Abstract
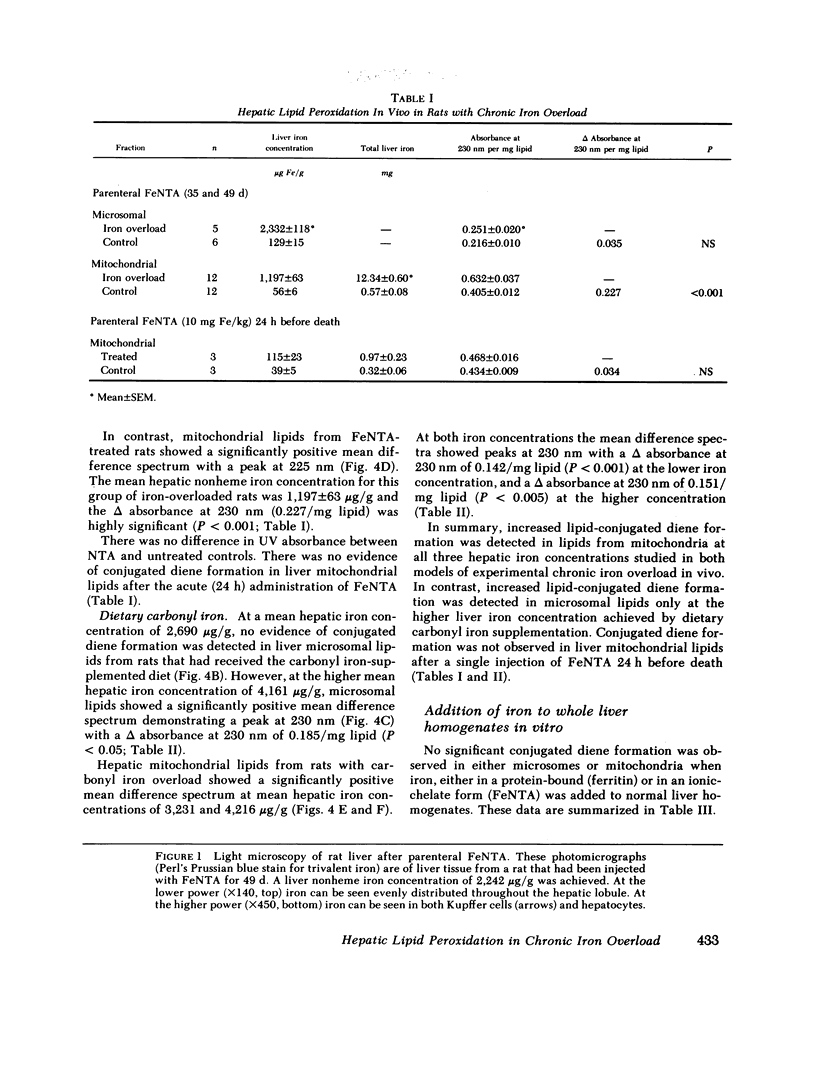
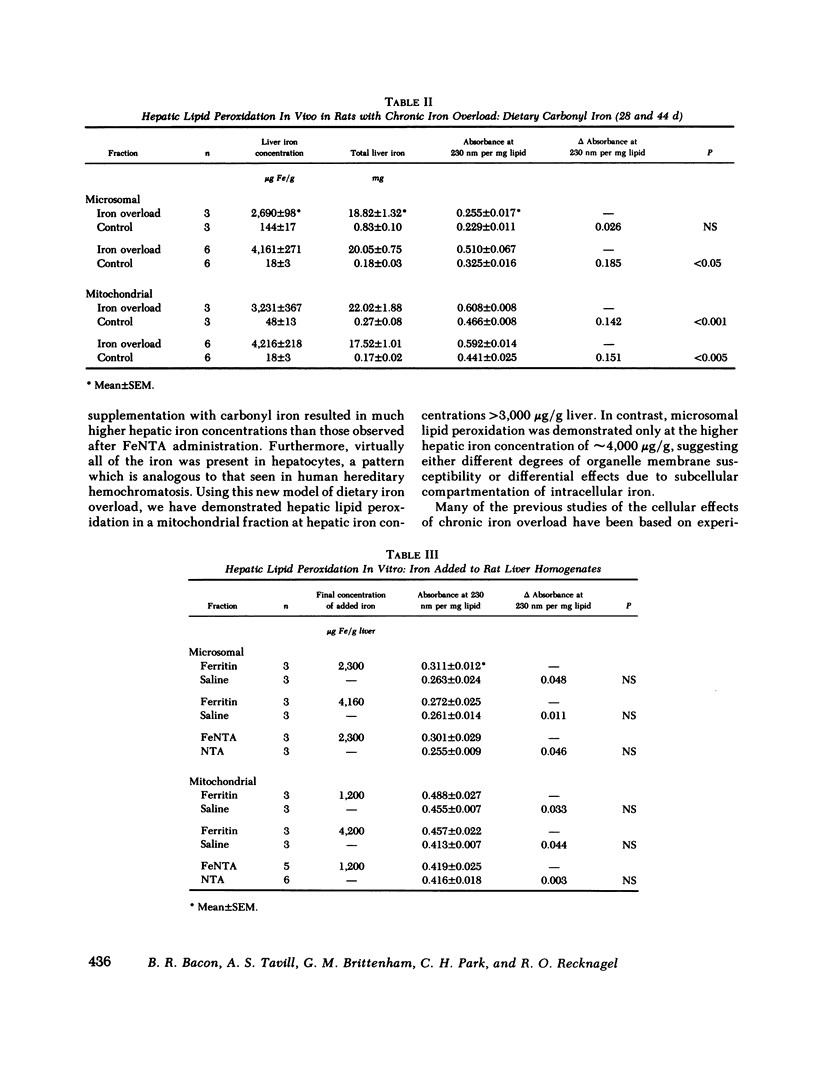
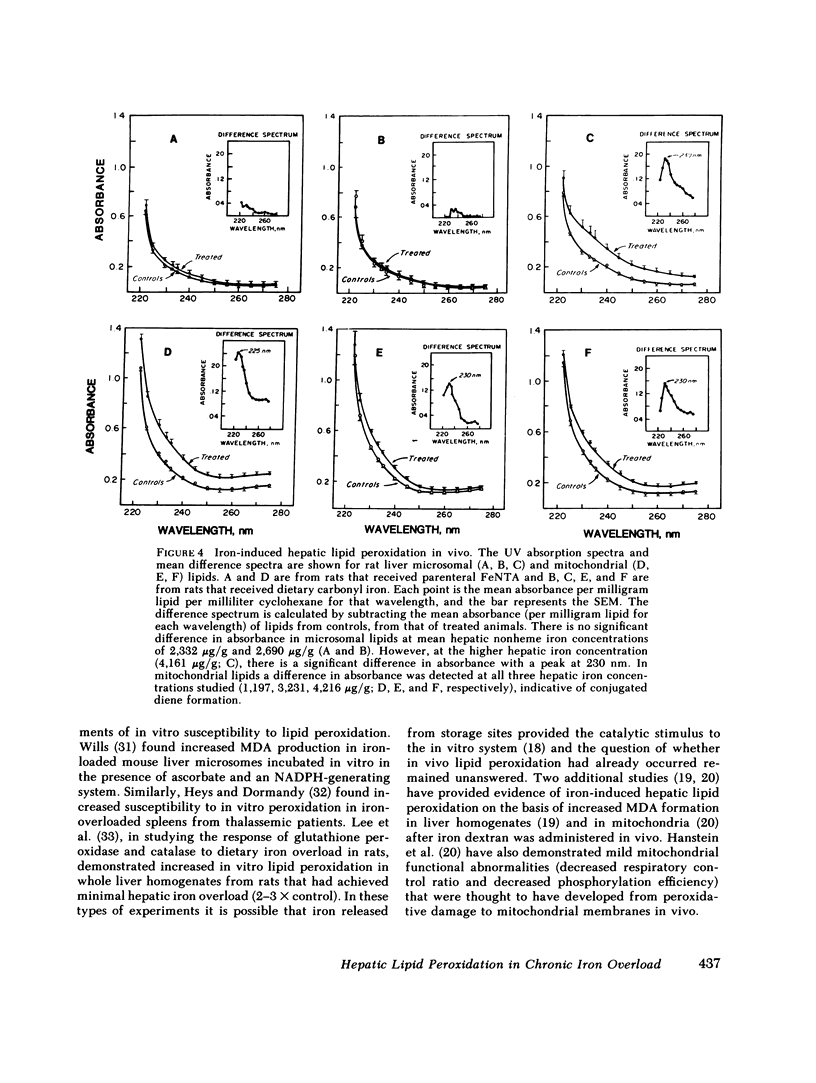
Peroxidative decomposition of cellular membrane lipids is a postulated mechanism of hepatocellular injury in parenchymal iron overload. In the present study, we looked for direct evidence of lipid peroxidation in vivo (as measured by lipid-conjugated diene formation in hepatic organelle membranes) from rats with experimental chronic iron overload. Both parenteral ferric nitrilotriacetate (FeNTA) administration and dietary supplementation with carbonyl iron were used to produce chronic iron overload. Biochemical and histologic evaluation of liver tissue confirmed moderate increases in hepatic storage iron. FeNTA administration produced excessive iron deposition throughout the hepatic lobule in both hepatocytes and Kupffer cells, whereas dietary carbonyl iron supplementation produced greater hepatic iron overload in a periportal distribution with iron deposition predominantly in hepatocytes. Evidence for mitochondrial lipid peroxidation in vivo was demonstrated at all three mean hepatic iron concentrations studied (1,197, 3,231, and 4,216 micrograms Fe/g) in both models of experimental chronic iron overload. In contrast, increased conjugated diene formation was detected in microsomal lipids only at the higher liver iron concentration (4,161 micrograms Fe/g) achieved by dietary carbonyl iron supplementation. When iron as either FeNTA or ferritin was added in vitro to normal liver homogenates before lipid extraction, no conjugated diene formation was observed. We conclude that the presence of conjugated dienes in the subcellular fractions of rat liver provide direct evidence of iron-induced hepatic mitochondrial and microsomal lipid peroxidation in vivo in two models of experimental chronic iron overload.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barry M., Flynn D. M., Letsky E. A., Risdon R. A. Long-term chelation therapy in thalassaemia major: effect on liver iron concentration, liver histology, and clinical progress. Br Med J. 1974 Apr 6;2(5909):16–20. doi: 10.1136/bmj.2.5909.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford A., Williams R. Long term results of venesection therapy in idiopathic haemochromatosis. Q J Med. 1976 Oct;45(180):611–623. [PubMed] [Google Scholar]
- Bonkowsky H. L., Healey J. F., Sinclair P. R., Sinclair J. F., Pomeroy J. S. Iron and the liver. Acute and long-term effects of iron-loading on hepatic haem metabolism. Biochem J. 1981 Apr 15;196(1):57–64. doi: 10.1042/bj1960057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Volatile hydrocarbon and carbonyl products of lipid peroxidation: a comparison of pentane, ethane, hexanal, and acetone as in vivo indices. Lipids. 1979 Dec;14(12):989–995. doi: 10.1007/BF02533435. [DOI] [PubMed] [Google Scholar]
- Dougherty J. J., Croft W. A., Hoekstra W. G. Effects of ferrous chloride and iron dextran on lipid peroxidation in vivo in vitamin E and selenium adequate and deficient rats. J Nutr. 1981 Oct;111(10):1784–1796. doi: 10.1093/jn/111.10.1784. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- Frank H., Hintze T., Bimboes D., Remmer H. Monitoring lipid peroxidation by breath analysis: endogenous hydrocarbons and their metabolic elimination. Toxicol Appl Pharmacol. 1980 Dec;56(3):337–344. doi: 10.1016/0041-008x(80)90066-6. [DOI] [PubMed] [Google Scholar]
- GOLBERG L., MARTIN L. E., BATCHELOR A. Biochemical changes in the tissues of animals injected with iron. 3. Lipid peroxidation. Biochem J. 1962 May;83:291–298. doi: 10.1042/bj0830291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, GEBICKI J. M., HOFFSTEN P. E., WEINSTEIN J., SCOTT A. Swelling and lysis of rat liver mitochondria induced by ferrous ions. J Biol Chem. 1963 Feb;238:828–835. [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts is a feasible source of hydroxy radicals in vivo. Biochem J. 1982 Aug 1;205(2):461–463. doi: 10.1042/bj2050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein W. G., Heitmann T. D., Sandy A., Biesterfeldt H. L., Liem H. H., Muller-Eberhard U. Effects of hexachlorobenzene and iron loading on rat liver mitochondria. Biochim Biophys Acta. 1981 Dec 18;678(3):293–299. doi: 10.1016/0304-4165(81)90106-9. [DOI] [PubMed] [Google Scholar]
- Heys A. D., Dormandy T. L. Lipid peroxidation in iron-overloaded spleens. Clin Sci (Lond) 1981 Mar;60(3):295–301. doi: 10.1042/cs0600295. [DOI] [PubMed] [Google Scholar]
- Hochstein P., Nordenbrand K., Ernster L. Evidence for the involvement of iron in the ADP-activated peroxidation of lipids in microsomes and mitochondria. Biochem Biophys Res Commun. 1964;14:323–328. doi: 10.1016/s0006-291x(64)80004-8. [DOI] [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977 Sep;50(3):433–439. [PubMed] [Google Scholar]
- Kornbrust D. J., Mavis R. D. Microsomal lipid peroxidation. I. Characterization of the role of iron and NADPH. Mol Pharmacol. 1980 May;17(3):400–407. [PubMed] [Google Scholar]
- Lee Y. H., Layman D. K., Bell R. R., Norton H. W. Response of glutathione peroxidase and catalase to excess dietary iron in rats. J Nutr. 1981 Dec;111(12):2195–2202. doi: 10.1093/jn/111.12.2195. [DOI] [PubMed] [Google Scholar]
- MCKNIGHT R. C., HUNTER F. E., Jr, OEHLERT W. H. MITOCHONDRIAL MEMBRANE GHOSTS PRODUCED BY LIPID PEROXIDATION INDUCED BY FERROUS ION. I. PRODUCTION AND GENERAL MORPHOLOGY. J Biol Chem. 1965 Aug;240:3439–3445. [PubMed] [Google Scholar]
- May M. E., Parmley R. T., Spicer S. S., Ravenel D. P., May E. E., Buse M. G. Iron nitrilotriacetate--induced experimental diabetes in rats. J Lab Clin Med. 1980 Apr;95(4):525–535. [PubMed] [Google Scholar]
- McKnight R. C., Hunter F. E., Jr Mitochondrial membrane ghosts produced by lipid peroxidation induced by ferrous ion. II. Composition and enzymatic activity. J Biol Chem. 1966 Jun 25;241(12):2757–2765. [PubMed] [Google Scholar]
- Peters T. J., Seymour C. A. Acid hydrolase activities and lysosomal integrity in liver biopsies from patients with iron overload. Clin Sci Mol Med. 1976 Jan;50(1):75–78. doi: 10.1042/cs0500075. [DOI] [PubMed] [Google Scholar]
- Powell L. W., Bassett M. L., Halliday J. W. Hemochromatosis: 1980 update. Gastroenterology. 1980 Feb;78(2):374–381. [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. Quantitative estimation of peroxidative degeneration of rat liver microsomal and mitochondrial lipids after carbon tetrachloride poisoning. Exp Mol Pathol. 1966 Oct;5(5):413–426. doi: 10.1016/0014-4800(66)90023-2. [DOI] [PubMed] [Google Scholar]
- Selden C., Owen M., Hopkins J. M., Peters T. J. Studies on the concentration and intracellular localization of iron proteins in liver biopsy specimens from patients with iron overload with special reference to their role in lysosomal disruption. Br J Haematol. 1980 Apr;44(4):593–603. doi: 10.1111/j.1365-2141.1980.tb08714.x. [DOI] [PubMed] [Google Scholar]
- Tappel A. L., Dillard C. J. In vivo lipid peroxidation: measurement via exhaled pentane and protection by vitamin E. Fed Proc. 1981 Feb;40(2):174–178. [PubMed] [Google Scholar]
- WILLIAMS R., SCHEUER P. J., SHERLOCK S. The inheritance of idiopathic haemochromatosis. A clinical and liver biopsy study of 16 families. Q J Med. 1962 Jul;31:249–265. [PubMed] [Google Scholar]
- Waller R. L., Recknagel R. O. Determination of lipid conjugated dienes with tetracyanoethylene-14C: significance for study of the pathology of lipid peroxidation. Lipids. 1977 Nov;12(11):914–921. doi: 10.1007/BF02533311. [DOI] [PubMed] [Google Scholar]
- Wills E. D. Effects of iron overload on lipid peroxide formation and oxidative demethylation by the liver endoplasmic reticulum. Biochem Pharmacol. 1972 Jan 15;21(2):239–247. doi: 10.1016/0006-2952(72)90274-2. [DOI] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. General considerations. Biochem J. 1969 Jun;113(2):315–324. doi: 10.1042/bj1130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. Relationship of hydroxylation to lipid peroxide formation. Biochem J. 1969 Jun;113(2):333–341. doi: 10.1042/bj1130333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. The role of non-haem iron. Biochem J. 1969 Jun;113(2):325–332. doi: 10.1042/bj1130325. [DOI] [PMC free article] [PubMed] [Google Scholar]