, Mnie-Filali O, Arnt J, Hertel P, Haddjeri N. (2009). Effects of bifeprunox and aripiprazole on rat serotonin and dopamine neuronal activity and anxiolytic behaviour. J Psychopharmacol 23:177–189. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. (2009). Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H, Manji HK. (2006). Cellular Plasticity Cascades: Genes-To-Behavior Pathways in Animal Models of Bipolar Disorder. Biol Psychiatry 59:1160–1171. [DOI] [PubMed] [Google Scholar]
- Einat H, Yuan P, Szabo ST, Dogra S, Manji HK. (2007). Protein kinase C inhibition by tamoxifen antagonizes manic-like behavior in rats: implications for the development of novel therapeutics for bipolar disorder. Neuropsychobiology 55:123–131. [DOI] [PubMed] [Google Scholar]
- Frau R, Orrù M, Puligheddu M, Gessa GL, Mereu G, Marrosu F, Bortolato M. (2008). Sleep deprivation disrupts prepulse inhibition of the startle reflex: reversal by antipsychotic drugs. Int J Neuropsychop 11:947–955. [DOI] [PubMed] [Google Scholar]
- Friedman E, Wang HY, Levinson D, Connell TA, Singh H. (1993). Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biol Psychiatry 33:520–525. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Pani L, Fadda P, Fratta W. (1995). Sleep deprivation in the rat: an animal model of mania. Eur Neuropsychopharmacol 5(Suppl):89–93. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Geddes JR. (2003). Latest maintenance data on lithium in bipolar disorder. Eur Neuropsychopharmacol 13(Suppl 2):S51–55. [DOI] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Manji HK. (2007). Strain differences in lithium attenuation of d-amphetamine-induced hyperlocomotion: a mouse model for the genetics of clinical response to lithium. Neuropsychopharmacology 32:1321–1333. [DOI] [PubMed] [Google Scholar]
- Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szymusiak R, McGinty D. (2005). Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci 22:2111–2116. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Friedman E. (1999). Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disord 1:81–86. [DOI] [PubMed] [Google Scholar]
- Hahn C–G, Umapathy C, Wang H–Y, Koneru R, Levinson DF, Friedman E. (2005). Lithium and valproic acid treatments reduce PKC activation and receptor-G protein coupling in platelets of bipolar manic patients. J Psychiatr Res 39:355–363. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Lipinski JF, Frankenburg FR, Grochocinski VJ, Kupfer DJ. (1988). Electroencephalographic sleep in mania. Arch Gen Psychiatry 45:267–273. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Lipinski JF, Keck PE, Jr, Aizley HG, Lukas SE, Rothschild AJ, Waternaux CM, Kupfer DJ. (1992). Polysomnographic characteristics of young manic patients. Comparison with unipolar depressed patients and normal control subjects. Arch Gen Psychiatry 49:378–383. [DOI] [PubMed] [Google Scholar]
- Jensen JB, Mørk A. (1997). Altered protein phosphorylation in the rat brain following chronic lithium and carbamazepine treatments. Eur Neuropsychopharmacol 7:173–179. [DOI] [PubMed] [Google Scholar]
- Jouvet D, Vimont P, Delorme F. (1964). Study of selective deprivation of the paradoxal phase of sleep in the cat. J Physiol Paris 56:381. [PubMed] [Google Scholar]
- Kato TA, Monji A, Yasukawa K, Mizoguchi Y, Horikawa H, Seki Y, Hashioka S, Han Y–H, Kasai M, Sonoda N, Hirata E, Maeda Y, Inoguchi T, Utsumi H, Kanba S. (2011). Aripiprazole inhibits superoxide generation from phorbol-myristate-acetate (PMA)-stimulated microglia in vitro: implication for antioxidative psychotropic actions via microglia. Schizophr Res 129:172–182. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Tottori K, Uwahodo Y, Hirose T, Miwa T, Oshiro Y, Morita S. (1995). 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharm Exp Ther 274:329–336. [PubMed] [Google Scholar]
- Klempin F, Babu H, De Pietri Tonelli D, Alarcon E, Fabel K, Kempermann G. (2010). Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front Mol Neurosci 3 pii:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J, Garland KA, Scaffidi A, Headey B, Anderson R, de Castella A, Fitzgerald P, Davis SR. (2006). A pilot study of hormone modulation as a new treatment for mania in women with bipolar affective disorder. Psychoneuroendocrinology 31:543–547. [DOI] [PubMed] [Google Scholar]
- Lenox RH, Wang L. (2003). Molecular basis of lithium action: integration of lithium-responsive signaling and gene expression networks. Mol Psychiatry 8:135–144. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. (2006). Global burden of disease and risk factors. 1st ed. Washington, DC: World Bank Publications. [PubMed] [Google Scholar]
- Machado-Vieira R, Luckenbaugh DA, Soeiro-de-Souza MG, Marca G, Henter ID, Busnello JV, Gattaz WF, Zarate CA., Jr (2013). Early improvement with lithium in classic mania and its association with later response. J Affect Disord 144:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Soeiro-De-Souza MG, Richards EM, Teixeira AL, Zarate CA., Jr (2014). Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: Developing treatments using an integrated translational approach. World J Biol Psychiatry 15:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. (1999). Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry 46:1328–1351. [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Nomikos GG, Panagis G. (2009). Effects of mood stabilizers on brain reward processes in rats: studies using the intracranial self-stimulation paradigm. Eur Neuropsychopharmacol 9:205–214. [DOI] [PubMed] [Google Scholar]
- Mnie-Filali O, Faure C, Lambás-Señas L, El Mansari M, Belblidia H, Gondard E, Etiévant A, Scarna H, Didier A, Berod A, Blier P, Haddjeri N. (2011). Pharmacological blockade of 5-HT7 receptors as a putative fast acting antidepressant strategy. Neuropsychopharmacology 36:1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary OF, O’Connor RM, Cryan JF. (2012). Lithium-induced effects on adult hippocampal neurogenesis are topographically segregated along the dorso-ventral axis of stressed mice. Neuropharmacology 62:247–255. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2007). The rat brain in stereotaxic coordinates. 6th Edition. Amsterdam: Academic Press/Elsevier. [Google Scholar]
- Plante DT, Winkelman JW. (2008). Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psych 165:830–843. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. (1978). “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol 51(3):291–294. [DOI] [PubMed] [Google Scholar]
- Sabioni P, Baretta IP, Ninomiya EM, Gustafson L, Rodrigues ALS, Andreatini R. (2008). The antimanic-like effect of tamoxifen: behavioural comparison with other PKC-inhibiting and antiestrogenic drugs. Prog Neuropsychopharmacol Biol Psychiatry 32:1927–1931. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, Zarate CA., Jr (2010). The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry 71:1488–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Sapin E, Lapray D, Bérod A, Goutagny R, Léger L, Ravassard P, Clément O, Hanriot L, Fort P, Luppi P–H. (2009). Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLOS ONE 4:e4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Huang J, Klein PS, Manji HK. (2008). Cellular plasticity cascades in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology 33:110–133. [DOI] [PubMed] [Google Scholar]
- Shimazaki Y, Nishiki T, Omori A, Sekiguchi M, Kamata Y, Kozaki S, Takahashi M. (1996). Phosphorylation of 25-kDa synaptosome-associated protein. Possible involvement in protein kinase C-mediated regulation of neurotransmitter release. J Biol Chem 271:14548–14553. [DOI] [PubMed] [Google Scholar]
- Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, Kaang BK. (2003). Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J Neurochem 85:872–881. [DOI] [PubMed] [Google Scholar]
- Steed E, Jones CA, McCreary AC. (2011). Serotonergic involvement in methamphetamine-induced locomotor activity: a detailed pharmacological study. Behav Brain Res 220:9–19. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. (2008). Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 64:293–301. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Machado-Vieira R, Yuan P, Wang Y, Wei Y, Falke C, Cirelli C, Tononi G, Manji HK, Du J. (2009). Glutamate receptors as targets of protein kinase C in the pathophysiology and treatment of animal models of mania. Neuropharmacology 56:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanti A, Westphal WP, Girault V, Brizard B, Devers S, Leguisquet AM, Surget A, Belzung C. (2013). Region-dependent and stage-specific effects of stress, environmental enrichment, and antidepressant treatment on hippocampal neurogenesis. Hippocampus 23:797–811. [DOI] [PubMed] [Google Scholar]
- Tsuchiya KJ, Byrne M, Mortensen PB. (2003). Risk factors in relation to an emergence of bipolar disorder: a systematic review. Bipolar Disord 5:231–242. [DOI] [PubMed] [Google Scholar]
- Tung A, Takase L, Fornal C, Jacobs B. (2005). Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience 134:721–723. [DOI] [PubMed] [Google Scholar]
- Verret L, Léger L, Fort P, Luppi P–H. (2005). Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur J Neurosci 21:2488–2504. [DOI] [PubMed] [Google Scholar]
- Wang HY, Markowitz P, Levinson D, Undie AS, Friedman E. (1999). Increased membrane-associated protein kinase C activity and translocation in blood platelets from bipolar affective disorder patients. J Psychiatr Res 33:171–179. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Sack DA, Rosenthal NE. (1987). Sleep reduction as a final common pathway in the genesis of mania. Am J Psych 144:201–204. [DOI] [PubMed] [Google Scholar]
- Yamamori S, Sugaya D, Iida Y, Kokubo H, Itakura M, Suzuki E, Kataoka M, Miyaoka H, Takahashi M. (2014). Stress-induced phosphorylation of SNAP-25. Neurosci Lett 561:182–187. [DOI] [PubMed] [Google Scholar]
- Yildiz A, Guleryuz S, Ankerst DP, Ongür D, Renshaw PF. (2008). Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry 65:255–263. [DOI] [PubMed] [Google Scholar]
- Young JW, Henry BL, Geyer MA. (2011). Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol 164:1263–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. (2007). A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neurosci Biobehav Rev 31:882–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Quiroz J, Jolkovsky L, Luckenbaugh DA, Manji HK. (2007). Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord 9:561–570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.