Abstract
Transcranial direct current stimulation is a noninvasive technique that has been experimentally tested for a number of psychiatric and neurological conditions. Preliminary observations suggest that this approach can indeed influence a number of cellular and molecular pathways that may be disease relevant. However, the mechanisms of action underlying its beneficial effects are largely unknown and need to be better understood to allow this therapy to be used optimally. In this review, we summarize the physiological responses observed in vitro and in vivo, with a particular emphasis on cellular and molecular cascades associated with inflammation, angiogenesis, neurogenesis, and neuroplasticity recruited by direct current stimulation, a topic that has been largely neglected in the literature. A better understanding of the neural responses to transcranial direct current stimulation is critical if this therapy is to be used in large-scale clinical trials with a view of being routinely offered to patients suffering from various conditions affecting the central nervous system.
Keywords: inflammation, neurogenesis, long-term potentiation
Introduction
Transcranial direct current stimulation (tDCS) is a noninvasive experimental therapy used to stimulate the brain with externally applied direct current electric fields (DCEFs). The promising clinical outcomes obtained in various conditions coupled with the fact that this approach is safe, well tolerated, inexpensive, and simple to administer has catalyzed the popularity of tDCS and its potential use in routine clinical practice. To date, it has been tested to treat aspects of stroke (Sohn et al., 2013), multiple sclerosis (Ferrucci et al., 2014), Parkinson’s disease (Benninger et al., 2010), schizophrenia (Andrade, 2013), and depression (Dell’Osso et al., 2012). Despite accumulating evidence supporting the efficacy of tDCS as a treatment option for these conditions, there is only one single Phase III trial currently taking place (http://www.prnewswire.com/news-releases/soterix-medical-inc-announces-phase-3-clinical-trial-for-depression-comparing-tdcs-lte-against-antidepressant-drug-escitalopram-229718911.html); all previous trials having been conducted to confirm safety and targeted end-points in small cohorts. Sizable studies will thus be critical to confirm its true effectiveness for specific disorders.
However, the impact of DCEF on cellular elements has been recognized for nearly a century (Ingvar, 1920), and DCEF is well known to be involved in numerous physiological processes such as wound healing and embryogenesis. Despite the fact that DCEF is also recognized to influence phenotypic and functional parameters such as the morphology, orientation, migration, growth, and metabolism of several mammalian cells, including neurons and neural stem cells (McCaig et al., 2005), little is known about the mechanisms of action that govern these effects.
In this review, we summarize the current state of knowledge regarding the cellular and molecular mechanisms of action of DCEFs, as revealed in vitro and in animal studies. By so doing, we provide a comprehensive understanding of the impact of tDCS on cells of the central nervous system, which includes the molecular cascades known to be affected by it to the more global physiological responses associated with this manipulation.
The Basics of TDCS
Amongst all existing brain stimulation therapies, tDCS is the only one that uses DCEF to stimulate the brain. A weak current is conveyed via electrodes positioned on the scalp; the stimulation electrode is located above the region of interest and the reference electrode placed elsewhere on the body (eg, the contralateral orbit or the deltoid muscle) (Nitsche et al., 2008). The anode or the cathode can be used to stimulate the brain, with anodal stimulation generally augmenting neuronal excitability, whereas cathodal stimulation produces the opposite effect (Cambiaghi et al., 2010; Fritsch et al., 2010; Kabakov et al., 2012). In both cases, the current induces a sustainable response in the form of a long-term potentiation (LTP)- or long-term depression (LTD)-like plasticity. However, it is now also becoming clear that this relationship is more complex than once thought, in that anodal tDCS can actually lead to decreased excitability when the stimulation time is increased (Monte-Silva et al., 2013), and cathodal tDCS can lead to increased excitability when intensity is augmented (Batsikadze et al., 2013). Thus, the relationship between the stimulation and neural response is not dependent on just the electrode type but also the length and strength of the stimulation applied through it.
To date, tDCS has been primarily recognized and used for its localized cortical LTP- and LTD-like effects (Ranieri et al., 2012), but recent animal studies have revealed that tDCS (1–4.16 A/m2) can also affect subcortical structures, such as the red nucleus, medial longitudinal fascicle (Bolzoni et al., 2013a, 2013b), and thalamus, as shown in variations of regional cerebral blood flow (Lang et al., 2005). However, it has yet to be demonstrated whether these changes result from the direct influence of the applied DCEF or if they are driven by increased excitability of the cortical neurons connecting to these deeper structures (Im et al., 2012; Bolzoni et al., 2013a).
Effects of DCEFs on Membrane Polarity
One of the most accepted effects of tDCS is its ability to modify neuronal membrane polarity and, by so doing, its threshold for action potential generation (Nitsche and Paulus, 2001; Liebetanz et al., 2002; Stagg and Nitsche, 2011). As in ephaptic coupling, which consists of extrasynaptic communication between cells via extracellular electric fields (EFs) (such as local field potentials), tDCS does not trigger action potentials but most likely affects the spike timing of individual neurons receiving suprathreshold inputs (Anastassiou et al., 2011). In clinical studies, the explanation for this is thought to simply reflect the depolarization of neurons during anodal stimulation and hyperpolarization during cathodal stimulation (Brunoni et al., 2012; Nitsche et al., 2012). However, in the EF, each feature of a single cell is differentially affected. Structural components of cellular elements (eg, neurite, nucleus, etc.) at the cathode are subject to depolarization, whereas those facing the anode are more prone to hyperpolarization (Bedlack et al., 1992; Bikson et al., 2004; Arlotti et al., 2012; Rahman et al., 2013).
The changes in the cell firing rate, leading to an overall modulation of cortical excitability (Cambiaghi et al., 2010), were initially hypothesized to derive from somatic membrane polarization rather than dendritic or axonal polarization (Liebetanz et al., 2006b). This has been suggested to be because of higher Na+ channel density in the soma than in the apical dendrite, or its proximity to the axon hillock (Liebetanz et al., 2006b). However, recent findings in rat hippocampal slices have shown that the excitatory or inhibitory effects of DCS are determined by the orientation of the axons in the EF (Kabakov et al., 2012), supporting the importance of the presynaptic modulation of neurons put forward by Purpura and McMurty (1965), namely that DCS works at the level of synaptic inputs and not just action potential generation in the efferent neuron per se (Kabakov et al., 2012). In this study, measurements of the paired-pulse facilitation and the field excitatory postsynaptic potential to direct current stimulation of the hippocampus suggested that cathodal DCS inhibits field excitatory postsynaptic potential and increases the paired-pulse ratio, with opposite effects for anodal stimulation. Interestingly, if the anodal stimulation is too strong, the effects are cancelled, an observation that may relate to it being able to hyperpolarize the membrane when delivered in this way (Kabakov et al., 2012; Rahman et al., 2013). Another example has been described with the primary motor cortex, where the afferent axonal synaptic input (Figure 1) can be facilitated by anodal tDCS (Rahman et al., 2013), leading to the increase in motor evoked potentials (Liebetanz et al., 2002; Stagg and Nitsche, 2011). Indeed, variations of paired-pulse recordings, when simultaneously applied to tDCS in free-moving rabbits, further support the notion that the changes are due to presynaptic modifications (Marquez-Ruiz et al., 2012). Even if the modulation is likely dependent on the orientation of the neurons in the EF, the polarization of neuronal subcompartments is difficult to establish in complex brain structures such as the primary motor cortex (Radman et al., 2009; Rahman et al., 2013), as the axons and dendrites forming synapses are not all oriented in the same direction.
Figure 1.
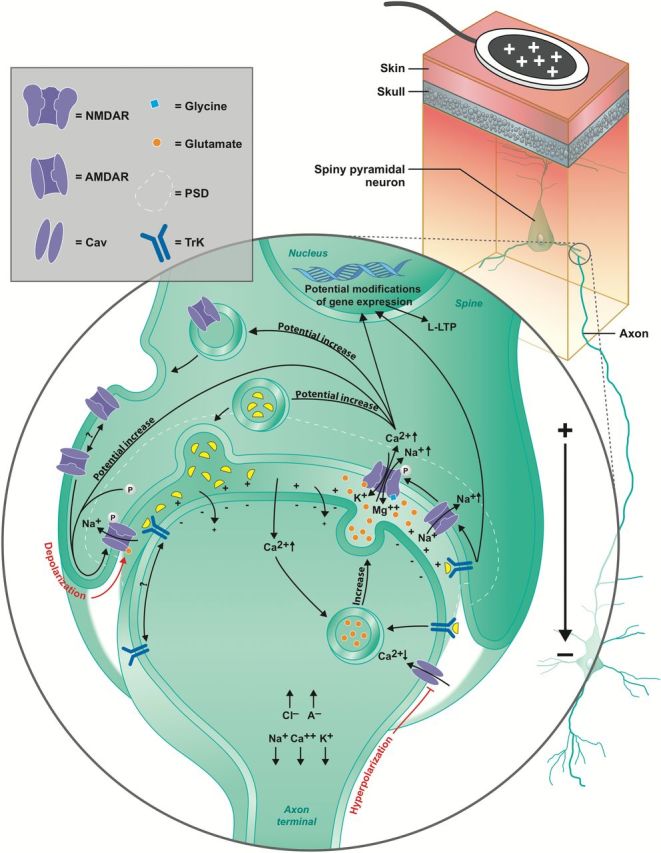
Putative molecular mechanisms of action of anodal transcranial direct current stimulation (tDCS). Schematic illustrating the effects of anodal tDCS on the synapses of pyramidal neurons in the primary motor cortex. Note that cathodal tDCS is not represented, as it largely generates the opposite effects of anodal tDCS, except for the mechanisms involving brain-derived neurotrophic factor (BDNF), which is described below. Anodal tDCS hyperpolarizes the membrane of the axon terminal facing the anode (Bikson et al., 2004). Despite the hyperpolarization, there is greater neurotransmitter release, which is caused by an increase in intracellular Ca2+ in response to anodal tDCS, whereas a decrease of Ca2+ leads to lower neurotransmitter release (Perret et al., 1999; Stagg et al., 2009a).
Tropomyosin-receptor kinase (Trk) receptors may also be attracted to the synapse in anodal tDCS (if the presynaptic synapse faces the skull, as illustrated here) (McCaig et al., 2000; Viard et al., 2004). The activation of Trk receptors suggests a role for BDNF in anodal tDCS, which further increases the probability of synaptic vesicle docking and neurotransmitter release (Pozzo-Miller et al., 1999).
Direct current electric fields (DCEFs) also directly affect the postsynaptic neuron by depolarizing (basal dendrites and soma, represented in the figure) or hyperpolarizing (apical dendrites) the membrane in anodal tDCS, or the opposite with cathodal tDCS (Kabakov et al., 2012), which further facilitates/inhibits α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)- and N-methyl-d-aspartate receptor (NMDAR)-mediated ionic changes.
Overall, with long-term potentiation (LTP) responses, there is an upregulation of neurotransmitter release that facilitates the opening of AMPARs and indirectly that of NMDARs (Derkach et al., 2007). The opposite is true for long-term depression (LTD). The Ca2+ influx has been demonstrated to increase AMPAR phosphorylation and their incorporation into the membrane (Opazo et al., 2010). Ca2+ further increases the release of neurotrophic factors into the synaptic cleft and its absence decreases it (Neal and Guilarte, 2010). Once activated, postsynaptic Trk receptor induces later phase LTP (L-LTP) and favors the opening of NMDARs, which also promotes L-LTP, whereas the opposite is involved in cathodal tDCS, promoting later phase LTD (L-LTD) (Minichiello, 2009). Both L-LTP and L-LTD are dependent on modifications of gene expression (Frey et al., 1996; Smolen, 2007). PSD, postsynaptic domain; Cav; voltage-gated calcium channel.
Putative Molecular Mechanisms of tDCS
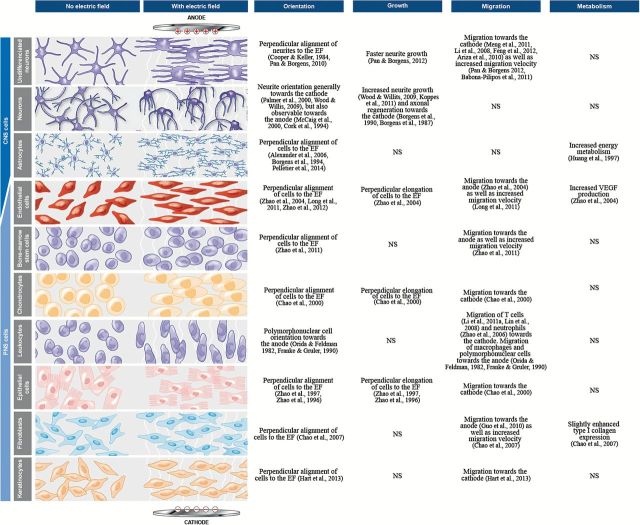
DCEFs can also govern cell migration, a phenomenon referred to as electrotaxis (McCaig et al., 2005; Zhao, 2009), as well cell orientation (growth cone direction), differentiation, and metabolism, the responses of which vary depending on the cell type (summarized in Figure 2). However, although the mechanisms of action underlying these effects remain unknown, there is evidence to suggest that the changes in the orientation and speed of cell migration and neurite growth could be explained, at least in part, by localized shifts of intracellular Ca2+ (Palmer et al., 2000; Mycielska and Djamgoz, 2004). Linked to this is the asymmetrical relocalization of receptors within the membrane brought about by DCEFs (McLaughlin and Poo, 1981; McCaig et al., 2005). In many cell types, membrane receptors, such as acetylcholine receptors and the tropomyosin-receptor-kinase (Trk) families, move and accumulate at one end of an EF to cause an electrotaxis effect (McCaig et al., 2005). In neurites, this may then contribute to the long-term neuromodulation observed in structures targeted by the tDCS treatment (McCaig et al., 2000) (Figure 1). However, other receptors at the synapse may also be involved in this response, as the size of the DCS-induced LTP is influenced by the orientation of the dendrites in a DCEF (Kabakov et al., 2012) and N-methyl-d-aspartate receptors (NMDARs) (Figure 1) (Liebetanz et al., 2002). This facilitation is greatest when the postsynaptic membranes, on soma or dendrites, are depolarized (Kabakov et al., 2012). This is seen when it is closest to the negative end of the field and under such circumstances, this also facilitates the opening of voltage-dependent ionic channels and NMDAR activation (by removal of the blocking Mg2+ ions) (Kampa et al., 2004). There are also other contributing factors to this neuromodulatory action, including changes in brain-derived neurotrophic factor (BDNF) expression. Although approximately 0.75V/m anodal DCS increases the peak amplitude of the excitatory postsynaptic potentials, it is completely absent in slices from BDNF knockout mice or when the TrkB receptor is blocked (Fritsch et al., 2010). In individuals expressing the BDNF Val66Met polymorphism, which affects the release of BDNF, motor skill acquisition after a 5-day tDCS treatment is significantly lower than in healthy volunteers (Fritsch et al., 2010). This polymorphism further abolishes changes of spinal cord excitability usually observed during stimulation (Lamy and Boakye, 2013), all of which highlights the importance of this growth factor in DCEF-mediated changes at the synapse. Further support for this comes from a study in which DCS was applied to brain slices of the motor cortex and the LTP-like effects observed were found to be partly dependent on the activation of TrkB, the main receptor of BDNF. The activation of this receptor is necessary to initiate LTP but does not seem to be needed to sustain or promote LTP-associated plasticity (Fritsch et al., 2010).
Figure 2.
Cell types and their responses to transcranial direct current stimulation (tDCS).
Most neurons change their growth direction in an EF, which is further associated with increases in the number of dendritic spines, as shown in vitro (Figure 2). To date, the best-known effects of tDCS are on the sustained modulation of neuronal excitability measured by motor evoked potential and functional magnetic resonance imaging in humans (Nitsche et al., 2008). In mice, motor evoked potential can be facilitated or suppressed, for up to 10 minutes, using anodal and cathodal tDCS, respectively (Cambiaghi et al., 2010). This has also been confirmed in rats by measuring the changes in blood flow after tDCS (Takano et al., 2011; Wachter et al., 2011) and by electrophysiological measures in awake rabbits (Marquez-Ruiz et al., 2012).
Other factors and neurotransmitters are also involved. Following tDCS delivered over the primary motor cortex, γ-aminobutyric acid levels (measured by magnetic resonance spectroscopy) are further reduced in healthy human volunteers (Stagg et al., 2009a). Blockade of serotonin reuptake increases LTP induced by anodal tDCS of the motor cortex and reverses cathodal LTD into LTP (Nitsche et al., 2009), whereas D2 antagonists abolish cathodal and delay anodal tDCS-induced plasticity in healthy volunteers (Nitsche et al., 2006), demonstrating the importance of dopamine and serotonin in human tDCS. Interestingly, stimulation of the rat frontal cortex significantly enhances extracellular striatal dopamine but only in the context of cathodal tDCS (Tanaka et al., 2013). In mouse models of ischemic stroke, there is an increase of lactate after anodal tDCS and a decrease of glutamate concentration and levels of NR2B (a subunit for NMDAR) after cathodal tDCS (Peruzzotti-Jametti et al., 2013) (Table 1). Taken together, the release of neurotrophic factors (eg, BDNF), the growth and orientation of dendritic spines, and the release of a number of neurotransmitters support a role for tDCS in neuronal plasticity, all of which may be mediated through NMDARs.
Table 1.
Summary of tDCS Studies Conducted in Normal Animals and Animal Models of Disease
| References | Species | Gender | Age and/or Weight | Disease models | Areas of stimulation | Charge density (A/m2) | Number of days x sessions/day | Results/observations |
|---|---|---|---|---|---|---|---|---|
| Liebetanz et al., 2006a | Wistar rats | M | 332–422 g | Cortical spreading depression | Unilateral parietal cortex | 28.50 | 1 x 1 | Anodal tDCS increases CSD spreading velocity. |
| Liebetanz et al., 2006b | Wistar rats | M | 245–309 g | Epilepsy | Unilateral parietal cortex | 28.57 or 57.14 | 1 x 1 | Cathodal tDCS shows anticonvulsive properties. |
| Fregni and Pascual- Leone, 2007 | Wistar rats | M | 358–373 g | Cortical spreading depression | Unilateral parietal cortex | 28.5 | 1 x 1 | Anodal tDCS increases CSD spreading velocity preconditioned by 1-Hz repetitive electrical stimulations. |
| Schweid et al., 2008 | Cats | M | 2.9–3.2 kg | - | Unilateral visuoparietal cortex | 5 | 1 x 1 | Cathodal tDCS induces decreased performance for static visual targets presented in the contrastimulated visual hemifield. |
| Ben Taib and Manto, 2009 | Sprague-Dawley rats | M | 280–400 g | Hemi-cerebellectomy | Unilateral motor cortex | 51.20 | 1 x 1 | Anodal tDCS antagonizes motor cortex hypoexcitability induced by high-frequency stimulation of the interpositus nucleus. |
| Liebetanz et al., 2009 | Wistar rats | M&F | 286–334 g | - | Unilateral frontal cortex | 0.286 to 285.7 | 1 x 1 | Threshold for tissue damage using cathodal tDCS established at 142.9 A/m2. |
| Kim et al., 2010 | Sprague-Dawley rats | NS | 5 wk | Ischemia (unilateral MCAO) | Unilateral visual cortex | 1.26 | 14 x 1 | Anodal tDCS have neuroprotective effects on neural axons following infarct. |
| Cambiaghi et al., 2010 | C57BL/6 mice | F | 10–14wk/ 25–30 g | - | Unilateral primary motor cortex | 55.50 | 1 x 1 | Anodal tDCS increases motor evoked potential. Cathodal tDCS decreases it. |
| Wachter et al., 2011 | Sprague-Dawley rats | M | ~310 g | - | Unilateral middle cerebral artery territory | 7.14, 14.29, or 28.57 | 1 x 1 | Anodal tDCS increases cerebral blood flow. Cathodal tDCS decreases it. Higher current density results in more distinct effects. |
| Takano et al., 2011 | Sprague-Dawley rats | M | ~288 g | - | Bilateral frontal cortex (with electrode placement on the midline) | 1.60 and 16 | 1 x 1 | Anodal tDCS increases fMRI signal intensity in the frontal cortex and nucleus. accumbens. |
| Cambiaghi et al., 2011 | C57BL/6 mice | F | 8–12 wk | - | Unilateral primary motor cortex | 55.50 | 1 x 1 | Increase (anodal) or decrease (cathodal) in size of visual evoked potentials for 10min after tDCS. |
| Dockery et al., 2011 | Long-Evans rats | M | 250–325 g | - | Unilateral frontal cortex | 57.14 | 1 x 1 | Long-term benefits of frontal cathodal tDCS when paired with training on working memory and skill learning of a novel task. |
| Kamida et al., 2011 | Wistar rats | M | 23 d | Epilepsy | Unilateral motor cortex | 57.10 | 14 x 1 | Anodal tDCS has neuroprotective effects on hippocampal cells and reduces the granular and CA3 mossy fiber sprouting. Further reduces convulsions and rescues cognitive impairments. |
| Li et al., 2011b | Sprague-Dawley rats | F | NS | Parkinson’s disease (unilateral 6-OHDA lesion) | Unilateral primary motor cortex | 11.43 or 22.86 | 1 x 1 | Anodal tDCS abolishes the ipsilateral bias in a corridor test (effect of 1 d). |
| Yoon et al., 2012 | Sprague-Dawley rats | M | 6wk/220– 280 g | Ischemia (unilateral MCAO) | Unilateral at ischemic borders (established by MRI) | 28.20 | 5 x 1 | Anodal tDCS increases MAP-2 and GAP-43 staining in both lesioned and intact brain. |
| Marquez-Ruiz et al., 2012 | New Zealand white albino rabbits | NS | 2.3–2.7 kg | - | Unilateral somatosensory cortex | 3.70 | 1 x 1 | Anodal tDCS increases evoked potential. Cathodal tDCS decreases it. Lasting effects are observed only after cathodal tDCS. Both types of stimulation modify thalamo-cortical synapses at the presynaptic site. tDCS modulates the sensory perception process of associative learning. A1R activation are necessary for cathodal-evoked LTD. |
| Spezia Adachi et al., 2012 | Wistar rats | M | 250–300 g | Chronic inflammation (intraplantar injections of CFA) | Bilateral parietal cortex | 33.40 | 8 x 1 | Anodal tDCS has antinociceptive properties. |
| Rueger et al., 2012 | Wistar rats | M | 290–330 g | - | Unilateral motor cortex | 142.90 | 5 x 1 or 10 x 1 | Anodal and cathodal tDCS increase the number of Iba1+ cells. Cathodal tDCS increases the number of proliferating cells and He3+ neural stem cells in the cortex. |
| Jiang et al., 2012 | Wistar rats | M | 4–5 mo | Ischemia (unilateral MCAO) | Unilateral visual cortex | 1.26 | (3.7 or 14) x 1 | Anodal tDCS improves motor functions. Increased density of dendritic spines and decreased pannexin-1 mRNA levels. |
| Spezia Adachi et al., 2012 | Sprague-Dawley rats | M | 60 d/180– 230 g | Chronic stress-induced pain | Bilateral parietal cortex (with electrode placement on the midline) | 33.40 | 8 x 1 | Anodal tDCS has antinociceptive effects and reduces TNF-α level in the hippocampus (serum levels unchanged). |
| Zobeiri and van Luijtelaar, 2013 | WAG/Rij rats | M | 6 mo/322– 364 g | Genetic model of absence epilepsy | Bilateral perioral region of the somatosensory cortex (use of 2 independent electrodes) | 28.57 and 42.86 | 1 x 4 | Reduced number of slow-wave discharges during and after cathodal tDCS. Increased sub-delta and delta waves in the motor cortex suggest the hyperpolarization of cortical cells. |
| Tanaka et al., 2013 | Sprague-Dawley rats | M | 9 wk | - | Bilateral frontal cortex (with electrode placement on the midline) | 32 | 1 x 1 | Cathodal, but not anodal stimulation, increases extracellular striatal dopamine levels. |
| Bolzoni et al., 2013b | Cats | NS | 2.2–3.4 kg | - | Unilateral sensorimotor cortex | 1 or 2.50 | 1 x several | Anodal tDCS facilitates the activation of rubrospinal and reticulospinal neurons. |
| Bolzoni et al., 2013a | Sprague Dawley & Wistar rats | M&F | 200–300 g | - | Unilateral sensorimotor cortex | 4.16 | 1 x (5 to 7) | Firing of subcortical structures (medial longitudinal fascicle and red nucleus) is facilitated by cathodal tDCS and depressed by anodal tDCS. |
| Peruzzotti- Jametti et al., 2013 | C57/BL6 mice | M | 8–10wk/ 20–22 g | Ischemia (unilateral MCAO) | Left parietal area | 55.0 | 1 x 2 | Cathodal tDCS decreases the number of Iba+ and CD45+ cells at the infarct site, reduces the infarct size, decreases the number of caspase-3+ cells in the cortex and striatum, and reduces glutamate and NR2B levels in the cortex. Anodal tDCS increases the infarct size, exacerbates cortical hemorrhages and disruptions of the blood brain barrier, increases the number of caspase-3+ cells in the cortex and striatum, and increases lactate levels in the cortex. |
| Pedron et al., 2014 | Swiss mice | F | 4 mo | Nicotine abstinence in addicted mice | Unilateral frontal cortex | 57.14 | 5 x 2 | Anodal tDCS has antidepressant properties, improves working memory, and reduces conditioned place preference for nicotine in normal animals. In nicotine-addicted mice, it reduces locomotor activity, depression-related behavior, and addictive behaviors. |
Abbreviations: 6-OHDA, 6-hydroxydopamine; A1R, Adenosine A1 receptor; CA3, cornu ammonis 3; CFA, complete Freund’s adjuvant; CSD, cortical spreading depression; fMRI, functional magnetic resonance imaging; LTD, long-term depression; GAP-43, growth associated protein-43; Iba1, ionized calcium-binding adapter molecule; MAP-2, microtubule-associated protein-2; MCAO, middle cerebral artery occlusion, mRNA, messenger ribonucleic acid; NR2B, N-methyl D-aspartate receptor aubtype 2B; NS, not specified; tDCS, transcranial direct current stimulation; TNF-α = tumor necrosis factor α.
The Effects of tDCS on Other Neural and Inflammatory Processes
Although tDCS can affect synaptic processes, it is also clear that DCEFs generated in the stimulated cerebral tissue (Nitsche et al., 2008; Rahman et al., 2013) can influence physiological processes, including inflammation, neurogenesis, neuroplasticity, and angiogenesis (Figure 3; Table 1).
Figure 3.
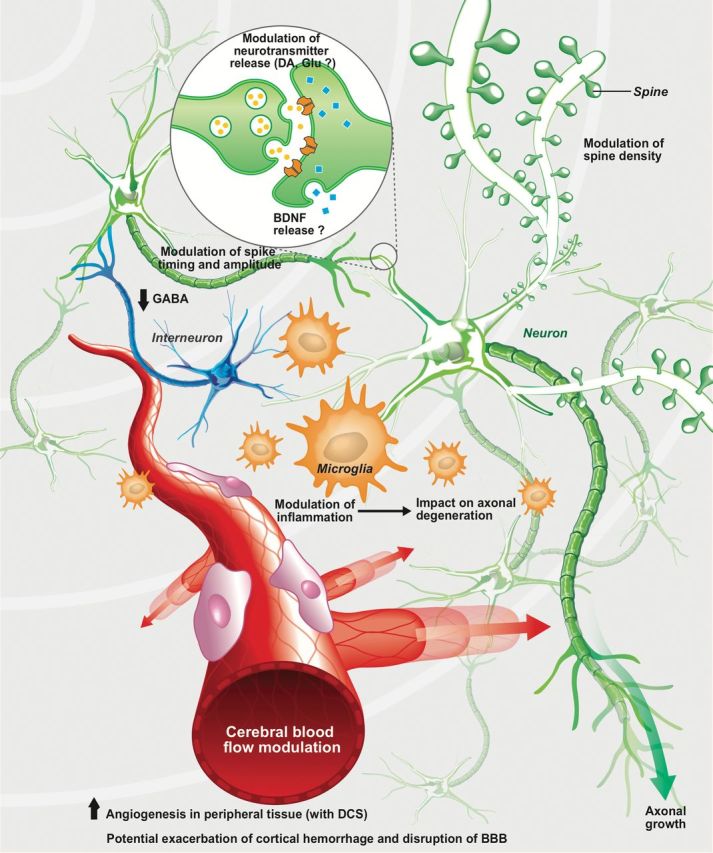
Putative cellular mechanisms of action of anodal transcranial direct current stimulation (tDCS). Anodal tDCS has been demonstrated to increase the amplitude and reduce the timing of glutamatergic neuronal firing (the opposite effect is observed for cathodal stimulation) (Cambiaghi et al., 2010; Hunter et al., 2013). In contrast, the firing of interneurons is decreased in both anodal and cathodal tDCS in healthy human subjects, as suggested by decreased γ-aminobutyric acid (GABA) release (Stagg et al., 2009a). Glutamate is also reduced by cathodal tDCS in healthy human subjects, whereas dopamine has been reported to be increased in normal rats with such therapy (Stagg et al., 2009a; Tanaka et al., 2013). Increases in growth associated protein-43 (GAP-43), a protein synthesized during axonal growth, and microtubule-associated protein-2 (MAP-2), involved in dendritic remodeling along with an increase in dendritic density, has been reported in stimulated brain structures in rats with ischemic lesions, which further suggests that anodal tDCS may have neuroprotective as well as neurorestorative properties (Yoon et al., 2012). There are, however, no data on their modulation by cathodal tDCS. In ischemic mice, cathodal tDCS also decreases ionized calcium-binding apater molecule-1 (Iba1+), CD45+, and caspase-3+ cell numbers, whereas the opposite is seen with anodal tDCS (Peruzzotti-Jametti et al., 2013). In a rat model of chronic stress-induced pain, tumor necrosis factor-α (TNF-α) is also downregulated by anodal tDCS, but an increase in the number of Iba1+ cells is observed in both anodal and cathodal tDCS at high intensity in normal rats (Spezia Adachi et al., 2012). Angiogenesis and increases in vascular endothelial growth factor (VEGF) levels have been reported in peripheral tissues exposed to DCEF (Bai et al., 2011). Finally, anodal tDCS in ischemic mice may also exacerbate cortical hemorrhage and provoke the disruption of the blood-brain barrier (BBB; Peruzzotti-Jametti et al., 2013). Taken together, all of this suggests that tDCS affects a number of physiological processes in both the central and peripheral nervous systems that may be relevant to its effects in disease states. AP, action potential; BDNF, brain-derived neurotrophic factor; DA, dopamine; Glu, glutamate.
Effects of tDCS on inflammation
DCEFs have demonstrated significant effects on the inflammatory response both in the central and peripheral nervous systems. For example, in vitro DCEFs can accelerate and polarize the migration of several types of peripheral immune cells, including lymphocytes (Li et al., 2011a), monocytes (Lin et al., 2008), neutrophils (Zhao et al., 2006), macrophages (Orida and Feldman, 1982), and polymorphonuclear cells (Franke and Gruler, 1990) (Figure 2). Cultured primary astrocytes as well as astrocytic cell lines (Pelletier et al., 2014) align perpendicularly to an EF (Borgens et al., 1994; Alexander et al., 2006) and have increased energy metabolism when stimulated by DCEFs (Huang et al., 1997). We have also recently observed that high-voltage EFs may provoke an inflammatory response in quiescent BV2 microglial cells, as shown by the increase in cyclooxygenase-2 expression (Pelletier et al., 2014). This is in agreement with studies in rats in which daily anodal (5 consecutive days) or cathodal stimulation over the primary motor cortex increases the number of cortical microglial cells on the ipsilateral side of the stimulation (Rueger et al., 2012) (Table 1). This, however, may be secondary to tissue damage, because despite the fact that the stimulation parameters chosen for this particular study were not reported to lead to apparent brain lesions, another study established that cathodal stimulation at this level can create discernable cortical lesions (Liebetanz et al., 2002), and it is this that drives the inflammatory response. In contrast, in mice stimulated with cathodal tDCS 30 minutes after a middle cerebral artery occlusion (model of ischemic stroke), the number of cortical Iba1+ and CD45+ (ie, microglial) cells was actually reduced (Peruzzotti-Jametti et al., 2013). This coupled to the reported decrease in hippocampal tumor necrosis factor-α levels 48 hours following the last of 8 daily anodal tDCS sessions of the parietal cortex in a rat model of chronic restraint stress (Spezia Adachi et al., 2012) suggests that there are both anti-inflammatory and pro-inflammatory effects of tDCS depending on EF direction and intensity (Figure 3).
Effects of tDCS on angiogenesis
In vitro application of DCS can accelerate the migration of endothelial cells to the anode (Zhao et al., 2004; Long et al., 2011) and their orientation (Zhao et al., 2004, 2012; Long et al., 2011) (Figure 2). In addition, when EFs exceeding 100V/m are applied, cultured endothelial cells not only elongate but secrete higher levels of vascular endothelial growth factor, nitric oxide, and interleukin-8 (Bai et al., 2011), all critical players in angiogenesis. This helps explain that when a DCEF is applied in vitro to an aortic ring dissected from rodents, vessel-like structures orient toward the anode (Song et al., 2007). Furthermore, DCS has been shown to increase capillary density in a rabbit model of myocardial infarction when the stimulation is applied directly on the epicardium (Zhang et al., 2011). These data all support the idea that tDCS can influence the vasculature and drive angiogenesis, although how this specifically relates to angiogenesis within the central nervous system is still unclear.
Effects of tDCS on Apoptosis, Neurogenesis, and Neuroplasticity
Apoptosis and Neurogenesis
DCS has been shown to affect apoptotic processes. In ischemic mice, tDCS significantly decreases the number of caspase-3 positive cells in the cortex and striatum 24 hours following cathodal stimulation but increases it when anodal stimulation is used (Peruzzotti-Jametti et al., 2013). We know from in vitro studies that anti-apoptotic proteins, namely apoptosis inhibitor 5, caspase 8, and Fas-associated death domain-like apoptosis regulator and the protein kinase C epsilon, are all upregulated in fibroblasts exposed to a 100-V/m stimulation (Jennings et al., 2008), which may help explain these data.
In addition to its effects on apoptosis, cathodal tDCS of the rat primary motor cortex for 10 consecutive days has been reported to increase (by 160%) the number of proliferating cells and neural stem cells within the stimulated region (Rueger et al., 2012). Although the impact of this type of stimulation on behavioral phenotypes was not investigated, it is important to remember that this study actually used a current intensity (142.9 A/m2) that greatly exceeded that being used for humans. Nevertheless, it should be noted that neural precursor cells exposed to DCEFs may not only increase in number but also preferentially migrate towards the cathode in vitro (Cooper and Keller, 1984; Li et al., 2008; Ariza et al., 2010; Meng et al., 2011; Feng et al., 2012), and this could be exploited to direct neural stem cell migration towards a lesion or damaged location.
Neurite Outgrowth
In vitro studies have further demonstrated that weak DCEFs applied to neurons can increase the total number of neurite branches at the cathode, which is decreased with anodal stimulation (McCaig et al., 2005). DCEFs can also induce more rapid neurite growth (Wood and Willits, 2009; Koppes et al., 2011) and modulate their orientation. Depending on the cell type, differentiation stage, or animal model used, neurites can be redirected towards the cathode (Patel and Poo, 1982; Erskine et al., 1995; Palmer et al., 2000; Rajnicek et al., 2006; Wood and Willits, 2009; Koppes et al., 2011), the anode (Cork et al., 1994), align perpendicularly to the EFs (Pan and Borgens, 2010) or not be affected at all (Cormie and Robinson, 2007) (Figure 2). In vivo, daily tDCS over a period of 2 weeks following ischemia in rats increases spine density in the remaining cells at the infarct site, which is further accompanied by improved motor function (Jiang et al., 2012). In addition, upregulation of MAP-2, a critical protein in dendritic outgrowth and remodeling, and GAP-43, a protein found in axonal growth cones, further support this specific effect of tDCS on dendritic as well as axonal regrowth following tDCS (Yoon et al., 2012). GAP-43 expression also raised following low-intensity DCEF stimulation of differentiated neurons in vitro (Pelletier et al., 2014), and when a cathodal stimulating electrode delivering a weak DCS is implanted into the hemi-lesioned spine of guinea pigs, there is increased axonal regrowth that further leads to the recovery of the cutaneous trunci muscle reflex created by this type of lesion (Borgens et al., 1987, 1990). These axons are also able to grow through the glial scar, something that is not observed in sham lesions. Finally, in paraplegic dogs, subcutaneous application of DCEF has been shown to lead to improvements in neurological measures such as recovery of deep and superficial pain sensation, proprioceptive reflexes, and locomotor ability (Borgens et al., 1993). Taken as a whole, DCEF seems to be able to induce robust axonal outgrowth in vitro and in vivo.
Can DCEF Observations Made in Vitro and in Small Animals Be Extrapolated to Humans?
In vitro and in vivo animal studies conducted thus far are too few to answer this question, especially given that the stimulation parameters are very different from one study to the other. This, of course, complicates the interpretation and the extrapolation of data to the clinical setting. Nevertheless, the use of in vitro models offers some advantages. Simpler models where the basic parameters can be controlled are ideal to dissect cellular and molecular mechanisms. For example, the use of brain slices permits simultaneous stimulation at different intensities, which would be difficult to perform in animals. It is also possible in these model systems to investigate various electrophysiological properties that would be otherwise impossible to observe in vivo, for example, cell behavior in EFs (eg, orientation in the field). The strength of working with isolated tissue is that one can control the direction of current flow and monitor its behavior at all times (Bikson et al., 2012). Certainly, in vitro observations must be interpreted with care as they may not always reflect what takes place in the more complex environment of the mature nervous system. Whenever possible, results should be confirmed by in vivo studies.
In vivo studies in small animals are also extremely valuable. In stimulation studies conducted in rodents, electrodes are usually fixed to the skull and because it has a low conductance, there is almost no diffusion of the electric current before it reaches the brain. The electrode size (2mm diameter) further allows one to stimulate a relatively specific area of the brain and to consistently stimulate the same region in the same or different animals of the same cohort. It also enables one to stimulate freely moving animals, bypassing potential artifacts created by anesthesia (Gersner et al., 2011). Importantly, there are now an infinite number of animal models of various pathological conditions. Despite the fact that these models do not perfectly mimic all aspects of disease, they do replicate several behavioral and pathological features that allow to study and better understand the various mechanisms driving anomalies and how these respond to tDCS interventions.
Lastly, we must keep in mind that the stimulating parameters reported in in vitro and in vivo studies are admittedly higher than those typically used in human tDCS, where stimulation amounts to approximately ≤1V/m, for a maximum of 0.28 A/m2 brain current density (Im et al., 2012). However, this does not invalidate preclinical data, and trying to replicate the exact parameters used in the clinic from small animals may not be that logical given the differences in brain size and cellular composition (Bikson et al., 2012). Despite the significantly higher current intensities used in small animals, several responses resemble those measured in humans. For example, tDCS applied to tobacco smokers led to reduced smoking (Fecteau et al., 2014), just as it has been associated with reduced addictive behavior in mice treated with nicotine (Pedron et al., 2014). tDCS treatment of Parkinson’s disease patients reveals long-term improvements of some motor features (Benninger et al., 2010), which has also been reported in the rat 6-hydroxydopamine (6-OHDA) lesion model of parkinsonism (Li et al., 2011b). Cathodal tDCS significantly attenuates epileptic discharge frequency in patients (Auvichayapat et al., 2013), with similar anticonvulsive properties also being observed in rats following cathodal tDCS (Liebetanz et al., 2006b). Finally, tDCS applied to stroke patients improves gait performance (Tahtis et al., 2014), hand dexterity, and selective attention (Au-Yeung et al., 2014), whereas motor and cognitive improvements were also seen in animal models of stroke (Kim et al., 2010).
An overview of the data available has shed light on the impact of tDCS on a number of biological phenomena. tDCS can generate LTP- and LTD-like effects and modulate cell morphology, orientation, migration, and growth. Although affecting primarily the cortex, it has the capacity to reach deeper structures, and its effects include neuroprotection, axogenesis, and neurogenesis as well and the modulation of inflammatory responses. Despite the inherent limitations of in vitro settings and animal models of disease, the information derived from these model systems is quite insightful and will help identify putative mechanisms of action of tDCS responsible for the clinical outcomes reported. However, Phase III clinical trials will be critical to unveil the true potential of this methodology as a treatment option.
Conclusion
Taken together, the data for tDCS hold promise for the treatment of diseases affecting the central nervous system. However, a significant amount of fundamental research still needs to be done to support the therapeutic usefulness of tDCS. Furthermore, stimulation dose response curves also need to be performed to identify the most effective conditions and thus optimize the therapy, as stimulation parameters are critical in determining outcome. At this stage, a deeper and better understanding of the mechanisms of action of noninvasive brain stimulation is necessary to unveil the true potential of tDCS in the clinical treatment of a range of neurological and psychiatric conditions.
Statement of Interest
None.
Acknowledgments
This work was supported by a pilot grant from Parkinson Society Canada to Francesca Cicchetti, who is also a recipient of a National Researcher career award from the Fonds de recherche du Québec en santé providing salary support and operating funds. The authors would like to sincerly thank Mr Gilles Chabot for artwork. The authors are entirely responsible for the scientific content of the paper.
References
- Alexander JK, Fuss B, Colello RJ. (2006). Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol 2:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiou CA, Perin R, Markram H, Koch C. (2011). Ephaptic coupling of cortical neurons. Nat Neurosci 14:217–223. [DOI] [PubMed] [Google Scholar]
- Andrade C. (2013). Once- to twice-daily, 3-year domiciliary maintenance transcranial direct current stimulation for severe, disabling, clozapine-refractory continuous auditory hallucinations in schizophrenia. J ECT 29:239–242. [DOI] [PubMed] [Google Scholar]
- Ariza CA, Fleury AT, Tormos CJ, Petruk V, Chawla S, Oh J, Sakaguchi DS, Mallapragada SK. (2010). The influence of electric fields on hippocampal neural progenitor cells. Stem Cell Rev 6:585–600. [DOI] [PubMed] [Google Scholar]
- Arlotti M, Rahman A, Minhas P, Bikson M. (2012). Axon terminal polarization induced by weak uniform DC electric fields: a modeling study. Conf Proc IEEE Eng Med Biol Soc 2012:4575–4578. [DOI] [PubMed] [Google Scholar]
- Auvichayapat N, Rotenberg A, Gersner R, Ngodklang S, Tiamkao S, Tassaneeyakul W, Auvichayapat P. (2013). Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul 6:696–700. [DOI] [PubMed] [Google Scholar]
- Au-Yeung SS, Wang J, Chen Y, Chua E. (2014). Transcranial direct current stimulation to primary motor area improves hand dexterity and selective attention in chronic stroke. Am J Phys Med Rehabil 10.1097/PHM.0000000000000127. [DOI] [PubMed] [Google Scholar]
- Babona-Pilipos R, Droujinine IA, Popovic MR, Morshead CM. (2011). Adult subependymal neural precursors, but not differentiated cells, undergo rapid cathodal migration in the presence of direct current electric fields. PLoS One 6:e23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Forrester JV, Zhao M. (2011). DC electric stimulation upregulates angiogenic factors in endothelial cells through activation of VEGF receptors. Cytokine 55:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. (2013). Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591:1987–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedlack RS, Jr., Wei M, Loew LM. (1992). Localized membrane depolarizations and localized calcium influx during electric field-guided neurite growth. Neuron 9:393–403. [DOI] [PubMed] [Google Scholar]
- Ben Taib NO, Manto M. (2009). Trains of transcranial direct current stimulation antagonize motor cortex hypoexcitability induced by acute hemicerebellectomy. J Neurosurg 111:796–806. [DOI] [PubMed] [Google Scholar]
- Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, Hallett M. (2010). Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. (2004). Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol 557:175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Reato D, Rahman A. (2012). Cellular and network effects of transcranial direct current stimulation: insights from animal models and brain slices. In: Frontiers in neuroscience, pp 55–91 Boca Raton, FL: CRC Press. [Google Scholar]
- Bolzoni F, Pettersson LG, Jankowska E. (2013a) Evidence for long–lasting subcortical facilitation by transcranial direct current stimulation in the cat. J Physiol 591:3381–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolzoni F, Baczyk M, Jankowska E. (2013b) Subcortical effects of transcranial direct current stimulation (tDCS) in the rat. J Physiol 15;591:4027–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgens RB, Blight AR, McGinnis ME. (1987). Behavioral recovery induced by applied electric fields after spinal cord hemisection in guinea pig. Science 238:366–369. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Blight AR, McGinnis ME. (1990). Functional recovery after spinal cord hemisection in guinea pigs: the effects of applied electric fields. J Comp Neurol 296:634–653. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Toombs JP, Blight AR, McGinnis ME, Bauer MS, Widmer WR, Cook JR., Jr (1993). Effects of applied electric fields on clinical cases of complete paraplegia in dogs. Restor Neurol Neurosci 5:305–322. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Shi R, Mohr TJ, Jaeger CB. (1994). Mammalian cortical astrocytes align themselves in a physiological voltage gradient. Exp Neurol 128:41–49. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. (2012). Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul 5:175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambiaghi M, Velikova S, Gonzalez-Rosa JJ, Cursi M, Comi G, Leocani L. (2010). Brain transcranial direct current stimulation modulates motor excitability in mice. Eur J Neurosci 31:704–709. [DOI] [PubMed] [Google Scholar]
- Cambiaghi M, Teneud L, Velikova S, Gonzalez-Rosa JJ, Cursi M, Comi G, Leocani L. (2011). Flash visual evoked potentials in mice can be modulated by transcranial direct current stimulation. Neuroscience 185:161–165. [DOI] [PubMed] [Google Scholar]
- Cooper MS, Keller RE. (1984). Perpendicular orientation and directional migration of amphibian neural crest cells in dc electrical fields. Proc Natl Acad Sci U S A 81:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork RJ, McGinnis ME, Tsai J, Robinson KR. (1994). The growth of PC12 neurites is biased towards the anode of an applied electrical field. J Neurobiol 25:1509–1516. [DOI] [PubMed] [Google Scholar]
- Cormie P, Robinson KR. (2007). Embryonic zebrafish neuronal growth is not affected by an applied electric field in vitro. Neurosci Lett 411:128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Osso B, Zanoni S, Ferrucci R, Vergari M, Castellano F, D’Urso N, Dobrea C, Benatti B, Arici C, Priori A, Altamura AC. (2012). Transcranial direct current stimulation for the outpatient treatment of poor-responder depressed patients. Eur Psychiatry 27:513–517. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. (2007). Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 8:101–113. [DOI] [PubMed] [Google Scholar]
- Dockery CA, Liebetanz D, Birbaumer N, Malinowska M, Wesierska MJ. (2011). Cumulative benefits of frontal transcranial direct current stimulation on visuospatial working memory training and skill learning in rats. Neurobiol Learn Mem 96:452–460. [DOI] [PubMed] [Google Scholar]
- Erskine L, Stewart R, McCaig CD. (1995). Electric field-directed growth and branching of cultured frog nerves: effects of aminoglycosides and polycations. J Neurobiol 26:523–536. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, Hone-Blanchet A, Fregni F, Boggio P, Ciraulo D, Pascual-Leone A. (2014). Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend 140:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JF, Liu J, Zhang XZ, Zhang L, Jiang JY, Nolta J, Zhao M. (2012). Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 30:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci R, Vergari M, Cogiamanian F, Bocci T, Ciocca M, Tomasini E, De Riz M, Scarpini E, Priori A. (2014). Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. NeuroRehabilitation 34:121–127. [DOI] [PubMed] [Google Scholar]
- Franke K, Gruler H. (1990). Galvanotaxis of human granulocytes: electric field jump studies. Eur Biophys J 18:335–346. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. (2007). Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol 3:383–393. [DOI] [PubMed] [Google Scholar]
- Frey U, Frey S, Schollmeier F, Krug M. (1996). Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol 490 (Pt 3):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersner R, Kravetz E, Feil J, Pell G, Zangen A. (2011). Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci 31:7521–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Peng L, Hertz L. (1997). Effects of a low-voltage static electric field on energy metabolism in astrocytes. Bioelectromagnetics 18:77–80. [DOI] [PubMed] [Google Scholar]
- Hubbard JI, Willis WD. (1962). Hyperpolarization of mammalian motor nerve terminals. J Physiol 163:115–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MA, Coffman BA, Trumbo MC, Clark VP. (2013). Tracking the neuroplastic changes associated with transcranial direct current stimulation: a push for multimodal imaging. Front Hum Neurosci 7:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im CH, Park JH, Shim M, Chang WH, Kim YH. (2012). Evaluation of local electric fields generated by transcranial direct current stimulation with an extracephalic reference electrode based on realistic 3D body modeling. Phys Med Biol 57:2137–2150. [DOI] [PubMed] [Google Scholar]
- Ingvar S. (1920). Reaction of cells to the galvanic current in tissue cultures. Exp Biol Med 17:198–199. [Google Scholar]
- Jennings J, Chen D, Feldman D. (2008). Transcriptional response of dermal fibroblasts in direct current electric fields. Bioelectromagnetics 29:394–405. [DOI] [PubMed] [Google Scholar]
- Jiang T, Xu RX, Zhang AW, Di W, Xiao ZJ, Miao JY, Luo N, Fang YN. (2012). Effects of transcranial direct current stimulation on hemichannel pannexin-1 and neural plasticity in rat model of cerebral infarction. Neuroscience 226:421–426. [DOI] [PubMed] [Google Scholar]
- Kabakov AY, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. (2012). Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus. J Neurophysiol 107:1881–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamida T, Kong S, Eshima N, Abe T, Fujiki M, Kobayashi H. (2011). Transcranial direct current stimulation decreases convulsions and spatial memory deficits following pilocarpine-induced status epilepticus in immature rats. Behav Brain Res 217:99–103. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Clements J, Jonas P, Stuart GJ. (2004). Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity. J Physiol 556:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim BK, Ko YJ, Bang MS, Kim MH, Han TR. (2010). Functional and histologic changes after repeated transcranial direct current stimulation in rat stroke model. J Korean Med Sci 25:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes AN, Seggio AM, Thompson DM. (2011). Neurite outgrowth is significantly increased by the simultaneous presentation of Schwann cells and moderate exogenous electric fields. J Neural Eng 8:046023. [DOI] [PubMed] [Google Scholar]
- Lamy JC, Boakye M. (2013). BDNF Val66Met polymorphism alters spinal DC stimulation-induced plasticity in humans. J Neurophysiol 110:109–116. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. (2005). How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci 22:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laste G, Caumo W, Adachi LN, Rozisky JR, de Macedo IC, Filho PR, Partata WA, Fregni F, Torres IL. (2012). After-effects of consecutive sessions of transcranial direct current stimulation (tDCS) in a rat model of chronic inflammation. Exp Brain Res 221:75–83. [DOI] [PubMed] [Google Scholar]
- Li L, El-Hayek YH, Liu B, Chen Y, Gomez E, Wu X, Ning K, Li L, Chang N, Zhang L, Wang Z, Hu X, Wan Q. (2008). Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells 26:2193–2200. [DOI] [PubMed] [Google Scholar]
- Li J, Nandagopal S, Wu D, Romanuik SF, Paul K, Thomson DJ, Lin F. (2011a) Activated T lymphocytes migrate toward the cathode of DC electric fields in microfluidic devices. Lab Chip 11:1298–1304. [DOI] [PubMed] [Google Scholar]
- Li Y, Tian X, Qian L, Yu X, Jiang W. (2011b) Anodal transcranial direct current stimulation relieves the unilateral bias of a rat model of Parkinson’s disease. Conf Proc IEEE Eng Med Biol Soc 2011:765–768. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125:2238–2247. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Fregni F, Monte-Silva KK, Oliveira MB, Amancio-dos-Santos A, Nitsche MA, Guedes RC. (2006a) After-effects of transcranial direct current stimulation (tDCS) on cortical spreading depression. Neurosci Lett 398:85–90. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Klinker F, Hering D, Koch R, Nitsche MA, Potschka H, Loscher W, Paulus W, Tergau F. (2006b) Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia 47:1216–1224. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA. (2009). Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol 120:1161–1167. [DOI] [PubMed] [Google Scholar]
- Lin SL, Li Y, Woolley AT, Lee ML, Tolley HD, Warnick KF. (2008). Programmed elution and peak profiles in electric field gradient focusing. Electrophoresis 29:1058–1066. [DOI] [PubMed] [Google Scholar]
- Long H, Yang G, Wang Z. (2011). Galvanotactic migration of EA.Hy926 endothelial cells in a novel designed electric field bioreactor. Cell Biochem Biophys 61:481–491. [DOI] [PubMed] [Google Scholar]
- Marquez-Ruiz J, Leal-Campanario R, Sanchez-Campusano R, Molaee-Ardekani B, Wendling F, Miranda PC, Ruffini G, Gruart A, Delgado-Garcia JM. (2012). Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc Natl Acad Sci U S A 109:6710–6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig CD, Sangster L, Stewart R. (2000). Neurotrophins enhance electric field-directed growth cone guidance and directed nerve branching. Dev Dyn 217:299–308. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. (2005). Controlling cell behavior electrically: current views and future potential. Physiol Rev 85:943–978. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Poo MM. (1981). The role of electro-osmosis in the electric-field-induced movement of charged macromolecules on the surfaces of cells. Biophys J 34:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Arocena M, Penninger J, Gage FH, Zhao M, Song B. (2011). PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp Neurol 227:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L. (2009). TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10:850–860. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA. (2013). Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 6:424–432. [DOI] [PubMed] [Google Scholar]
- Mycielska ME, Djamgoz MB. (2004). Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J Cell Sci 117:1631–1639. [DOI] [PubMed] [Google Scholar]
- Neal AP, Guilarte TR. (2010). Molecular neurobiology of lead (Pb(2+)): effects on synaptic function. Mol Neurobiol 42:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57:1899–1901. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. (2006). Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci 23:1651–1657. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimul 1:206–223. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Kuo MF, Karrasch R, Wachter B, Liebetanz D, Paulus W. (2009). Serotonin affects transcranial direct current-induced neuroplasticity in humans. Biol Psychiatry 66:503–508. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Muller-Dahlhaus F, Paulus W, Ziemann U. (2012). The pharmacology of neuroplasticity induced by non-invasive brain stimulation: building models for the clinical use of CNS active drugs. J Physiol. 590:4641–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. (2010). CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67:239–252. [DOI] [PubMed] [Google Scholar]
- Orida N, Feldman JD. (1982). Directional protrusive pseudopodial activity and motility in macrophages induced by extracellular electric fields. Cell Motil 2:243–255. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Messerli MA, Robinson KR. (2000). Neuronal galvanotropism is independent of external Ca(2+) entry or internal Ca(2+) gradients. J Neurobiol 45:30–38. [DOI] [PubMed] [Google Scholar]
- Pan L, Borgens RB. (2010). Perpendicular organization of sympathetic neurons within a required physiological voltage. Exp Neurol 222:161–164. [DOI] [PubMed] [Google Scholar]
- Patel N, Poo MM. (1982). Orientation of neurite growth by extracellular electric fields. J Neurosci 2:483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedron S, Monnin J, Haffen E, Sechter D, Van Waes V. (2014). Repeated transcranial direct current stimulation prevents abnormal behaviors associated with abstinence from chronic nicotine consumption. Neuropsychopharmacology 39:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier SJ, Lagace M, St-Amour I, Arsenault D, Cisbani G, Chabrat A, Fecteau S, Levesque M, Cicchetti F (2014). The morphological and molecular changes of brain cells exposed to direct current electric field stimulation. Int J Neuropsychopharmacol in press. [DOI] [PMC free article] [PubMed]
- Perret S, Cantereau A, Audin J, Dufy B, Georgescauld D. (1999). Interplay between Ca2+ release and Ca2+ influx underlies localized hyperpolarization-induced [Ca2+]i waves in prostatic cells. Cell Calcium 25:297–311. [DOI] [PubMed] [Google Scholar]
- Peruzzotti-Jametti L, Cambiaghi M, Bacigaluppi M, Gallizioli M, Gaude E, Mari S, Sandrone S, Cursi M, Teneud L, Comi G, Musco G, Martino G, Leocani L. (2013). Safety and efficacy of transcranial direct current stimulation in acute experimental ischemic stroke. Stroke 44:3166–3174. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. (1999). Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci 19:4972–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. (1965). Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 28:166–185. [DOI] [PubMed] [Google Scholar]
- Radman T, Ramos RL, Brumberg JC, Bikson M. (2009). Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul 2:215–228, 228 e211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M. (2013). Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 591:2563–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajnicek AM, Foubister LE, McCaig CD. (2006). Temporally and spatially coordinated roles for Rho, Rac, Cdc42 and their effectors in growth cone guidance by a physiological electric field. J Cell Sci 119:1723–1735. [DOI] [PubMed] [Google Scholar]
- Ranieri F, Podda MV, Riccardi E, Frisullo G, Dileone M, Profice P, Pilato F, Di Lazzaro V, Grassi C. (2012). Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol 107:1868–1880. [DOI] [PubMed] [Google Scholar]
- Rueger MA, Keuters MH, Walberer M, Braun R, Klein R, Sparing R, Fink GR, Graf R, Schroeter M. (2012). Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS One 7:e43776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweid L, Rushmore RJ, Valero-Cabre A. (2008). Cathodal transcranial direct current stimulation on posterior parietal cortex disrupts visuo-spatial processing in the contralateral visual field. Exp Brain Res 186:409–417. [DOI] [PubMed] [Google Scholar]
- Smolen P. (2007). A model of late long-term potentiation simulates aspects of memory maintenance. PLoS One 2:e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MK, Jee SJ, Kim YW. (2013). Effect of transcranial direct current stimulation on postural stability and lower extremity strength in hemiplegic stroke patients. Ann Rehabil Med 37:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Gu Y, Pu J, Reid B, Zhao Z, Zhao M. (2007). Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat Protoc 2:1479–1489. [DOI] [PubMed] [Google Scholar]
- Spezia Adachi LN, Caumo W, Laste G, Fernandes Medeiros L, Ripoll Rozisky J, de Souza A, Fregni F, Torres IL. (2012). Reversal of chronic stress-induced pain by transcranial direct current stimulation (tDCS) in an animal model. Brain Res 1489:17–26. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. (2011). Physiological basis of transcranial direct current stimulation. Neuroscientist 17:37–53. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, O’Shea J, Kincses ZT, Woolrich M, Matthews PM, Johansen-Berg H. (2009a) Modulation of movement-associated cortical activation by transcranial direct current stimulation. Eur J Neurosci 30:1412–1423. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. (2009b) Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29:5202–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahtis V, Kaski D, Seemungal BM. (2014). The effect of single session bi-cephalic transcranial direct current stimulation on gait performance in sub-acute stroke: a pilot study. Restor Neurol Neurosci, 10.3233/RNN-140393. [DOI] [PubMed] [Google Scholar]
- Takano Y, Yokawa T, Masuda A, Niimi J, Tanaka S, Hironaka N. (2011). A rat model for measuring the effectiveness of transcranial direct current stimulation using fMRI. Neurosci Lett 491:40–43. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Takano Y, Tanaka S, Hironaka N, Kobayashi K, Hanakawa T, Watanabe K, Honda M. (2013). Transcranial direct-current stimulation increases extracellular dopamine levels in the rat striatum. Front Syst Neurosci 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard P, Butcher AJ, Halet G, Davies A, Nurnberg B, Heblich F, Dolphin AC. (2004). PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci 7:939–946. [DOI] [PubMed] [Google Scholar]
- Wachter D, Wrede A, Schulz-Schaeffer W, Taghizadeh-Waghefi A, Nitsche MA, Kutschenko A, Rohde V, Liebetanz D. (2011). Transcranial direct current stimulation induces polarity-specific changes of cortical blood perfusion in the rat. Exp Neurol 227:322–327. [DOI] [PubMed] [Google Scholar]
- Wood MD, Willits RK. (2009). Applied electric field enhances DRG neurite growth: influence of stimulation media, surface coating and growth supplements. J Neural Eng 6:046003. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Oh BM, Kim DY. (2012). Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Res 1452:61–72. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liu ZT, He GX, Liu JP, Feng J. (2011). Low-voltage direct-current stimulation is safe and promotes angiogenesis in rabbits with myocardial infarction. Cell Biochem Biophys 59:19–27. [DOI] [PubMed] [Google Scholar]
- Zhao M. (2009). Electrical fields in wound healing: an overriding signal that directs cell migration. Semin Cell Dev Biol 20:674–682. [DOI] [PubMed] [Google Scholar]
- Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. (2004). Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci 117:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. (2006). Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 442:457–460. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Qin L, Reid B, Pu J, Hara T, Zhao M. (2012). Directing migration of endothelial progenitor cells with applied DC electric fields. Stem Cell Res 8:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobeiri M, van Luijtelaar G. (2013). Noninvasive transcranial direct current stimulation in a genetic absence model. Epilepsy Behav 26:42–50. [DOI] [PubMed] [Google Scholar]