Abstract
Background:
Previous studies indicated a systemic deregulation of the pro-/antiinflammatory balance in subjects after 6 months of a first psychotic episode. This disruption was reexamined 12 months after diagnosis to identify potential risk/protective factors and associations with symptom severity.
Methods:
Eighty-five subjects were followed during 12 months and the determination of the same pro-/antiinflammatory mediators was carried out in plasma and peripheral blood mononuclear cells. Multivariate logistic regression analyses were used to identify risk/protective factors. Multiple linear regression models were performed to detect the change of each biological marker during follow-up in relation to clinical characteristics and confounding factors.
Results:
This study suggests a more severe systemic pro-/antiinflammatory deregulation than in earlier pathological stages in first psychotic episode, because not only were intracellular components of the inflammatory response increased but also the majority of soluble elements. Nitrite plasma levels and cyclooxygenase-2 expression in peripheral blood mononuclear cells are reliable potential risk factors and 15d-prostaglandin-J2 plasma levels a protection biomarker. An interesting relationship exists between antipsychotic dose and the levels of prostaglandin-E2 (inverse) and 15d-prostaglandin-J2 (direct). An inverse relationship between the Global Assessment of Functioning scale and lipid peroxidation is also present.
Conclusions:
Summing up, pro-/antiinflammatory mediators can be used as risk/protection biomarkers. The inverse association between oxidative/nitrosative damage and the Global Assessment of Functioning scale, and the possibility that one of the targets of antipsychotics could be the restoration of the pro-/antiinflammatory balance support the use of antiinflammatory drugs as coadjuvant to antipsychotics.
Keywords: first-episode psychosis, inflammation, biomarker, oxidative damage, risk/protective factors, antipsychotics
Introduction
The onset of psychotic illness typically occurs in late adolescence or early adulthood. This first episode of psychosis (FEP) is suffered by about 3% of the population (Perälä et al., 2007) and its clinical evolution tends to chronicity. Up to 80% of the patients relapse during the next 5 years after a FEP, with major risk to become resistant to treatment. Complete remission occurs in only one-third of the patients (Huber et al., 2008).
The FEP population represents a unique opportunity to evaluate biological, clinical, and functional outcomes of psychotic disorders: research at the onset of illness avoids the effect of confounding variables (Khan et al., 2008; Bernardo et al., 2013) and early intervention mitigates progression and improves prognosis (Linszen et al., 2001).
Despite all the great efforts made involving basic and clinical science, we are still far from a complete understanding of the pathophysiology of the disorder. Not surprisingly, <50% of patients respond to initial treatments (Lewis et al., 2006). In the past 15 years, a great deal of interest has been focused on immune/inflammatory alterations and the oxidative/nitrosative consequences as key pathophysiological mechanisms involved (Müller and Schwarz, 2008; Flatow et al., 2013). Although most of the scientific evidence has been found in established schizophrenia, some studies indicate subtle alterations in inflammatory/immune mediators and oxidative/nitrosative stress at the very beginning of the disease (Borovcanin et al., 2012; Martínez-Cengotitabengoa et al., 2012). Moreover, neuroimaging studies demonstrate that neuroinflammation is already present in early stages of schizophrenia (Pasternak et al., 2012).
In contrast, fewer studies have focused on the role of antiinflammatory signaling pathways, the counterbalancing mechanisms that control the potentially deleterious proinflammatory mediators (García-Bueno et al., 2008). Recent data show a clear systemic misbalance in some pro-/antiinflammatory mediators in patients with schizophrenia (Martínez-Gras et al., 2011). Also, the increase of antiinflammatory cytokines (transforming growth factor beta and interleukin 10) seems to be a mechanism elicited by several antipsychotics (Meyer et al., 2011).
Recently, Flamm-PEPs, a multicenter study inside the Spanish national network for mental health research, presented the first integrated results about the imbalance of pro-/antiinflammatory components in peripheral blood mononuclear cells (PBMCs) in FEP patients (García-Bueno et al., 2013). In particular, the proinflammatory pathway studied was the canonical one triggered by the activation of the nuclear transcription factor κB (NFκB) (Bierhaus et al., 2001; Madrigal et al., 2001). NFκB activates specific DNA sequences in the promoter of target genes, such as those that codify for the inflammatory enzymes inducible nitric oxide synthase (iNOS) and the isoform 2 of cyclooxygenase (COX2). The overactivation of the enzymes produces an increase in oxidative and nitrosative mediators (ie, stable NO metabolites such as NO− x, prostaglandins such as PGE2), which can cause the depletion of endogenous antioxidant defences and attack membrane phospholipids, causing cell damage by lipid peroxidation (García-Bueno et al., 2008). On the other hand, endogenous counterbalancing mechanisms are activated in response to inflammatory/immune stimuli, such as peroxisome proliferator activated receptors (PPARs). These nuclear receptors (mainly their gamma isoform, PPARγ; Heneka and Landreth, 2007) act as ligand-dependent transcription factors, decreasing the expression of proinflammatory genes. Interestingly, several COX-derived products, such as the prostaglandin 15-deoxy-PGJ2 (15d-PGJ2), act as endogenous antiinflammatory agents by targeting PPARγ (García-Bueno et al., 2005). Thus, the 15d-PGJ2/PPARγ pathway is an endogenous compensatory mechanism. This pathway could also be pharmacologically stimulated, representing not only a potential biomarker but also an important new candidate therapeutic target in those neuropsychiatric disorders in which inflammation may be playing a role.
In short, the above-cited first study of Flamm-PEPs (García-Bueno et al., 2013) also showed a decrease in the 15d-PGJ2/PPARγ pathway, demonstrating a proinflammatory phenotype in patients during their first 6 months after diagnosis. The disruption of this physiological balance is again reexamined in the present follow-up study 12 months after the inclusion of the patients in order to (1) analyze the evolution of the balance described in FEP, (2) identify potential risk/protective factors, (3) recognize associations between pro-/antiinflammatory mediators and symptoms’ severity during follow-up, and finally (4) by means of multiple linear regression models, to analyze the change of every biological marker during follow-up in relation to clinical characteristics and confounding factors.
Methods and Materials
Subjects (see supplementary Material 1 for complete details)
A total of 117 FEP subjects and 106 gender-, race-, and age-matched healthy controls were included in the baseline Flamm-PEPs study (García-Bueno et al., 2013). From the initial sample, 85 FEP subjects were followed during the next 6 months (maximum 12 months after inclusion). Baseline inclusion criteria for patients were: (1) age: 7 to 45 years; (2) duration of the psychotic symptoms: <1 year; and (3) speak Spanish correctly. The exclusion criteria were: (1) mental retardation (IQ <70 together with impaired functioning); (2) history of traumatic head injury with loss of consciousness; and (3) history of organic disease with mental repercussions. Having designed a real-life patient, naturalistic study, substance use, or having suicidal ideation were not exclusion criteria (Bernardo et al., 2013). Controls were selected from the same geographic areas. Inclusion criteria were: (1) same gender as patients; (2) similar age (±10%); (3) similar paternal socioeconomic status, allowing ±1 level in the Hollingshead-Redlich scale (Hollingshead and Redlich, 1958); (4) no past or present psychiatric disorder per DSM-IV criteria; (5) speak Spanish correctly; and (6) no history of psychotic disorder among first-degree relatives. The exclusion criteria for controls were the same as for patients.
Neither the patients nor the controls presented ongoing infections, fever, allergies, or other serious medical conditions or were receiving immunosuppressive drugs or vaccinations for at least 6 months prior to inclusion in the study or antiinflammatory-analgesics in the 2 days previous to blood extraction. Clinical assessment of patients and controls included a complete medical history, physical examination, electrocardiogram, and laboratory tests (Bernardo et al., 2013).
Expert clinicians used the Spanish translation of the DSM-IV semistructured diagnostic interview to establish diagnosis in adults (First and Spitzer, 1999) and the Kiddie-Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version for subjects younger than 18 years old (Ulloa et al., 2006). There was a broad age of inclusion to allow the inclusion of early-onset psychotic patients (Bernardo et al., 2013). Medical records and interviews with relatives were used as other sources of information to establish the onset of positive symptoms. The onset of the FEP was defined as the first week with the Positive and Negative Syndrome Scale (PANSS) items P1, P3, P5, P6, or G9 scoring ≥4 (García-Bueno et al., 2013). Global Assessment of Functioning scale (GAF) is a numerical scale (0–100) that measures functionality of patients based on work and social adjustment (Hall, 1995). The duration of untreated psychosis (DUP) was defined as the number of days elapsed between this onset and the beginning of the first antipsychotic treatment. DUBM was defined as the number of days elapsed between the onset of the FEP and blood sampling.
After complete description of the study to the subjects, written informed consent was obtained. The study was approved by the Ethics and Clinical Research Boards of all the hospitals involved in the study.
Specimen Collection and Preparation (see supplementary Material 1 for complete details)
Venous blood samples (10mL) were collected between 8:00 and 10:00 am after overnight fasting. Blood tubes were centrifuged (641 g x 10 minutes, 4ºC). The resultant plasma samples were stored at −80ºC. The rest of the sample was diluted in culture medium (RPMI 1640, Invitrogen), and a gradient with Ficoll-Paque (GE Healthcare) was used to isolate mononuclear cells by centrifugation (800 g x 40 minutes, room temperature). The PBMC layer was aspired and resuspended in RPMI and centrifuged (1116 g x 10 minutes, room temperature). The supernatant was removed and the mononuclear cell enriched pellet was stored at −80 ºC.
Biochemical Determinations in Plasma (see supplementary Material 1 for complete details)
Prostaglandins, the COX by-products PGE2 and 15d-PGJ2, were determined by enzyme immunoassay (Cayman Chemicals and DRG Diagnostics, respectively). Nitrites (NO− 2), the final and stable product of NO, were determined by using the Griess method. Lipid peroxidation was determined by Thiobarbituric Acid Reactive Substances (TBARS) assay (Cayman Chemicals, Tallin, Estonia), and cotinine levels, the major degradation product of nicotine metabolism, were determined by enzyme immunoassay (Cozart).
Biochemical Determinations in PBMC (see supplementary Material 1 for complete details)
PBMC samples were first fractionated in cytosolic and nuclear extracts. Determination of proinflammatory p65 NFκB subunit and ant-inflammatory PPARγ respective transcriptional activities were carried out in nuclear extracts from PMBC.
NFκB Activity
Activation of NFκB occurs by enzymatic degradation of the bound inhibitory protein (IκBα), allowing movement from cytoplasm to the nucleus where they bind to consensus κB sequences in DNA. The presence of p65 subunit in cell nuclei is considered an index of activity measured with a transcription factor assay (Cayman Chemicals, Tallin, Estonia).
PPARγ Transcription Factor
PPARγ transcription factor activity was determined using ELISA-based kits (Cayman Chemicals, Tallin, Estonia).
Western-Blot (WB) Analysis
Protein levels of IκBα, COX2, and iNOS were quantified by WB in cytosolic extracts from PBMCs. Protein levels of PPARγ were quantified in nuclear extracts. The housekeeping gene β-actin was used as loading control. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control for PPARγ (blots shown in the respective figures). For clarity, 2 WBs are presented in the figures representative of all the samples studied (in each different gel, n=3 per group control or FEP).
Statistical Analysis (see supplementary Material 1 for complete details)
Differences between baseline characteristics for patients and controls were assessed using chi-square, t test, or nonparametric Mann-Whitney U tests according to the distribution and scales of the variables. The effect of psychotropic medication was evaluated with linear regression models for each biomarker. To calculate the association between FEP and the level of biological markers, 5 hierarchical logistic regression models were used in which we gradually controlled for potential confounders (age, gender, body mass index [BMI], cannabis use per month, and cotinine levels). Multiple linear regression analysis was used to analyze the change in each biological marker depending on the change in demographic (gender, age, BMI), clinical variables (DUP, DUBM, GAF), antipsychotic medication (defined daily dose [DDD]), and cannabis and tobacco (cotinine).
Results
Demographic and Clinical Features
Table 1 presents the demographic and clinical characteristics of the FEP patients and the healthy control group. There were 32 subject from the total 117 FEP subjects who did not follow the 6-month period of study. Because of these early drop-outs, there were differences in the socioeconomic status between the groups that were not present at baseline.
Table 1.
Demographic and Clinical Characteristics
| Characteristic | Patients Baseline (N=85) | Patients Follow-up (N=85) | Controls (N=106) |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y | 24.89±6.26 | 25.21±6.03 | 25.18±6.79 |
| Sex, n (%) | |||
| Male | 60 (70.6) | — | 70 (66.0) |
| Female | 25 (29.4) | — | 36 (34.0) |
| Psychiatric history | |||
| Socioeconomic status | |||
| High | 16 (18.8) | — | 14 (13.2) |
| Medium-high | 7 (8.2) | — | 17 (16.0) |
| Medium | 30 (35.3)* | — | 54 (50.9) |
| Medium-low | 24 (28.2) | — | 19 (17.9) |
| Low | 8 (9.4)* | — | 2 (1.9) |
| Ethnic group | |||
| Caucasian | 79 (92.9) | — | 96 (90.6) |
| Gipsy | 1 (1.2) | — | 0 (0) |
| Maghrebian | 1 (1.2) | — | 2 (1.9) |
| Asian | 1 (1.2) | — | 0 (0) |
| Caribbean | 1 (1.2) | — | 0 (0) |
| Hispanic | 2 (2.4) | — | 6 (5.7) |
| Other | 0 (0) | — | 2 (1.9) |
| Age of psychosis onset | 24.37±5.92 | — | — |
| DUP, d | 68.58±77.28 | — | — |
| Diagnosis – no. (%) | |||
| Affective Psychosis | 17 (20.0) | 17 (20.0) | — |
| Non-affective Psychosis | 68 (80.0) | 68 (80.0) | — |
| Psychopathology score | |||
| PANSS | |||
| Total | 51.72±19.23 | 40.29±23.67 | — |
| Positive | 10.67±5.38 | 8.01±5.03 | — |
| Negative | 14.58±6.26 | 11.76±8.15 | — |
| General | 26.47±9.75 | 20.52±11.99 | — |
| Young | 1.45±3.25 | 1.39±3.57 | — |
| Montgomery-Asberg | 6.51±6.52 | 6.02±6.94 | |
| Overall functioning score (GAF) | 68.6±13.88 | 72.08±17.23 | — |
| Antipsychotic medication, n (%) | |||
| Risperidone | 30 (35.3) | 21 (25.9) | — |
| Olanzapine | 9 (10.6) | 6 (7.4) | — |
| Aripiprazole | 9 (10.6) | 15 (18.5) | — |
| Paliperidone | 7 (3.7) | 5 (6.2) | — |
| Clozapine | 5 (5.9) | 6 (7.4) | — |
| Quetiapine | 6 (7.1) | 5 (6.2) | — |
| Ziprasidone | 2 (2.4) | 2 (2.4) | — |
| None | 17 (8.9) | 21 (25.9) | — |
| Defined daily dose of chlorpromazine equivalents, mg | 282.19±253.04 | 298.06±303.16 | — |
| Lithium use, n (%) | 8 (9.4) | 7 (8.4) | — |
| Body mass index | 24.83±3.81* | 24.65±5.73 * | 23.14±3.16 |
| Cannabis use, n (%) | 18 (21.18) | 4 (5.1) * | 14 (16.0) |
| Cannabis use per month, n cigarettes | 4.71±19.36 | 1.09±6.37 | 1.15±6.36 |
| Tobacco use, n (%) | 49 (57.6) | 54 (65.1)* | 25 (29.4) |
| Tobacco use per month, n cigarettes | 238.9±259.08 | 241.98±254.11* | 45.38 (119.31) |
| Cotinine, ng/mL | 86.73±84.46 | 97.27±84.50* | 26.28±49.31 |
Abbreviations: DUP, duration of untreated psychosis; GAF, Global Assessment of Functioning scale.
* P<.05. Comparison between patients: follow-up and healthy controls.
Inflammatory and Oxidative/Nitrosative Stress Markers in Control and FEP Patients
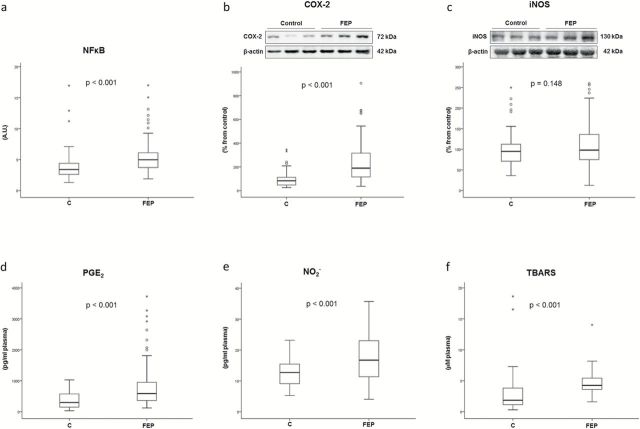
Figure 1 shows mean differences (SD) in inflammatory and oxidative/nitrosative stress markers in control and FEP patients. NFκB is a master regulator of the inflammatory and oxidative/nitrosative status of a cell. In nuclear extracts from PMBCs, its activity is increased in FEP samples (supplementary Material 2 and Figure 1a). Similarly, COX2 expression was significantly higher in FEP subjects, although the expression of iNOS was not different between groups (supplementary Material 2 and Figure 1b-c). At this particular stage of the disease, the plasma levels of PGE2, NO− 2, and TBARS were increased in FEP (supplementary Material 2 and Figure 1d-f, respectively). The numerical data for WB analysis from all subjects have been included in supplementary Table S3.
Figure 1.
Mean differences (SD) in proinflammatory biomarkers between first episode of psychosis (FEP) and controls (univariate analysis). (a) Nuclear transcription factor κB (NFκB) activity in peripheral blood mononuclear cell (PBMC) nuclear extracts from FEP patients (n = 85) and controls (n = 35). (b) Western-blot analysis of isoform 2 of cyclooxygenase (COX2) protein (patients n = 85, controls, n = 88); (c) Inducible nitric oxide synthase (iNOS) in PBMC cytosolic extracts from FEP patients (n = 85) and controls (n = 88); and (d) PGE2 (patients, n = 85; controls, n = 104). (e) Plasma levels of nitrites (NO− 2) (patients, n = 85; controls, n = 61). (f) Thiobarbituric acid reactive substances (TBARS) (patients, n = 85; controls, n = 104). AU, arbitrary units. Two-tailed nonparametric Mann-Whitney U test was used. ° represents an atypical value and * an extreme value.
Antiinflammatory Markers in Control and FEP Patients
Figure 2 shows mean differences (SD) in antiinflammatory and oxidative/nitrosative stress markers in control and FEP patients. IκBα in cytosolic extracts was not decreased in patients compared with healthy subjects (supplementary Material 2; Figure 2a). However, plasma levels of the antiinflammatory 15d-PGJ2 were significantly lower in FEP (supplementary Material 2; Figure 2b).
Figure 2.
Mean differences (SD) on antiinflammatory biomarkers. (a) Western-blot analysis of bound inhibitory protein (IκBα) in peripheral blood mononuclear cell (PBMC) cytosolic extracts (patients, n = 85; controls, n = 88); (b) Plasma levels of prostaglandin 15d-PGJ2 (patients, n = 85; controls, n = 104). (c) Western-blot analysis of peroxisome proliferator activated receptor γ (PPARγ; patients, n = 85; controls, n = 16). (d) Transcriptional activity of PPARγ (patients, n = 85; controls, n = 87) in PBMC nuclear extracts. AU, arbitrary units. Two-tailed t test was assessed for PPARγ protein levels, and for the rest of variables, 2-tailed nonparametric Mann-Whitney U test was used. ° represents an atypical value and * an extreme value.
Given that 15d-PGJ2 acts as an endogenous ligand for PPARγ, which is considered a potent antiinflammatory transcription factor, we analyzed its expression and transcriptional activity. WB analysis revealed lower expression of PPARγ in patients (supplementary Material 2; Figure 2c). Moreover, its transcriptional activity was also reduced in the patient group (supplementary Material 2; Figure 2d).
Multiple Logistic Regression Analysis
In the final multiple logistic regression model, 6 of 10 biological markers studied were significantly associated with followed FEP after controlling for all possible confounders (Table 2; supplementary Material 4). The resulting equation allows the estimation of the individual probability of FEP during a 6-month follow-up by substituting values of the biological levels and the demographic and clinical variables: log (P/1−P)=7.783+[0.005xPGE2]+[0.017xCOX2]+[0.369xNO-2]+[0.182xTBARS]+[−0.832xPPARγ act]+[−0.023x15d-PGJ2]+[0.062xgender (male)]+[−0.194xage]+[−0.070xBMI]+[−0.014xcannabis use per month]+[0.034xcotinine] (Hosmer et al., 2013).
Table 2.
Multivariate Logistic Regression Analysis
| B | SE | Wald | OR | CI 95% | P | |
|---|---|---|---|---|---|---|
| PGE2 | 0.005 | 0.002 | 3.721 | 1.005 | 1.000–1.009 | .054 |
| COX2 | 0.017 | 0.007 | 6.509 | 1.017 | 1.004–1.031 | .011 |
| NO-2 | 0.369 | 0.163 | 5.137 | 1.447 | 1.051–1.991 | .023 |
| TBARS | 0.182 | 0.162 | 1.269 | 1.200 | 0.874–1.649 | .260 |
| PPARγ act | −0.832 | 0.631 | 1.741 | 0.435 | 0.126–1.498 | .187 |
| 15d-PGJ2 | −0.023 | 0.007 | 10.099 | 0.977 | 0.963–0.991 | .001 |
Abbreviations: B, median; COX-2: cyclooxygenase 2; CI, confidence interval; NO-2, nitrites; OR, odds ratio; PGE2, prostaglandin E2; PPARg act, peroxisome proliferator activated receptors activity; SE, standard error; TBARS, thiobarbituric acid reactive substances; 15d-PGJ2, prostaglandin 15-deoxy-PGJ2.
Association between FEP and level of biomarker. All the biomarkers were analyzed together and adjusted for age, gender, body mass index, cannabis use per month, and cotinine level. The bold values in the table represent the values reaching statistical significance (P<.05).
Among the proinflammatory markers, the highest OR observed was for NO− 2 (OR=1.447), meaning that for each unit increased of this biomarker, the risk of FEP increased by 44.7% [(e0.369x1−1)x100] after controlling for remaining biological markers and all possible confounders (P=.023). Similarly, the results were 20% for TBARS (not significant), 1.7% for COX2 (P=.011), and 0.5% for PGE2 (not significant).
Among the antiinflammatory markers, the PPARγ activity had the lowest OR observed (OR=0.435), meaning that the risk decreased by 56.5% [(e−0.832x1−1)x100] for each unit of increased biomarker after controlling for remaining biological markers and all possible confounders, although it was not significant (P=.187). Similarly, the result for 15d-PGJ2 was 2.3% (P=.001).
Multiple Linear Regression Analysis
By means of multiple linear regression analysis, it is possible to predict the change of each biological marker from baseline to the follow-up point (6 to 12 months) controlling for a set of explanatory variables: gender, age, BMI, cannabis use, plasma cotinine levels, DDD of chlorpromazine equivalents, the DUP and the GAF scale score, and the days from diagnostic to the blood sampling for basal values (DUBM). Four (PGE2, COX2, NO− 2, and TBARS) of the 6 proinflammatory and oxidative/nitrosative biological markers studied and 2 (15dPGJ2 and PPARγ activity) of the 4 antiinflammatory markers studied were significantly associated with some of the explanatory variables (Table 3).
Table 3.
Differences (Mean ± SD) in the Biomarker Levels between Baseline (6 Months After Diagnosis) and 1-Year Follow-Up Patients
| Marker | Patients Baseline (N=85) | Patients Follow-Up (N=85) | Statistics | P |
|---|---|---|---|---|
| iNOS -WBc- | 131.44±51.43 | 109.05±52.89 | Z=−2.47 | .013 |
| COX2 -WBc- | 161.08±164.82 | 230.60±169.86 | Z=−3.13 | .002 |
| NFκB -act- | 12.13±23.75 | 5.52±2.75 | Z=−0.20 | .845 |
| PGE2 -sol- | 522.29±770.33 | 870.02±741.96 | Z= −4.58 | <.001 |
| NO- 2 -sol- | 14.94±6.20 | 17.61±7.34 | Z=−1.88 | .060 |
| TBARS -sol- | 3.65±3.81 | 4.50±1.75 | Z=−3.69 | <.001 |
| IκBα -WBc- | 86.36±49.97 | 104.21±82.09 | Z=−0.73 | .468 |
| 15d-PGJ2 -sol- | 571.25±154.15 | 408.89±150.69 | Z=−5.72 | <.001 |
| PPARγ -WBn- | 79.45±33.51 | 55.94±49.09 | Z=−2.35 | .019 |
| PPARγ -act- | 1.29±0.93 | 1.09±0.55 | Z=−1.11 | .267 |
Abbreviations: act, activity assay in nuclear extracts; sol: plasma levels of soluble compounds; COX-2, cyclooxygenase 2; IkBa, inhibitor of the NFkB alpha; iNOS, inducible nitric oxide synthase; NFκB, nuclear transcription factor κB; NO-2, nitrites; PGE2, prostaglandin E2; PPARg, peroxisome proliferator activated receptor gamma; TBARS, thiobarbituric acid reactive substances; 15d-PGJ2, prostaglandin 15-deoxy-PGJ2.
Nonparametric Wilcoxon signed-rank test was used. The bold values in the table represent the values reaching statistical significance (P<.05). Analyses carried out in WB: protein expression, determined by western blot in PBMC (WBc: in cytoplasmatic fraction; WBn: in nuclear fraction). See Methods for details.
Inflammatory and Oxidative/Nitrosative Stress Markers
The increased plasma levels of PGE2 from baseline to follow-up appeared negatively related to DDD (for each DDD unit increased during this period, PGE2 will decrease 0.733 units during follow-up, once controlling the effect of the increase in the other explanatory variables; Table 4). Also, PGE2 will decrease 3.387 units for each increase in 1 unit of cotinine plasma levels and will increase 0.714 units DUBM. COX2 expression was also related to cotinine: it will increase 0.672 units for each unit of cotinine level increased from basal to follow-up. The increase in nitrite levels also appeared related to the explanatory variables chosen: NO− 2 increased by 7.451 units more in female patients than in male patients (Kleinbaum et al., 2008).
Table 4.
Multiple Linear Regression Analysis
| PGE2 | COX2 | NO- 2 | TBARS | 15d-PGJ2 | PPARγ act. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ß | P | ß | P | ß | P | ß | P | ß | P | ß | P | |
| Gender (ref. female) | −45.635 | .805 | −37.395 | .426 | 7.451 | .015 | 1.793 | .083 | −31.095 | .625 | −0.179 | .632 |
| Age | −5.010 | .759 | −0.462 | .915 | −0.042 | .870 | 0.108 | .232 | −11.284 | .052 | 0.075 | .048 |
| Body mass index | −1.898 | .932 | 5.596 | .281 | −0.038 | .903 | −0.217 | .073 | 2.463 | .743 | −0.141 | .093 |
| Antipsychotic DDD | −0.733 | .028 | 0.148 | .085 | 0.006 | .236 | −0.001 | .604 | 0.333 | .006 | 0.000 | .888 |
| DUP | 1.020 | .462 | 0.187 | .578 | 0.019 | .355 | −0.013 | .080 | −0.025 | .957 | 0.003 | .274 |
| GAF | −3.401 | .660 | 2.490 | .206 | 0.122 | .263 | −0.120 | .005 | 0.783 | .763 | −0.033 | .024 |
| Cannabis use per month | −0.619 | .890 | −1.296 | .215 | −0.190 | .008 | 0.015 | .531 | 0.994 | .510 | −0.003 | .714 |
| Cotinine plasma levels | −3.387 | .007 | 0.672 | .046 | 0.001 | .955 | 0.001 | .927 | 0.503 | .225 | 0.002 | .343 |
| DUBM | 0.714 | .017 | −0.047 | .500 | 0.001 | .837 | 0.005 | .002 | −0.057 | .563 | 0.000 | .555 |
Abbreviations: ß, linear regression coefficient; BMI, body mass index; DDD, defined daily dose of antipsychotic chlorpromazine equivalents; DUBM, number of days elapsed between the onset of the FEP and blood samplings; DUP, duration of untreated psychosis; GAF, Global Assessment of Functioning scale.
Change of each biological marker (from baseline to the follow-up point) depending on demographic and clinical variables. The bold values in the table represents the values reaching statistical significance (P<.05).
The increase of TBARS plasma levels from basal to follow-up, the final marker of systemic oxidative/nitrosative stress, also appeared related to clinical status: for each unit of GAF scale increase, TBARS decreases 0.12 units. Finally, for DUBM, TBARS will increase 0.005 units.
Antiinflammatory Markers
Plasma levels of 15d-PGJ2 from baseline to the follow-up were positively related to DDD (for each DDD unit during this period, 15d-PGJ2 will increase 0.333 units during follow-up, once controlling the effect of the increase in the other explanatory variables; Table 4). Finally, the activity of PPARγ will decrease 0.033 units for each point in GAF score and increases 0.75 units for each year of age.
Discussion
In this follow-up study, we have strengthened the evidence of systemic inflammatory alterations in FEP patients. Previously, with this same cohort of patients, we described phenotypical differences in proinflammatory mediators at the cellular machinery level in PBMCs, but the resultant soluble elements were not significantly altered (García-Bueno et al., 2013). However, 6 months later, some of the soluble elements analyzed already appeared significantly altered (PGE2, TBARS, 15d-PGJ2), suggesting the existence of a deeper deregulation of the pro/antiinflammatory balance (supplementary Table S5).
The multivariate logistic regression analysis identified the plasma levels of the marker of oxidative/nitrosative damage NO− 2 and the COX2 expression in PBMCs as the most reliable potential risk factors. Also, plasma levels of the antiinflammatory 15d-PGJ2 might be used as a potential protection factor for FEP.
The current longitudinal approach allowed the analysis of the changes of every biological marker between 6 and 12 months after the inclusion of the patients in relation to the changes of clinical characteristics and confounding factors. Especially remarkable are the associations found between the antipsychotic dose and the change in the plasma levels of PGE2 (inverse) and 15d-PGJ2 (direct). Indeed, these results suggest that one of the therapeutic mechanisms of antipsychotics may be the restoration of the pro-/antiinflammatory balance disrupted in FEP. It is worth noting that 25.9% of the FEP patients recruited here were receiving medication with risperidone, an atypical antipsychotic drug that normalizes increased inflammatory mediators (cytokines and inflammatory PGs) (Sugino et al., 2009; Adkins et al., 2012) and restores antiinflammatory pathways in murine models of neuroinflammation (MacDowell et al., 2013). In our conditions, the increase of inflammation during follow-up can be due to the fact that antipsychotics are not able to completely reverse the inflammation associated with the disease, and this circumstance could justify the potential use of antiinflammatory drugs as coadjuvant to antipsychotics for the treatment of the psychotic disease (Sommer et al., 2012).
Other results deserving attention are the opposite relationships found between cotinine levels and COX-2 protein expression in PBMCs and PGE2 plasma levels. Nicotine effects on the cyclooxygenase pathway in an inflammatory context are complex and sometimes contradictory, depending of the nicotine levels reached, subtype of receptor activated, the route of administration of nicotine, and whether there is a subjacent pathological state (Piao et al., 2009; Gonçalves et al., 2011; Hurley and Tizabi, 2013). Our paradoxical results need to be further explored to analyze the effects of nicotine on the antiinflammatory α7 nicotinic acetylcholine receptor (Hurst et al., 2013) or on the expression and enzymatic activity of tissue-specific PGE2 synthases, such as microsomal-PGE-synthase-1. Our results support the idea that cigarette smoking is a potent confounding factor to control for when the inflammation/innate immune system is studied.
Regarding other potential confounding factors, the results described here suggest that the alterations in the pro-/antiinflammatory balance in FEP patients are not related to changes in BMI in both time-points studied (see supplemental Table S6). In addition, NO− 2 plasma levels increased more in female than in male patient subjects between both time points studied, age was directly related to PPARγ activity, and cannabis use per month was inversely related to NO− 2 plasma levels. Further studies are needed to corroborate whether these relationships are particular for PEPs or can be extended to other states of psychotic disease. From a clinical point of view, the inverse relationship found between the GAF scale and TBARS plasma levels is particularly relevant and provides scientific evidence of a direct role for oxidative/nitrosative stress cellular damage in the general symptomatology of FEP and possibly in other disorders with psychotic symptoms. In a comparable cohort of patients, it has been reported that cognitive deficits are related to oxidative stress (Martínez-Cengotitabengoa et al., 2012).
As previously described, the longitudinal design of this study allowed to analyze the evolution of the inflammatory response taking place in FEP patients. The comparison of the data obtained in the 2 selected time-points illustrates the complex and continuous dynamic changes of the inflammatory response in the natural course of incipient psychosis. This complexity might be one of the causes of controversy regarding the specific role of inflammatory mediators in the pathophysiology of the different types of psychotic disorders (Meyer et al., 2011) and the difficulties in the design and proper use of treatment strategies based on classical antiinflammatory drugs, producing in most cases only relatively modest improvements in clinical symptoms in the early stage of schizophrenia (Sommer et al., 2012).
The results of the multivariate analysis show potential risk/protective factors common in both baseline and follow-up visits. This is the case of COX2 protein levels in PBMCs as a risk factor and 15d-PGJ2 plasma levels as a putative protection factor. However, other plausible biomarkers have lost their validity (ie, IκBα). These results illustrate how one biomarker could be useful in a limited phase of the natural course of the disease (status biomarker), but others are more general for all the phases of the disease (trait biomarker), as has been shown for specific cytokines (Miller et al., 2011).
The reformulation of the traditional conception of the psychotic illness, being now considered as a heterogeneous disorder with multisystemic impact in addition to its psychiatric expression (Kirkpatrick, 2009), is clearly supported by these data. Recent studies compared the same immune biomarkers (eg, cytokines such as IFNγ) in plasma and postmortem brain tissue from control and subjects with schizophrenia and found similar alterations in both compartments, validating the concept that schizophrenia can be studied through systemic biomarkers (Harris et al., 2012). A recent review suggested a link between peripheral inflammatory/immune processes and MRI-detected anomalies in the brain (Frodl and Amico, 2014), and studies in postmortem brain tissue from subjects with schizophrenia showed increased levels of some of the pro-/antiinflammatory and oxidative/nitrosative stress markers studied here (NFκB, COX2, TBARS) (Rao et al., 2013). Our data might serve to elucidate whether the alterations of these inflammatory risk/protection biomarkers could have etiological relevance and not only utility for diagnosis and monitoring the evolution of the disease (Bergink et al., 2013).
The present study identifies vulnerability conditions related to PBMC pro-/antiinflammatory pathways in FEP patients. One special feature of the study is that it includes a wide spectrum of biochemical markers in both PBMCs and plasma, allowing to establish in-depth relationships between multiple components of the signalling pathways. In addition, we have used the DUBM variable that enhances the accuracy of the values obtained in each biomarker. Thus, this variable is especially relevant in the particular case of the proinflammatory/oxidative stress biomarkers PGE2 and TBARS, which significantly increase depending on the days between diagnosis and the moment of blood extraction. Additional studies that analyze inflammatory/immune biomarkers in psychiatric disease should take into account and control for this variable.
Limitations
The present results may also explain one possible limitation reported in our first study (García-Bueno et al., 2013) about the role of stress in the observed changes: stress exposure could be one of the factors implicated in the onset of FEP (Holtzman et al., 2013), and stress could contribute to the observed shift towards enhanced pro- vs antiinflammatory signalling (García-Bueno et al., 2005). However, current results indicate a clear evolution after 1 year (not only were intracellular components of the inflammatory response increased, but also the majority of resultant proinflammatory soluble elements and the oxidative/nitrosative stress markers). Also, the deregulation of the balance is permanent in chronic schizophrenic inpatients with a 13-year history of psychosis (Martínez-Gras et al., 2011). This evolution suggests that the inflammatory process is not merely due to acute stress exposure in the beginning of FEP.
Control subjects were not followed 6 months after baseline as patients were, and this could be considered a major limitation. When this study was designed, our hypothesis was that the pro-/antiinflammatory mediators analyzed did not significantly change in 6 months in control subjects. However, this possibility exists, and the interpretation of the results obtained 6 months after baseline with regards to control subjects should be done with caution. The main objective of the experimental design made was to study the intra-subject evolution of the disease between both time points.
It is worth noting that 75% of the FEP patients included in our study were under atypical antipsychotic treatment, and there is increasing evidence on the potential antiinflammatory effects of antipsychotics (Sugino et al., 2009; Miller et al., 2011; MacDowell et al., 2013). Nevertheless, we tried to control the possible confounding effect of antipsychotic treatment through a multiple linear regression analysis.
In addition, each antipsychotic group and lithium have very different impacts on metabolic signalling (weight gain), and with the approximation made with the chlorpromazine equivalents, their differential profile could be lost. To resolve this issue, we analyzed if there are differences in weight gain between basal and follow-up time points studied and between the different antipsychotic groups and lithium, respectively (supplementary Tables S6-8) and there were no significant differences.
Finally, it would be necessary to determine if the peripheral changes found in inflammatory-related molecules in FEP subjects could also take place at a central level.
Conclusion
In conclusion, this follow-up study corroborates the existence of a deregulated systemic pro-/antiinflammatory balance in FEP, which becomes more severe during the initial 1 year after the diagnosis. The multivariate analysis applied shows potential risk/protective factors in both time points studied (COX-2 protein levels in PBMCs and 15d-PGJ2 plasma levels) that can be considered trait markers and other specific state biomarkers (initial: IκBα; 1-year evolution: NO− 2 and TBARS). Striking results come from the multiple linear regression approach, which shows how one of the targets of antipsychotic treatment may be the restoration of the inflammatory balance. Finally, from a clinical point of view, the inverse relationship found between the final product of oxidative/nitrosative cellular damage TBARS and the GAF scale is especially relevant in order to justify the development of antioxidant/antiinflammatory treatment strategies not only for established schizophrenia but also for earlier stages of a psychotic disorder.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
The authors provide full disclosure of any and all biomedical financial interests. The authors declare that there are not conflicts of interest.
Supplementary Material
Acknowledgments
This work was supported by CIBERSAM Intramural Projects 2010 (P02): Flamm-PEPs, Inflammatory alterations in schizophrenia: search of biological markers in first psychotic episodes, Spanish Ministry of Economy and Competiveness, Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias and also by PI 10/0123 to J.C.L. and PI 1100325 to M. Bernardo.
References
- Adkins DE, Khachane AN, McClay JL, Aberg K, Bukszár J, Sullivan PF, van den Oord EJ. (2012). SNP-based analysis of neuroactive ligand-receptor interaction pathways implicates PGE2 as a novel mediator of antipsychotic treatment response: data from the CATIE study. Schizophr Res 135:200–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink V, Gibney SM, Drexhage HA. (2013). Autoimmunity, inflammation and psychosis: a search for peripheral markers. Biol Psychiatry S0006–3223(13)00946–3. [DOI] [PubMed] [Google Scholar]
- Bernardo M, Bioque M, Parellada M, Saiz Ruiz J, Cuesta MJ, Llerena A, Sanjuán J, Castro-Fornieles J, Arango C, Cabrera B; PEPs Group (2013). Assessing clinical and functional outcomes in a gene-environment interaction study in first episode of psychosis (PEPs). Rev Psiquiatr Salud Ment 6:4–16. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. (2003). A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A 100:1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovcanin M, Jovanovic I, Radosavljevic G, Djukic Dejanovic S, Bankovic D, Arsenijevic N, Lukic ML. (2012). Elevated serum level of type-2 cytokine and low IL-17 in first episode psychosis and schizophrenia in relapse. J Psychiatr Res 46:1421–1426. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, eds (1999). Entrevista clínica estructurada para los trastornos del eje-I del DSM-IV. Barcelona: Masson. [Google Scholar]
- Flatow J, Buckley P, Miller BJ. (2013). Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Amico F. (2014). Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Prog Neuropsychopharmacol Biol Psychiatry 48:295–303.32. [DOI] [PubMed] [Google Scholar]
- García-Bueno B, Madrigal JL, Lizasoain I, Moro MA, Lorenzo P, Leza JC. (2005). Peroxisome proliferator-activated receptor gamma activation decreases neuroinflammation in brain after stress in rats. Biol Psychiatry 57: 885–894. [DOI] [PubMed] [Google Scholar]
- García-Bueno B, Caso JR, Leza JC. (2008). Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev 32:1136–1151. [DOI] [PubMed] [Google Scholar]
- García-Bueno B, Bioque M, Mac-Dowell KS, Barcones MF, Martínez-Cengotitabengoa M, Pina-Camacho L, Rodríguez-Jiménez R, Sáiz PA, Castro C, Lafuente A, Santabárbara J, González-Pinto A, Parellada M, Rubio G, García-Portilla MP, Micó JA, Bernardo M, Leza JC. (2013). Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull 2013; e-pub ahead of print 13 March 2013; PMID:23486748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves RB, Coletta RD, Silvério KG, Benevides L, Casati MZ, da Silva JS, Nociti FH., Jr (2011). Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. 60:409–424. [DOI] [PubMed] [Google Scholar]
- Hall RC. (1995). Global assessment of functioning. A modified scale. Psychosomatics. 36:267–275. [DOI] [PubMed] [Google Scholar]
- Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, Bahn S. (2012). Comparison of peripheral and central schizophrenia biomarker profiles. PLoS One 7:e46368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Landreth GE. (2007). PPARs in the brain. Biochim Biophys Acta 1771:1031–1045. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC, eds (1958). Social class and mental illness. New York: Wiley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman CW, Trotman HD, Goulding SM, Ryan AT, Macdonald AN, Shapiro DI, Brasfield JL, Walker EF. (2013). Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience 249:172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, Sturdivant RX. (2013). Applied logistic regression. 3rd ed. New York: John Wiley & Sons. [Google Scholar]
- Huber CG, Naber D, Lambert M. (2008). Incomplete remission and treatment resistance in first-episode psychosis: definition, prevalence and predictors. Expert Opin Pharmacother 9:2027–2038. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Tizabi Y. (2013). Neuroinflammation, neurodegeneration, and depression. Neurotox Res. 23:131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst R, Rollema H, Bertrand D. (2013). Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 137:22–54. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, Gheorghe MD, Rybakowski JK, Galderisi S, Libiger J, Hummer M, Dollfus S, López-Ibor JJ, Hranov LG, Gaebel W, Peuskens J, Lindefors N, Riecher-Rössler A, Grobbee DE; EUFEST study group (2008). Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 371:1085–1097. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B. (2009). Schizophrenia as a systemic disease. Schizophr Bull 35:381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE, Nizam A. (2008). Applied regression analysis and other multivariable methods. 4th ed. Thomson: Belmont, CA. [Google Scholar]
- Lewis DA, González-Burgos G. (2006). Pathophysiologically based treatment interventions in schizophrenia. Nature Med 12:1016–1022. [DOI] [PubMed] [Google Scholar]
- Linszen D, Dingemans P, Lenior M. (2001). Early intervention and a five year follow up in young adults with a short duration of untreated psychosis: ethical implications. Schizophr Res 51:55–61. [DOI] [PubMed] [Google Scholar]
- MacDowell KS, García-Bueno B, Madrigal JL, Parellada M, Arango C, Micó JA, Leza JC. (2013). Risperidone normalizes increased inflammatory parameters and restores anti-inflammatory pathways in a model of neuroinflammation. Int J Neuropsychopharmacol 16:121–135. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Boscá L, Leza JC. (2001). Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J Neurochem 76:532–538. [DOI] [PubMed] [Google Scholar]
- Martínez-Cengotitabengoa M, Mac-Dowell KS, Leza JC, Micó JA, Fernandez M, Echevarría E, Sanjuan J, Elorza J, González-Pinto A. (2012). Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr Res 137:66–72. [DOI] [PubMed] [Google Scholar]
- Martínez-Gras I, Pérez-Nievas BG, García-Bueno B, Madrigal JL, Andrés-Esteban E, Rodríguez-Jiménez R, Hoenicka J, Palomo T, Rubio G, Leza JC. (2011). The anti-inflammatory prostaglandin 15d-PGJ2 and its nuclear receptor PPARgamma are decreased in schizophrenia. Schizophr Res 128:15–22. [DOI] [PubMed] [Google Scholar]
- Meyer U. (2011). Anti-inflammatory signaling in schizophrenia. Brain Behav Immun 25:1507–1518. [DOI] [PubMed] [Google Scholar]
- Meyer U, Schwarz MJ, Müller N. (2011). Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther 132:96–110. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. (2011). Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. (2008). A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: schizophrenia and major depression as inflammatory CNS disorders. Eur Arch Psychiatry Clin Neurosci 258 Suppl 2:97–106. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin CF, Bouix S, Seidman LJ, Goldstein JM, Woo TU, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M. (2012). Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci 32:17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perälä J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsä E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppä T, Härkänen T, Koskinen S, Lönnqvist J. (2007). Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry 64:19–28. [DOI] [PubMed] [Google Scholar]
- Piao WH, Campagnolo D, Dayao C, Lukas RJ, Wu J, Shi FD. (2009). Nicotine and inflammatory neurological disorders. Acta Pharmacol Sin 30:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Kim HW, Harry GJ, Rapoport SI, Reese EA. (2013). Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr Res 147:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, de Witte L, Begemann M, Kahn RS. (2012). Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry 73:414–419. [DOI] [PubMed] [Google Scholar]
- Sugino H, Futamura T, Mitsumoto Y, Maeda K, Marunaka Y. (2009). Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry 33:303–307. [DOI] [PubMed] [Google Scholar]
- Ulloa RE, Ortiz S, Higuera F, Nogales I, Fresán A, Apiquian R, Cortés J, Arechavaleta B, Foulliux C, Martínez P, Hernández L, Domínguez E, de la Peña F. (2006). [Interrater reliability of the Spanish version of Schedule for Affective Disorders and Schizophrenia for School-Age Children--Present and Lifetime version (K-SADS-PL)]. Actas Esp Psiquiatr 34:36–40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.